Abstract
Background:
Platelet activation with subsequent neutrophilic adherence to the vasculature initiates ischemia-reperfusion injury. We hypothesized that higher plasma P-selectin levels reflecting platelet activation would therefore be associated with primary graft dysfunction (PGD) after lung transplantation.
Methods:
In a prospective, multicenter cohort study of 376 patients who had undergone lung transplantation between 2002 and 2007, we measured soluble P-selectin levels before lung transplantation and at 6 and 24 h after lung reperfusion in 20 patients with grade III PGD (Pao2/fraction of inspired oxygen, < 200 mm Hg [with alveolar infiltrates seen on chest radiographs]) at 72 h after transplantation and 61 control subjects without PGD.
Results:
Higher postoperative soluble P-selectin levels were associated with an increased risk of PGD at 72 h after transplantation (odds ratio [OR] per 1 natural log increase in soluble P-selectin at 6 h after lung allograft reperfusion, 3.5; 95% confidence interval [CI], 1.01 to 11.8; p = 0.048) and at 24 h after lung allograft reperfusion (OR, 4.8; 95% CI, 1.4 to 16.1; p = 0.01). Higher preoperative mean pulmonary artery pressure and the use of cardiopulmonary bypass were also associated with an increased risk of PGD.
Conclusion:
Higher postoperative soluble P-selectin levels were associated with an increased risk of PGD at 72 h following lung transplantation.
Primary graft dysfunction (PGD) is a form of acute lung injury (ALI) occurring immediately after lung transplantation due to ischemia-reperfusion injury.1–4 The incidence of PGD ranges from 10 to 25%, and PGD is the leading cause of early posttransplantation morbidity and mortality.1–3,5–7 The risk factors for PGD are varied, involving the donor, recipient, and the processes of cold ischemia and reperfusion of the lung allografts.8,9
Prior studies10–13 have demonstrated that the adhesion of neutrophils to the pulmonary vascular endothelium, diapedesis, and infiltration into the vessel wall are key initial steps in ALI and PGD, leading to the capillary leak that characterizes these syndromes. Activated platelets play an integral role in the tethering and activation of neutrophils with eventual firm adherence to the vascular wall and transit to the interstitial and alveolar spaces. P-selectin is mobilized to the platelet surface from α granules and to the endothelial cell membrane from Weibel-Palade bodies and serves as a receptor to a variety of ligands before being shed in the plasma in soluble form.14 Although soluble P-selectin therefore may be of platelet or endothelial origin, it is considered a reliable and valid measure of platelet activation.15,16 Animal models of ALI show elevations in soluble P-selectin levels,11 suggesting that platelet activation is present. P-selectin may have mechanistic importance in PGD as well because P-selectin blockade (or P-selectin knockout) protected the lung allograft after ischemia and reperfusion in a mouse model.17
We have previously shown18 that soluble P-selectin levels, CD40 ligand levels, and platelet-leukocyte aggregates (the most sensitive measure of platelet activation) increase in parallel after lung transplantation without cardiopulmonary bypass (CPB) compared to nontransplant thoracic surgery, indicating that soluble P-selectin in these patients is most likely shed from platelet sources in response to lung implantation. However, to our knowledge, no previous studies have addressed the relationship between platelet activity and PGD. In the current study, we hypothesized that platelet activation, as measured by increased soluble P-selectin levels, would be associated with a higher risk of PGD in lung transplantation recipients.
Materials and Materials
We performed a case-control study nested in a prospective cohort of 376 patients undergoing a first lung transplantation at seven centers in the United States (see Appendix for institutions and investigators). The study sample consisted of 20 randomly selected case patients with PGD grade III and 61 control subjects without PGD (PGD grade 0) enrolled between 2002 and 2007 with sufficient plasma. PGD case patients had diffuse alveolar infiltrates involving the lung allografts and, in the case of a single lung transplant, sparing the native lung, as seen on chest radiographs; Pao2/fraction of inspired oxygen (Fio2) ratio of < 200 mm Hg; and no other secondary cause of graft dysfunction identified, all at 72 h after transplantation. This definition has been validated previously.1,2,4,7 Control subjects were patients from the cohort with clear lung allografts as determined by chest radiography (PGD grade 0) at 72 h after transplantation (see online supplement for details).
Informed consent for this study was obtained prior to organ transplantation. Blood samples were obtained in citrated tubes (Vacutainer; Becton Dickinson; Franklin Lakes, NJ) before transplantation, and at 6 h and 24 h after reperfusion of the lung allograft. Samples were centrifuged within 30 min of collection, and plasma samples were stored at −80°C. Clinical variables were categorized and defined using methods published previously.3 Soluble P-selectin levels were measured in a central laboratory with an ultrasensitive, solid-phase sandwich, enzyme-linked immunosorbent assay (R&D Systems; Minneapolis, MN) [see online supplement for details].
