Abstract
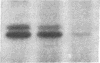
Binding of polyvalent antigens to IgE present in Fc epsilon receptors on the surface of mast cells and the RBL-2H3 cell line triggers the exocytotic release of allergic mediators. Preincubation of RBL-2H3 cells with cholera toxin (CT) was found to potentiate greater than or equal to 2- to 3-fold the rate and final amount of antigen-induced secretion of [3H]serotonin and N-acetyl beta-D-glucosaminidase. This was accompanied by a more variable increase in the initial rate of antigen-triggered formation of inositol phosphates. The holotoxin was required for potentiation, as neither the A nor the B subunit was effective when added separately. Four observations indicate that cAMP was not the primary effector of the augmentation of secretion caused by CT: (i) culture conditions were found in which CT caused large increases in secretion but very modest (or no) increases in cAMP; (ii) under other conditions, progressive increase in [CT] caused a maximum 2.5- to 3-fold increase in cAMP followed by a return to basal levels, whereas the secretory response saturated and remained stable; (iii) permeant cAMP analogs consistently enhanced secretion at low doses and inhibited at higher doses, but the peak enhancement was always much less than that achieved by an optimal dose of CT; (iv) the selective phosphodiesterase inhibitor Ro 20-1724 exhibited similar biphasic dose-response curves, the maximum enhancement again being small compared to that caused by CT itself. Both in vitro and in vivo, CT catalyzed transfer of ADP-ribose from NAD to two membrane proteins that comigrated on NaDodSO4/polyacrylamide gel electrophoresis with two CT substrates in other cell types, and these were identified by immunoblotting as Gs alpha. These results suggest that ADP-ribosylation of a cholera toxin substrate potentiates IgE-mediated secretion from RBL-2H3 cells by a largely cAMP-independent route.
Full text
PDF
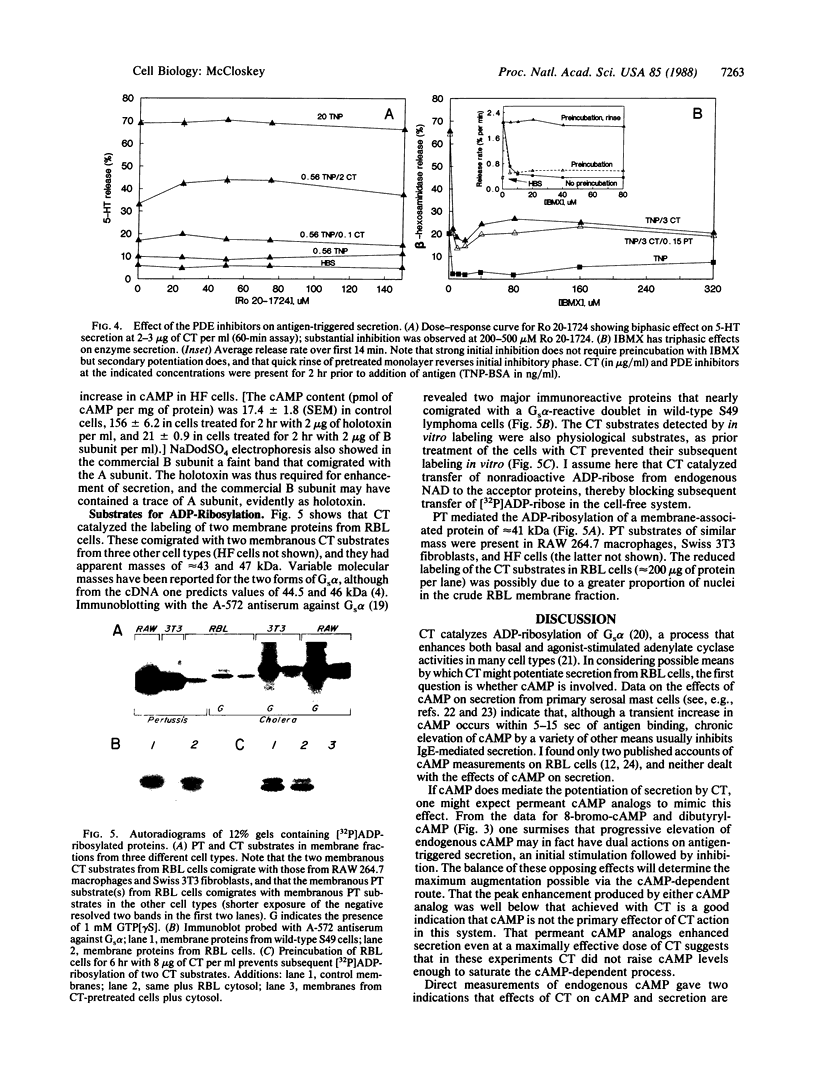

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksamit R. R., Backlund P. S., Jr, Cantoni G. L. Cholera toxin inhibits chemotaxis by a cAMP-independent mechanism. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7475–7479. doi: 10.1073/pnas.82.22.7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm E., Bloom G. D. Cyclic nucleotide involvement in histamine release from mast cells--a reevaluation. Life Sci. 1982 Jan 18;30(3):213–218. doi: 10.1016/0024-3205(82)90501-x. [DOI] [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Beaven M. A., Rogers J., Moore J. P., Hesketh T. R., Smith G. A., Metcalfe J. C. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7129–7136. [PubMed] [Google Scholar]
