Summary
Over the past three decades many techniques for expressing exogenous genes in a variety of cells and cell lines have been developed. Exogenous gene expression in macrophages has lagged behind that of other nonhematopioetic cells. There are many reasons for this, but most are due to technical difficulties associated with transfecting macrophages. As professional phagocytes, macrophages are endowed with many potent degradative enzymes that can disrupt nucleic acid integrity and make gene transfer into these cells an inefficient process. This is especially true of activated macrophages which undergo a dramatic change in their physiology following exposure to immune or inflammatory stimuli. Viral transduction of these cells has been hampered because macrophages are end-stage cells that generally do not divide; therefore, some of the vectors that depend on integration into a replicative genome have met with limited success. Furthermore, macrophages are quite responsive to “danger signals,” and therefore several of the original viral vectors that were used for gene transfer induced potent anti-viral responses in these cells making these vectors inappropriate for gene delivery. Many of these difficulties have been largely overcome, and relatively high efficiency gene expression in primary human or murine macrophages is becoming more routine. In the present chapter we discuss some of the gene expression techniques that have met with success and review the advantages and disadvantages of each.
Keywords: Adenovirus, DEAE-dextran, Electroporation, Lentivirus, Nucleoporation, Transduction, Transfection, Retrovirus, Vector
1. Introduction
Macrophages are cells that participate in a number of important immunological processes, including phagocytosis, antigen processing and presentation, and the secretion of a wide variety of mediators that can influence host defense. A fundamental property of macrophages is their ability to accumulate in affected tissue and form an inflammatory focus there. The accumulation of macrophages at inflammatory sites has been associated with many pathogenetic settings, including infectious granulomas, atherosclerosis, rheumatoid arthritis, tumors, etc. Macrophages have great potential as drug delivery vehicles because of their ability to home to inflamed tissue. This potential has led to attempts to design macrophage-specific gene delivery systems that allow the efficient expression of therapeutic genes in macrophages. Thus, the delivery of exogenous gene products using genetically modified macrophages is an area with great therapeutic and/or immunomodulatory potential.
The process of introducing nucleic acid across the host cell membrane by mechanical or physical disruption is termed “transfection.” This is distinct from “transduction,” a process for delivery of nucleic acid into cells by viral vectors, which we will discuss subsequently. Most of the original transfection techniques enjoyed only limited success with macrophages. This is due to the requirement of fairly high gene copy numbers required to efficiently transfect cells and the relatively high degree of toxicity associated with the process whereby the host cell membrane is made permeable to DNA. However, because nonviral-based gene transfer methods continue to have some conspicuous advantages over viral-based transduction, efforts continue to be devoted to improving these methods. The various transfection methods used to introduce foreign DNA into mammalian cells include: DEAE-dextran, calcium phosphate coprecipitation, cationic lipid vehicles, and physical disruption of the host cell membranes by electroporation or nucleofection. All these approaches have been used with varying degrees of success on macrophages.
In the 1960s, Pagano and his colleagues used DEAE-dextran for nucleic acid transfer into mammalian cells (1, 2). DEAE-dextran is a cationic polymer that can interact with negatively charged nucleic acids. The overall positive charge of the polymer in the nucleic acid/DEAE-dextran complex promotes its interaction with the negatively charged cell membrane, facilitating endocytosis. The calcium phosphate coprecipitation-mediated transfection method was introduced by Graham and van der Eb (3). This method involves mixing the nucleic acid with calcium chloride, dropping this mixture in a controlled fashion into a buffered saline/phosphate solution, and then incubating the final mixture to generate a precipitate. The precipitate is then dispersed onto cells and taken up via endocytosis. This transfection technique gained popularity due to its simplicity and low cost. Another advantage of this technique was the discovery that the concentrations of calcium phosphate used for this technique could inhibit host cell nucleases, protecting exogenous DNA from extensive degradation (4). Although these two methods have been fairly widely used for the transfection of various cultured cell lines, their utility for monocytes and macrophages is limited (5). Using the macrophage-like cell line RAW264.7, Thompson and colleagues (6) made a systematic comparison of different transfection methods and concluded that DEAE-dextran and calcium phosphate coprecipitation had the lowest efficiency and the highest rate of interassay variability.
In 1980, a group led by Papahadjopoulos first reported the successful delivery of DNA into a monkey cell line by using unilamellar phospholipid vesicles (7). Later this technique was refined due to the application of a novel synthetic cationic lipid: N -[1-(2,3-dioleyloxy) propyl]- N,N,N-trimethylammonium chloride (DOTMA). In comparison with DEAE-dextran and calcium phosphate coprecipitation, liposome-mediated transfection or “lipofection” had 5-fold to as much as 100-fold greater efficiency of delivery, depending on the cell tested (8). The advantages of lipofection included the simplicity of the methodology and the fact that both transient and stable transfections could be similarly accomplished using this technique. Another conspicuous advantage was that liposome/nucleic acid complexes could be administered in vivo to animals and humans for therapeutic purposes (9).
Liposomes are typically formed by mixing a cationic lipid with a neutral lipid. The positively charged cationic lipid interacts with the negatively charged nucleic acid to form a liposome/nucleic acid complex, which can be taken up by cells via endocytosis. After entering the cells, liposome/nucleic acid complexes appear within endosomes. The endosomes release their contents into cytoplasmic compartments and the nucleus (10). The release of nucleic acids from the endosome is believed to be facilitated by the neutral lipid in the liposome/nucleic acid complex. Some neutral lipids may also assist fusion of the complex with the outer cell membrane to enhance the lipofection efficiency (11). Recent refinements of the liposome technology have led to the development of uniform-sized micelles to deliver DNA with greater efficiency and reproducibility. Many lipid-based reagents are commercially available, including LIPOFECTAMINE2000® from Invitrogen Corporation and the FUGENE® Transfection Reagents from Roche Applied Sciences. Additional components can be added to these basic formulas to more efficiently condense the nucleic acid, or to facilitate their release from endosomes (ExGen® 500 reagent from Fermanta). The FUGENE® reagents have been used to successfully transfect the mouse macrophage-like cell line, RAW264.7 (http://www.roche-applied-science.com), and the human macrophage-line cell line, U937 (12). These methods are quite straightforward and commercially available. Detailed instructions are provided by the manufacturer.
The use of recombinant viruses to infect mammalian cells has emerged as one of the preferred means to deliver exogenous genes to mammalian cells. Adenoviruses, adeno-associated viruses, and retroviruses/lentiviruses have all proven to have utility for gene transfer. All these methods carry the conspicuous advantages of high efficiency gene transfer into mammalian cells, which can result in high expression of the gene product. However, all these systems have some potential disadvantages that must be addressed before they enjoy routine use. The immunogenicity of adenoviruses is a limitation that is being overcome in many of the newer generation viruses. The genotoxicity associated with retroviral integration continues to be an area of concern (13–15). Clinical gene therapy trials with retroviral vectors were dealt a serious blow in 2003 when serious side effects in gene therapy for X-SCID patients were revealed (14, 16). Thus, significant hurdles in vector selection have been cleared but several still remain before large clinical trials can be initiated. Optimization of these viral infection methods to deliver and express genes in hard-to-transfect cells is still in progress, and this is particularly exciting for gene delivery into macrophages.