Statistical Analysis
Data were summarized as the mean ± SD and median (interquartile range [IQR]), as appropriate. Unpaired t tests, rank sum tests, signed rank tests, and χ2 or Fisher exact tests were used to compare PGD case patients with non-PGD control subjects. Correlations were assessed using Spearman rank correlation coefficients.
Bivariate and multivariate logistic regression models were used to assess the relationship between soluble P-selectin levels at baseline, 6 h, and 24 h postoperatively and the occurrence of PGD at 72 h after lung transplantation. Included in the multivariate analysis were potential confounding variables with p values < 0.20 in bivariate analyses. Potential confounders were included in the models one at a time; significant confounding was indicated by a change in the coefficient of the term for soluble P-selectin > 20%. Mean pulmonary artery pressure (mPAP) at anesthesia induction was considered in quartiles with indicator variables in the regression models; missing data were coded as such in this analysis. Soluble P-selectin levels were log transformed for the regression analysis. Power calculations are provided in the online supplement.
All statistical comparisons were performed using a statistical software package (Stata, version 10.0; Stata Corp; College Station, TX). This research protocol was approved by the institutional review boards at each of the participating centers.
Results
There were 81 patients in the study sample (Table 1). The mean age of the patients was 53 ± 12 years, 33 patients (41%) were women, and 72 patients (89%) were non-Hispanic white. Most patients had undergone lung transplantation for COPD, and 25 patients (31%) received CPB.
Table 1.
Characteristics of the Study Sample
| Characteristics | No. | Data |
|---|---|---|
| Recipient variables | ||
| Age, yr | 81 | 53 ± 12 |
| Female sex | 81 | 33 (41) |
| Race/ethnicity | 81 | |
| Non-Hispanic white | 72 (89) | |
| Black | 8 (10) | |
| Hispanic | 1 (1) | |
| Other | 1 (1) | |
| Diagnosis | 81 | |
| COPD | 37 (46) | |
| Diffuse parenchymal lung disease | 28 (35) | |
| Cystic fibrosis | 10 (12) | |
| Other | 6 (7) | |
| Donor variables | ||
| Age, yr | 81 | 31 ± 14 |
| Female sex | 81 | 34 (42) |
| Race/ethnicity | 81 | |
| Non-Hispanic white | 61 (75) | |
| Black | 8 (10) | |
| Hispanic | 10 (12) | |
| Other | 2 (2) | |
| Surgical variables | ||
| Bilateral transplant | 81 | 50 (62) |
| Use of CPB | 81 | 25 (31) |
| mPAP at induction, mm Hg | 61 | 29 ± 12 |
| Ischemic time, min | 77 | 293 (240–352) |
| Packed RBCs during first 24 h, mL | 81 | 250 (0–1,000) |
| Platelets during first 24 h, mL | 81 | 0 (0–0) |
| Fresh frozen plasma during first 24 h, mL | 81 | 0 (0–450) |
Values are given as the mean ± SD, No. (%), or median (IQR).
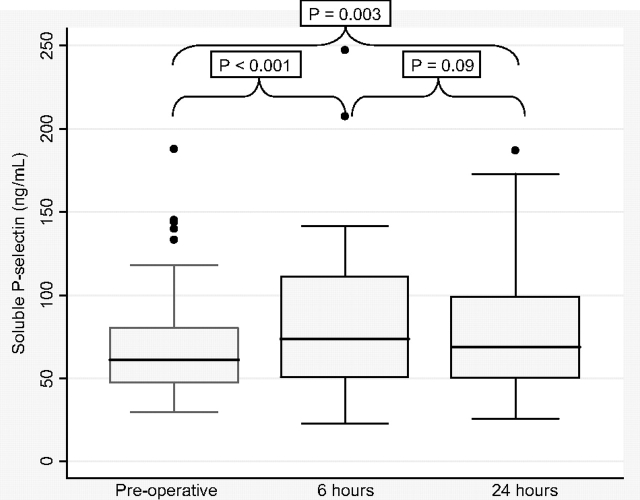
Soluble P-selectin levels before lung transplantation and at 6 and 24 h after reperfusion of the lung allografts are shown in Figure 1. One patient had a missing preoperative P-selectin level, two had missing levels at 6 h, and one had a missing level at 24 h. In the entire study sample, median soluble P-selectin levels significantly increased from baseline (61 ng/mL [IQR, 47 to 80 ng/mL]) to 6 h (74 ng/mL [IQR, 50 to 111 ng/mL], p < 0.001 vs baseline) and to 24 h (69 ng/mL [IQR, 50 to 99 ng/mL], p = 0.003 vs baseline) after reperfusion.
Figure 1.
