Abstract
Background:
Pulmonary hypertension (PH) is a common complication of sarcoidosis that is associated with increased mortality. The pathogenesis of PH in sarcoidosis is uncertain, and the role of pulmonary arterial hypertension (PAH)-specific therapies remains to be determined.
Methods:
We conducted a retrospective study of patients with sarcoidosis and PH at two referral centers. New York Heart Association (NYHA) functional class, exercise capacity, hemodynamic data, pulmonary function tests, and survival were collected and analyzed.
Results:
Twenty-two sarcoidosis patients treated with PAH-specific therapies were identified. After a median of 11 months of follow-up, NYHA class was improved in nine subjects. Mean 6-min walk distance (n = 18) increased by 59 m (p = 0.032). Patients with a higher FVC experienced a greater increment in exercise capacity. Among 12 patients with follow-up hemodynamic data, mean pulmonary artery pressure was reduced from 48.5 ± 4.3 to 39.4 ± 2.8 mm Hg (p = 0.008). The 1- and 3-year transplant-free survival rates were 90% and 74%, respectively.
Conclusions:
PAH-specific therapy may improve functional class, exercise capacity, and hemodynamics in PH associated with sarcoidosis. Prospective, controlled trials of PAH therapies for sarcoidosis are warranted to verify this apparent benefit. Mortality among the study population was high, highlighting the need for urgent evaluation at a lung transplant center.
Keywords: bosentan, prostacyclin, pulmonary hypertension, sarcoidosis, sildenafil
Pulmonary hypertension (PH) is a serious complication of sarcoidosis. The exact prevalence is not clear with a reported range of 1 to 28%.1 Among patients listed for lung transplantation in the United States, 74% had PH documented by right heart catheterization.2 Sarcoidosis is classified under the miscellaneous category (group 5) and considered separately from other respiratory disorders, such as interstitial and obstructive lung diseases (group 3), in the Venice 2003 clinical classification of PH.3 The basis for this is the relatively frequent occurrence of severe PH in sarcoidosis patients, even in the absence of overt parenchymal lung disease4,5 and the potential for direct pulmonary vascular involvement by the inflammatory process. Indeed, granulomatous vascular lesions are extremely common.6,7
The presence of PH in sarcoidosis patients is associated with persistent dyspnea,8 reduced exercise capacity,9 and increased oxygen requirements.2 Moreover, it appears to be an independent predictor of mortality.10,11 There are sparse data regarding the treatment of PH associated with sarcoidosis. Reports12–17 of therapeutic responses to corticosteroids and other immunosuppressants are limited and have yielded variable results. Several efficacious pharmacologic agents are now available for the treatment of pulmonary arterial hypertension (PAH).18 Whether these drugs are useful in PH that is associated with sarcoidosis or other lung diseases remains to be determined.19 Small case series have described favorable outcomes with epoprostenol,20 bosentan,8 and sildenafil21 therapy. We performed a retrospective analysis to describe our experience with PAH-specific therapies in patients with sarcoidosis-associated PH.
Materials and Methods
Patients from two regional referral centers (Johns Hopkins University; Baltimore, MD; and Inova Fairfax Hospital; Falls Church, VA) were studied. The study was approved by the respective institutional review boards of the two institutions. Records were reviewed to identify patients with sarcoid-associated PH who had been treated with PAH-specific therapy. All patients with sarcoidosis and PH confirmed by right-heart catheterization who had received PAH-specific therapy, including sildenafil, bosentan, or any prostanoid analog, were included in the study.
PH was defined as a mean pulmonary artery pressure (MPAP) ≥ 25 mm Hg with normal left ventricular systolic function by echocardiography and a pulmonary artery wedge pressure (PAWP) ≤ 15 mm Hg. Patients were excluded if they had evidence of another cause for PH such as HIV infection, connective tissue disease, portal hypertension, congenital or valvular heart disease, obstructive sleep apnea, thromboembolic disease, or a history of anorexigen use. All decisions regarding the diagnostic evaluation and treatment of patients found to have sarcoidosis-associated PH were made by expert clinicians on an individual patient basis independently at both institutions.
