Abstract
In skeletal muscle and tendon the extracellular matrix confers important tensile properties and is crucially important for tissue regeneration after injury. Musculoskeletal tissue adaptation is influenced by mechanical loading, which modulates the availability of growth factors, including growth hormone (GH) and insulin-like growth factor-I (IGF-I), which may be of key importance. To test the hypothesis that GH promotes matrix collagen synthesis in musculotendinous tissue, we investigated the effects of 14 day administration of 33–50 μg kg−1 day−1 recombinant human GH (rhGH) in healthy young individuals. rhGH administration caused an increase in serum GH, serum IGF-I, and IGF-I mRNA expression in tendon and muscle. Tendon collagen I mRNA expression and tendon collagen protein synthesis increased by 3.9-fold and 1.3-fold, respectively (P < 0.01 and P= 0.02), and muscle collagen I mRNA expression and muscle collagen protein synthesis increased by 2.3-fold and 5.8-fold, respectively (P < 0.01 and P= 0.06). Myofibrillar protein synthesis was unaffected by elevation of GH and IGF-I. Moderate exercise did not enhance the effects of GH manipulation. Thus, increased GH availability stimulates matrix collagen synthesis in skeletal muscle and tendon, but without any effect upon myofibrillar protein synthesis. The results suggest that GH is more important in strengthening the matrix tissue than for muscle cell hypertrophy in adult human musculotendinous tissue.
Introduction
Locomotive and supportive functions are dependent upon the transmission from muscle to the skeleton of force via intramuscular connective tissue and tendon. Collagen is the most important protein in conferring the required mechanical properties (Kjaer, 2004). The adaptation of matrix tissue to mechanical load is probably mediated inter alia by growth factors, of which growth hormone (GH) and insulin-like growth factor-I (IGF-I) are among the most important. IGF-I has a well characterized muscle growth-promoting effect in loaded animal muscle due both to circulating IGF-I secreted by the liver under GH control and after local production of a number of IGF-I variants (Goldspink, 1999; Adams et al. 2007). Furthermore, muscle-specific overexpression of IGF-I in transgenic mice (Musaro et al. 2001) and acute ectopic expression of IGF-I by electroporation result in muscle hypertrophy (Alzghoul et al. 2004). Although these findings suggest involvement of GH and IGF-I in activity-dependent muscle plasticity, direct evidence for such a role has never been demonstrated in adult human muscle.
In animal tendon explants there is a dose-dependent effect of IGF-I on cell proliferation and collagen synthesis (Abrahamsson et al. 1991; Banes et al. 1995; Murphy & Nixon, 1997) and cognate effects can be seen after GH treatment of various length in muscle and tendon of rats, sheep and pigs (Pell & Bates, 1987; Kyparos et al. 2002; Choy et al. 2005). In patients with acromegaly, high blood concentrations of GH and IGF-I are associated with increased thickness and content of collagen rich tissue (Gonc & Kandemir, 2007; Zgliczynski et al. 2007), but the dynamics of this process have not been studied in detail. In hypopituitary men, who received GH treatment, there is a consequent rise in the expression of mRNA for both IGF-I and collagen 3A1 in skeletal muscle (Sjogren et al. 2007), but no studies so far have directly determined the possible effects of GH/IGF-I on local collagen protein production in human musculotendinous tissue.
Mechanical loading of muscle and tendon increases expression of both IGF-I and collagen mRNA (Olesen et al. 2006; Heinemeier et al. 2007a), but no direct coupling between GH/IGF-I and collagen synthesis determined dynamically has been demonstrated after increases in loading.
Although it has been shown that recombinant human GH (rhGH) administration has no effect on human muscle size and muscle protein synthesis (Rennie, 2003) it is nevertheless commonly assumed by coaches and athletes that this is not true. A perception of increased performance of explosive events is almost certainly mistaken (Liu et al. 2008); however, there may be subjective changes in kinaesthesia due both to the perception of oedema and possibly increased muscle stiffness due to growth of collagen tissue. This, together with increased collagen mass observed in patients with acromegaly (Gonc & Kandemir, 2007; Zgliczynski et al. 2007), reinforced our interest in the effects of rhGH on collagen metabolism in the musculoskeletal system.
In the present study, we wished to test the major hypothesis that manipulating blood concentration of GH and IGF-I, as well as tissue IGF-I mRNA expression, would increase collagen mRNA expression and collagen protein synthesis in skeletal muscle and tendon. Furthermore, we wished to check the previous report of a lack of stimulation of human muscle protein synthesis (Yarasheski et al. 1992) by measuring the synthesis of myofibrillar protein. We aimed to fulfill our objectives by studying the responses of healthy young men to 2 weeks of rhGH administration.
Methods
Healthy volunteers and GH administration
Ten healthy, sedentary men (30 ± 2 years) were recruited for the study. All were non-smoking, not taking any medication, and judged healthy based on routine medical examination and medical history. The participants gave written informed consent to a protocol adhering to the Declaration of Helsinki, which was approved by the Ethics Committee of Copenhagen and Frederiksberg (KF (01) 258765). The study was carried out as a double-blinded, randomized, placebo-controlled crossover trial. rhGH (Norditropin, Novo Nordisk, Bagsvaerd, Denmark) was administered by daily subcutaneous injection in the thigh for 14 consecutive days. rhGH dosage was 33.3 μg kg−1 day−1 on days 1–7 and 50 μg kg−1 day−1 on days 8–14. During each supplementation period, the participants visited the laboratory twice to detect possible side effects and to ensure compliance. The two periods were separated by a 5 months washout period.
Exercise
All participants did one bout of leg extension exercise (Technogym, Super Executive Line, Gambottola, Italy) with concentric unilateral load of the quadriceps muscle and patella tendon. Prior to the study, one-leg one repetition maximum (1 RM) was determined, and the workload was defined as 10 × 10 repetitions at 70% of 1 RM.
Tissue biopsy procedures
Biopsies were taken 24 h after exercise with the subjects in a post-absorptive state (10 h fasting). The sample sites were prepared with local anaesthetics (lidocaine, 1%), and tendon and muscle was sampled from both the exercised and the rested leg. Tendon samples were taken from the patella tendon (Bard Magnum Biopsy Instrument, C.R. Bard, Inc., Covington, GA, USA) with a 14 g needle (Movin, 2000). The total wet weight of each tendon sample was from 5 to 10 mg per biopsy. Muscle was sampled from the vastus lateralis (m. quadriceps) muscle using a 5 mm Bergström needle with suction (Bergstrom, 1975). The total wet weights of the muscle samples were from 50 to 80 mg. Tendon and muscle samples were frozen in liquid nitrogen and stored at −80°C.
