Abstract
Aims
Although studies of the accuracy of heart failure (HF) classification scoring systems are available, few have examined their performance when restricted to self-reported items.
Methods and results
We evaluated the association between a simplified version of the Gothenburg score, a validated HF score comprised of cardiac and pulmonary signs and symptoms and medication use, and incident HF hospitalizations in 15 430 Atherosclerosis Risk in Communities (ARIC) Study participants. Gothenburg scores (range: 0–3) were constructed using self-reported items obtained at study baseline (1987–89). Incident HF hospitalization over 14.7 years of follow-up was defined as the first identified hospitalization with an ICD-9 discharge code of 428 (n = 1668). Self-reported Gothenburg scores demonstrated very high agreement with the original metric comprised of self-reported and clinical measures and were directly associated with incident HF hospitalizations: [score = 1: hazard rate ratio (HRR) = 1.23 (1.07–1.42); score = 2: HRR = 2.17 (1.92–2.43); score = 3: HRR = 3.98 (3.37–4.70)].
Conclusion
In a population-based cohort, self-reported Gothenburg criteria items were associated with hospitalized HF over a prolonged follow-up time. The association was also consistent across groups defined by sex and race, suggesting that this simple score deserves further study as a screening tool for the identification of individuals at high risk of HF in resource-limited settings.
Keywords: Epidemiology, Heart failure, Gothenburg score, Surveillance
Introduction
Heart failure (HF) is a common, costly, disabling, and often fatal disorder that affects approximately 14 million Europeans1 and 5.7 million Americans.2 Although treatment can reduce considerable morbidity and mortality attributed to HF,3,4 approximately half of those with HF die within 5 years after diagnosis.5,6 The changing demography of the world's population, including recent estimates suggesting that 2 billion persons will be aged 60 or greater in 2050, further underscore the worldwide public health burden posed by HF.7
The need for accurate and standardized HF classification criteria operable for therapeutic trials and epidemiologic studies prompted the development of several HF scoring systems including Framingham,8 Gothenburg,9,10 NHANES-I,11 and Boston.12 All scores share the same basic format of incorporating HF signs, symptoms, and clinical indices (to which various weights may be applied), although the presence of rales is the only criterion included in all scores. Three of the questionnaires, Framingham, Gothenburg, and NHANES-I, were designed for epidemiologic studies; the others were developed for drug trials.13
Although studies have contrasted the accuracy of HF scoring systems for HF classification,13 few have examined the performance of parsimonious scoring systems restricted to items obtained through self report. In particular, criteria that are simple to administer may facilitate screening of those at risk of future HF hospitalization, a salient question since HF consumes 1–2% of healthcare resources in developed countries.14 Thus, we evaluated the association between Gothenburg scores based on self-reported items and incident HF hospitalizations in the cohort of the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study
The ARIC Study includes an ongoing population-based cohort of 15 792 Caucasian and African American males and females aged 45–64 years at study baseline from four US communities in Maryland, Minnesota, Mississippi, and North Carolina. Participants were recruited in 1987–89 to examine cardiovascular and pulmonary disease, patterns of medical care, and disease variation over time.15 Standardized physical examinations and interviewer-administered questionnaires were conducted at baseline, and at three follow-up examinations that occurred at 3-year intervals. Participant follow-up through annual telephone interviews, review of hospitalization records, and vital status is ongoing. The investigation conforms with the principles outlined in the Declaration of Helsinki, the Institutional Review Board at each participating institution approved the ARIC Study and all participants provided informed consent before each examination.
All cardiovascular risk factors were assessed at study baseline. Left ventricular hypertrophy (LVH) was measured by electrocardiogram (ECG) using Cornell criteria.16 Prevalent diabetes (defined as fasting plasma glucose ≥6.93 mmol/L, non-fasting glucose ≥11.0 mmol/L, or self-reported use of diabetes medications or physician diagnosis of diabetes), hypertension (defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or self-reported use of medications for blood pressure control), and obesity [body mass index (BMI) ≥30 kg/m2] were examined as modifiers of the relationship between self-reported Gothenburg score at baseline and incident hospitalized HF.
Gothenburg score
The Gothenburg score was introduced in 1987 as a HF screening tool.9,10 The score is derived from cardiac signs and symptoms, pulmonary signs and symptoms, and therapies for HF, and used to classify patients into four groups with increasing likelihood of having HF (Table 1). The criteria were validated in several studies and shown to have a sensitivity of 83.5 and a specificity of 80.9 for classifying HF in a random sample of health care recipients.13
Table 1.
