Abstract
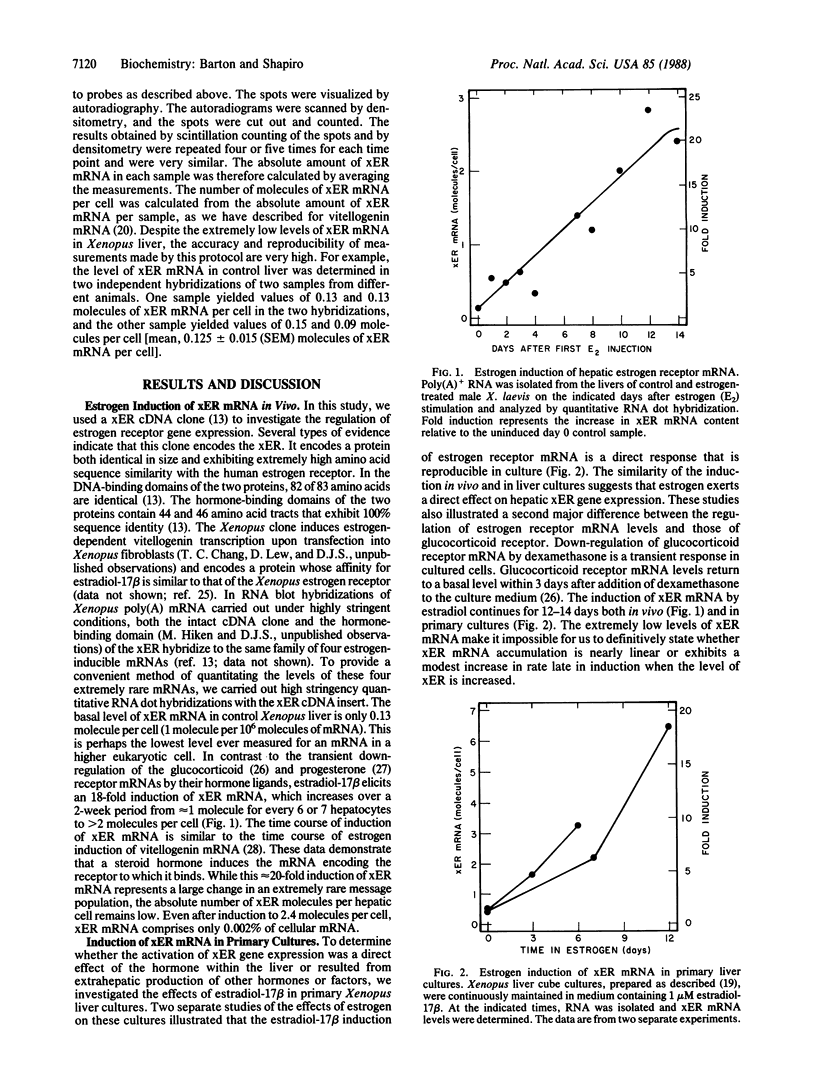
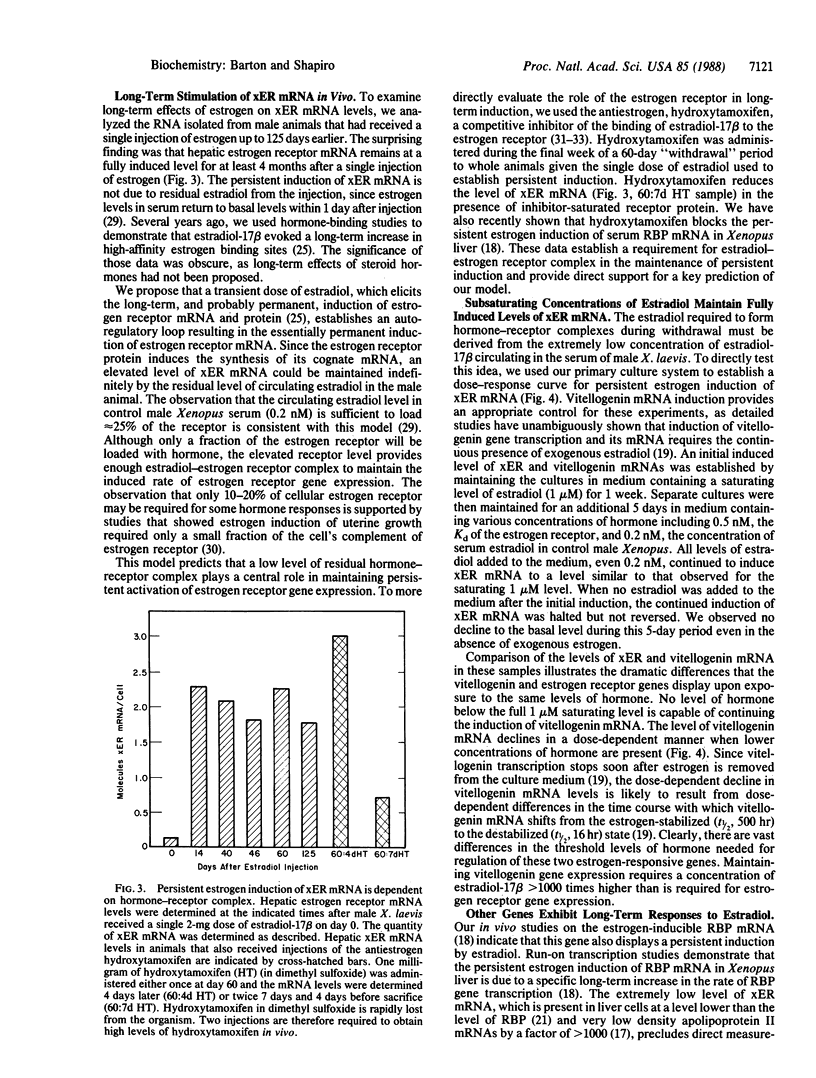
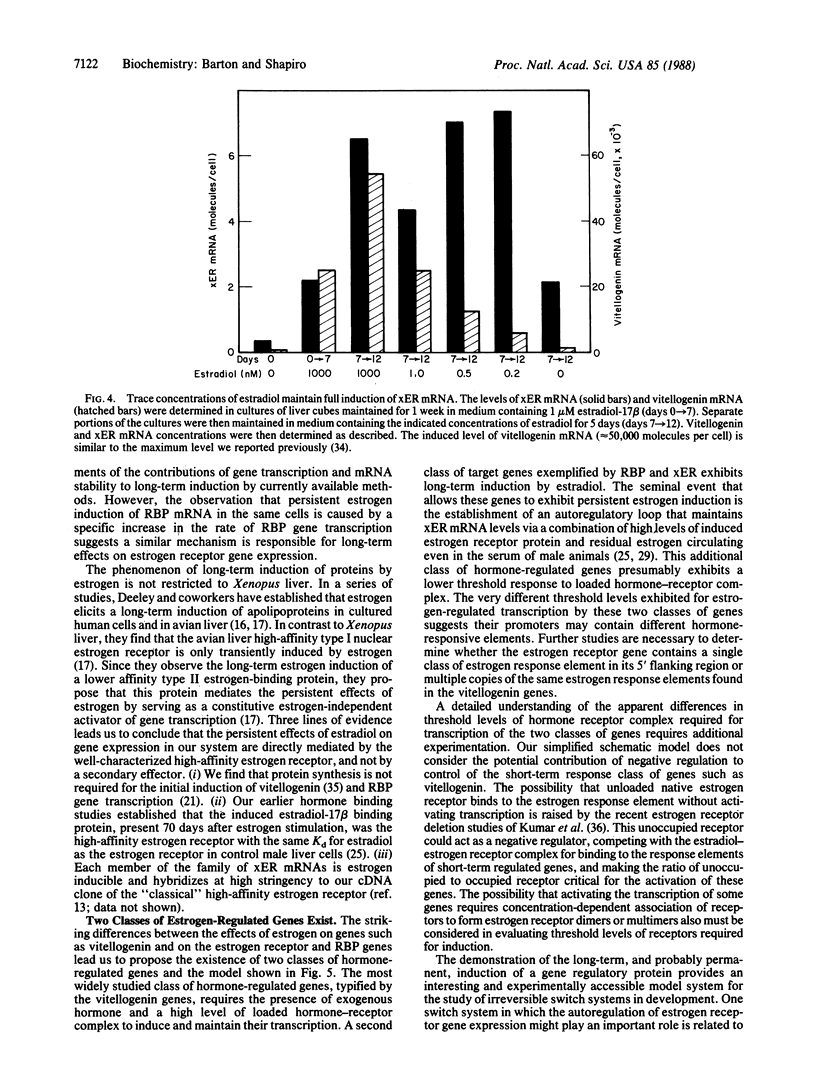
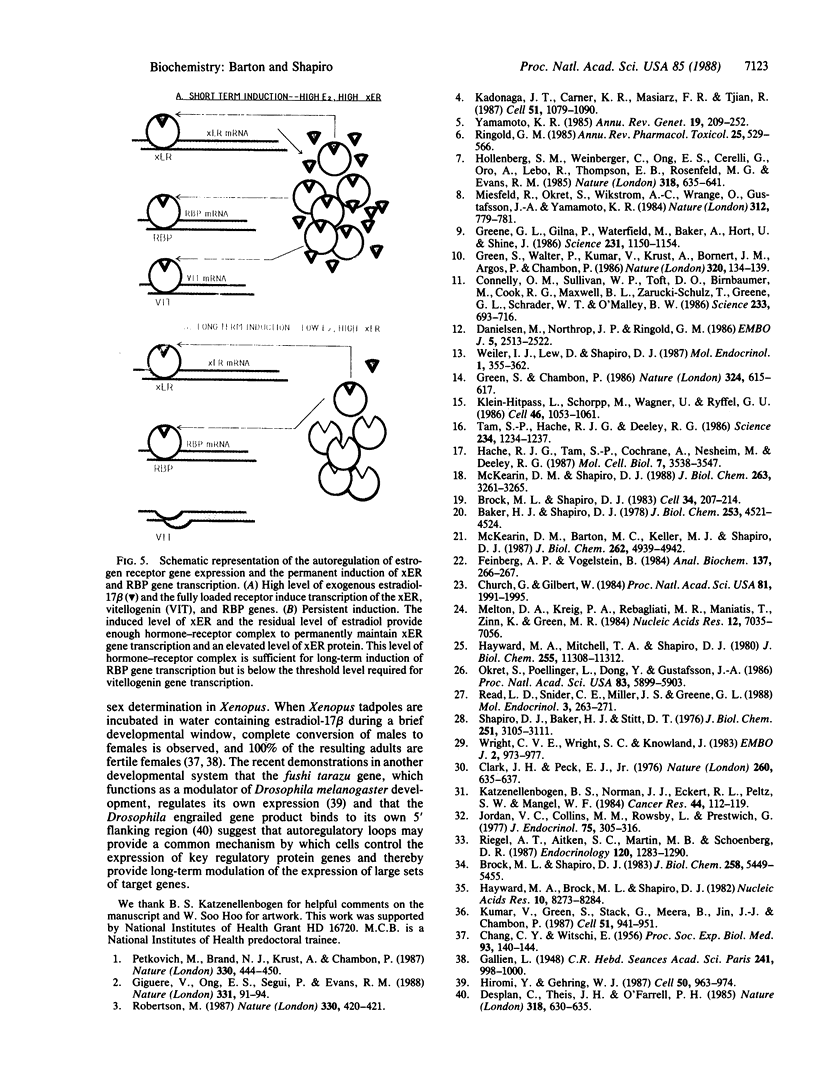
A single transient dose of estradiol-17 beta is sufficient to elicit the permanent induction of hepatic estrogen receptor mRNA, which is induced 18-fold (from 0.13 to 2.4 molecules per cell) and then remains fully induced for at least 125 days. In primary liver cultures, extremely low concentrations of estradiol-17 beta, which are below the Kd of the Xenopus laevis estrogen receptor, maintain persistent induction of estrogen receptor mRNA but not of estrogen-inducible vitellogenin mRNA. These data and the ability of the antiestrogen, hydroxytamoxifen, to reverse persistent induction of estrogen receptor mRNA, support a model in which transient doses of estradiol-17 beta induce the estrogen receptor and thereby establish an autoregulatory loop. The low levels of estradiol-17 beta normally circulating in male X. laevis and the elevated level of receptor provide sufficient hormone-receptor complex to permanently maintain the induced level of expression of the estrogen receptor gene. The permanent induction of the estrogen receptor may be the regulatory switch that results in the persistent expression of a recently identified class of proteins that exhibit long-term responses to estrogen.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker H. J., Shapiro D. J. Rapid accumulation of vitellogenin messenger RNA during secondary estrogen stimulation of Xenopus laevis. J Biol Chem. 1978 Jul 10;253(13):4521–4524. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen regulates the absolute rate of transcription of the Xenopus laevis vitellogenin genes. J Biol Chem. 1983 May 10;258(9):5449–5455. [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- CHANG C. Y., WITSCHI E. Genic control and hormonal reversal of sex differentiation in Xenopus. Proc Soc Exp Biol Med. 1956 Oct;93(1):140–144. doi: 10.3181/00379727-93-22688. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature. 1985 Dec 19;318(6047):630–635. doi: 10.1038/318630a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Giguère V., Yang N., Segui P., Evans R. M. Identification of a new class of steroid hormone receptors. Nature. 1988 Jan 7;331(6151):91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. A superfamily of potentially oncogenic hormone receptors. Nature. 1986 Dec 18;324(6098):615–617. doi: 10.1038/324615a0. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Gilna P., Waterfield M., Baker A., Hort Y., Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986 Mar 7;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Haché R. J., Tam S. P., Cochrane A., Nesheim M., Deeley R. G. Long-term effects of estrogen on avian liver: estrogen-inducible switch in expression of nuclear, hormone-binding proteins. Mol Cell Biol. 1987 Oct;7(10):3538–3547. doi: 10.1128/mcb.7.10.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. A., Brock M. L., Shapiro D. J. Activation of vitellogenin gene transcription is a direct response to estrogen in Xenopus laevis liver. Nucleic Acids Res. 1982 Dec 20;10(24):8273–8284. doi: 10.1093/nar/10.24.