Patient demographics, sarcoidosis radiographic stage, and pulmonary function test results obtained closest to the date of the initial right heart catheterization were recorded. Pulmonary hemodynamics, 6-min walk distance (6MWD) and New York Heart Association (NYHA) functional class were collected at baseline and at the most recent follow-up assessment after the initiation of PAH-specific therapy. The 6-min walk test was performed at both institutions according to standard American Thoracic Society criteria22 by trained, dedicated staff. Survival was ascertained by chart review, telephone contact, and the Social Security Death Index.
Statistical Analysis
Data are expressed as the mean ± SEM. The paired Student t test was used to compare mean values for initial and posttreatment hemodynamics and 6MWD. The analyses were performed using patients with baseline and at least one follow-up data point for the variable being analyzed. After the data were collected, an exploratory statistical analysis was performed to look for relationships between baseline variables and outcome after therapy. The unpaired Student t test was used to assess changes in 6MWD and hemodynamics with treatment between patients grouped according to baseline lung function test results and radiographic stage. Correlation coefficients were determined and linear regression was performed to look for relationships among changes in 6MWD, hemodynamics, and pulmonary function test results. The Mann-Whitney test and Spearman correlations were used to examine the relationship between nominal and continuous variables and ordinal and continuous variables, respectively. Time to death or lung transplantation was characterized with the Kaplan-Meier method. Overall survival was determined by censoring transplanted subjects at the time of transplant.
Results
Patient Characteristics
Twenty-seven patients were identified, 5 of whom had a PAWP > 15. These latter patients were excluded, and therefore 22 patients were included in the analysis. The demographic and clinical features of the group are shown in Table 1. Sarcoidosis was confirmed by biopsy in 21 patients. In one case, the diagnosis was made based on typical clinical findings. The majority of patients (68%) had advanced pulmonary fibrosis (radiographic stage IV disease). Pulmonary function testing revealed moderate restriction and a profoundly reduced diffusing capacity of the lung for carbon monoxide (Dlco). Two subjects had a combined obstructive/restrictive ventilatory defect as indicated by an FEV1/FVC ratio < 0.7 and a decreased total lung capacity. Twenty subjects had moderate-to-marked functional limitations (NYHA class III-IV). The degree of PH was severe with an average MPAP of 46 mm Hg. Fifteen patients required supplemental oxygen at an average flow rate of 2.3 L/min. All but one patient had been treated with corticosteroids but had been judged by the treating physician to have clinically significant PH despite receiving adequate immunosuppressive therapy. Six patients were also treated with methotrexate, two with plaquenil, and one with azathioprine.
Table 1.
Demographic, Clinical, and Hemodynamic Characteristics of 22 Patients at Pretreatment Baseline*
| Characteristics | Values |
|---|---|
| Age at PH diagnosis, yr | 47.5 ± 1.1 |
| Female gender | 17 (77.3) |
| African-American race | 22 (100) |
| Sarcoidosis radiographic stage | |
| 0 | 3 (13.6) |
| I | 0 (0.0) |
| II | 3 (13.6) |
| III | 1 (4.5) |
| IV | 15 (68.2) |
| NYHA class | |
| 1 | 0 (0.0) |
| 2 | 2 (9.1) |
| 3 | 17 (77.3) |
| 4 | 3 (13.6) |
| Hemodynamics | |
| Right atrial pressure, mm Hg | 10.1 ± 1.6 |
| MPAP, mm Hg | 46.1 ± 2.7 |
| Cardiac output, L/min | 4.2 ± 0.4 |
| PVR, dyne . s . cm−5 | 810 ± 89.1 |
| PAWP, mm Hg | 9.8 ± 0.7 |
| Pulmonary function test results | |
| FEV1, % predicted | 51.2 ± 3.7 |
| FVC, % predicted | 53.6 ± 3.3 |
| FEV/FVC ratio, % | 80.4 ± 2.0 |
| TLC, % predicted | 60.2 ± 2.6 |
| Dlco, % predicted | 38.1 ± 4.9 |
| Supplemental oxygen use | 15 |
| Oxygen flow, L/min | 2.3 ± 0.4 |
| Oxygen saturation, % | 94.6 ± 0.54 |
| 6MWD, m | 200.2 ± 32 |
*Values are given as mean ± SEM or No. (%). TLC = total lung capacity.