2. Materials
2.1. Cell Culture
RPMI-1640-10: Roswell Park Memorial Institute-1640 (RPMI-1640) (Mediatech, Inc., cellgro.bizatomic.net) supplemented with 10% fetal bovine serum (FBS, HyClone, http://www.HyClone.com), 10 mmol/L glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin (penicillin and streptomycin can be replaced by 50 µg/mL gentamicin sulfate). Storage at 4°C.
DMEM/F12-10: Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 (Ham) (1:1) (DMEM/F12) (GIBCO 10565, Invitrogen, Carlsbad, California 92008, http://www.invitrogen.com) supplemented with 10% fetal bovine serum (FBS, HyClone, http://www.HyClone.com), 10 mmol/L glutamine, 100 IU/mL penicillin, and 100 µg/mL streptomycin (penicillin and streptomycin can be replaced by 50 µg/mL gentamicin sulfate). Storage at 4°C.
L-929-conditioned medium: L-929 is a murine fibroblast cell line used as a source of M-CSF for culture of bone marrow-derived macrophages. L-929 cells (L-929 cells, CCL-1™, ATCC, http://www.atcc.org) are cultured in DMEM/F12-10 until confluent. L-929-conditioned medium is then harvested at confluence. Cells are removed by centrifugation and supernatants are stored at −80°C until use.
Cellstripper™ Nonenzymatic cell dissociation solution (Mediatech, Inc., cellgro.bizatomic.net). Storage at room temperature.
Falcon® Petri dishes (100 × 15 mm Style) (Becton Dickenson Labware).
All mammalian cell lines and primary cells are cultured in their corresponding media in an atmosphere of 5% CO2 at 37°C.
2.2. Macrophage Cell Lines and Bone Marrow-Derived Macrophages
THP-1 (TIB-202™, ATCC, http://www.atcc.org) and U937 (CRL-1593.2™, ATCC, http://www.atcc.org) are cultured in RPMI-1640-10.
RAW 264.7 (TIB-71™, ATCC, http://www.atcc.org) and J774A.1 (TIB-67™, ATCC, http://www.atcc.org) are cultured in DMEM/F12-10.
Bone marrow-derived macrophages: mouse bone marrow is flushed from the femurs and tibias of mice at 6–10 weeks of age. The cells are plated in petri dishes in DMEM/F12-10 supplemented 20% L-929-conditioned medium. Cells are fed on days 2 and 5. On day 7, cells are removed from petri dishes and cultured on tissue culture dishes in DMEM/F12-10. On the next day, cells are subjected to experiments.
2.3. Electroporation Apparatus
BioRad Gene Pulser® II apparatus are obtained from BioRad Laboratory (http://www.biorad.com).
Electroporation cuvettes (gap width of 4 mm) can be obtained from numerous vendors including BioRad Laboratory.
2.4. Nucleofection
A Nucleofector device is purchased from Amaxa Biosystem (Amaxa GmbH, Cologne, Germany. http://www.amaxa.com).
Cell Line Nucleofector™ Kit T (VCA-1002, Amaxa GmbH, Cologne, Germany. http://www.amaxa.com) is used for the transfection of bone marrow-derived murine macrophages as described in Subheading 3. Storage conditions are listed in the accompanying manual.
2.5. Adenoviral Transduction
A method described by He et al. (17) has been widely accepted as a fast and easy alternative to the traditional methods of generating recombinant adenovirus. This system has been improved and commercialized as the AdEasy™ System by many companies including Stratagene® (http://www.stratagene.com), QBiogene® (http://www.qbiogene.com), and the ATCC® (http://www.atcc.org). Storage conditions are listed in the accompanied manual.
AdEasy™ vectors are pShuttle, pShuttle-CMV, and pAdEasy-1. The pAdEasy-1 vector is devoid of E1 and E3 regions so that the recombinant virus will not replicate in cells other than complementing cells, such as human embryonic kidney 293 (HEK293).
BJ5183 (recA+) (BJ5183-AD1 Electrocompetent Cells, http://www.stratagene.com) is used for in vivo homologous recombination, and DH5 a (MAX Efficiency® DH5 a™ Chemically Competent Cells, Invitrogen, http://www.invitrogen.com) is used for scale-up production of viral vectors. Storage at −80°C.
EndoFree® Plasmid Maxi kit is obtained from QIAGEN (Cat. No.: 12362, QIANGEN, http://www1.qiangen.com). Storage conditions are listed in the accompanied manual.
HEK293 cells (CRL-1573™, ATCC, http://www.atcc.org) are used as a host cell to generate recombinant adenovirus and are cultured DMEM/F12-10.
Adeno-X™ Rapid Titer Kit is supplied from Takara Bio Company (Cat. No.: 631028, Clontech, http://www.clontech.com). Storage conditions are listed in the accompanied manual.
2.6. Viral Transduction by Retroviruses and Lentiviruses
The ViraPower™ Lentiviral Expression System (Invitrogen, Carlsbad, California 92008, http://www.invitrogen.com) is loosely based on the HIV-1 strain NL4-3. This system contains Invitrogen’s Directional TOPO and Gateway Lentiviral vectors that can accommodate up to 6 kb of inserted exogenous gene. The packaging mix contains three supercoiled packaging plasmids (gag/pol, rev, and VSV-G envelop) that are needed for helper functions and supplying viral proteins in trans. Also contained in this system are Stbl3 E. coli cells for transformation and the 293FT packaging cell line that can facilitate production of high-titer virus. The system also contains EmGFP lentiviral expression vectors for assessment of transfection and transduction efficiency and HT1080 cells for titering viral stock.
HT1080 is a human fibrosarcoma cell line (CCL-121™, ATCC, http://www.atcc.org) and culture in DMEM/F12-10.
Polybrene® can be obtained from Sigma-Aldrich (Catalog No. H9268, http://www.sigma-aldrich.com).
G418 is used to maintain regular culture of 293FT cells and can be obtained from Invitrogen (Geneticin™, Invitrogen, http://www.invitrogen.com).
Blasticidin and Zeocin™ can be obtained from InvivoGen (http://www.invivogen.com).
Lipofectamine™ 2000 is the product from Invitrogen (http://www.invitrogen.com). Fugene™ 6 transfection reagent is supplied from Roche Applied Science (http://www.roche.com).
3. Methods
3.1. Electroporation
Electroporation is an established technique that has been used in a variety of applications, including but certainly not limited to introducing exogenous nucleic acids into cells. In 1982, Neumann et al. applied the electroporation technique to introduce DNA into mouse fibroblasts (18, 19). Potter and his colleagues later improved the technique to introduce DNA into a broad range of established cell lines (20, 21). Electroporation has the unique advantage over methods that involve endocytosis and/or phagocytosis since direct uptake of DNA bypasses the lysosomal compartment, limiting the possibility of enzymatic degradation. During the electroporation process, cells are exposed to several electric pulses of varying intensity and duration, creating a trans-membrane potential across the poorly conducting cell membrane. Once this trans-membrane potential reaches a certain threshold, which is proportional to the cell radius and the applied electric field strength, the molecular structure of the membrane will be rearranged to create hydrophilic pores that are permeable to DNA. These pores can be transient in nature and resealed to preserve the integrity of the cell. This process is known as reversible electroporation. An excessive electric field can cause a trans-membrane potential exceeding a tolerable threshold, resulting in pores that cannot be resealed, leading to the destruction of the cell.