Box-and-whisker plots of soluble P-selectin levels preoperatively and at 6 and 24 h after lung allograft reperfusion. Horizontal line = median; box = 25th and 75th percentiles; whiskers = adjacent values.
Among those PGD and non-PGD patients who did not receive CPB during the transplantation procedure (n = 56), there was still a significant median increase in soluble P-selectin levels from baseline (60 ng/mL [IQR, 46 to 76 ng/mL]) to 6 h (69 ng/mL [IQR, 45 to 104 ng/mL], p = 0.004 vs baseline) and possibly to 24 h (66 ng/mL [IQR, 45 to 95 ng/mL], p = 0.09 vs baseline) after reperfusion. Focusing only on the non-PGD control group (with or without CPB) [n = 61], we again found that median soluble P-selectin levels increased from baseline (60 ng/mL [IQR, 46 to 79 ng/mL]) to 6 h (70 ng/mL [IQR, 46 to 109 ng/mL], p = 0.002 vs baseline) and possibly to 24 h (63 ng/mL [IQR, 45 to 97 ng/mL], p = 0.07 vs baseline) after reperfusion.
Patients with diffuse parenchymal lung disease in the entire study sample may have had higher median soluble P-selectin levels before transplantation than patients with COPD (64 ng/mL [IQR, 59 to 87 ng/mL] vs 56 ng/mL [IQR, 38 to 69 ng/mL], respectively; p = 0.06) Although mPAP at anesthesia induction for lung transplantation was not associated with soluble P-selectin levels at baseline in the entire study sample (r = 0.02; p = 0.93), there were significant direct associations between mPAP at anesthesia induction and soluble P-selectin levels at 6 and 24 h after lung transplantation in the entire study sample and in those who did not receive CPB (Fig 2, 3). In those patients who received CPB, there were no associations between CPB time and soluble P-selectin levels at any time point (data not shown).
Figure 2.
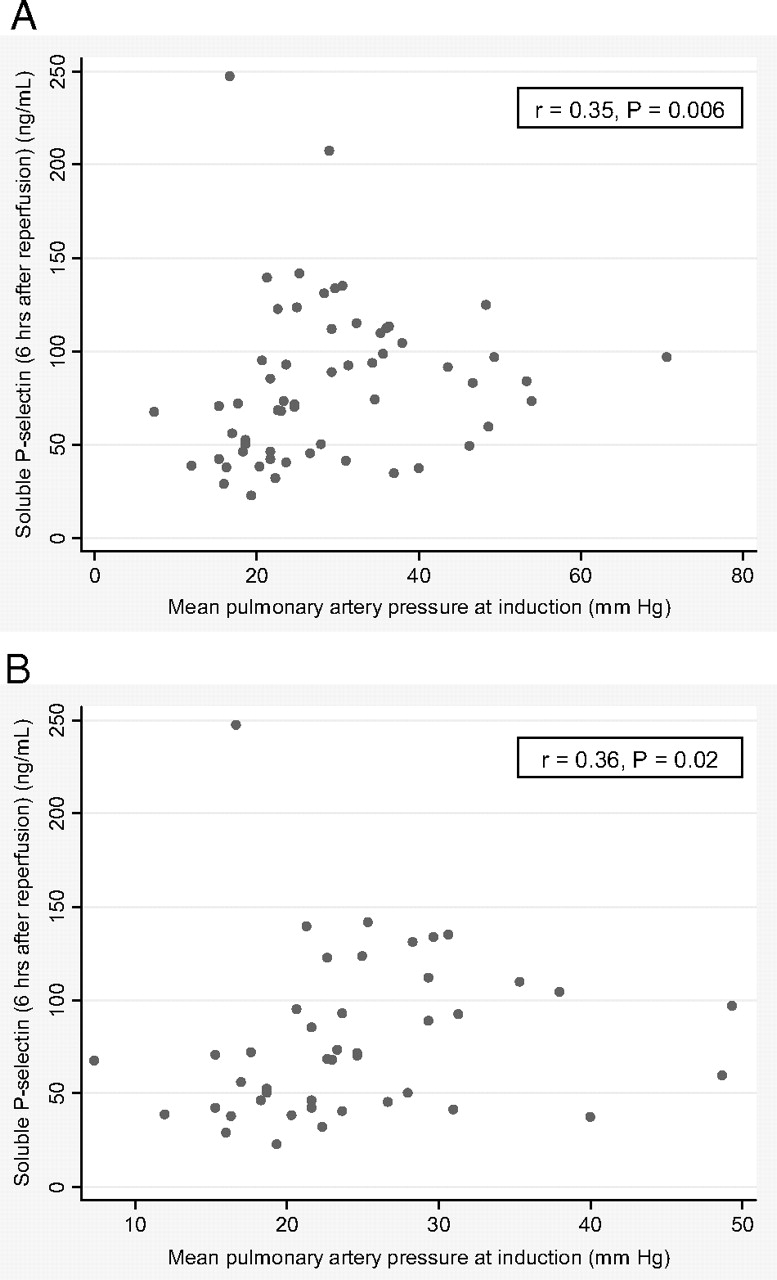
Scatterplots of soluble P-selectin levels at 6 h after lung allograft reperfusion vs mPAP at induction. A: all patients (n = 59). B: patients not receiving CPB (n = 44).