Gothenburg score items and the ascertainment method at the ARIC Study baseline examination
| Signs and symptoms | Source |
Question | |
|---|---|---|---|
| Cardiac | |||
| Prevalent CHD | Self-report | Positive response to the question: Has a doctor ever said you had a heart attack? | |
| Angina | Self-report |
|
|
| Oedema | Self-report | Positive responses to both of the following questions:
|
|
| Nocturnal dyspnoea | Self-report | Positive response to the question: ‘Have you ever been awakened at night by trouble breathing?’ | |
| Rales | Physical exam | N/A | |
| Atrial fibrillation | ECG | N/A | |
| Pulmonary | |||
| Chronic bronchitis | Self-report | Positive response to the question: ‘Have you ever had chronic bronchitis?’ | |
| Chronic asthma | Self-report | Positive response to the question: ‘Have you ever had chronic asthma?’ | |
| Cough, phlegm, or wheezing | Self-report | Positive response to any of the following questions:
|
|
| Rhonchi | Physical exam | N/A | |
| Therapy | |||
| Digitalis | Self-report | Medication codes (312xxx) | |
| Diuretics | Self-report | Medication codes (37xxxx) | |
ARIC, Atherosclerosis Risk in Communities Study; CHD, coronary heart disease; ECG, electrocardiogram.
Scores for the full Gothenburg criterion were assigned in this study at baseline from standardized physical examinations and interviewer-administered questionnaires as part of the ARIC protocol. Rhonchi and rales were detected during physical examination by a nurse or physician's assistant and atrial fibrillation was ascertained during central processing of digitized ECG records. All other signs and symptoms, as well as prevalent coronary heart disease (CHD) or angina, were reported by participants on interviewer-administered questionnaires (Table 1). Participants were assigned a Gothenburg score = 0 if they had no signs or symptoms in all categories. A Gothenburg score = 1 was assigned to participants positive for only cardiac signs and symptoms, a score = 2 corresponds to positive responses in the cardiac and symptoms category and either the pulmonary signs and symptoms or therapy categories. Those with score = 3 were positive in all three categories (cardiac, pulmonary, and therapy). Of note, each score component is represented by a binary indicator that does not discriminate between one or multiple positive responses for a given category. Self-reported Gothenburg scores were assigned based only on the self-reported signs and symptoms (i.e. excluding rales, rhonchi, and atrial fibrillation on ECG) without modification of the rest of the scoring. Hereafter, the full Gothenburg score (including both physical signs and self-reported items) is referred to as the ‘original’ and the reduced set of self-reported items is referred to as 'self-reported'.
Outcome
Events were ascertained through yearly interviews of cohort members during which all hospitalizations were reported as well as active surveillance of local hospital discharge lists to identify cohort hospitalizations with cardiovascular disease diagnoses. Incident HF hospitalizations were defined as a participant's first recorded hospitalization with an International Classification of Diseases, Ninth Revision (ICD-9) discharge code = 428 in any position. Heart failure hospitalizations were not validated for the presence of acute, decompensated HF by a review of the pertinent hospital records. Information on hospitalizations preceding the ARIC baseline examinations was unavailable. Follow-up time was calculated as time from study enrolment and completion of the Gothenburg questionnaire to either incident HF hospitalization, loss to follow-up, death, or 31 December 2004, the last date of complete hospitalization data.
Statistical analysis
We excluded a total of 362 participants from the analysis: 83 participants with prevalent HF, defined as a positive response to the question ‘were any of the medications you took during the past two weeks for heart failure’, 48 participants who reported a race other than African American or Caucasian, and 231 participants who had insufficient data at baseline to assign a Gothenburg score. Race- and sex-specific age-standardized incidence rates (IRs) of hospitalized HF were calculated using age in 5-year categories. Hazard rate ratios (HRRs) were estimated using Cox regression. The assumption of proportional hazards was confirmed by visual examination of survival plots. All statistical modelling was performed using SAS v9.1.3 (Cary, NC, USA).