Muscle and tendon mRNA measurements
The amount of mRNA for collagen α1(I) (COL1A1), collagen α1(III) (COL3A1), IGF-IEa and IGF-IEc was measured with real-time reverse transcriptase (RT) PCR (Table 1). Tendon and muscle were homogenized in TriReagent (Molecular Research Centre, Cincinnati, OH, USA) using a bead-mixer with steel beads (Biospec Products, Bartlesville, OK, USA). Following homogenization, bromo-chloropropane (Molecular Research Centre) was added in order to separate the samples into an aqueous and an organic phase. Glycogen was added to the tendon samples to improve RNA precipitation. Following isolation of the aqueous phase, RNA was precipitated using isopropanol, washed in ethanol and dissolved in RNAse-free water. All tissue samples were weighed prior to RNA extraction. Muscle RNA concentrations were determined by spectroscopy and tendon RNA concentrations were determined using RiboGreen assay (Molecular Probes, Eugene, OR, USA). Good RNA quality was ensured by gel electrophoresis, which was combined with Northern blotting with radioactive probes for 28S and 18S ribosomal RNA for the smaller tendon samples.
Table 1.
Primers for real-time PCR
| MRNA | Sense | Antisense | GenBank Acc. No. |
|---|---|---|---|
| IGF-IEa# | GACATGCCCAAGACCCAGAAGGA | CGGTGGCATGTCACTCTTCACTC | NM_00618.3 |
| IGF-IEc# | GCCCCCATCTACCAACAAGAACAC | CGGTGGCATGTCACTCTTCACTC | NM_001111283.1 |
| COL1A1 | GGCAACAGCCGCTTCACCTAC | GCGGGAGGACTTGGTGGTTTT | Z74615.1 |
| COL3A1 | CACGGAAACACTGGTGGACAGATT | ATGCCAGCTGCACATCAAGGAC | NM_000090.3 |
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT | NM_002046.3 |
| RPLP0 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG | NM_053275.3 |
The IGF-IEc specific 49 base-pair insertion (Hameed et al. 2002) is discriminated by an insertion-specific sense primer (IGF-IEc) versus an insertion point overlapping primer (last two 3′ bases) sense primer (IGF-IEa).
Synthesis of complementary DNA (cDNA) was performed using the Omniscript reverse transcriptase (Qiagen, Hilden, Germany) on 500 ng of muscle RNA and Sensiscript reverse transcriptase (Qiagen) on 30 ng of tendon RNA, both in 20 μl. For each target mRNA, 0.25 μl cDNA was amplified in 25 μl Quantitect SYBR Green Master Mix (Qiagen) with specific primers (100 nm each, Table 1) on a real-time PCR machine (MX3000P, Stratagene, La Jolla, CA, USA). The thermal profile was 95°C, 10 min → (95°C, 15 s → 58°C, 30 s → 63°C, 90 s) × 50 → 95°C, 60 s → 55°C, 30 s → 95°C, 60 s. Signal intensity was acquired at the 63°C step and the threshold cycle (Ct) values were related to a standard curve made with the cloned PCR product. Specificity was confirmed by melting curve analysis after amplification (the 55°C to 95°C step). We analysed the tissue samples for both IGF-IEa and IGF-IEc. A 49 base-pair insertion in IGF-IEc makes this isoform distinguishable from IGF-IEa. In the IGF-IEa assay the sense primer was placed over the insertion point ensuring that the last two bases fitted downstream of the insertion point. This creates a single base mismatch between IGF-IEa and IGF-IEc in the 3′ end (…GGA-3′ in IGF-IEa versus…GTA-3′ in IGF-IEc), which should inhibit amplification from IGF-IEc cDNA (Wu et al. 2009). Indeed, there was no amplification of the 49 base-pair larger IGF-IEc isoform in the IGF-IEa assay, based on agarose gel and melting curve analysis. For the IGF-IEc assay, the sense primer was placed in the IGF-IEc specific insertion. The large ribosomal protein P0 (RPLP0) mRNA, which was stably expressed relative both to GAPDH mRNA and total RNA (data not shown), was chosen as internal control.
Collagen and myofibrillar protein fractional synthesis rate (FSR)
Measurement of collagen and myofibrillar protein FSR was carried out according to previous techniques (Babraj et al. 2002). Plasma proline was used to establish the natural abundance of [15N] proline and [1-13C] proline, thereby eliminating the need for a pre-infusion basal tissue biopsy. To label collagen protein in muscle and tendon, a flooding dose of [15N] proline or [1-13C] proline (0.75–1 g labelled proline (>99 atoms%, Cambridge Isotope Laboratory, Andover, MA, USA) plus 3–3.25 g unlabelled proline (AppliChem, Darmstadt, Germany), 4 g total) was given. Blood samples were drawn every 10–30 min during the 2 h experiment to establish area under the curve (AUC) for the precursor. For extraction of proteins, tendon tissue was ground in liquid nitrogen to a fine powder, re-suspended in extraction buffer (0.02 m Tris-HCl, 0.15 m NaCl, 0.1 m EDTA, 0.1% Triton X-100, pH 7.4), and centrifuged (1600 g, 4°C) to pellet the collagen. The pellets were washed twice with 70% ethanol. Muscle tissue was ground in liquid nitrogen and re-suspended in extraction buffer. The homogenate was centrifuged (1600 g, 4°C), the supernatant removed and the myofibrillar/collagen pellet re-suspended in 0.3 m NaOH at 37°C for 20 min. The soluble myofibrillar protein and the insoluble collagen were separated by centrifugation. The myofibrillar fraction was precipitated using 1 m perchloric acid and the pellet washed twice with 70% ethanol. For protein hydrolysis and gas-chromatography mass-spectrometry, protein from all sources was hydrolysed in 6 m HCl at 110°C overnight and the amino- and imino-acids purified using cation exchanger (Dowex 50WX8, Biorad, Copenhagen, Denmark) and eluted by 2 m NH4OH. The amino- and imino-acids were derivatized as their N-acetyl-N-propyl (NAP) esters. NAP amino- and imino-acids were analysed by capillary gas-chromatography–combustion–isotope-ratio mass-spectrometer (GC-C-IRMS) (Delta-plus XL, Thermo Finnigan, Bremen, Germany); separation was achieved on a 30 m × 0.25 mm, 1.5 μm film DB 1701 capillary column (J&W Scientific, Agilent Technologies, USA). Plasma proline enrichments were analysed as their t-butyldimethylsilyl derivative by gas-chromatography mass-spectrometry (Trace DSQ, Thermo Fisher Scientific, Hemel Hempstead, UK) using a EC-1, 30 m × 0.25 mm, 0.25 μm column (Econocap, Grace, Deerfield, USA). The rate of tissue protein synthesis was calculated according to the precursor-product principle as: Fractional protein synthesis (FSR% h−1) =ΔEp× 100/AUCp. ΔEp is the change in proline labelling over time in tissue protein and AUCp is the area under the curve of venous proline labelling with time in hours.