Response to PAH Therapy
The initial PAH-specific therapy was sildenafil in 9 patients, bosentan in 12 patients, and IV epoprostenol in 1 patient. Combination therapy was employed in eight patients because of an inadequate response to monotherapy, and the therapy combinations used included sildenafil plus bosentan in three patients, sildenafil plus inhaled iloprost in two patients, sildenafil plus bosentan plus inhaled iloprost in two patients, and epoprostenol plus bosentan in one patients. The posttreatment data reported here were obtained while subjects were receiving their final PH regimen at the time of the last follow-up.
Functional Class and 6MWD
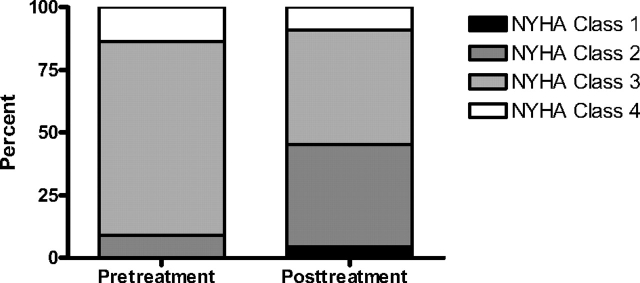
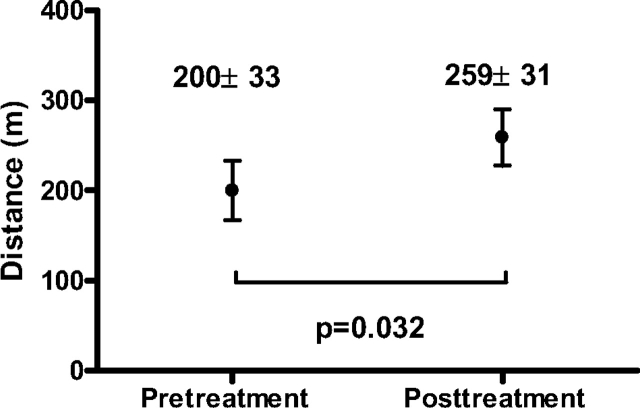
After a median duration of 11 months of therapy (range, 5.2 to 46.6 months), NYHA class had improved by one level in six subjects and by two levels in three subjects. Functional class deteriorated by one level in 2 patients and was unchanged in the remaining 11 patients (Fig 1). Posttreatment 6MWD values were available for 18 patients after a median treatment duration of 14.7 months (range, 13 days to 52.5 months). As shown in Figure 2, there was a statistically significant increase in 6MWD of 59 m (p = 0.032). Eleven patients (61%) showed a ≥ 10% increase in the 6MWD, whereas 5 patients (28%) showed a ≥ 10% decrement.
Figure 1.
NYHA functional classification at baseline and after treatment with PAH-specific therapy (n = 22).
Figure 2.
6MWD before and after treatment with PAH-specific therapy (n = 18).