Electroporation-based gene transfection into macrophages has received mixed reviews. Since electroporation bypasses lysosomes and causes cell membrane permeabilization that can inhibit phagosomal acidification (22), the potential problems associated with the degradation of DNA by macrophage lysosomal nucleases are minimized. Several groups have successfully used electroporation to introduce exogenous DNA into macrophage-like cell lines. However, the use of electroporation on primary macrophages appears to have been met with more limited success. For example, Stacey et al. found that despite the successful transfection of the macrophage-like RAW264.7 cell line via electroporation, a similar application to primary bone marrow-derived macrophages was much less efficient (23). In many instances, electroporation of primary macrophages results in excessive cell death, regardless of source (synthetic, genomic, or plasmid) or sequence of the DNA.
The following procedures are successfully applied for transfection of macrophage cell lines such as RAW 264.7 cells.
Log-phased cells are split 24 h before electroporation to ensure that cells are growing and healthy. Before electroporation, the cells are centrifuged and then resuspended in fresh medium (RPMI-1640) without fetal calf serum or other additives, such as penicillin and streptomycin.
Approximately 4 × 106 cells in a 0.25-mL volume are added to the cuvette (4 mm) and then mixed with DNA. The mixture is allowed to stand at room temperature for 10 min (see Note 1).
Gene Pulser® II apparatus is set for 950 µF and the Pulse Controller for 200 Ω Efficient transfection can be achieved at a voltage of 250 V. Normally by these parameters, a clump of dead cells will be noticeable (see Note 2).
The electroporated cells are removed from the dead cells and transferred into prewarmed complete medium (see Note 3). The cells are plated in 24 wells of a 48-well plate and allowed to recover overnight.
After overnight recovery, the media is removed and replenished with a fresh media, at which time the cells are ready for experimental studies.
3.2. Nucleofection
Nucleofection is a technology developed by Amaxa GmbH (Cologne, Germany). This proprietary technology includes two components: (1) a Nucleofector device that can deliver unique preprogrammed electrical parameters for electroporation, and (2) solutions that are specific for the individual cell types that are targeted for transfection. By varying these combinations, this technology can efficiently deliver DNA into a variety of cell types. The DNA rapidly travels to the nucleus, after which expression of transfected genes can be detected within a short period of time. In 2003, Martinet et al. reported that transfection efficiencies up to 80% without significant cell toxicity could be achieved on human U937 and THP-1 cells (24). Lenz et al. reported similar transfection efficiencies of human monocyte-derived dendritic cells (25). Others have not experienced a similar level of success with nucleofection. For example, nucleofection of DNA into the murine macrophage-like J774A.1 cells resulted in a substantial amount of apoptosis with little exogenous gene expression (26). This group did, however, see up to 75% transfection efficiency when using mRNA rather than DNA.
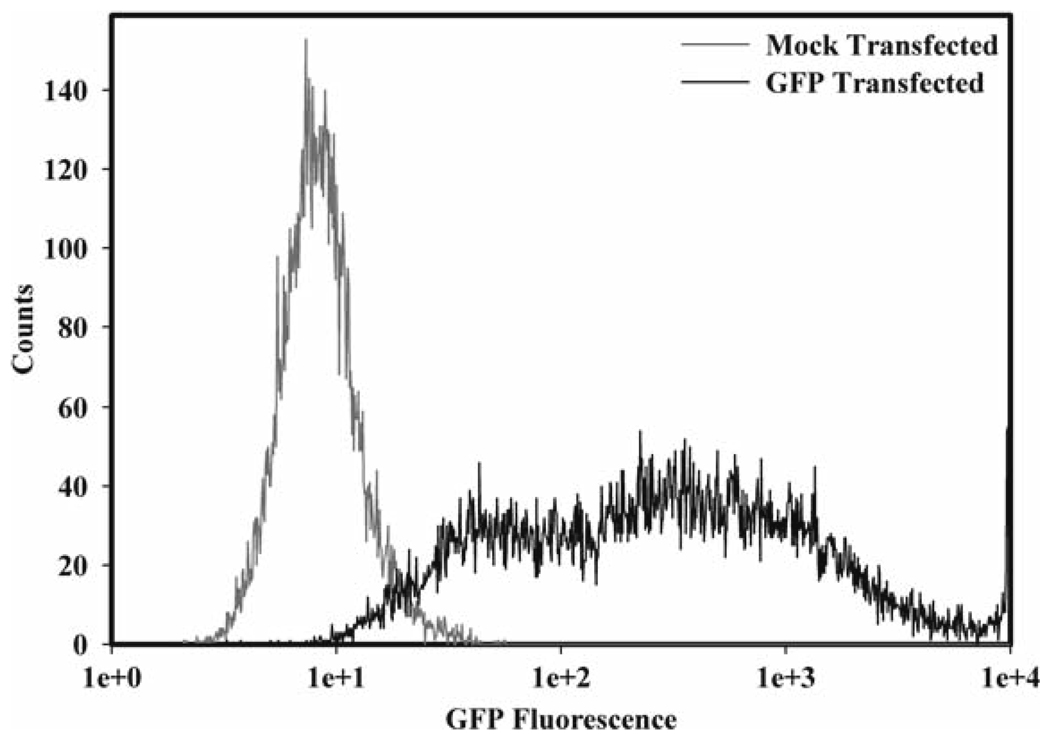
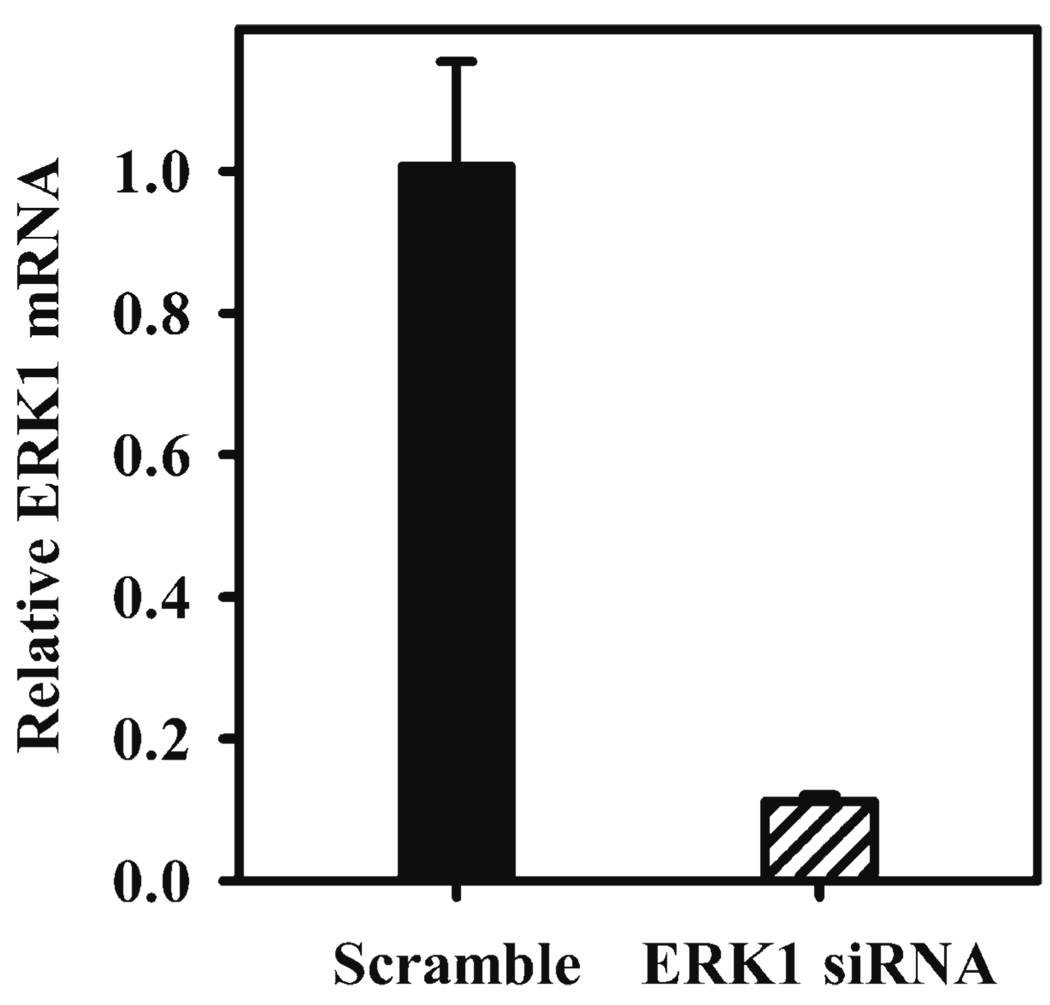
We have used the following nucleofection procedures to efficiently transfer DNA into primary mouse bone marrow-derived macrophages and RAW264.7 cells (27, 28). Using GFP expression as a readout, we obtained transfection efficiencies of approximately 90% in primary murine macrophages (Fig. 1) (27). We have also found this technique to be particularly effective for the delivery of siRNA into primary macrophages. Using siRNA to the MAPKs we were able to achieve a near complete knockdown of ERK mRNA levels (Fig. 2) and ERK kinase activity in primary murine macrophages (28).