Hormone assays
Blood samples drawn from the antecubital vein were separated (3200 g, 4°C) and serum was stored at −80°C. Serum GH (sGH) and serum IGF-I (sIGF-I) concentrations were determined by a time-resolved immuno-fluorometric assay (TR-IFMA, Perkin Elmer, Turku, Finland). Serum IGFBP-1 (sIGFBP-1) was determined by an in-house radioimmunoassay as described previously (Westwood et al. 1994). Serum IGFBP-3 (sIGFBP-3) was measured by commercially available immunoradiometric assay (IRMA, BioSource Europe, Nivelles, Belgium). The hormone assays have intra- and inter-assay coefficients of variation below 5 and 10%, respectively.
Statistics
Using SAS (Statistical Analysis System, SAS Institute Inc., Cary, NC, USA) the mixed linear model procedure was conducted for the effect of factors and interaction on tissue mRNA expression and protein FSR. Factors were rhGH/placebo, exercise/rest and period 1/period 2. Outcome was difference between groups with confidence interval (CI) and significance level. Subgroup/post hoc analysis was not conducted. mRNA data were log-transformed before statistical analysis and are presented as geometric mean ± back-transformed s.e.m. Differences were considered significant when P < 0.05. For serum hormones, differences between rhGH vs. placebo were tested with paired t tests at the individual time points. Pearson's correlation analysis was used to determine baseline correlation between systemic IGF-I/local IGF-I mRNA and collagen mRNA. Subject characteristics are presented as mean ±s.d. All other results are presented as mean ±s.e.m.
Results
All 10 participants completed both rhGH and placebo trial periods, and were fully compliant with regard to rhGH/placebo administration according to GH-administration diaries and sGH and sIGF-I measurements.
Systemic GH and IGF-I
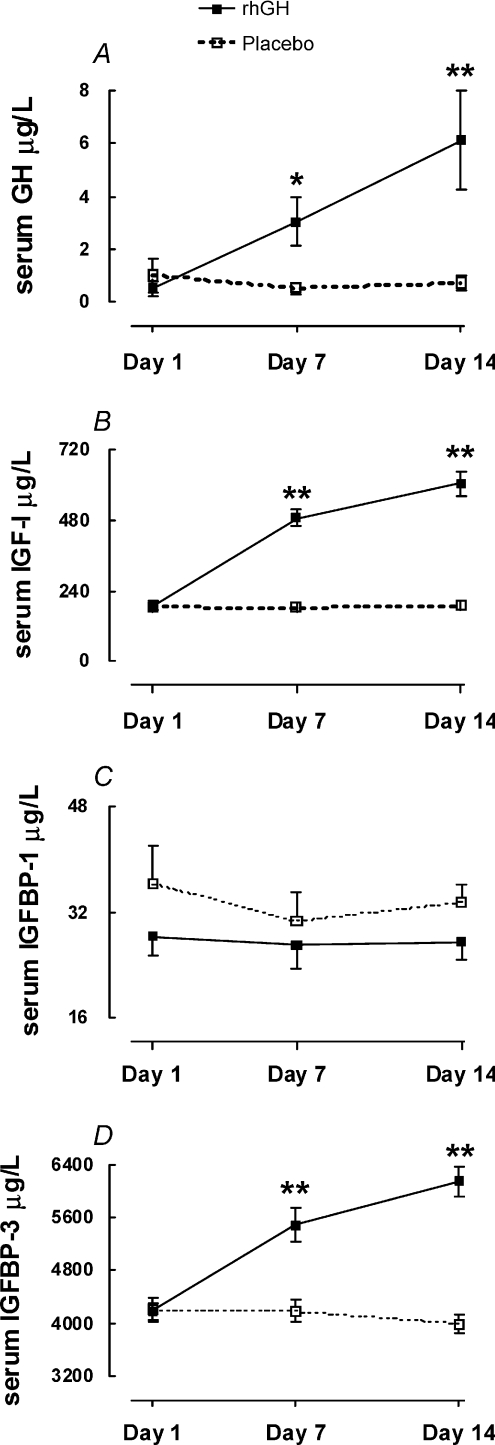
sGH was increased by 5.8-fold (95% CI: 1.3; 10; P= 0.02) on day 7 and by 8.5-fold (95% CI: 3.0; 14; P= 0.01) on day 14 of rhGH supplementation (Fig. 1A). sIGF-I was increased by 2.7-fold (95% CI: 2.3; 3.1; P < 0.01) on day 7 and by 3.2-fold (95% CI: 2.7; 3.7; P < 0.01) on day 14 of rhGH supplementation (Fig. 1B). sIGFBP-1 did not respond to rhGH supplementation (Fig. 1C), whereas sIGFBP-3 increased by 1.3-fold (95% CI: 1.1; 1.5; P < 0.01) on day 7 and by 1.5-fold (95% CI: 1.4; 1.7; P < 0.01) on day 14 of rhGH supplementation (Fig. 1D).
Figure 1. Circulating concentrations of GH, IGF-I, IGF-BP-1 and IGF-BP-3 in young male participants (n= 10) that were studied over 14 days in a crossover design with rhGH/placebo supplementation.