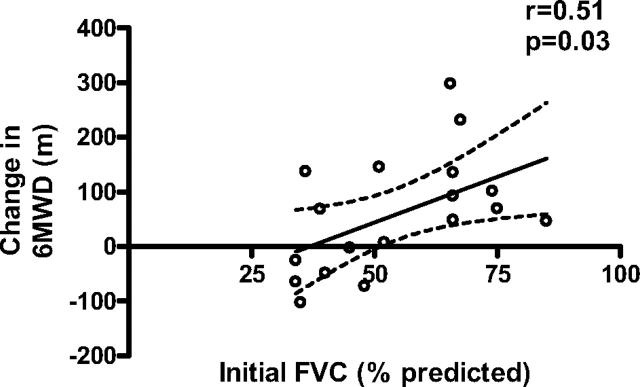
We explored the relationship between pulmonary function test variables and the change in 6MWD in response to PAH therapy. Patients with an FVC above the median value of 51% predicted (n = 9) had a dramatic mean improvement in 6MWD (114 ± 31.4 m) compared with no change in those with an FVC below the median (n = 9; 3.5 ± 31 m; p = 0.02 for difference in the change in 6MWD between the two groups). Nearly identical results were obtained when subjects were divided according to FEV1. Furthermore, linear regression analysis revealed a significant correlation between the change in the 6MWD and baseline FVC as shown in Figure 3. However, there were some patients with significant restrictive disease that nonetheless appeared to have a salutary response to therapy. For example, two patients with initial FVCs of 36% and 51% predicted experienced an increase in 6MWD of 137 and 145 m, respectively, whereas the FVC remained stable. No relationship was observed between Dlco and the response to therapy. There was also no significant difference between the change in 6MWD between those patients with radiographic stage 4 disease (n = 13; 37.3 ± 21.6 m) vs those with stage 0 to 3 disease (n = 5; 115 ± 70.4 m; p = 0.18). No difference in the clinical response to therapy could be detected between the different PH agents (data not shown). Follow-up pulmonary function tests showed no significant changes.
Figure 3.
Linear regression analysis of the initial percent predicted FVC and change from baseline in the 6MWD after treatment with PAH-specific therapy (n = 18).
Hemodynamic Response
Posttreatment hemodynamic data were available for 12 patients. These were obtained after a median duration of 15.2 months (range, 3 to 47 months). As shown in Table 2, the average MPAP fell by 9 mm Hg accompanied by no change in cardiac output or PAWP, yielding a significant reduction in pulmonary vascular resistance (PVR). A nonsignificant trend for lower right atrial pressure was also noted.
Table 2.
Hemodynamics Before and After Treatment With PAH-Specific Therapy*
| Variables | No. | Pretreatment | Posttreatment | p Value |
|---|---|---|---|---|
| MPAP, mm Hg | 12 | 48.5 ± 4.3 | 39.4 ± 2.8 | 0.008 |
| Cardiac output, L/min | 11 | 4.3 ± 0.66 | 4.6 ± 0.36 | 0.791 |
| Right atrial pressure, mm Hg | 11 | 9.9 ± 2.5 | 7.0 ± 1.6 | 0.214 |
| PAWP, mm Hg | 10 | 9.4 ± 0.88 | 11.4 ± 1.8 | 0.275 |
| PVR, dyne . s . cm−5 | 10 | 890.8 ± 105 | 540.6 ± 91.8 | 0.011 |
*Values are given as the mean ± SEM, unless otherwise indicated.
Survival
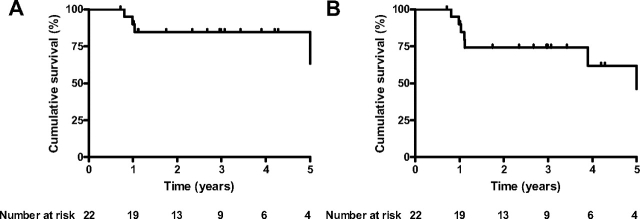
During the observation period, four patients died and six patients underwent lung transplantation. The Kaplan-Meier survival curves for the population are displayed in Figure 4.
Figure 4.
Kaplan-Meier curves showing overall survival with transplanted patients censored at time of transplant (left, A) and time to death or transplant (right, B).
Safety of PAH Therapy
All of the agents used were generally well tolerated with no serious adverse events attributable to any of the drugs. No patient discontinued therapy because of side effects, including liver function abnormalities with bosentan use. At the last follow-up, 15 patients required supplemental oxygen at a mean flow rate of 3.2 ± 0.6 L/min. The mean arterial oxygen saturation was 95 ± 0.7% (difference was not significant compared with baseline values).
Discussion
PH-specific therapy with agents such as epoprostenol, sildenafil, and bosentan improves hemodynamics, exercise tolerance, and, in the case of epoprostenol, mortality in patients with PAH.23,24 Patients with primary lung disease, such as sarcoidosis, however, have not been included in clinical trials of these drugs. PH in this population has been linked to functional impairment and mortality. Whether the treatment of PH secondary to sarcoidosis could result in improved morbidity and mortality remains to be determined. We have reported here on the largest series to date of therapy in patients with sarcoidosis-related PH.