All growth media is warmed to 37°C. Prepare cell culture plates containing growth media and preincubate in a 37°C/5% CO2 incubator. Bone marrow-derived macrophages are removed from the femurs of mice and grown for 6–7 days in CSF until ready for use. A total of approximately 5 × 106 cells are used per Nucleofection. Cells are centrifuged at 300 × g for 10 min at room temperature and resuspended at a concentration of 5 × 106 cells/mL in PBS. One milliliter of cells is added to 1.5-mL Eppendorf® tubes and centrifuged for 10s at 7,000 × g by Eppendorf microcentrifuge.
PBS is gently aspirated from each tube, and the pellets are resuspended in 100 µl of room temperature Nucleofection media (avoiding bubbles) along with 3–4 µg of plasmid or siRNA at a final concentration of 10–1,000 nM (in 1–2 m L) and gently mixed. The mixture is incubated at room temperature for approximately 5 min.
Cells are gently resuspended using plastic pipettes and transferred to a Nucleofection cuvette. It is critical to avoid the formation of bubbles during resuspension and transfer.
The cuvette is placed in the Nucleofector and the T-20 program is run. After the program ends, the cuvette is removed and 1 mL of prewarmed growth media is added. The cuvette is recapped and gently tapped to allow cell debris to rise to the top. Cells are gently resuspended using a plastic pipette, being careful to avoid cell debris that has risen to the top.
Depending on the application, cells may be counted and directly transferred to preincubated growth media, or if multiple transfections are performed using the same plasmid, cells may be combined, then counted, and then transferred to preincubated growth media.
Growth medium is changed the following morning at which time gene expression and/or transfection efficiency may be assessed. After the medium is changed, the transfected cells are cultured for an additional 24 h before gene expression is assessed for either knockdown or gain of function transfections (see Note 4).
Fig. 1.
GFP transfection of bone marrow-derived macrophages (BMM Φ). 5 × 106 cells at day 6 (BMM Φ) were subject to nucleofection using program T-20, cell line kit-T, and 3.5 µg of pmaxGFP plasmid (GFP) or no DNA (Mock). GFP expression was assessed by flow cytometry.
Fig. 2.
ERK1 mRNA after nucleofection with ERK1-specfic siRNA. 5 × 106 cells at day 6 (BMM Φ) were subject to nucleofection using an ERK1-specific siRNA (black bar) or nonspecific scramble dsRNA (hatched bar) at a final concentration of 100 nM. ERK1 mRNA levels were assessed 48 h after nucleofection by real-time PCR.
3.3. Adenovirus Transduction
Adenoviruses are virions that have a double-stranded DNA genome with sizes ranging from 26 to 45 kb. Adenoviruses can replicate in either quiescent or dividing cells after infection. A relatively high level of protein expression can be achieved following transduction, and estimates of as high as 35% of total cellular protein have been reported (29, 30). Since transduced genes remain epichromosomal, activation or inactivation of host genes following integration is avoided. Postinfection viability of the host cells remains at nearly 100% for most mammalian cell types; thus, it is well tolerated. For these reasons recombinant adenovirus is the vector of choice for many protein overexpression studies.
Most adenoviruses utilize the ubiquitously expressed coxackie and adenovirus receptor (CAR) for initial attachment and integrinalpha-V glycoproteins for internalization. The heparan sulfate glycosaminoglycan receptor has also been shown to be involved in the binding of adenoviruses. These features lead to gene delivery in nontarget tissues and make the prospect of systemic gene delivery less plausible. Modifications to refine the specificity in attachment of adenovirus are under development, and these are likely to be especially important for macrophage transduction. Since macrophages do not express the high-affinity adenovirus receptor and only low levels of alpha-V-beta 5 integrin (31, 32); transduction efficiency in these cells is sometimes low. The development of vectors that are targeted to specific receptors on macrophages has begun to address this limitation for in vitro transduction (33). It should be noted, however, that the infection of alveolar macrophages by adenoviruses in vivo appears to proceed reasonably well. This may be due to the presence of surfactant proteins in the lung that may enhance viral uptake (34, 35.
Adenoviruses can be highly immunogenic and induce proinflammatory cytokine production (36). Additionally they induce dendritic cells to become mature antigen-presenting cells (37). In early clinical trials, an Ad5-based gene delivery vector was associated with a fatal systemic inflammatory response (38). Such problems have largely been addressed by engineering nonimmunogenic vectors that are devoid of viral-coding sequences (gutless) and/or engineered viruses that coexpress immunomodulatory molecules (39).
The adeno-associated viruses (AAV) are single-stranded nonpathogenic DNA parvoviruses with a genome of roughly 4.7 kb. AAV needs the presence of an adenovirus or a herpes simplex virus coinfection in order to fulfill its life cycle. In the absence of these helper viruses, AAV integrates into human chromosome 19 via specific AAVS1 site to establish latency (40). This property makes them superior to other viral methods, especially retroviruses, which randomly insert into host chromosomes. There are two open reading frames (ORFs) that encode viral proteins: the left one encodes the proteins essential for replication and the right one encodes the structural proteins for the capsid. Palindromic inverted elements that flank the two ends of the genome are the minimal cis-acting elements necessary for the integration and rescue of the viral genome during the latent stage. These elements are also essential for the replication of the viral genome and packaging into the capsid (41, 42). AAV infection begins with virus binding to the cell surface via its primary receptor, heparan sulfate proteoglycans (HSPG), followed by viral internalization, intracellular trafficking, nuclear entry, and virion disassembly and release of a single-stranded DNA template for second-strand DNA synthesis. Fibroblast growth factor receptor 1 and alpha-V-beta 5 integrin have been identified as coreceptors for AAV infection (43). In addition, adeno-associated viruses can use O-linked or N-linked sialic acids for cell binding. Different AAV serotypes exhibit different tissue or cell tropisms, which may be exploited for the development of AAV tissue or cell-specific-targeting vectors. The tropism of AAV can also be engineered through modification of the capsid. A chimeric capsid protein containing the variable region of an antibody against CD34 was used to specifically target hematopoietic progenitor cells (44).