Data are means ±s.e.m.*Difference between rhGH supplementation and placebo P < 0.05. **Difference between rhGH and placebo P < 0.01.
Design
The crossover design necessitated two biopsies from each patella tendon. In order to avoid a carry-over effect from the first to the second tendon biopsy, the two biopsies were separated by 5 months and the anatomical locations of the biopsies were separated by at least 1 cm. There was, however, a significant carry-over effect of the repeated biopsy (referred to as period-effect in the text) on the concentration of collagen I and III mRNA in tendon (both P < 0.01). Although interesting in itself, this required additional statistical evaluation. The period-effect interacted significantly with exercise for both collagen I and III mRNA (P < 0.01), making interpretation of the individual effect of period and exercise on tendon collagen mRNA complicated. There was no period-effect on tendon IGF-IEa mRNA (P= 0.26) but period-effect and exercise interacted significantly for tendon IGF-IEa mRNA (P= 0.01). Most importantly, there was no interaction between rhGH supplementation and period-effect (P > 0.14) or rhGH supplementation and exercise (P > 0.81) for either collagen I, collagen III or IGF-IEa mRNA. This shows that the effect of rhGH treatment on mRNA for collagen I, III and IGF-IEa in tendon was not influenced by the level of period-effect or by exercise. Data for tendon collagen I, III and IGF-IEa mRNA expression are presented as differences between placebo and rhGH supplementation only (Figs 2A, and 3A and B). Essentially, there was no period-effect on tendon collagen protein FSR (P= 0.45) or on any of the measured variables in skeletal muscle (P > 0.25), where biopsies were taken more than 5 cm apart.
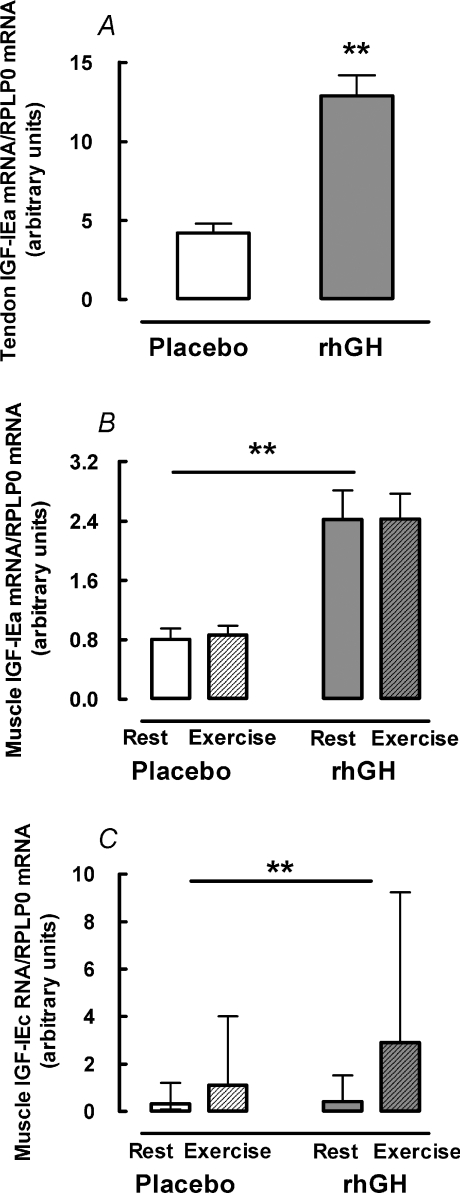
Figure 2. Local IGF-I isoform mRNA expression in human skeletal muscle and tendon.
Young male participants (n= 10) were studied over 14 days in a crossover design with rhGH/placebo supplementation. Data for exercise effect on tendon mRNA is not shown due to repeated biopsy effect (see text for details). Data are geometric means ± back-transformed s.e.m. A, tendon IGF-IEa. B, muscle IGF-IEa. C, muscle IGF-IEc. **Difference between rhGH and placebo P < 0.01.
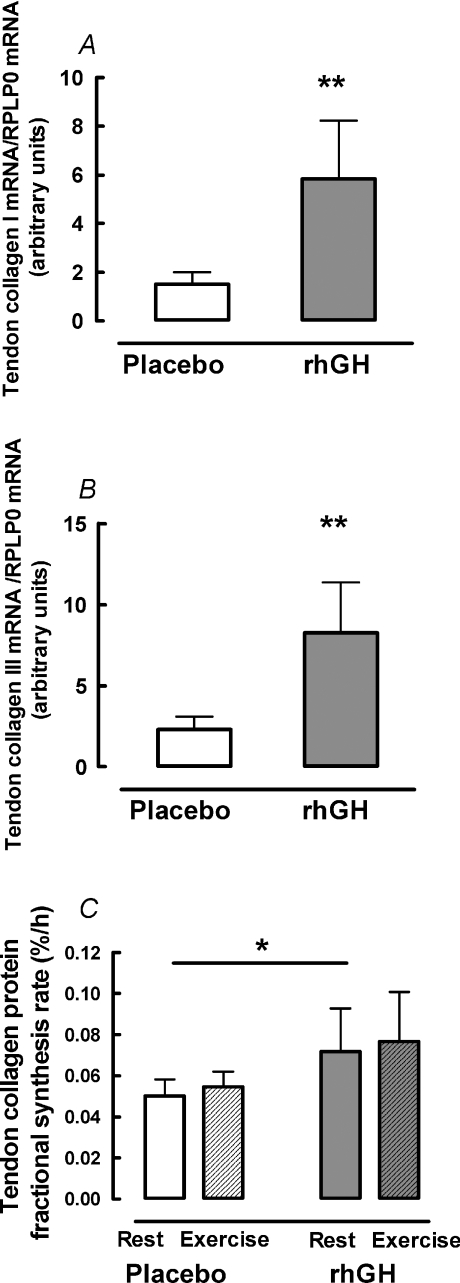
Figure 3. Tendon collagen mRNA expression and protein fractional synthetic rate.
Young male participants (n= 10) were studied over 14 days in a crossover design with rhGH/placebo supplementation. Data for exercise effect on tendon mRNA are not shown due to repeated biopsy effect (see text for details). Data are geometric means ± back-transformed s.e.m. A, collagen I mRNA. B, collagen III mRNA. C, protein fractional synthesis rate. *Difference between rhGH and placebo P < 0.05. **Difference between rhGH and placebo P < 0.01.