The patients in our study demonstrated a significant improvement in the 6MWD, and more than one third experienced a reduction in NYHA functional class. Among those with follow-up hemodynamic data, significant improvements in MPAP and PVR were observed. Baseline pulmonary function test results showed moderate-to-severe restriction, and those patients with a more preserved baseline FVC showed a superior response to treatment with PAH-specific therapy.
Approximately half of the patients in our study population had advanced lung disease. The presence of pulmonary fibrosis would be expected to portend a lower likelihood of response to pulmonary vasodilator therapy. Indeed, this appeared to be validated by our data, which showed that those patients with more severe restrictions derived less overall benefit.
The pathogenesis of sarcoidosis-related PH is poorly understood. Diverse pathologic processes including fibrotic destruction of the pulmonary parenchyma, vascular remodeling related to chronic hypoxia, and intravascular or extravascular granuloma invasion or compression of pulmonary arteries likely contribute to a final common clinical presentation of PH. The absence of expected radiographic and pathologic findings in some patients with sarcoidosis and PH have led several authors16,25 to propose alternative mechanisms such as a pulmonary vasculopathy characterized by endothelial dysfunction. An imbalance in vasoconstrictor and vasodilatory mediators among patients with sarcoidosis and PH has also been shown. Increased levels of the potent vasoconstrictor endothelin-1 have been described in BAL fluid26 and serum27 in patients with sarcoidosis-related PH. Another study16 reported evidence of increased pulmonary production of thromboxane A2 during a period of vasodilator-induced increase in pulmonary blood flow.
This evidence of endothelial dysfunction, pulmonary vasoconstriction, and vascular remodeling similar to that found in idiopathic PAH support the potential utility of PAH-specific therapies in sarcoidosis. Favorable hemodynamic responses to acute and long-term administration of vasodilator drugs in this population have been demonstrated. Fisher et al20 observed an average reduction in PVR of 45% in response to short-term epoprostenol administration among seven patients. Preston and coworkers25 described eight patients with radiographic stage III or IV disease, four of whom had an acute ≥ 20% decrease in MPAP with the use of inhaled nitric oxide. Five patients receiving long-term therapy (2 to 6 months) with pulse inhaled nitric oxide had improvements in the 6MWD, and three patients had improvements in their NYHA functional class.25
Studies of long-term PAH therapy in patients with sarcoidosis and PH have thus far been limited to a few retrospective case reports and small case series. In one series,20 five patients with an average MPAP of 58 mm Hg were treated with continuous IV epoprostenol. After a mean duration of 29 months, NYHA functional class decreased by one level in four patients and by two levels in one patient. However, treatment was complicated by death in one patient and by acute respiratory failure in another, as well as by the typical side effects of epoprostenol. Baughman and colleagues8 reported on five patients who had been treated with bosentan for at least 4 months. The initial MPAP for the group was 50 mm Hg with a follow-up value (three patients) of 35 mm Hg. Treatment with sildenafil was evaluated in 12 patients being considered for lung transplantation in Denmark. Follow-up right-heart catheterization data in nine patients after a median duration of 4 months of therapy demonstrated a significant decrease in MPAP (mean decrease, 48 ± 15 to 39 ± 13 mm Hg; p = 0.03) and an increase in the cardiac output (mean increase, 4.2 ± 1.2 to 5.7 ± 1.8 L/min; p = 0.01). There was no significant change in the 6MWD (mean change, 238 ± 126 to 264 ± 149 m; p = 0.81).21
Our study has several important limitations and caveats. It is a descriptive, retrospective, observational study that reports on the experience of two centers treating sarcoidosis-associated PH. Our description of the course of treatment of a group of treated patients was intended to generate hypotheses for future well-designed prospective trials. Therefore, it should not be construed as a recommendation or guidance for therapy in these patients.