Figure 3.
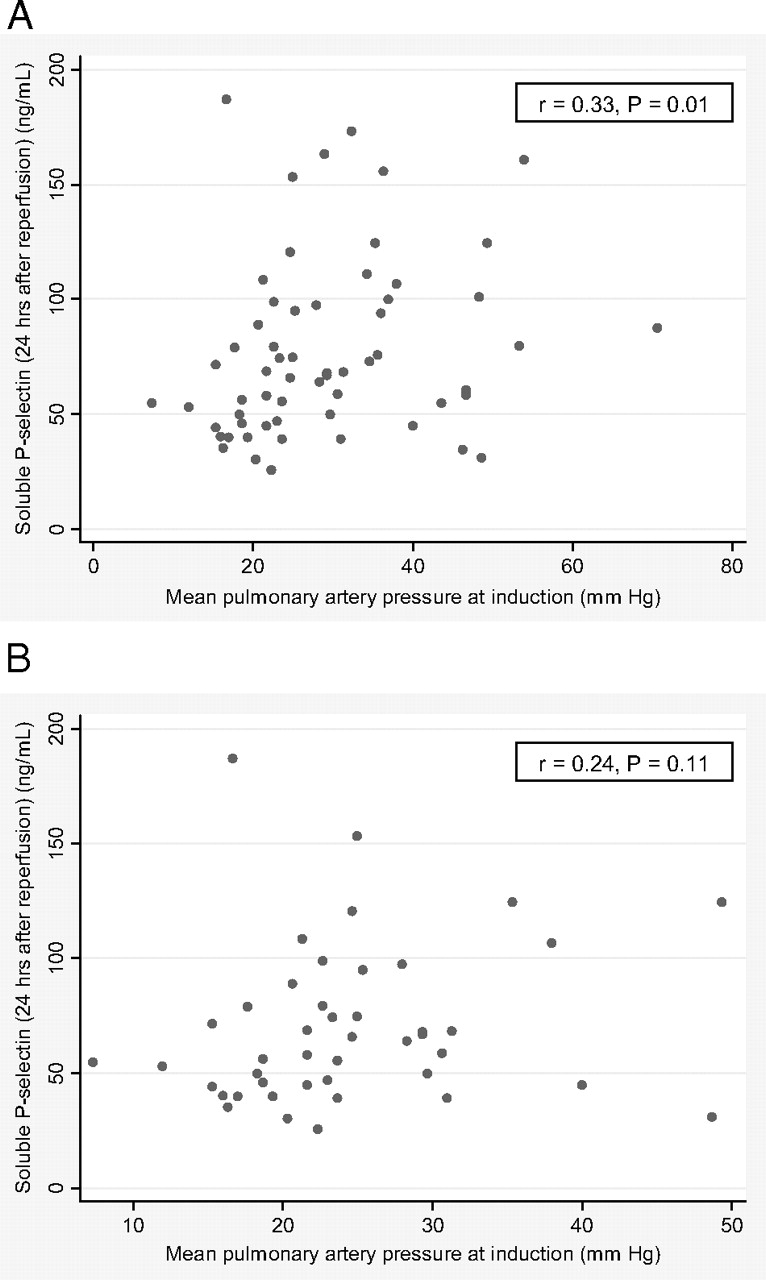
Scatterplots of soluble P-selectin levels at 24 h after lung allograft reperfusion vs mPAP at induction. A: all patients (n = 60). B: patients not receiving CPB (n = 44).
We then compared PGD case patients to non-PGD control subjects (Table 2). PGD case patients tended to be younger than control subjects; however, there were no sex differences between the groups. PGD case patients were less likely to be non-Hispanic white and more likely to be black than control subjects. Case patients with PGD were more likely to have undergone transplantation for diffuse parenchymal lung disease than for COPD. PGD case patients had higher mPAP levels at induction, had more frequently used CPB, and had required transfusion of more blood products during transplantation and during the first 24 h postoperatively.
Table 2.