Tendon
Tendon IGF-IEa mRNA increased 3.0-fold (95% CI: 2.3; 3.9; P < 0.01) after rhGH supplementation (Fig. 2A). IGF-IEc mRNA concentration was below the detection limit in 30% of the tendon biopsies and in the remaining samples there was no effect of rhGH (P= 0.31) or exercise (P= 0.91) (not shown). rhGH supplementation increased collagen I mRNA by 3.9-fold (95% CI: 2.0; 7.8; P < 0.01) and collagen III mRNA by 3.6-fold (95% CI: 2.0; 6.4; P < 0.01) (Fig. 3A and B). Tendon collagen protein FSR was 1.3−fold (95% CI: 1.1; 1.5; P= 0.02) higher after rhGH supplementation than placebo (Fig. 3C). Prior exercise did not affect collagen mRNA (data not shown due to repeated biopsy effect) or collagen protein FSR (P > 0.44) and there was no interaction between rhGH and exercise (P= 0.95) either for collagen mRNA or protein synthesis (Fig. 3).
Muscle
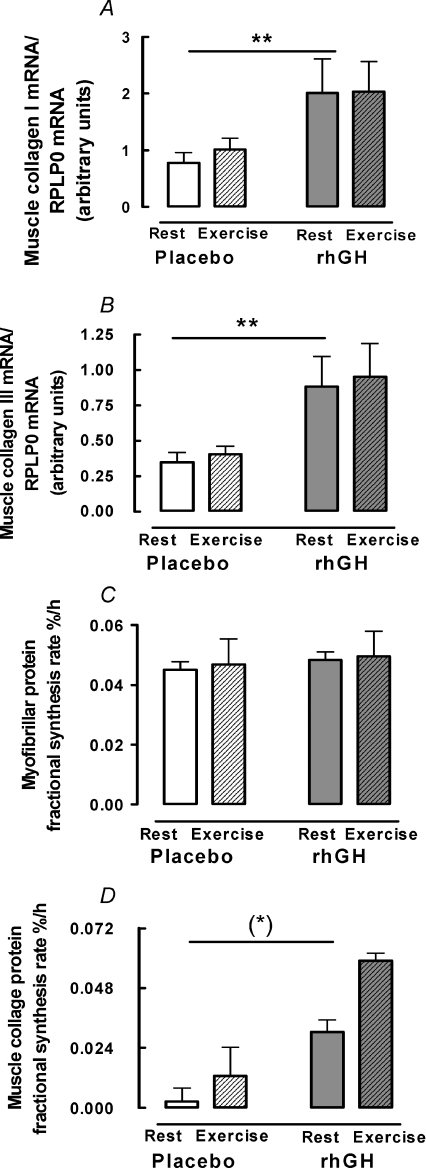
In muscle, IGF-IEa mRNA was 2.9-fold higher (95% CI: 2.1; 3.9; P < 0.01) and IGF-IEc mRNA was 5.0-fold higher (95% CI: 3.3; 7.5; P < 0.01) with rhGH supplementation relative to placebo (Fig. 2B and C). There was no effect of exercise on IGF-IEa (P= 0.72) or IGF-IEc (P= 0.23) in muscle and no interaction between rhGH and exercise on either isoform (P > 0.74) (Fig. 2B and C). Relative to placebo, rhGH supplementation increased mRNA for collagen I by 2.3-fold (95% CI: 1.3; 4.0; P < 0.01) and collagen III by 2.5-fold (95% CI: 1.5; 3.9; P < 0.01) (Fig. 4A and B). Although not statistically significant, a tendency towards a 5.8-fold (95% CI: 0.9; 11.8; P= 0.06) increase in muscle collagen protein FSR was observed following rhGH supplementation (Fig. 4D). Exercise did not affect collagen I mRNA (P= 0.44), collagen III mRNA (P= 0.47) or collagen protein FSR (P > 0.10) and there was no interaction between rhGH supplementation and exercise for any collagen mRNA or collagen protein FSR (P > 0.49) (Fig. 4). Muscle myofibrillar protein FSR was not affected by rhGH (P= 0.75) or exercise (P= 0.10) and there was no interaction between rhGH and exercise (P= 0.49) (Fig. 4C).
Figure 4. Muscle collagen mRNA expression and protein fractional synthesis rate, and muscle myofibrillar protein fractional synthesis rate.
Young male participants (n= 10) were studied over 14 days in a crossover design with rhGH/placebo supplementation. Data are mean ±s.e.m. A, collagen I mRNA. B, collagen III mRNA. C, myofibrillar protein fractional synthesis rate. D, collagen protein fractional synthesis rate. (*) Difference between rhGH and placebo P= 0.06. **Difference between rhGH and placebo P < 0.01.
IGF-I–collagen correlation
Local tissue IGF-I mRNA expression correlated significantly with collagen I mRNA expression in both tendon (r2= 0.48, P= 0.03) and muscle (r2= 0.33, P= 0.02) (not shown). Serum IGF-I did not correlate significantly with collagen I mRNA expression in either tendon (r2= 0.34, P= 0.08) or muscle (r2= 0.11, P= 0.20) (not shown).
Discussion
The major finding in the present study is that increased GH availability causes increased expression of collagen mRNA and also increased collagen protein synthesis in connective tissue in human skeletal muscle and tendon, but has no effect on myofibrillar protein synthesis. Moreover, it was demonstrated that serum IGF-I, as well as tissue IGF-I mRNA expression was related to serum GH titre. These findings demonstrate that GH caused a rise in matrix collagen synthesis of skeletal muscle and tendon, but did not reveal any effect on myofibrillar protein synthesis.
Effect of GH on systemic and local IGF-I
As expected, sIGF-I and sIGFBP-3 availability was related to sGH titre. Supplementation with rhGH, which was gradually increased over time, led to a gradual increase in sIGF-I/IGFBP-3 following the pattern of the sGH increase (Fig. 1). This observation is of fundamental importance for the interpretation of the results as a whole, because it confirmed that the rhGH supplementation was sufficient to stimulate the GH/IGF-I axis.