Results
Agreement between the original and self-reported Gothenburg scores at baseline was very high in the combined population (Table 2) and across subpopulations defined by sex and race (data not shown). Prevalence estimates for the clinically measured signs and symptoms excluded from the self-reported Gothenburg score were generally low: atrial fibrillation on ECG and rales were identified in 31 (0.2%) and 105 (0.7%) participants, respectively (Table 3). Although rhonchi was more frequent (3.9%), its exclusion did not affect score assignment appreciably, as it frequently co-occurred with other pulmonary signs and symptoms (55.7%) or occurred in the absence of cardiac signs and symptoms (27.0%).
Table 2.
Agreement between original and self-reported Gothenburg score among 15 336 ARIC Study participants, 1987–1989a
| Self-reported Gothenburg score |
|||||
|---|---|---|---|---|---|
| Original Gothenburg score | 0 | 1 | 2 | 3 | Total |
| 0 | 9123 | 0 | 0 | 0 | 9123 |
| 1 | 24 | 2647 | 0 | 0 | 2671 |
| 2 | 41 | 62 | 2768 | 0 | 2871 |
| 3 | 8 | 0 | 25 | 638 | 671 |
| Total | 9196 | 2709 | 2793 | 638 | 15 336 |
ARIC, Atherosclerosis Risk in Communities Study.
aScore: 0, no signs or symptoms; 1, cardiac signs and symptoms only; 2, cardiac signs and symptoms and either pulmonary signs and symptoms or therapy; 3, cardiac and pulmonary signs and symptoms and therapy.
Table 3.
Baseline characteristics of ARIC Study participants (N = 15 430) by Gothenburg score, 1987–1989a
| Baseline characteristics | Gothenburg score |
|||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| (N = 9268; 60%) | (N = 2726; 18%) | (N = 2798; 18%) | (N = 638; 4%) | |
| Age (years) | 54.0 (5.7) | 53.9 (5.7) | 54.6 (5.8) | 55.5 (5.6) |
| African American | 24.8 | 27.8 | 29.6 | 37.8 |
| Female | 46.7 | 69.1 | 67.4 | 72.4 |
| Current smoker | 25.7 | 19.5 | 32.4 | 31.0 |
| Current alcohol consumer | 60.5 | 49.9 | 49.4 | 41.6 |
| ≤ High school education | 52.5 | 56.8 | 64.1 | 68.6 |
| BMI (kg/m2) | 26.9 (4.6) | 28.2 (5.7) | 29.1 (6.3) | 31.1 (6.8) |
| SBP (mm Hg) | 120.8 (18.4) | 120.4 (19.2) | 122.8 (19.4) | 125.1 (19.8) |
| DBP (mm Hg) | 73.8 (11.1) | 73.1 (11.5) | 73.5 (11.4) | 74.9 (11.8) |
| Hypertension | 30.5 | 27.2 | 46.1 | 78.5 |
| Diabetes | 9.3 | 10.2 | 17.7 | 27.2 |
| LVH present on ECG | 1.7 | 2.4 | 3.0 | 4.7 |
| Atrial fibrillation on ECG | 0.18 | 0.03 | 0.25 | 0.94 |
| Rales | 0.61 | 0.37 | 1.3 | 0.63 |
| Rhonchi | 3.4 | 5.4 | 6.1 | 6.1 |
ARIC, Atherosclerosis Risk in Communities Study; BMI, body mass index; CHD, coronary heart disease; DBP, diastolic blood pressure; ECG, electrocardiogram; LVH, left ventricular hypertrophy; SBP, systolic blood pressure.
aData are percentages for dichotomous characteristics and means (standard deviation) for continuous variables.
There were 1668 incident HF hospitalizations over a mean follow-up time of 14.7 years. Incident HF hospitalizations occurred on average 9.6 years (Figure 1) after the baseline examination (range: 0.03–17.9 years). Almost half (44%) of ARIC participants who survived their first HF hospitalization were re-admitted or died within 6 months of discharge.
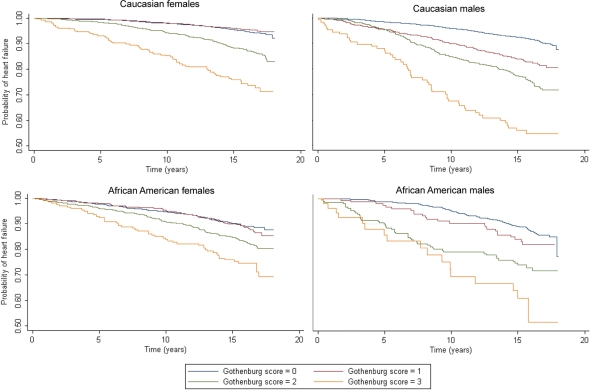
Figure 1.