Characteristics of PGD Case Patients and Control Subjects
| Characteristics | No. | Case Patients (n = 20) | Control Subjects (n = 61) | p Value |
|---|---|---|---|---|
| Recipient variables | ||||
| Age, yr | 81 | 49 ± 14 | 54 ± 12 | 0.18 |
| Female sex | 81 | 8 (40) | 25 (42) | 0.94 |
| Race/ethnicity | 81 | 0.02 | ||
| Non-Hispanic white | 15 (75) | 57 (93) | ||
| Black | 5 (25) | 3 (5) | ||
| Hispanic | 1 (2) | |||
| Diagnosis | 81 | 0.03 | ||
| COPD | 5 (25) | 32 (52) | ||
| Diffuse parenchymal lung disease | 12 (60) | 16 (26) | ||
| Cystic fibrosis | 1 (5) | 9 (15) | ||
| Other | 2 (10) | 4 (7) | ||
| Donor variables | ||||
| Age, yr | 81 | 30 ± 13 | 32 ± 14 | 0.69 |
| Female sex | 81 | 8 (40) | 25 (41) | 0.94 |
| Race/ethnicity | 81 | 0.30 | ||
| Non-Hispanic white | 18 (90) | 43 (71) | ||
| Black | 8 (13) | |||
| Hispanic | 2 (10) | 8 (13) | ||
| Other | 2 (3) | |||
| Surgical variables | ||||
| Bilateral transplant | 81 | 13 (65) | 37 (61) | 0.73 |
| Use of CPB | 81 | 14 (70) | 11 (18) | < 0.001 |
| mPAP at induction, mm Hg | 61 | 40 ± 14 | 25 ± 9 | < 0.001 |
| Ischemic time, min | 77 | 286 (257–375) | 300 (235–352) | 0.50 |
| Packed RBCs during first 24 h, mL | 81 | 875 (0–1,500) | 250 (0–750) | 0.05 |
| Platelets during first 24 h, mL | 81 | 0 (0–622) | 0 (0–0) | < 0.001 |
| Fresh frozen plasma during first 24 h, mL | 81 | 363 (0–1,053) | 0 (0–0) | 0.002 |
Values are given as the mean ± SD, No. (%), or median (IQR), unless otherwise indicated.
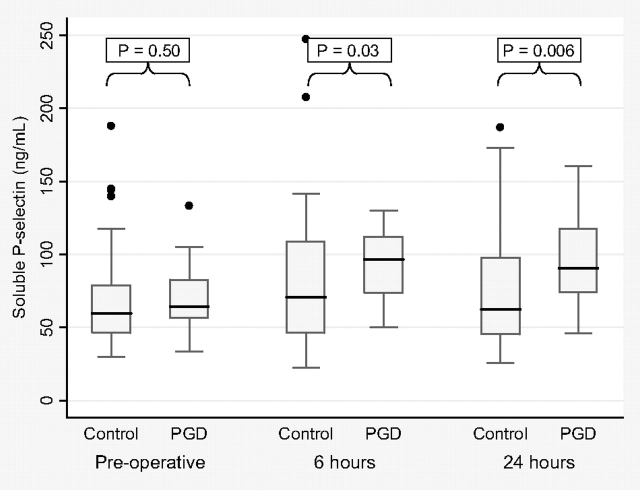
Case patients with PGD had median soluble P-selectin levels at baseline that were similar to those of control subjects without PGD (64 ng/mL [IQR, 56 to 83 ng/mL] vs 60 ng/mL [IQR, 46 to 79 ng/mL], respectively; p = 0.50) [Fig 4]. However, case patients with PGD had higher median soluble P-selectin levels than non-PGD control subjects at 6 h (97 ng/mL [IQR, 73 to 112 ng/mL] vs 70 ng/mL [IQR, 46 to 109 ng/mL], respectively; p = 0.03) and at 24 h (91 ng/mL [IQR, 74 to 118 ng/mL] vs 63 ng/mL [IQR, 45 to 97 ng/mL], respectively; p = 0.006) after lung reperfusion.
Figure 4.
Box-and-whisker plots of soluble P-selectin levels for PGD case patients and control subjects preoperatively and at 6 and 24 h after lung allograft reperfusion. Horizontal line = median; box = 25th and 75th percentiles; whiskers = adjacent values.
Higher soluble P-selectin levels at 6 h after lung allograft reperfusion were associated with an increased risk of PGD (odds ratio [OR] per 1 natural log increase in soluble P-selectin level, 3.5; 95% confidence interval [CI], 1.01 to 11.8; p = 0.048), as they were at 24 h after lung reperfusion (OR per 1 natural log increase in soluble P-selectin level, 4.8; 95% CI, 1.4 to 16.1; p = 0.01) [Table 3]. Adjustment for a variety of variables, including sex, race, or pulmonary diagnosis, did not affect the results. Similarly, there was no change in the findings after adjustment for the use of blood products, such as packed RBCs, platelets, or fresh frozen plasma. The associations between soluble P-selectin levels and PGD case status seemed to be explained in part by the use of CPB because adjustment for this factor significantly reduced the effect estimates of soluble P-selectin levels.
Table 3.