Specifically, our patients were a select group who were receiving care for advanced lung disease or were awaiting lung transplantation at tertiary care facilities, who were treated with PAH-specific therapy, so our observations may not apply to all patients with sarcoidosis. Decisions regarding the diagnosis and treatment of PH were made by expert clinicians on an individual patient basis and not by any standardized clinical protocol or algorithm. Data were not available for patients who had PH but were not treated with PAH therapy. The treatment was not blinded, and there was no control group. Thus, our results are subject to selection and treatment bias.
Complete data were not available for all patients included in the study. Follow-up 6MWD values were missing from four subjects, and the hemodynamic response to therapy could be assessed in only half of the cohort. Nevertheless, taken together with the observed improvement in functional class in nine patients, our data suggest the possibility of a clinically important response to PAH-specific therapy. Another limitation of our data are that the assessment of oxygenation in our study was limited to pulse oximetry and supplemental oxygen flow rates. PAH-specific therapies have a variable effect on ventilation/perfusion mismatch in patients with sarcoidosis, and changes in the oxygen requirement could be relevant to future trials.28 Though the mean supplemental oxygen flow rate increased slightly after treatment, no statistically significant change was identified, and the number of data points was too small to allow an adequate assessment of changes in oxygenation with therapy.
Importantly, six patients in our sample underwent lung transplantation, and four died without undergoing transplantation during the follow-up period. These observations emphasize the severity of illness in patients with PH secondary to sarcoidosis. Given the currently available data, the importance of expeditious referral to a lung transplant center for evaluation cannot be overemphasized. In fact, under the current lung allocation system in the United States, the presence of PH in sarcoidosis patients dramatically raises the lung allocation score.29
In summary, we found a favorable clinical and hemodynamic response to PAH-specific therapy among selected patients with sarcoidosis-related PH. Although our sample size was limited, it is nonetheless the largest study to date in patients with sarcoidosis- associated PH. Despite the small number of patients, there was a demonstrable benefit in exercise capacity assessed by the change in the 6MWD. Our study suggests that the clinical characteristics of those patients most likely to respond to therapy are those with FVCs > 50% of predicted values. Therefore, the presence of severe pulmonary fibrosis appears to be associated with a lower likelihood of a response to therapy. We believe that the results of our study, along with those of previous reports, support the potential utility of PAH therapy in patients with PH associated with sarcoidosis. Such treatment could decrease morbidity and mortality, and potentially delay the need for transplantation among appropriately selected patients. Prospective controlled studies of PAH agents are needed to confirm this apparent benefit and to best define in which patients such therapy might be indicated.
Abbreviations:
- Dlco
diffusing capacity of the lung for carbon monoxide
- MPAP
mean pulmonary artery pressure
- NYHA
New York Heart Association
- PAH
pulmonary arterial hypertension
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- 6MWD
6-min walk distance
Footnotes
Funding/Support: This research was supported in part by the Intramural Research Program of the National Institutes of Health; the National Heart, Lung, and Blood Institute; and Clinical Center, Critical Care Medicine Department.