McEacher et al. demonstrated a correction of a murine model of Gaucher disease by the intravenous administration of adeno-associated virus vector that contained the human glucocerebrosidase gene (45). Efficient gene knockdown of HIV-1-transactivating region (TAR) in human hematopoietic stem cells by transduction with AAV was demonstrated (46). In this work, antisense RNA resulted in a continuous inhibition of viral replication without selective pressure. Thus, although not covered here in detail, adeno-associated viruses remain a viable alternative for the delivery of exogenous genes into macrophages.
The following procedures are described for adenovirus-mediated transduction of genes into macrophages:
The gene of interest is cloned into a shuttle vector. Shuttle vectors that are devoid of a promoter allow the insertion of the gene of interest under control of its own promoter. Alternatively, vectors containing strong constitutive promoters, such as CMV or EF-1 (elongation factor-1) promoter, are available and allow for high level of gene and protein expression (see Note 5).
To achieve homologous recombination of recombinant adenovirus, the shuttle vector containing the gene of interest has to be linearized by Pme I and dephosphorylated. One microgram of linearized recombinant transfer vector and 0.1 µg of the pAdEasy-1 vector are then cotransfected into BJ5183 bacterial cells by electroporation. A control transformation lacking the pAdEasy-1 vector should be included.
Typical parameters for electroporation of BJ5183 bacterial cells are: 25 µF of capacity, 200 Ω of resistance and 2.5 kV for a Bio-Rad instrument, 5 Ω of resistance, and 2.5 kV with 50 µF capacity for a BTX instrument.
After recovery at 37°C for 1 h with 1 mL SOC medium, the electroporated mixture is plated onto three LB plates in the presence of antibiotic for 24 h at 37°C, with 100, 300, and 600 µl on each. Approximately twenty of the smallest colonies are picked up and individually grown in LB medium with antibiotic. Restriction enzyme analysis is performed on mini-prepared plasmid DNA to identify appropriate recombinants.
Selected recombinants can be amplified in an electrocompetent recA-strain of E. coli, such as DH5α. The procedure is similar to that described earlier for BJ5183. Recombinant plasmid DNA is purified by EndoFree® Plasmid Maxi kit (QIAGEN). Purified plasmid is then linearized by Pac I and purified through phenol/chloroform extraction and EtOH precipitation under sterile conditions. Five micrograms of purified linearized recombinant plasmid is used for transfection into HEK293 cells.
Approximately 1 × 106 HEK293 cells are plated on a 60-mm-diameter culture dish in DMEM with 5% FBS for 24 h before transfection, by which time they will reach ~70% confluency (see Note 6). Cells are changed with fresh medium at least 1 h before transfection. Transfection can be accomplished by lipofection or nucleoporation (see Note 7). The transfected cells are then collected 7–10 days later by scraping cells off the dish and subsequent centrifugation. The pelleted cells are resuspended in a small volume of media and subject to three freeze/thaw cycles. After centrifugation at maximum speed for 10 min, the supernatants are collected as the original viral stock (see Note 8). One milliliter of the collected supernatant is generally ready to infect 3 × 106 cells to amplify virus (see Note 9). The remaining original stock solution is stored at −80°C (see Note 10).
To obtain high-titer viral stocks, HEK293 cells are infected at a multiplicity of infection (MOI) of 0.1–1 as determined in 8 and grown for 4 days, at which time virus is collected as described earlier. The process is repeated 1–3 times with a final repeat using a total of 5 × 108 HEK293 cells and an MOI of 1–5. Lysis of 50% HEK293 will be found between the third and fifth day following infection. At the fifth day postinfection, the viruses are harvested and purified using a double cesium chloride gradient (see Note 11), as previously described (47). This is the virus preparation used to transducer macrophages.
A viral particle titration should be performed before transduction of the final target macrophages. Protocols used for titration can be divided into two types: physical methods (optical viral unit or viral particle) and biological ones. A fast, easy, and reliable biological assay developed by Bewig and Schmidt (48) and commercialized by Clontech Laboratories, Inc. (http://www.clontech.com) as the Adeno-X™ Rapid Titer Kit can be used. Alternatively, other methods can be used to fit the needs of each laboratory.
Human macrophages, particularly alveolar macrophages, are known to have low expression levels of the high-affinity coxsackie-adenovirus receptor (CAR). Thus, in addition to keeping cells in optimal condition and using viral titers as high as possible, special steps are needed to facilitate adenovirus infection. Viral particles in prewarmed medium without serum are mixed with cholesterol at 3 µg/mL (Sigma-Aldrich, St. Louis, MO) and incubated at 37°C for 30 min (49). Macrophages are washed and resuspended at 5 × 106 cells/mL in complete medium typically with M-CSF (20 ng/mL) and 10% FCS. 5 × 105 cells are transferred into a nonadherent tube (Cat. No. 466982, Nunc, Roskilde, Denmark) and infected with the cholesterol/adenovirus mixture at an MOI of 100:1. The mixture is then centrifuged at 2,000 × g at 37°C for 2 h. After infection, the cells are washed three times with serum-free medium, and then complete medium is added to the culture (50). After culture for 24–48 h, successful delivery of genes of interest can be examined by methods such as Western blot, flow cytometry, quantitative PCR, etc. as suitable for individual purposes.
3.4. Transduction by Retroviruses and Lentiviruses
Over the past decade, retroviruses have been used as a means to deliver genes into mammalian cells. Retroviruses are named for their ability to reverse-transcribe their RNA genome into DNA after infection. The general limitation associated with retroviral transduction is that they typically integrate only into a dividing genome, and therefore they cannot be used on terminally differentiated, nondividing cells, such as macrophages. The lentiviruses, however, are a subgroup of retroviruses that integrate into the genomes of nondividing cells. Consequently, these viruses have the most potential for macrophage gene delivery. All retroviruses have three coding domains: the gag domain that generates the matrix-, capsid-, and nucleoproteins; the pol domain that yields the viral protease, reverse transcriptase, and integrase; and the env domain that encodes envelope glycoprotein. The life cycle of retroviruses can be simply defined as follows: binding of viruses to their receptors, entry and internalization, reverse transcription, assembly of preintegration complex, nuclear transportation, transcription of integrated virus, translation from spliced mRNA, virion assembly, and exit from the cell.
Lentiviruses (e.g., HIV) are naturally attractive candidates as vectors for gene expression in primary nondividing macrophages. In 1995, a team led by Verma and Trono first detailed the construction of a harmless HIV-derived lentiviral vector that was able to deliver genes into a variety of cells, including human primary macrophages (51). These authors used an HIV-derived lentivirus-based luciferase vector pseudotyped with the VSV G protein to transduce human monocyte-derived primary macrophages. A significant increase in luciferase activity could be detected in an envelope-dependent fashion. The possibility that the vector transduced a small portion of proliferating macrophages was ruled out by mutating Vpr and MA, two virion proteins that are central for HIV infection of primary macrophages. Since then, numerous studies using this or a modified delivery system have been reported. Rossi et al. demonstrated that similar HIV-1-based lentiviral vectors could mediate efficient gene transfer into primary murine macrophages and mature B lymphocytes without the participation of HIV-1 accessory proteins Nef, Vpr, Vif, and Vpu (52). The lentiviral vector-mediated method was used to genetically modify monocytes so that the autocrine and paracrine production of GM-CSF and IL-4 could be used to differentiate human CD14+ monocytes into immature dendritic cells (53).