An important finding was that local IGF-I mRNA expression increased in both tendon and muscle in response to rhGH supplementation. The finding that elevating GH not only increases IGF-I systemically but also results in an elevated expression of IGF-IEa mRNA locally in tendon tissue in man is, to our knowledge, novel and supports the observation of GH-induced rise in mRNA for both IGF-I and collagen in skeletal muscle of hypopituitary men (Sjogren et al. 2007). It is also consonant with the described effect of GH in several animal tissues (Butler & Le Roith, 2001) and it is supported by earlier observations made in human skeletal muscle after 5 and 12 weeks of rhGH supplementation in elderly subjects (Hameed et al. 2004). The finding that IGF-IEc is present in human tendon, albeit in small amounts, is novel and is supported by observations in rats (Heinemeier et al. 2007b).
GH/IGF-I effect on connective tissue
A primary observation was that the GH/IGF-I axis appeared to be closely involved in regulation of the connective tissue supporting skeletal muscle. Administration of rhGH increased collagen I and III mRNA 2-fold in both tendon and muscle, and collagen protein FSR 1.3-fold and 6-fold in tendon and muscle, respectively (Figs 3 and 4).
Reports showing a 2-fold increase in the concentration of markers for collagen synthesis (pro-collagen III pro-peptide (PIIIP)) in human adults following GH supplementation (Longobardi et al. 2000; Powrie et al. 2007) and appropriately altered PIIIP concentrations in acromegalic and growth hormone-deficient (GHD) patients (Ueland et al. 2006; Gonc & Kandemir, 2007) are compatible with our observations of increased collagen mRNA expression and synthesis after GH elevation. These studies support the view that GH stimulates collagen synthesis; nevertheless, as serum PIIIP only indirectly reflects whole body collagen synthesis, such observations are clearly inconclusive regarding changes in the collagen matrixes in muscle and tendon. Other results from animal work support our conclusions (Wilson et al. 1995), although intramuscular GH injections did not affect tendon collagen synthesis in pigs until after 3 months (Choy et al. 2005).
Importance of IGF-I signalling
The observed effect of rhGH supplementation on collagen synthesis was possibly mediated by IGF-I signalling. A significant correlation between local IGF-I mRNA and collagen mRNA in both muscle and tendon was demonstrated, whereas no significant correlations between sGH or sIGF-I and collagen expression were observed in the rhGH-supplemented participants. This suggests a role for local IGF-I signalling, a notion that is supported by animal studies, showing that circulating IGF-I has little effect on overall growth in mice (Sjogren et al. 1999), while locally produced IGF-I is a prerequisite for GH stimulatory effects in muscle and cartilage (Schlechter et al. 1986; Kim et al. 2005). IGF-I is known to induce collagen synthesis in animal tendon explants (Abrahamsson et al. 1991; Banes et al. 1995; Murphy & Nixon, 1997), and in mice that over-express human IGF-I locally it was demonstrated that the collagen content was elevated in heart muscle (Delaughter et al. 1999). These findings are in accordance with our results and support the view that IGF-I rather that GH directly is responsible for the elevated collagen expression and protein synthesis seen in the present study (Figs 3 and 4).
Muscle fibrillar protein synthesis
In contrast to the effect on connective tissue, elevated GH did not increase myofibrillar protein synthesis. With rhGH supplementation, both circulating and local IGF-I was increased by 3-fold but this did not enhance myofibrillar protein FSR (Fig. 4). This finding is in contrast to the well-established positive effect of GH and IGF-I on muscle protein and strength found in animals with a large growth potential (Musaro et al. 2001; Quinn et al. 2007), but it is in concert with previous human experiments where no effect on muscle strength and protein FSR was observed (Yarasheski et al. 1993; Blackman et al. 2002; Lange et al. 2002; Berggren et al. 2005; Ehrnborg et al. 2005). So rather than causing muscle fibre growth, GH/IGF-I appear to stimulate the supporting connective tissue that would help force transmission from the contracting muscle fibres to the bone.
Exercise effect
Based on previous observations of a positive correlation between mechanical loading and collagen synthesis in human muscle and tendon (Miller et al. 2005; de Boer et al. 2007), we attempted to introduce a local exercise-induced stimulation of protein synthesis. This was done to study a possible interaction between the effects of exercise and IGF-I status, as suggested in vitro (Banes et al. 1995). Although the positive effect of GH on local collagen production persisted during exercise, with rhGH supplementation there was somewhat surprisingly no effect of exercise per se on collagen or myofibrillar protein synthesis and no interactions between GH and exercise. The explanation probably lies in the exercise mode. In our study we aimed at giving a well-defined bout of concentric resistance exercise with high load with a known potential to increase mixed muscle protein synthesis (Phillips et al. 1997; de Boer et al. 2007). However, the total number of repetitions was only 100, in contrast to approximately 2000 light load repetitions in the study by Miller et al. (2005), and may therefore have been insufficient to create a robust exercise-induced response on collagen synthesis. In this context, our findings suggest that the total number of repetitions, rather than the load, is important in eliciting a response in collagen and myofibrillar protein synthesis measured 24 h post exercise.
Clinical perspectives
In this study, just 14 days of rhGH supplementation in healthy individuals increased collagen synthesis by up to 6-fold without causing any side effects. An increase of this magnitude holds clinical perspectives in relation to traumatic musculoskeletal injuries, where the collagen matrix inevitably is damaged (James et al. 2008). In animals, local and systemic application of IGF-I has been shown to increase both tendon collagen content and force-to-failure in relation to both overuse and acute injuries (Dahlgren et al. 2002; Provenzano et al. 2007), and similar observations are made in animal skin and bone (Dunaiski & Belford, 2002; Andreassen & Oxlund, 2003). Very few studies have investigated the effects of GH or IGF-I treatment in relation to human musculoskeletal tissue injuries. Tentatively indicative of a beneficial effect of GH treatment is a case control study with six patients, in whom application of a mixture of growth factors, including IGF-I, increased foot range of motion and decreased recovery time after Achilles tendon rupture (Sanchez et al. 2007). However, more convincing evidence is given in a placebo-controlled trial with 406 patients, in whom a significantly shorter time to healing of closed fractures was observed after high-dose GH treatment (Raschke et al. 2007). This is consonant with the increased collagen synthesis in muscle and tendon we observed after GH treatment given in similar high doses.