Logistic Regression Models for the Association of P-selectin Levels at 6 and 24 h With PGD
| Variables | OR for PGD per 1 ln Increase in P-selectin at 6 h After Reperfusion | 95% CI | p Value | OR for PGD per 1 ln Increase in P-selectin at 24 h After Reperfusion | 95% CI | p Value |
|---|---|---|---|---|---|---|
| Unadjusted | 3.5 | 1.01–11.8 | 0.048 | 4.8 | 1.4–16.1 | 0.01 |
| Adjusted for | ||||||
| Recipient age | 2.9 | 0.8–10.9 | 0.11 | 4.4 | 1.2–16.0 | 0.02 |
| Recipient sex | 4.8 | 1.4–16.7 | 0.01 | 4.8 | 1.4–16.7 | 0.01 |
| Recipient diagnosis | 4.8 | 1.1–20.6 | 0.04 | 5.1 | 1.4–19.2 | 0.02 |
| Recipient race/ethnicity | 4.1 | 1.1–14.7 | 0.03 | 6.9 | 1.8–27.3 | 0.005 |
| mPAP at induction | 3.8 | 1.2–18.7 | 0.07 | 4.8 | 1.2–18.7 | 0.025 |
| Bilateral transplant | 3.5 | 1.0–12.0 | 0.05 | 4.9 | 1.4–17.1 | 0.01 |
| Use of CPB | 2.1 | 0.5–8.8 | 0.29 | 2.9 | 0.7–11.3 | 0.13 |
| Ischemic time | 3.2 | 0.9–11.5 | 0.07 | 4.2 | 1.2–14.8 | 0.03 |
| Packed RBCs during first 24 h | 3.7 | 1.0–13.2 | 0.04 | 5.1 | 1.5–17.6 | 0.01 |
| Platelets during first 24 h | 4.9 | 1.4–16.9 | 0.06 | 4.9 | 1.4–16.9 | 0.01 |
| Fresh frozen plasma during first 24 h | 2.9 | 0.8–10.4 | 0.10 | 4.5 | 1.3–15.6 | 0.02 |
ln = natural log.
Discussion
PGD contributes significantly to overall morbidity and is the major cause of early death following lung transplantation. We have shown that higher soluble P-selectin levels were associated with the occurrence of PGD in this case-control study nested within a large multicenter cohort of patients undergoing lung transplantation. Although increased mPAP and CPB are traditional risk factors for PGD (and were risk factors in our study), we have shown that increased soluble P-selectin levels and platelet activation may explain the mechanism of these associations. We have previously characterized PGD in terms of coagulation/fibrinolysis, endothelial dysfunction, inflammation, and oxidative stress.19–21 However, these are the first data showing the importance of increased platelet activation in patients with PGD following human lung transplantation.
Platelets contribute to the course of ischemia-reperfusion injury in patients who have undergone transplants of other solid organs, such as the liver22; however, there is less known about this process in the lung.23 Okada et al24 showed that the accumulation of platelets in preserved and transplanted rat lungs was associated with the degree of reperfusion injury. Platelet adherence also resulted in capillary congestion. Other studies17,25 of animal models of ischemia-reperfusion injury and ALI have not only documented platelet-mediated leukocyte infiltration in the lungs, but also specifically identified platelet-derived P-selectin as the key molecule in this process. One study17 utilized a rat model of orthotopic left lung transplantation after 6 h of hypothermic preservation. The administration of blocking anti-P-selectin antibody before reperfusion reduced pulmonary vascular resistance, increased Pao2, and improved survival. P-selectin knockout mice subjected to 30 min of pulmonary ischemia followed by reperfusion also showed significant reductions in neutrophil infiltration, increases in Pao2, and prolonged survival compared to wild-type mice. A model of lung ischemia-reperfusion in rabbits confirmed the rolling and adherence of platelets mediated by platelet P-selectin in the pulmonary vasculature after reperfusion.25
Zarbock et al26 have shown that platelets play an important role in the accumulation of neutrophils in the intravascular, interstitial, and intraalveolar spaces of the lung in ALI. Platelet depletion in this model prevented abnormalities in gas exchange, accumulation of neutrophils in all three compartments, and protein leak. Antibodies against P-selectin prevented the formation of circulating platelet-neutrophil aggregates, decreased neutrophils in BAL fluid, improved the Pao2/Fio2 ratio, and prolonged survival in this model. In addition, wild-type mice with ALI and reconstituted with bone marrow from P-selectin knockout mice (thus permitting endothelial P-selectin but not megakaryocyte P-selectin expression) demonstrated better gas exchange and decreased neutrophil accumulation in the intravascular, interstitial, and intraalveolar spaces of the lung compared to wild-type mice with intact bone marrow. Another study27 showed that platelet P-selectin was critical to the proinflammatory phenotype of endothelial dysfunction induced by high-tidal volume ventilation.