The subgroup lentivirus includes human immunodeficiency viruses (HIV-1 and HIV-2), simian immunodeficiency viruses, feline immunodeficiency virus (FIV), equine anemia infectious virus, and maedi-visna virus. All these viruses can integrate into the genome of nondividing cells, which makes them attractive delivery vehicles for the expression of exogenous genes or small interfering RNAs. Many optimized lentiviral systems such as the FIV-based vector have been developed to circumvent safety hurdles for potential application in human subjects. All of them are based on a genetically split gene expression design. The essential viral elements include the minimal LV packaging helper proteins (gag-pol genes), the LV transfer vector RNA containing the transgene expression cassette, and heterologous glycoproteins. The following procedure is based on an HIV-1-based vector system developed by Invitrogen (http://www.invitrogen.com) for academic applications (see Note 12). The HIV-1-based system is well developed and characterized and been proven to be successful for use on primary macrophages (51).
The procedure can be divided into five basic steps: (a) cloning the gene of interest into the lentiviral vector; (b) transforming E. coli and isolating plasmid DNA; (c) testing the construct by transient transfection; (d) harvesting and titering the viral stock; and (e) transducing macrophages with virus.
TOPO-technology-based vectors are designed to facilitate PCR-based cloning using a proofreading polymerase. Taq polymerase should not be used due to the inhibitory effect of 3′-A on the D-TOPO reaction. An excess of PCR product inhibits the D-TOPO reaction, thus 1:1 ratio of insert:vector should generally be maintained.
All retroviral vectors have two LTRs with 180 bp of repeats; thus, they can recombine in bacteria, easily resulting in smaller products. To reduce the possible occurrence of inappropriate recombination between the LTRs, Stbl3 E. coli cells are used for transformation. The choice of LB plates with 100 µg/mL ampicillin also reduces recombination. Only small colonies are picked for subsequent minipreparation, as recombination leads to loss of most of the plasmid, conferring a growth advantage and favoring the formation of the large colonies. EDTA should be included in the miniprep solution to kill Stbl3 E. coli cells containing a thermostable periplasmic endonuclease. Phenol/chloroform extraction is added to denature the enzyme. Restriction endonuclease analysis is performed to determine whether unwanted recombinants exist in the preparation.
The 293FT cell line is derived from HEK293 cells. Both these cells contain a stably integrated T antigen, but the 293FT cells have the added convenience of rapid growth in culture. Plasmids with an SV40 ori are not appropriate since it can be recognized by T antigen and lead to cell death. Transient transfection of the newly constructed viral vector and packaging vectors (packaging mix) in 293FT cells can be performed by using Lipofectamine 2000™ (Invitrogen) or Fugene™ 6 (Roche). Multinucleated syncytia should be found after transfection since VSV-G glycoproteins (packaging mix) can cause cells to fuse. Cells should be at approximately 90% confluence by plating 5 × 106 cells per 10 cm2 1 day before transfection in complete media.
Virus-containing supernatants are collected 48–72 h after transfection. Due to the infectious nature of the viruses, BSL-2 guidelines should be followed. The supernatants are centrifuged at 1,800 g at 4°C for 15 min and then filtered through a 0.45-µm filter. The virus can be kept in the medium used to culture 293 cells (in the absence of G418) in small aliquots at −80°C. The virus can also be concentrated by ultracentrifugation as previously described (54).
Viral titering is done on HT1080 cells (human fibrosarcoma cell line from ATCC: http://www.atcc.org). Tenfold serial dilutions of lentiviral stock are prepared and transduced into HT1080 cells in the presence of Polybrene (Sigma, Catalog No. H9268). Twenty-four hours after viral transduction, cells are trypsinized from 6-well culture plates and expanded to 100-mm plates. Twenty-four hours later, 10 µg/mL blasticidin or 100 µg/mL zeocin are added for selection. One week after blasticidin selection or 3 weeks after zeocin, crystal violet staining is carried out to visualize plaques. Cells are washed with PBS, then treated with 1 mL of crystal violet solution (1% crystal violet in 10% ethanol) for 10 min at room temperature, and then washed with PBS twice. The infected plaques are visible to the naked eye for counting to determine the viral titer of the original collected viral supernatant.
Cell lines or primary macrophages and monocytes are obtained and maintained as described earlier. One day before transduction, macrophages are plated so that they will be approximately 30% confluent the next day. Virus solutions with a range of MOI from 1 to 100 are prepared in cell culture media with or without Polybrene or DEAE-dextran (see Note 13). Virus should be kept at a ratio no greater than 1:2 in the final volume of cell culture medium. The final volume in a well of 24-well plates should not exceed 250 µl. The lower the volume, the better. Twenty-four hours after incubation with virus, culture super-nantants are removed and fresh culture medium is added. For nonadherent monocytes, on the day of transduction cells are plated at a density so that they will not need to be subcultured for 3 days. The cells are plated in a small volume (100 µl for 24-well plates). Virus solutions with different MOI are added and mixed well with the cells. The final volume should be kept under 200 µl in total. Cells and viruses are mixed every couple of hours at least twice during the daytime and incubated at 37°C for an additional 24 h. Fresh medium (100 µl) is then added, or virus-containing medium is removed by centrifugation and replaced with fresh culture medium. Expression of inserted genes can be monitored beginning at day 2 and continuing until 7 days post-transduction (see Note 14).
Footnotes
Precooling the cuvette and cells before electroporation is not necessary.
The BioRad Gene Pulser II apparatus generates exponential pulses by the Pulse Trac™ waveform delivery system for optimal transfection in an electroporation cuvette. Other electroporation devices that generate square wave pulses such as BTX® molecular delivery system (http://www.btxonline.com) have also been well established for the transfection of mammalian cells including human and mouse macrophage cell lines. The parameters are: 260 V and three pulses of 10 ms with a pulse length set to BCM 820 when 4-mm gap cuvette is used. Ten micrograms of DNA in 0.4 mL volume with 5 × 106 cells in total is used. These parameters are also applicable to human THP-1 and U937 cell lines.
It is common to lose up to 50% of the cells during the electroporation procedure.
We have achieved a transfection efficiency of approximately 90% using this technique on primary bone marrow macrophages (Fig. 1).
The CMV promoter may not be suitable for the expression of inserted genes in rodent cells due to methylation-dependent downregulation (55). EF-1 (elongation factor-1) promoter or other macrophage-specific promoters are alternative choices.
To avoid the occurrence of replication competent adenoviruses (RCAs), passage numbers of HEK293 cells should not be higher than four.
Addition of GeneJammer (Stratagene: http://www.stratagene.com) during the adenovirus infection can lead to increases in both the total numbers of infected cells and the level of transgene expression per cell in the absence of CAR (56).
In order to avoid the generation of the unwanted recombinants, extraction of recombinant adenovirus plasmid from HEK293 cells should be performed as quickly as possible using the minipreparation procedure for low copy plasmid. Furthermore, a recombinant adenovirus plasmid with a large size insertion is genetically not stable in a recA+ strain such as BJ5183.