Financial/nonfinancial disclosures: Dr. Nathan has received lecture/consultant fees from Gilead, United Therapeutics, and Actelion. Dr. Ahmad has received consultant fees from Actelion. Dr. Zaiman has received clinical research support from Actelion. Dr. Hassoun has received clinical research support from Actelion, Gilead, Pfizer, and United Therapeutics. Dr. Girgis has received clinical research support from Pfizer, United Therapeutics, and Lilly/ICOS; and lecture/consultant fees from Gilead and Actelion. Drs. C.F. Barnett, Bonura, Shlobin, Osei, Moller, and S.D. Barnett have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
For editorial comment see page 1410
References
- 1.Shigemitsu H, Nagai S, Sharma OP. Pulmonary hypertension and granulomatous vasculitis in sarcoidosis. Curr Opin Pulm Med. 2007;13:434–438. doi: 10.1097/MCP.0b013e328273bc5c. [DOI] [PubMed] [Google Scholar]
- 2.Shorr AF, Helman DL, Davies DB, et al. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25:783–788. doi: 10.1183/09031936.05.00083404. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Galie N, Rubin LJ, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Nunes H, Humbert M, Capron F, et al. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulica R, Teirstein AS, Kakarla S, et al. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128:1483–1489. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 6.Rosen Y, Moon S, Huang CT, et al. Granulomatous pulmonary angiitis in sarcoidosis. Arch Pathol Lab Med. 1977;101:170–174. [PubMed] [Google Scholar]
- 7.Takemura T, Matsui Y, Saiki S, et al. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol. 1992;23:1216–1223. doi: 10.1016/0046-8177(92)90288-e. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP, Engel PJ, Meyer CA, et al. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:108–116. [PubMed] [Google Scholar]
- 9.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–213. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 10.Arcasoy SM, Christie JD, Pochettino A, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. 2001;120:873–880. doi: 10.1378/chest.120.3.873. [DOI] [PubMed] [Google Scholar]
- 11.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. 2003;124:922–928. [PubMed] [Google Scholar]
- 12.Rodman DM, Lindenfeld J. Successful treatment of sarcoidosis-associated pulmonary hypertension with corticosteroids. Chest. 1990;97:500–502. doi: 10.1378/chest.97.2.500. [DOI] [PubMed] [Google Scholar]
- 13.Mangla A, Fisher J, Libby DM, et al. Sarcoidosis, pulmonary hypertension, and acquired peripheral pulmonary artery stenosis. Cathet Cardiovasc Diagn. 1985;11:69–74. doi: 10.1002/ccd.1810110110. [DOI] [PubMed] [Google Scholar]
- 14.Davies J, Nellen M, Goodwin JF. Reversible pulmonary hypertension in sarcoidosis. Postgrad Med J. 1982;58:282–285. doi: 10.1136/pgmj.58.679.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damuth TE, Bower JS, Cho K, et al. Major pulmonary artery stenosis causing pulmonary hypertension in sarcoidosis. Chest. 1980;78:888–891. doi: 10.1378/chest.78.6.888. [DOI] [PubMed] [Google Scholar]
- 16.Barst RJ, Ratner SJ. Sarcoidosis and reactive pulmonary hypertension. Arch Intern Med. 1985;145:2112–2114. [PubMed] [Google Scholar]
- 17.Gluskowski J, Hawrylkiewicz I, Zych D, et al. Effects of corticosteroid treatment on pulmonary haemodynamics in patients with sarcoidosis. Eur Respir J. 1990;3:403–407. [PubMed] [Google Scholar]
- 18.Puri A, McGoon MD, Kushwaha SS. Pulmonary arterial hypertension: current therapeutic strategies. Nat Clin Pract Cardiovasc Med. 2007;4:319–329. doi: 10.1038/ncpcardio0890. [DOI] [PubMed] [Google Scholar]
- 19.Girgis RE, Mathai SC. Pulmonary hypertension associated with chronic respiratory disease. Clin Chest Med. 2007;28:219–232. doi: 10.1016/j.ccm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130:1481–1488. doi: 10.1378/chest.130.5.1481. [DOI] [PubMed] [Google Scholar]
- 21.Milman N, Burton CM, Iversen M, et al. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant. 2008;27:329–334. doi: 10.1016/j.healun.2007.11.576. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich CG, Geier A, Lammert F. Bosentan for pulmonary hypertension. N Engl J Med. 2002;347:292–294. [PubMed] [Google Scholar]
- 24.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 25.Preston IR, Klinger JR, Landzberg MJ, et al. Vasoresponsiveness of sarcoidosis-associated pulmonary hypertension. Chest. 2001;120:866–872. doi: 10.1378/chest.120.3.866. [DOI] [PubMed] [Google Scholar]
- 26.Reichenberger F, Schauer J, Kellner K, et al. Different expression of endothelin in the bronchoalveolar lavage in patients with pulmonary diseases. Lung. 2001;179:163–174. doi: 10.1007/s004080000058. [DOI] [PubMed] [Google Scholar]
- 27.Letizia C, Danese A, Reale MG, et al. Plasma levels of endothelin-1 increase in patients with sarcoidosis and fall after disease remission. Panminerva Med. 2001;43:257–261. [PubMed] [Google Scholar]
- 28.Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 29.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]