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward M. A., Mitchell T. A., Shapiro D. J. Induction of estrogen receptor and reversal of the nuclear/cytoplasmic receptor ratio during vitellogenin synthesis and withdrawal in Xenopus laevis. J Biol Chem. 1980 Dec 10;255(23):11308–11312. [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Norman M. J., Eckert R. L., Peltz S. W., Mangel W. F. Bioactivities, estrogen receptor interactions, and plasminogen activator-inducing activities of tamoxifen and hydroxy-tamoxifen isomers in MCF-7 human breast cancer cells. Cancer Res. 1984 Jan;44(1):112–119. [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- McKearin D. M., Barton M. C., Keller M. J., Shapiro D. J. Estrogen induces transcription of the Xenopus laevis serum retinol-binding protein gene. J Biol Chem. 1987 Apr 15;262(11):4939–4942. [PubMed] [Google Scholar]
- McKearin D. M., Shapiro D. J. Persistent estrogen induction of hepatic Xenopus laevis serum retinol binding protein mRNA. J Biol Chem. 1988 Mar 5;263(7):3261–3265. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Okret S., Poellinger L., Dong Y., Gustafsson J. A. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Read L. D., Snider C. E., Miller J. S., Greene G. L., Katzenellenbogen B. S. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol Endocrinol. 1988 Mar;2(3):263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- Riegel A. T., Aitken S. C., Martin M. B., Schoenberg D. R. Differential induction of hepatic estrogen receptor and vitellogenin gene transcription in Xenopus laevis. Endocrinology. 