Good management of the first amplification of viral stock or use of low passage viral stock is critical because they contain the lowest possible ratio of RCA/recombinants. The frequency of RCA occurrence is about one revertant per 107 viruses.
Nyberg-Hoffman virus storage buffer (Tris 10 mM, pH 8.0, with 2 mM MgCl2 and 4% sucrose) allows the viral particles to be stored at concentrations up to 1 × 1013 viral particles/mL without precipitation.
Cesium chloride purification is essential for in vivo gene delivery because it can remove contaminants that can elicit an immune response in vivo.
Other Lentiviral vector-based systems are also available through commercial vendors, such as a series of feline lentiviral vectors supplied by SBI (http://www.sbi.com) and a lenti-siRNA vector from GenScript (http://www.genscript.com). Feline or other nonhuman lentiviral vectors-based gene delivery systems are particularly suitable for gene therapy in human subjects (57).
Polybrene (hexadimethrine bromide) is a small positively charged molecule that can enhance transduction by retroviruses because it can reduce repulsion between sialic acid-containing molecules and neutralize surface charge to allow the viral glycoproteins to bind more efficiently to their receptors. However, polybrene must be used with caution, particularly for cells derived from hematopoietic precursors because of its toxicity to T and B cells. DEAE-dextran is an alternative, but both reagents should be titrated before being added to macrophages or monocytes. Titration of polybrene generally begins at 1 m g/mL, and DEAE-dextran at 3 µg/mL. Inclusion of polybrene or DEAE-dextran generally increases transduction efficiency by twofold (58).
Because macrophages are particularly adept at sensing ‘danger’ and migrating to areas of infection or tissue damage, these cells become potential drug delivery vehicles (63). The development of innovative new methods for introducing exogenous macromolecules into macrophages is not only useful for basic bench experimentation, but has also led to the design of new protocols to be applied to clinical gene therapy or drug delivery. Chemical and lipofection methods do not have the concern of the host immune response that is often associated with viral infection. Adenovirus-based gene delivery may have the disadvantage of transient expression of the transduced gene, and it can lead to some side effects in vivo, such as hepatotoxicity (59). Furthermore, the repeated administration of adenovirus will often result in unwanted host immune responses. Lentivirus-based constructs have the advantages of macrophage-specific targeting and continuous expression of the transduced gene due to integration into chromosomal DNA. Thus, lentivirus-based systems are quite promising for both research and clinical application. However, dangers associated with HIV genotoxicity continue to be major limitations for the use of these vectors. Importantly, many new and refined vectors are under development to obviate many of these concerns. New methods, including the use of nanoparticles with cell-specific-targeting moieties, have been reported (60). Osmotic delivery of small interfering RNA (siRNA) (61) and even the use of bacterial ghosts loaded with DNA have been used as methods to efficiently transfer nucleic acids into macrophages (62). The goal is to obtain high transfection efficiency and cell specificity so that genetic manipulation of macrophages will become a routine task.
References
- 1.McCutchan JH, Pagano JS. Enhancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethyl-laminoethyl-dextran. J Natl Cancer Inst. 1968;41:351–357. [PubMed] [Google Scholar]
- 2.Vaheri A, Pagano JS. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965;27:434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]
- 3.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 4.Loyter A, Scangos GA, Ruddle FH. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci U S A. 1982;79:422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mack KD, Wei R, Elbagarri A, Abbey N, McGrath MS. A novel method for DEAE-dextran mediated transfection of adherent primary cultured human macrophages. J Immunol Methods. 1998;211:79–86. doi: 10.1016/s0022-1759(97)00194-4. [DOI] [PubMed] [Google Scholar]
- 6.Thompson CD, Frazier-Jessen MR, Rawat R, Nordan RP, Brown RT. Evaluation of methods for transient transfection of a murine macrophage cell line, RAW 264.7. Biotechniques. 1999;27:824–830. 832. doi: 10.2144/99274rr05. [DOI] [PubMed] [Google Scholar]
- 7.Fraley R, Subramani S, Berg P, Papahad-jopoulos D. Introduction of liposome-encapsulated SV40 DNA into cells. J Biol Chem. 1980;255:10431–10435. [PubMed] [Google Scholar]
- 8.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wanz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glorioso JC, Huang L, Dunbar C, Felgner PL. Highlights from the third annual ASGT meeting. American Society of Gene Therapy. Mol Ther. 2000;2:96–100. doi: 10.1006/mthe.2000.0108. [DOI] [PubMed] [Google Scholar]
- 10.Friend DS, Papahad-jopoulos D, Debs RJ. Endocytosis and intracellular processing accompanying transfection mediated by cationic liposomes. Biochim Biophys Acta. 1996;1278:41–50. doi: 10.1016/0005-2736(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 11.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidyleth-anolaminein cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Wang JM, Gong WH, Mukaida N, Young HA. Differential regulation of chemokine gene expression by 15-de-oxy-delta 12,14 prostaglandin J2. J Immunol. 2001;166:7104–7111. doi: 10.4049/jimmunol.166.12.7104. [DOI] [PubMed] [Google Scholar]
- 13.Baum C, von Kalle C, Staal FJ, Li Z, Fehse B, Schmidt M, et al. Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther. 2004;9:5–13. doi: 10.1016/j.ymthe.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Hacein-Bey-Abina S, von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 15.von Kalle C, Fehse B, Layh-Schmitt G, Schmidt M, Kelly P, Baum C. Stem cell clonality and genotoxicity in hematopoietic cells: gene activation side effects should be avoidable. Semin Hematol. 2004;41:303–318. doi: 10.1053/j.seminhematol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Engelman A. The ups and downs of gene expression and retroviral DNA integration. Proc Natl Acad Sci U S A. 2005;102:1275–1276. doi: 10.1073/pnas.0409587101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong TK, Neumann E. Electric field mediated gene transfer. Biochem Biophys Res Commun. 1982;107:584–587. doi: 10.1016/0006-291x(82)91531-5. [DOI] [PubMed] [Google Scholar]
- 20.Potter H, Weir L, Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electro-poration. Proc Natl Acad Sci U S A. 1984;81:7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988;174:361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- 22.Lukacs GL, Rotstein OD, Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990;265:21099–21107. [PubMed] [Google Scholar]
- 23.Stacey KJ, Ross IL, Hume DA. Electroporation and DNA-dependent cell death in murine macrophages. Immunol Cell Biol. 1993;71:75–85. doi: 10.1038/icb.1993.8. [DOI] [PubMed] [Google Scholar]
- 24.Martinet W, Schrijvers DM, Kockx MM. Nucleofection as an efficient nonviral transfection method for human monocytic cells. Biotechnol Lett. 2003;25:1025–1029. doi: 10.1023/a:1024157508492. [DOI] [PubMed] [Google Scholar]