Kaplan–Meier curves of incident heart failure hospitalization by self-reported Gothenburg score, stratified by gender and race.
Participants with higher Gothenburg scores at baseline were more likely to be African American, female, have educational achievement of high school or less, to be current smokers, and be slightly older (Table 3). Mean BMI, the prevalence of diabetes, and the presence of LVH on ECG all showed a monotonic increase across increasing Gothenburg scores. The majority of participants with a Gothenburg score = 3 were hypertensive at baseline (78.5%).
Age-adjusted IRs of hospitalized HF were directly associated with self-reported Gothenburg scores (Table 4). The association also varied by sex; estimated IRs were highest for males, but were comparable across race after stratification by sex. Self-reported Gothenburg scores were also directly associated with HRRs of incident hospitalized HF (Table 4). However, HRRs associated with self-reported Gothenburg score = 3 were greatest for Caucasian males and females, in contrast to IR estimates that were highest among male participants, reflecting the higher estimated IRs of HF observed among African American participants with Gothenburg score = 0.
Table 4.
Incidence rates per 1000 person-years and hazard rate ratios of heart failure hospitalization by self-reported Gothenburg scores, race, and sex among 15 430 ARIC Study participants, 1987–2004
| Score | All participants | Caucasians |
African Americans |
||
|---|---|---|---|---|---|
| Males (N = 5316) | Females (N = 5989) | Males (N = 1558) | Females (N = 2567) | ||
| Incidence rate (95% CI)a | |||||
| 0 | 5.40 (5.01, 5.79) | 5.82 (5.19, 6.44) | 3.63 (3.09, 4.17) | 7.87 (6.51, 9.23) | 7.11 (5.85, 8.37) |
| 1 | 6.73 (5.92, 7.53) | 11.51 (9.38, 13.64) | 3.1 (2.33, 3.87) | 13.43 (8.59, 18.27) | 8.46 (6.46, 10.46) |
| 2 | 10.48 (9.42, 11.54) | 15.47 (12.86, 18.07) | 8.04 (6.63, 9.46) | 17.98 (12.5, 23.46) | 9.56 (7.36, 11.76) |
| 3 | 20.99 (17.87, 24.1) | 33.95 (22.92, 44.97) | 16.63 (12.59, 20.68) | 32.27 (16.44, 48.1) | 18.67 (13.25, 24.08) |
| Hazard rate ratio (95% CI)b | |||||
| 0 | 1 | 1 | 1 | 1 | 1 |
| 1 | 1.23 (1.07, 1.42) | 2.01 (1.62, 2.48) | 0.86 (0.65, 1.15) | 1.60 (1.06, 2.41) | 1.18 (0.87, 1.59) |
| 2 | 2.17 (1.92, 2.43) | 2.99 (2.48, 3.61) | 2.29 (1.85, 2.84) | 2.66 (1.91, 3.70) | 1.61 (1.24, 2.10) |
| 3 | 3.98 (3.37, 4.70) | 5.69 (4.19, 7.73) | 4.96 (3.74, 6.59) | 4.27 (2.57, 7.10) | 2.71 (1.94, 3.77) |
ARIC, Atherosclerosis Risk in Communities study; CI, confidence interval; HF, heart failure; IR, incidence rate.
aAge standardized and presented per 1000 person-years.
bAge adjusted.
When examining the components of the Gothenburg criteria, the prevalence of HF therapy (digitalis and diuretic) at ARIC Study baseline (18.5%; 2642 self-reported diuretic use, 117 reported digitalis use, and 92 reported diuretic and digitalis use) was lower than the prevalence of cardiac (39.9%) and pulmonary (30.4%) signs and symptoms. However, combinations of criteria that included therapy were associated with the highest relative rates of incident HF hospitalizations when all combinations of the three criteria were evaluated in a multivariable model (Table 5). As expected, estimated HRRs of incident HF hospitalization were highest for populations with positive responses to all three Gothenburg criteria.
Table 5.