Conclusion
In conclusion, we found that rhGH administration caused a rise in matrix collagen synthesis in skeletal muscle and tendon, but had no effect on myofibrillar protein synthesis. The observed increase in both systemic IGF-I and tissue IGF-I mRNA suggests IGF-I signalling. Thus, GH/IGF-I apparently reinforces the supporting collagen framework around muscle fibres rather than the muscle contractile apparatus per se in adult skeletal muscle. GH/IGF-I may be more biologically important for strengthening the supportive matrix in tissues than for muscle cell hypertrophy in adult human musculotendinous tissue.
Acknowledgments
We thank the participants for their time and commitment to the study. Additionally, we thank Ann-Marie Sedstrøm, Ann-Christina Reimann, Kirsten Nyborg and Anna Selby for their technical assistance. This work was supported by the Danish Medical Research Council (271-07-0742), The Danish National Research Board, Novo-Nordisk Foundation, Lundbeck Foundation, The Nordea Foundation, Danish Anti-Doping Foundation, Team Denmark (33241979-06), the Eva and Henry Frænkels Memorial Foundation, UK BBSRC (BB/X510697/1 and BB/C516779/1) and the EC EXEGENESIS program, and the Clinical Institute at Aarhus University Hospital and Danish Rheumatism Association.
Glossary
Abbreviations
- 1 RM
1 repetition maximum
- AUC
area under the curve
- cDNA
complementary DNA
- CI
confidence interval
- COL1A1
collagen α1(I)
- COL3A1
collagen α1(III)
- Ct
threshold cycle
- FSR
fractional synthesis rate
- GAPDH
glyceralaldehyde-3-phosphate dehydrogenase
- GC-C-IRMS
gas-chromatography-combustion-isotope-ratio-mass-spectrometer
- GH
growth hormone
- GHD
growth hormone deficient
- IGF-I
insulin-like growth factor-I
- IGF-IEa
insulin-like growth factor-IEa
- IGF-IEc
insulin-like growth factor-IEc
- IGFBP
IGF-binding protein
- NAP
N-acetyl- N -propyl
- PIIIP
pro-collagen III pro-peptide
- rhGH
recombinant human GH
- RPLP0
large ribosomal protein P0
- sGH
serum GH
- sIGF-I
serum IGF-IEa
- sIGFBP
serum IGFBP
Author contributions
The experiments were performed at the Institute of Sports Medicine, Bispebjerg Hospital, Center of Healthy Aging, Faculty of Health Sciences, University of Copenhagen, Bispebjerg Bakke 23, DK-2400 Copenhagen NV, Denmark. All the authors have contributed to conception, design, analysis, interpretation of data, drafting and revising the manuscript, as well as to the final approval of the version to be published.
References
- Abrahamsson SO, Lundborg G, Lohmander LS. Recombinant human insulin-like growth factor-I stimulates in vitro matrix synthesis and cell proliferation in rabbit flexor tendon. J Orthop Res. 1991;9:495–502. doi: 10.1002/jor.1100090405. [DOI] [PubMed] [Google Scholar]
- Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J Appl Physiol. 2007;103:1644–1654. doi: 10.1152/japplphysiol.00669.2007. [DOI] [PubMed] [Google Scholar]
- Alzghoul MB, Gerrard D, Watkins BA, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18:221–223. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- Andreassen TT, Oxlund H. Local anabolic effects of growth hormone on intact bone and healing fractures in rats. Calcif Tissue Int. 2003;73:258–264. doi: 10.1007/s00223-002-2074-6. [DOI] [PubMed] [Google Scholar]
- Babraj J, Cuthbertson DJ, Rickhuss P, Meier-Augenstein W, Smith K, Bohe J, et al. Sequential extracts of human bone show differing collagen synthetic rates. Biochem Soc Trans. 2002;30:61–65. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Tsuzaki M, Hu P, Brigman B, Brown T, Almekinders L, Lawrence WT, Fischer T. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech. 1995;28:1505–1513. doi: 10.1016/0021-9290(95)00098-4. [DOI] [PubMed] [Google Scholar]
- Berggren A, Ehrnborg C, Rosen T, Ellegard L, Bengtsson BA, Caidahl K. Short-term administration of supraphysiological recombinant human growth hormone (GH) does not increase maximum endurance exercise capacity in healthy, active young men and women with normal GH-insulin-like growth factor I axes. J Clin Endocrinol Metab. 2005;90:3268–3273. doi: 10.1210/jc.2004-1209. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- Butler AA, Le Roith D. Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol. 2001;63:141–164. doi: 10.1146/annurev.physiol.63.1.141. [DOI] [PubMed] [Google Scholar]
- Choy VE, Kyparos A, Vailas AC, Crenshaw TD, Martinez DA. The biphasic response of porcine tendon to recombinant porcine growth hormone. Growth Horm IGF Res. 2005;15:39–46. doi: 10.1016/j.ghir.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dahlgren LA, Van Der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002;20:910–919. doi: 10.1016/S0736-0266(02)00009-8. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, et al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585:241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. doi: 10.1096/fasebj.13.14.1923. [DOI] [PubMed] [Google Scholar]
- Dunaiski V, Belford DA. Contribution of circulating IGF-I to wound repair in GH-treated rats. Growth Horm IGF Res. 2002;12:381–387. doi: 10.1016/s1096-6374(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Ehrnborg C, Ellegard L, Bosaeus I, Bengtsson BA, Rosen T. Supraphysiological growth hormone: less fat, more extracellular fluid but uncertain effects on muscles in healthy, active young adults. Clin Endocrinol (Oxf) 2005;62:449–457. doi: 10.1111/j.1365-2265.2005.02240.x. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323–334. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonc EN, Kandemir N. Long-term effects of growth hormone (GH) on bone mineral status and bone turnover markers in patients with isolated GH deficiency and multiple pituitary hormone deficiency. Clin Endocrinol (Oxf) 2007;66:672–677. doi: 10.1111/j.1365-2265.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- Hameed M, Harridge SD, Goldspink G. Sarcopenia and hypertrophy: a role for insulin-like growth factor-1 in aged muscle? Exerc Sport Sci Rev. 2002;30:15–19. doi: 10.1097/00003677-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Hameed M, Lange KH, Andersen JL, Schjerling P, Kjaer M, Harridge SD, Goldspink G. The effect of recombinant human growth hormone and resistance training on IGF-I mRNA expression in the muscles of elderly men. J Physiol. 2004;555:231–240. doi: 10.1113/jphysiol.2003.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007a;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M. Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and -tendon: differential effects of specific contraction types. J Appl Physiol. 2007b;102:573–581. doi: 10.1152/japplphysiol.00866.2006. [DOI] [PubMed] [Google Scholar]