- 25.Lenz P, Bacot SM, Frazier-Jessen MR, Feldman GM. Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett. 2003;538:149–154. doi: 10.1016/s0014-5793(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 26.Van De Parre TJ, Martinet W, Schrijvers DM, Herman AG, De Meyer GR. mRNA but not plasmid DNA is efficiently transfected in murine J774A.1 macrophages. Biochem Biophys Res Commun. 2005;327:356–360. doi: 10.1016/j.bbrc.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 2006;281:26041–26050. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas M, Zhang X, Prasanna V, Mosser DM. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 2005;175:469–477. doi: 10.4049/jimmunol.175.1.469. [DOI] [PubMed] [Google Scholar]
- 29.Garnier A, Cote J, Nadeau I, Kamen A, Massie B. Scale-up of the adenovirus expression system for the production of recombinant protein in human 293S cells. Cytotechnology. 1994;15:145–155. doi: 10.1007/BF00762389. [DOI] [PubMed] [Google Scholar]
- 30.Wurm F, Bernard A. Large-scale transient expression in mammalian cells for recombinant protein production. Curr Opin Biotechnol. 1999;10:156–159. doi: 10.1016/s0958-1669(99)80027-5. [DOI] [PubMed] [Google Scholar]
- 31.Kaner RJ, Worgall S, Leopold PL, Stolze E, Milano E, Hidaka C, et al. Modification of the genetic program of human alveolar macrophages by adenovirus vectors in vitro is feasible but inefficient, limited in part by the low level of expression of the coxsackie/adenovirus receptor. Am J Respir Cell Mol Biol. 1999;20:361–370. doi: 10.1165/ajrcmb.20.3.3398. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Endo RI, Nemerow GR. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De SK, Venkateshan CN, Seth P, Gajdusek DC, Gibbs CJ. Adenovirus-mediated human immunodeficiency virus-1 Nef expression in human monocytes/macrophages and effect of Nef on down modulation of Fcgamma receptors and expression of monokines. Blood. 1998;91:2108–2117. [PubMed] [Google Scholar]
- 34.Harrod KS, Trapnell BC, Otake K, Korfhagen TR, Whitsett JA. SP-Aenhances viral clearance and inhibits inflammation after pulmonary adenoviral infection. Am J Physiol. 1999;277(3 Pt 1):L580–L588. doi: 10.1152/ajplung.1999.277.3.L580. [DOI] [PubMed] [Google Scholar]
- 35.Zsengeller Z, Otake K, Hossain SA, Berclaz PY, Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol. 2000;74:9655–9667. doi: 10.1128/jvi.74.20.9655-9667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morelli AE, Larregina AT, Ganster RW, Zahorchak AF, Plowey JM, Takayama T, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Philpott NJ, Nociari M, Elkon KB, Falck-Pedersen E. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc Natl Acad Sci U S A. 2004;101:6200–6205. doi: 10.1073/pnas.0308368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone D, Lieber A. New serotypes of adenoviral vectors. Curr Opin Mol Ther. 2006;8:423–431. [PubMed] [Google Scholar]
- 39.Thummala NR, Ghosh SS, Lee SW, Reddy B, Davidson A, Horwitz MS, et al. A non-immunogenic adenoviral vector, coexpressing CTLA4Ig and bilirubin-uridine-diphosphoglucuronateglucuronosyltransferase permits long-term, repeatable transgene expression in the Gunn rat model of Crigler–Najjar syndrome. Gene Ther. 2002;9:981–990. doi: 10.1038/sj.gt.3301729. [DOI] [PubMed] [Google Scholar]
- 40.Kotin RM, Linden RM, Berns KI. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berns KI, Hauswirth WW. Adeno-associated viruses. Adv Virus Res. 1979;25:407–449. doi: 10.1016/s0065-3527(08)60574-6. [DOI] [PubMed] [Google Scholar]
- 42.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 43.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 44.Yang Q, Mamounas M, Yu G, Kennedy S, Leaker B, Merson J, et al. Development of novel cell surface CD34-targeted recombinant adenoassociated virus vectors for gene therapy. Hum Gene Ther. 1998;9:1929–1937. doi: 10.1089/hum.1998.9.13-1929. [DOI] [PubMed] [Google Scholar]
- 45.McEachern KA, Nietupski JB, Chuang WL, Armentano D, Johnson J, Hutto E, et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J Gene Med. 2006;8:719–729. doi: 10.1002/jgm.901. [DOI] [PubMed] [Google Scholar]
- 46.Santat L, Paz H, Wong C, Li L, Macer J, Forman S, et al. Recombinant AAV2 transduction of primitive human hematopoietic stem cells capable of serial engraftment in immune-deficient mice. Proc Natl Acad Sci U S A. 2005;102:11053–11058. doi: 10.1073/pnas.0502902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker TC, Noel RJ, Coats WS, Gómez-Foix AM, Alam T, Gerard RD, et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43(Pt A):161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 48.Bewig B, Schmidt WE. Accelerated titering of adenoviruses. Biotechniques. 2000;28:870–873. doi: 10.2144/00285bm08. [DOI] [PubMed] [Google Scholar]
- 49.Worgall S, Worgall TS, Kostarelos K, Singh R, Leopold PL, Hackett NR, et al. Free cholesterol enhances adenoviral vector gene transfer and expression in CAR-deficient cells. Mol Ther. 2000;1:39–48. doi: 10.1006/mthe.1999.0013. [DOI] [PubMed] [Google Scholar]
- 50.Mayne GC, Borowicz RA, Greeneklee KV, Finlay-Jones JJ, Williams KA, Hart PH. Centrifugation facilitates transduction of green fluorescent protein in human monocytes and macrophages by adenovirus at low multiplicity of infection. J Immunol Methods. 2003;278:45–56. doi: 10.1016/s0022-1759(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 51.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 52.Rossi GR, Mautino MR, Morgan RA. High-efficiency lentiviral vector mediated gene transfer into murine macrophages and activated splenic B lymphocytes. Hum Gene Ther. 2003;14:385–391. doi: 10.1089/104303403321208989. [DOI] [PubMed] [Google Scholar]
- 53.Koya RC, Weber JS, Kasahara N, Lau R, Villacres MC, Levine AM, et al. Making dendritic cells from the inside out: lentiviral vector-mediated gene delivery of granulocyte-macrophage colony-stimulating factor and interleukin 4 into CD14+ monocytes generates dendritic cells in vitro. Hum Gene Ther. 2004;15:733–748. doi: 10.1089/1043034041648381. [DOI] [PubMed] [Google Scholar]
- 54.Reiser J. Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther. 2000;7:910–913. doi: 10.1038/sj.gt.3301188. [DOI] [PubMed] [Google Scholar]
- 55.Zarrin AA, Malkin L, Fong I, Luk KD, Ghose A, Berinstein NL. Comparison of CMV, RSV, SV40 viral and Vlambda1 cellular promoters in B and T lymphoid and non-lymphoid cell lines. Biochim Biophys Acta. 1999;1446:135–139. doi: 10.1016/s0167-4781(99)00067-6. [DOI] [PubMed] [Google Scholar]
- 56.Fouletier-Dilling CM, Bosch P, Davis AR, Shafer JA, Stice SL, Gugala Z, et al. Novel compound enables high-level adenovirus transduction in the absence of an adenovirus-specific receptor. Hum Gene Ther. 2005;16:1287–1297. doi: 10.1089/hum.2005.16.1287. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston JC, Sauter SL, et al. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest. 1999;104:R55–R62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiser J, Harmison G, Kluepfel-Stahl S, Brady RO, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci U S A. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 61.Aoki M, Ishii T, Kanaoka M, Kimura T. RNA interference in immune cells by use of osmotic delivery of siRNA. Biochem Biophys Res Commun. 2006;341:326–333. doi: 10.1016/j.bbrc.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 62.Paukner S, Kudela P, Kohl G, Schlapp T, Friedrichs S, Lubitz W. DNA-loaded bacterial ghosts efficiently mediate reporter gene transfer and expression in macrophages. Mol Ther. 2005;11:215–223. doi: 10.1016/j.ymthe.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]