1987 Apr;120(4):1283–1290. doi: 10.1210/endo-120-4-1283. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Steroid hormone regulation of gene expression. Annu Rev Pharmacol Toxicol. 1985;25:529–566. doi: 10.1146/annurev.pa.25.040185.002525. [DOI] [PubMed] [Google Scholar]
- Robertson M. Retinoic acid receptor. Towards a biochemistry of morphogenesis. Nature. 1987 Dec 3;330(6147):420–421. doi: 10.1038/330420a0. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Baker H. J., Stitt D. T. In vitro translation and estradiol-17beta induction of Xenopus laevis vitellogenin messenger RNA. J Biol Chem. 1976 May 25;251(10):3105–3111. [PubMed] [Google Scholar]
- Tam S. P., Haché R. J., Deeley R. G. Estrogen memory effect in human hepatocytes during repeated cell division without hormone. Science. 1986 Dec 5;234(4781):1234–1237. doi: 10.1126/science.3022381. [DOI] [PubMed] [Google Scholar]
- Weiler I. J., Lew D., Shapiro D. J. The Xenopus laevis estrogen receptor: sequence homology with human and avian receptors and identification of multiple estrogen receptor messenger ribonucleic acids. Mol Endocrinol. 1987 May;1(5):355–362. doi: 10.1210/mend-1-5-355. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Wright S. C., Knowland J. Partial purification of estradiol receptor from Xenopus laevis liver and levels of receptor in relation to estradiol concentration. EMBO J. 1983;2(6):973–977. doi: 10.1002/j.1460-2075.1983.tb01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]