Hazard rate ratios of incident heart failure hospitalization by self-reported Gothenburg score among 15 430 ARIC Study participants, 1987–2004
| Gothenburg score criteria | N events | Person-years | HRR (95% CI)a |
|---|---|---|---|
| None | 385 | 94 191 | 1 |
| One criterion | |||
| Cardiac | 270 | 40 629 | 1.59 (1.36, 1.86) |
| Pulmonary | 172 | 27 875 | 1.46 (1.22, 1.75) |
| Therapy | 131 | 13 330 | 2.06 (1.68, 2.51) |
| Two criteria | |||
| Cardiac and pulmonary | 274 | 25 419 | 2.63 (2.25, 3.07) |
| Cardiac and therapy | 195 | 13 843 | 3.06 (2.57, 3.65) |
| Pulmonary and therapy | 62 | 3951 | 3.47 (2.65, 4.54) |
| Three criteria | |||
| Cardiac, pulmonary, and therapy | 179 | 7914 | 5.16 (4.32, 6.18) |
CI, confidence interval; HF, heart failure; HRR, hazard rate ratio.
aAdjusted for age, sex, and race.
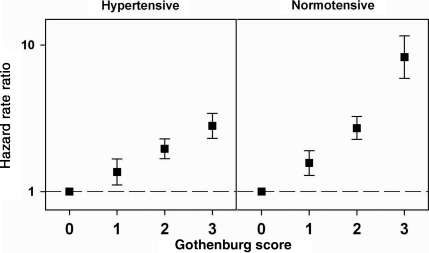
Baseline hypertension status modified the relationship between self-reported Gothenburg score and incident hospitalized HF (P < 0.0001), unlike obesity and diabetes (Figure 2, numeric estimates provided in Supplementary material online, Table S1). Hazard rate ratio estimates adjusted for age, sex, and race were consistently higher for participants who were normotensive at study baseline. The largest discrepancies were seen among participants with Gothenburg score = 3 [HRR (95% CI) for hypertensive participants: 2.81 (2.31, 3.42); normotensive participants: 8.27 (5.92, 11.54)].
Figure 2.
Adjusted hazard rate ratios of incident heart failure hospitalization by self-reported Gothenburg score, stratified by baseline hypertensive status.
Discussion
The burden of HF in economically developed countries has steadily increased over the past decades,17,18 reflecting numerous factors including the ageing populations19,20 and improved survival following myocardial infarctions.21,22 Patients with HF also have significantly greater functional limitations, in-home care needs, and nursing home admissions when compared with those with other cardiovascular diseases including CHD.23 Thus, identifying populations at long-term risk for future HF hospitalizations in outpatient/primary care settings could target proactive care before the syndrome and its associated disability worsen.
In this report, we evaluated the association between a simplified Gothenburg score, previously validated as a HF screening criterion, and incident hospitalized HF in a large, bi-racial US cohort. Specifically, we focused on the performance of the Gothenburg criteria restricted to self-reported items and showed that simple measurements reported by the study participants at baseline are associated with HF hospitalization over long-term follow-up. Moreover, the Gothenburg scores restricted to self-reported items demonstrated high agreement with the original score.
Two prior reports examined combinations of patient characteristics that can enable the identification of individuals at high risk of HF. Kannel et al.24 identified a risk score to predict HF cases over 38 years of follow-up in the Framingham Heart Study and a recent report estimated a risk score in the Health ABC study of elderly individuals over a mean follow-up of 6.5 years.25 Briefly, Kannel et al. included age, electrocardiographic LVH, cardiomegaly on chest x-ray film, heart rate, systolic blood pressure, vital capacity, diabetes mellitus, evidence of myocardial infarction, and valvular disease or hypertension. The nine HF predictors Health ABC investigators identified included prior CHD, systolic blood pressure, cigarette smoking, heart rate, age, LVH, and serum creatinine, albumin, and fasting glucose. Kannel et al. identified HF outcomes identified through clinic examinations, hospital admissions, and physician's records according to the Framingham criteria8 and Health ABC investigators classified first hospital admissions as incident HF based on criteria similar to those used in the Cardiovascular Health Study.26
The contrast with the parsimony of the Gothenburg score is readily apparent (Table 1), particularly considering the simple and non-invasive nature of the characteristics included in the score and its potential application as a screening tool in resource-limited settings. Emphasizing this point, we note the high degree of concordance between the original and self-reported scores. If replicated in other populations, our results are relevant in the context of several studies which suggested that HF misdiagnosis by primary care physicians is common.27,28
However, we make no direct comparisons with the properties of the HF prediction risk equations reported by the Framingham Heart Study and the Heath ABC investigators. Our study focused on the evaluation of an initial hospitalization with a HF discharge code, not otherwise validated for HF. Instead of deriving another HF risk prediction algorithm, we wish to highlight the potential advantages of a simple score such as the Gothenburg criterion constructed using self-reported items, as an initial assessment that can serve to identify populations who may benefit from clinical examination and/or measurements such as biomarkers or echocardiography.
