Abstract
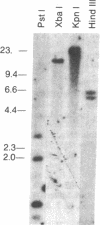
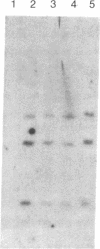
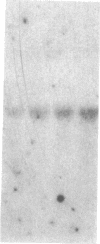
Adrenodoxin reductase is a mitochondrial flavoprotein that receives electrons from NADPH, thus initiating the electron-transport chain serving mitochondrial cytochromes P450. We have cloned and sequenced two human adrenodoxin reductase cDNAs that differ by the presence of six additional codons in the middle of one clone. The sequence in this region indicates that these six extra codons arise by alternative splicing of the pre-mRNA. Southern blot hybridization patterns of human genomic DNA cut with four restriction enzymes indicate that the human genome has only one gene for adrenodoxin reductase. Analysis of a panel of mouse-human somatic cell hybrids localized this gene to chromosome 17cen----q25. The alternatively spliced mRNA containing the six extra codons represents 10-20% of all adrenodoxin reductase mRNA. The expression of the adrenodoxin reductase gene may be stimulated by pituitary tropic hormones acting through cAMP, but its response is quantitatively much less than the responses of P450scc and adrenodoxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Holmberg I., Oftebro H., Pedersen J. I. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1980 Jun 10;255(11):5244–5249. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chen S. A., Besman M. J., Sparkes R. S., Zollman S., Klisak I., Mohandas T., Hall P. F., Shively J. E. Human aromatase: cDNA cloning, Southern blot analysis, and assignment of the gene to chromosome 15. DNA. 1988 Jan-Feb;7(1):27–38. doi: 10.1089/dna.1988.7.27. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Cloning and characterization of the bovine gene for steroid 21-hydroxylase (P-450c21). DNA. 1985 Jun;4(3):211–219. doi: 10.1089/dna.1985.4.211. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Voutilainen R., Mohandas T. K., Miller W. L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8962–8966. doi: 10.1073/pnas.83.23.8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. C., Picado-Leonard J., Haniu M., Bienkowski M., Hall P. F., Shively J. E., Miller W. L. Cytochrome P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(2):407–411. doi: 10.1073/pnas.84.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Blasio A. M., Voutilainen R., Jaffe R. B., Miller W. L. Hormonal regulation of messenger ribonucleic acids for P450scc (cholesterol side-chain cleavage enzyme) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human fetal adrenal cells. J Clin Endocrinol Metab. 1987 Jul;65(1):170–175. doi: 10.1210/jcem-65-1-170. [DOI] [PubMed] [Google Scholar]
- Golos T. G., Miller W. L., Strauss J. F., 3rd Human chorionic gonadotropin and 8-bromo cyclic adenosine monophosphate promote an acute increase in cytochrome P450scc and adrenodoxin messenger RNAs in cultured human granulosa cells by a cycloheximide-insensitive mechanism. J Clin Invest. 1987 Sep;80(3):896–899. doi: 10.1172/JCI113149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanukoglu I., Gutfinger T., Haniu M., Shively J. E. Isolation of a cDNA for adrenodoxin reductase (ferredoxin-NADP+ reductase). Implications for mitochondrial cytochrome P-450 systems. Eur J Biochem. 1987 Dec 15;169(3):449–455. doi: 10.1111/j.1432-1033.1987.tb13632.x. [DOI] [PubMed] [Google Scholar]
- Helms C., Graham M. Y., Dutchik J. E., Olson M. V. A new method for purifying lambda DNA from phage lysates. DNA. 1985 Feb;4(1):39–49. doi: 10.1089/dna.1985.4.39. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Yoshioka H., Yamane M., Gotoh O., Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986 May;83(9):2841–2845. doi: 10.1073/pnas.83.9.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate C. R., McNamara B. C., DiBartolomeis M. J. Control of steroid synthesis in adrenal fasciculata cells. Endocr Res. 1986;12(4):315–350. doi: 10.3109/07435808609035444. [DOI] [PubMed] [Google Scholar]
- John M. E., John M. C., Boggaram V., Simpson E. R., Waterman M. R. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4715–4719. doi: 10.1073/pnas.83.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. E., Gallant S., Brownie A. C. Actions of angiotensin II on aldosterone biosynthesis in the rat adrenal cortex. Effects on cytochrome P-450 enzymes of the early and late pathway. J Biol Chem. 1980 Apr 25;255(8):3442–3447. [PubMed] [Google Scholar]
- Lieberman S., Greenfield N. J., Wolfson A. A heuristic proposal for understanding steroidogenic processes. Endocr Rev. 1984 Winter;5(1):128–148. doi: 10.1210/edrv-5-1-128. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Levine L. S. Molecular and clinical advances in congenital adrenal hyperplasia. J Pediatr. 1987 Jul;111(1):1–17. doi: 10.1016/s0022-3476(87)80334-7. [DOI] [PubMed] [Google Scholar]
- Miller W. L. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988 Aug;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Morel Y., Picado-Leonard J., Wu D. A., Chang C. Y., Mohandas T. K., Chung B. C., Miller W. L. Assignment of the functional gene for human adrenodoxin to chromosome 11q13----qter and of adrenodoxin pseudogenes to chromosome 20cen----q13.1. Am J Hum Genet. 1988 Jul;43(1):52–59. [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J., Miller W. L. Cloning and sequence of the human gene for P450c17 (steroid 17 alpha-hydroxylase/17,20 lyase): similarity with the gene for P450c21. DNA. 1987 Oct;6(5):439–448. doi: 10.1089/dna.1987.6.439. [DOI] [PubMed] [Google Scholar]
- Picado-Leonard J., Voutilainen R., Kao L. C., Chung B. C., Strauss J. F., 3rd, Miller W. L. Human adrenodoxin: cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem. 1988 Mar 5;263(7):3240–3244. [PubMed] [Google Scholar]
- Sagara Y., Takata Y., Miyata T., Hara T., Horiuchi T. Cloning and sequence analysis of adrenodoxin reductase cDNA from bovine adrenal cortex. J Biochem. 1987 Dec;102(6):1333–1336. doi: 10.1093/oxfordjournals.jbchem.a122178. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1590–1594. doi: 10.1073/pnas.84.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Developmental and hormonal regulation of mRNAs for insulin-like growth factor II and steroidogenic enzymes in human fetal adrenals and gonads. DNA. 1988 Jan-Feb;7(1):9–15. doi: 10.1089/dna.1988.7.9. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Picado-Leonard J., DiBlasio A. M., Miller W. L. Hormonal and developmental regulation of adrenodoxin messenger ribonucleic acid in steroidogenic tissues. J Clin Endocrinol Metab. 1988 Feb;66(2):383–388. doi: 10.1210/jcem-66-2-383. [DOI] [PubMed] [Google Scholar]
- Voutilainen R., Tapanainen J., Chung B. C., Matteson K. J., Miller W. L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17 alpha-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab. 1986 Jul;63(1):202–207. doi: 10.1210/jcem-63-1-202. [DOI] [PubMed] [Google Scholar]
- Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem. 1984 Mar 25;259(6):3800–3804. [PubMed] [Google Scholar]
- Yanagibashi K., Haniu M., Shively J. E., Shen W. H., Hall P. The synthesis of aldosterone by the adrenal cortex. Two zones (fasciculata and glomerulosa) possess one enzyme for 11 beta-, 18-hydroxylation, and aldehyde synthesis. J Biol Chem. 1986 Mar 15;261(8):3556–3562. [PubMed] [Google Scholar]