We have previously shown18 that human lung transplantation itself is associated with platelet activation, even in the absence of traditional risk factors such as significant pulmonary hypertension and CPB. Our prospective cohort study18 of patients undergoing lung transplantation without CPB and patients undergoing nontransplantation thoracic surgery showed significant increases in levels of soluble P-selectin, CD40 ligand, and circulating platelet-monocyte conjugates in the lung transplantation patients compared to the thoracic surgery patients. Interestingly, the increases in these platelet markers in the lung transplantation recipients were associated with decreases in platelet count, suggesting that more platelet activation was associated with greater platelet consumption or trapping, presumably in the lung allografts. These findings suggest that the transplantation procedure leads to platelet activation, even in the absence of CPB. Importantly, the persistence of the association between soluble P-selectin levels and PGD in the current study, despite adjustment for the use of blood products, indicates that transfusion does not account for our findings.
Colombat et al28 have confirmed that platelet trapping occurs in the newly perfused lung allografts after human lung transplantation and has a significant impact on oxygenation and allograft function. These investigators performed lung sampling 15 to 30 min after reperfusion. Platelet aggregation in the venular plexus was more prominent in the lung transplantation samples than in samples of lung tissue from control subjects. The finding of P-selectin-stained platelets in the vascular lumen after lung transplantation was associated with a longer duration of mechanical ventilation, lower Pao2/Fio2 ratio, and pulmonary edema, as shown by chest radiography.
The association between pulmonary hypertension and PGD is complex and poorly understood. Some studies3 have suggested that only the diagnosis of pulmonary arterial hypertension itself increases the risk of PGD; other studies29 suggest that each higher increment of pulmonary artery pressure (from any underlying lung disease) translates to an increase in risk of PGD. In addition, because patients with significant pulmonary hypertension require CPB to undergo the transplantation procedure, it is difficult to tease out the respective causal roles. We showed that higher mPAP at induction was associated with higher soluble P-selectin levels both at 6 and 24 h after lung transplantation. This association appeared to persist even in those patients who did not require CPB, suggesting that preoperative pulmonary vascular disease results in platelet activation after lung transplantation independent of CPB. It is possible that long-standing pulmonary hypertension or right ventricular dysfunction may result in platelet defects leading to heightened susceptibility to an activated pulmonary vascular endothelium after transplantation. The compete lack of association between mPAP and soluble P-selectin levels at baseline suggests that some trigger is necessary to unmask this process.
There are several limitations to our study. First, PGD encompasses a spectrum of injury; we studied the extremes of this spectrum. Future prospective cohort studies should include patients with PGD grades I and II. Second, we intentionally did not match the control subjects to case patients in terms of pulmonary diagnosis or CPB use because such matching can introduce bias and actually decrease efficiency in certain situations. We did however adjust (or “control”) for differences in baseline covariates between case patients and control subjects in multivariate analyses, which is the preferable methodological approach in a study such as ours. Of course, the possibility of unmeasured or residual confounding exists.
In this study, we have provided the first evidence that elevated levels of soluble P-selectin are associated with PGD in human lung transplantation. These data and the strong body of evidence from animal models demonstrating that interference with P-selectin leads to attenuation of ischemia-reperfusion injury make targeting platelet activation a promising therapeutic approach in humans undergoing lung transplantation.
Acknowledgment:
We appreciate the technical assistance of Rebekah Boyle, MS, and April Perry, MT.
Abbreviations:
- ALI
acute lung injury
- CI
confidence interval
- CPB
cardiopulmonary bypass
- Fio2
fraction of inspired oxygen
- IQR
interquartile range
- mPAP
mean pulmonary artery pressure
- OR
odds ratio
- PGD
primary graft dysfunction
Appendix
Sites of and Participants in the Lung Transplant Outcomes Group
University of Alabama, Birmingham:
Keith Wille, MD; Joao de Andrade, MD; and Tonja Meadows, RN.
Columbia University:
David Lederer, MD; Selim Arcasoy, MD; Joshua Sonett, MD; Jessie Wilt, MD; Frank D'Ovidio, MD; Nadine Al-Naamani, MD; Robert Sorabella, BA; Catherine Forster, BA; Michael Koeckert, BA; Jeffrey Okun, BA; Nilani Ravichandran, FNP-C; Genevieve Reilly, FNP-C; and Debbie Rybak, BA.
Johns Hopkins University:
Jonathan Orens, MD; and Ashish Shah, MD.
University of Michigan:
Vibha Lama, MD, MS; Fernando Martinez, MD, MS; and Emily Galopin, BS.
University of Pennsylvania (Coordinating Site):
Jason D. Christie, MD, MS; Steven M. Kawut, MD, MS; Alberto Pocchetino, MD; Ejigayehu Demissie, MSN; Robert M. Kotloff, MD; Vivek N. Ahya, MD; Jeffrey Sager, MD, MS; Denis Hadjiliadis, MD, MHS; James C. Lee, MD; and Richard Aplenc, MD.