- Biffen M., Martin B. R. Polyphosphoinositide labeling in rat liver plasma membranes is reduced by preincubation with cholera toxin. J Biol Chem. 1987 Jun 5;262(16):7744–7750. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto K., Gill D. M. Cholera toxin activation of adenylate cyclase. Roles of nucleoside triphosphates and a macromolecular factor in the ADP ribosylation of the GTP-dependent regulatory component. J Biol Chem. 1980 Feb 25;255(4):1252–1258. [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D. Involvement of guanine nucleotide-binding protein in the gating of Ca2+ by receptors. Nature. 1983 Nov 3;306(5938):64–66. doi: 10.1038/306064a0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Lewis R. A., Austen K. F. Role of adenylate cyclase in immunologic release of mediators from rat mast cells: agonist and antagonist effects of purine- and ribose-modified adenosine analogs. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6800–6804. doi: 10.1073/pnas.77.11.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop F. C., Thomas D. D. Effect of cholera enterotoxin on calcium uptake and cyclic AMP accumulation in rat basophilic leukemia cells. Int J Biochem. 1984;16(3):275–280. doi: 10.1016/0020-711x(84)90100-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Levinson S. L., Blume A. J. Altered guanine nucleotide hydrolysis as basis for increased adenylate cyclase activity after cholera toxin treatment. J Biol Chem. 1977 Jun 10;252(11):3766–3774. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Maeyama K., Hohman R. J., Metzger H., Beaven M. A. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986 Feb 25;261(6):2583–2592. [PubMed] [Google Scholar]
- Maguire M. E., Erdos J. J. Inhibition of magnesium uptake by beta-adrenergic agonists and prostaglandin E1 is not mediated by cyclic AMP. J Biol Chem. 1980 Feb 10;255(3):1030–1035. [PubMed] [Google Scholar]
- Metzger H., Alcaraz G., Hohman R., Kinet J. P., Pribluda V., Quarto R. The receptor with high affinity for immunoglobulin E. Annu Rev Immunol. 1986;4:419–470. doi: 10.1146/annurev.iy.04.040186.002223. [DOI] [PubMed] [Google Scholar]
- Morita Y., Siraganian R. P. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J Immunol. 1981 Oct;127(4):1339–1344. [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Islet-activating protein, pertussis toxin, inhibits Ca2+-induced and guanine nucleotide-dependent releases of histamine and arachidonic acid from rat mast cells. FEBS Lett. 1984 Aug 6;173(2):414–418. doi: 10.1016/0014-5793(84)80816-9. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Warner J. A., Yancey K. B., MacGlashan D. W., Jr The effect of pertussis toxin on mediator release from human basophils. J Immunol. 1987 Jul 1;139(1):161–165. [PubMed] [Google Scholar]
- Weishaar R. E., Cain M. H., Bristol J. A. A new generation of phosphodiesterase inhibitors: multiple molecular forms of phosphodiesterase and the potential for drug selectivity. J Med Chem. 1985 May;28(5):537–545. doi: 10.1021/jm50001a001. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]