- James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Kim H, Barton E, Muja N, Yakar S, Pennisi P, Leroith D. Intact insulin and insulin-like growth factor-I receptor signalling is required for growth hormone effects on skeletal muscle growth and function in vivo. Endocrinology. 2005;146:1772–1779. doi: 10.1210/en.2004-0906. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Kyparos A, Orth MW, Vailas AC, Martinez DA. Growth and maturational changes in dense fibrous connective tissue following 14 days of rhGH supplementation in the dwarf rat. Growth Horm IGF Res. 2002;12:367–373. doi: 10.1016/s1096-6374(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Lange KH, Andersen JL, Beyer N, Isaksson F, Larsson B, Rasmussen MH, et al. GH administration changes myosin heavy chain isoforms in skeletal muscle but does not augment muscle strength or hypertrophy, either alone or combined with resistance exercise training in healthy elderly men. J Clin Endocrinol Metab. 2002;87:513–523. doi: 10.1210/jcem.87.2.8206. [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Olkin I, Friedlander A, Liu V, Roberts B, et al. Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med. 2008;148:747–758. doi: 10.7326/0003-4819-148-10-200805200-00215. [DOI] [PubMed] [Google Scholar]
- Longobardi S, Keay N, Ehrnborg C, Cittadini A, Rosen T, Dall R, et al. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. J Clin Endocrinol Metab. 2000;85:1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movin T. Tendon tissue sampling. Scand J Med Sci Sports. 2000;10:368–371. doi: 10.1034/j.1600-0838.2000.010006368.x. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Nixon AJ. Biochemical and site-specific effects of insulin-like growth factor I on intrinsic tenocyte activity in equine flexor tendons. Am J Vet Res. 1997;58:103–109. [PubMed] [Google Scholar]
- Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized IGF-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Olesen JL, Heinemeier KM, Haddad F, Langberg H, Flyvbjerg A, Kjaer M, Baldwin KM. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins, and collagen mRNA in mechanically loaded plantaris tendon. J Appl Physiol. 2006;101:183–188. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- Pell JM, Bates PC. Collagen and non-collagen protein turnover in skeletal muscle of growth hormone-treated lambs. J Endocrinol. 1987;115:R1–R4. doi: 10.1677/joe.0.115r001. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Powrie JK, Bassett EE, Rosen T, Jorgensen JO, Napoli R, Sacca L, et al. Detection of growth hormone abuse in sport. Growth Horm IGF Res. 2007;17:220–226. doi: 10.1016/j.ghir.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Ejandro-Osorio AL, Grorud KW, Martinez DA, Grindeland RE, Vailas AC, Vanderby R., Jr Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: evaluation of loaded and unloaded ligaments. BMC Physiol. 2007;7:2. doi: 10.1186/1472-6793-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Plymate SR. Muscle-specific overexpression of the type 1 IGF receptor results in myoblast-independent muscle hypertrophy via PI3K, and not calcineurin, signalling. Am J Physiol Endocrinol Metab. 2007;293:E1538–E1551. doi: 10.1152/ajpendo.00160.2007. [DOI] [PubMed] [Google Scholar]
- Raschke M, Rasmussen MH, Govender S, Segal D, Suntum M, Christiansen JS. Effects of growth hormone in patients with tibial fracture: a randomised, double-blind, placebo-controlled clinical trial. Eur J Endocrinol. 2007;156:341–351. doi: 10.1530/EJE-06-0598. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–51. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- Schlechter NL, Russell SM, Spencer EM, Nicoll CS. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proc Natl Acad Sci U S A. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren K, Leung KC, Kaplan W, Gardiner-Garden M, Gibney J, Ho KK. Growth hormone regulation of metabolic gene expression in muscle: a microarray study in hypopituitary men. Am J Physiol Endocrinol Metab. 2007;293:E364–E371. doi: 10.1152/ajpendo.00054.2007. [DOI] [PubMed] [Google Scholar]
- Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Fougner SL, Godang K, Schreiner T, Bollerslev J. Serum GH and IGF-I are significant determinants of bone turnover but not bone mineral density in active acromegaly: a prospective study of more than 70 consecutive patients. Eur J Endocrinol. 2006;155:709–715. doi: 10.1530/eje.1.02285. [DOI] [PubMed] [Google Scholar]
- Westwood M, Gibson JM, Davies AJ, Young RJ, White A. The phosphorylation pattern of insulin-like growth factor-binding protein-1 in normal plasma is different from that in amniotic fluid and changes during pregnancy. J Clin Endocrinol Metab. 1994;79(6):1735–1741. doi: 10.1210/jcem.79.6.7527409. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Rattray M, Thomas CR, Moreland BH, Schulster D. Growth hormone increases IGF-I, collagen I and collagen III gene expression in dwarf rat skeletal muscle. Mol Cell Endocrinol. 1995;115:187–197. doi: 10.1016/0303-7207(95)03690-3. [DOI] [PubMed] [Google Scholar]
- Wu J-H, Hong P-Y, Liu W-T. Quantitative effects of position and type of single mismatch on single base primer extension. J Microbiol Methods. 2009;77:267–275. doi: 10.1016/j.mimet.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Campbell JA, Smith K, Rennie MJ, Holloszy JO, Bier DM. Effect of growth hormone and resistance exercise on muscle growth in young men. Am J Physiol Endocrinol Metab. 1992;262:E261–E267. doi: 10.1152/ajpendo.1992.262.3.E261. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachweija JJ, Angelopoulos TJ, Bier DM. Short-term growth hormone treatment does not increase muscle protein synthesis in experienced weight lifters. J Appl Physiol. 1993;74:3073–3076. doi: 10.1152/jappl.1993.74.6.3073. [DOI] [PubMed] [Google Scholar]
- Zgliczynski W, Kochman M, Misiorowski W, Zdunowski P. In acromegaly, increased bone mineral density (BMD) is determined by GH-excess, gonadal function and gender. Neuro Endocrinol Lett. 2007;28:621–628. [PubMed] [Google Scholar]