Stanford University:
Ann Weinacker, MD; Ramona Doyle, MD; Susan Spencer Jacobs, MSN; and Val Scott, MSN.
Vanderbilt University:
Aaron Milstone, MD; Lorraine Ware, MD; E. Wesley Ely, MD, MPH; and Stacy Kelley-Blackburn, RN.
Footnotes
This research was supported by National Institutes of Health grants HL04243, HL067771, HL081332, HL087115, and HL081619.
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 2.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Carby M, Bag R, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction: part II. Definition: a consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 5.de Perrot M, Sekine Y, Fischer S, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 8.de Perrot M, Bonser RS, Dark J, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction: part III. Donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Barr ML, Kawut SM, Whelan TP, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction: part IV. Recipient-related risk factors and markers. J Heart Lung Transplant. 2005;24:1468–1482. doi: 10.1016/j.healun.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly SC, Haslett C, Dransfield I, et al. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994;344:215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- 11.Hirose M, Murai T, Kawashima H. Elevation of rat plasma P-selectin in acute lung injury. Biochim Biophys Acta. 2007;1772:382–389. doi: 10.1016/j.bbadis.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Sakamaki F, Ishizaka A, Handa M, et al. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med. 1995;151:1821–1826. doi: 10.1164/ajrccm.151.6.7539327. [DOI] [PubMed] [Google Scholar]
- 13.Bozza FA, Shah AM, Weyrich AS, et al. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am J Respir Cell Mol Biol. 2009;40:123–134. doi: 10.1165/rcmb.2008-0241TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furman MI, Benoit SE, Barnard MR, et al. Increased platelet reactivity and circulating monocyte-platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–358. doi: 10.1016/s0735-1097(97)00510-x. [DOI] [PubMed] [Google Scholar]
- 15.Abrams CS, Ellison N, Budzynski AZ, et al. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–138. [PubMed] [Google Scholar]
- 16.Hartwell DW, Mayadas TN, Berger G, et al. Role of P-selectin cytoplasmic domain in granular targeting in vivo and in early inflammatory responses. J Cell Biol. 1998;143:1129–1141. doi: 10.1083/jcb.143.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naka Y, Toda K, Kayano K, et al. Failure to express the P-selectin gene or P-selectin blockade confers early pulmonary protection after lung ischemia or transplantation. Proc Natl Acad Sci U S A. 1997;94:757–761. doi: 10.1073/pnas.94.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sternberg DI, Shimbo D, Kawut SM, et al. Platelet activation in the postoperative period after lung transplantation. J Thorac Cardiovasc Surg. 2008;135:679–684. doi: 10.1016/j.jtcvs.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covarrubias M, Ware LB, Kawut SM, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 20.Christie JD, Robinson N, Ware LB, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175:69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman SA, Wang L, Shah CV, et al. Plasma cytokines and chemokines in primary graft dysfunction post-lung transplantation. Am J Transplant. 2009;9:389–396. doi: 10.1111/j.1600-6143.2008.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porte RJ, Blauw E, Knot EA, et al. Role of the donor liver in the origin of platelet disorders and hyperfibrinolysis in liver transplantation. J Hepatol. 1994;21:592–600. doi: 10.1016/s0168-8278(94)80107-x. [DOI] [PubMed] [Google Scholar]
- 23.Ovechkin AV, Lominadze D, Sedoris KC, et al. Lung ischemia-reperfusion injury: implications of oxidative stress and platelet-arteriolar wall interactions. Arch Physiol Biochem. 2007;113:1–12. doi: 10.1080/13813450601118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada Y, Marchevsky AM, Zuo XJ, et al. Accumulation of platelets in rat syngeneic lung transplants: a potential factor responsible for preservation-reperfusion injury. Transplantation. 1997;64:801–806. doi: 10.1097/00007890-199709270-00002. [DOI] [PubMed] [Google Scholar]
- 25.Roberts AM, Ovechkin AV, Mowbray JG, et al. Effects of pulmonary ischemia-reperfusion on platelet adhesion in subpleural arterioles in rabbits. Microvasc Res. 2004;67:29–37. doi: 10.1016/j.mvr.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yiming MT, Lederer DJ, Sun L, et al. Platelets enhance endothelial adhesiveness in high tidal volume ventilation. Am J Respir Cell Mol Biol. 2008;39:569–575. doi: 10.1165/rcmb.2007-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombat M, Castier Y, Leseche G, et al. Early expression of adhesion molecules after lung transplantation: evidence for a role of aggregated P-selectin-positive platelets in human primary graft failure. J Heart Lung Transplant. 2004;23:1087–1092. doi: 10.1016/j.healun.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131:73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]