We note that of the short list of self-reported items considered, therapies for HF (restricted to digitalis and diuretics) were associated with the greatest relative rate of incident HF hospitalizations in the ARIC cohort. Some HF therapies commonly used at present (e.g. beta-blockers and ACE-inhibitors) were not in wide use at the time of ARIC's baseline examination or when the Gothenburg score was developed. Although few therapies are specific for HF in their indications, their inclusion in an updated list of self-reported items may be warranted. At this point, it is also unclear whether the greater relative rates of disease observed in ARIC participants who were normotensive at baseline reflect different pathways to the HF syndrome than in hypertensives, or a population in which the Gothenburg score performs better. Replication of these results is needed before interpretations are proposed.
We highlight several limitations of this study including the potential for inaccuracy associated with self-reported items included in the construction of the Gothenburg scores. The literature examining the accuracy of self-reported items such as those used to construct Gothenburg scores suggests moderate-to-high agreement with physician diagnoses and medical records,29–31 with most items being highly specific but with varying levels of sensitivity. Criteria that are moderately sensitive, but highly specific would generally lead to underestimation of Gothenburg scores and an attenuation of the association with incident hospitalized HF. However, the design of the Gothenburg score may minimize this concern, as each score component is represented by a binary indicator that does not discriminate between one or multiple positive responses for a given category.
We also point out that prevalent HF at baseline was identified, for exclusion, only based on participant self-report of the use of medication prescribed for HF. An examination of the timing of incident HF hospitalizations suggests that very few (<6%) HF hospitalizations occurred within 2 years of the baseline examination. Although we have no information on hospitalizations preceding the baseline ARIC examination, excluding from the analyses all events that occurred within 2 years of study enrolment did not visibly alter our results (data not shown).
We also note that we relied on 428 discharge codes to identify HF hospitalizations that were not validated for the presence of acute, decompensated HF by a review of the pertinent hospital records. Reports from the Women's Health Initiative Clinical Trial and Observational Study suggest moderate to good agreement between an ICD-9 428 hospital discharge codes and local adjudication; estimates of the sensitivity and specificity of ICD-9 code 428 were 78.3 and 98.1%, respectively.32 Analyses of incident HF events in our populations based on at least two hospitalizations listing a 428 discharge code in ARIC cohort participants (n = 900 events) were fully consistent with those presented herein (data not shown).
These limitations notwithstanding, our results suggest the potential applicability of the parsimonious Gothenburg criterion constructed using self-reported questionnaire items in population-based studies. The high agreement between the self-reported and the original score, their comparable relative rate estimates, and the consistency across groups defined by sex and race indicate that the self-reported score could be applied as a screening tool in resource-limited settings without compromising accuracy. Thus, the potential impact of these results warrants their prompt replication in other populations.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
This work was supported by National Heart, Lung, and Blood Institute contracts (grant numbers N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022).
Supplementary Material
Acknowledgements
The authors thank the staff of the ARIC Study for their important contributions.
Conflict of interest: none declared.
References
- 1.Davis RC, Hobbs FD, Kenkre JE, Roalfe AK, Hare R, Lancashire RJ, Davies MK. Prevalence of left ventricular systolic dysfunction and heart failure in high risk patients: community based epidemiological study. BMJ. 2002;325:1156. doi: 10.1136/bmj.325.7373.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 4.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 6.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. The prognosis of heart failure in the general population: The Rotterdam Study. Eur Heart J. 2001;22:1318–1327. doi: 10.1053/euhj.2000.2533. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. The world is fast ageing—have we noticed. Available at: http://www.who.int/ageing/en/index.html . [Google Scholar]
- 8.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson H, Svardsudd K, Caidahl K, Bjuro T, Larsson B, Welin L, Ohlson LO, Wilhelmsen L. Early heart failure in the population. The study of men born in 1913. Acta Med Scand. 1988;223:197–209. doi: 10.1111/j.0954-6820.1988.tb15788.x. [DOI] [PubMed] [Google Scholar]
- 11.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 12.Carlson KJ, Lee DC, Goroll AH, Leahy M, Johnson RA. An analysis of physicians' reasons for prescribing long-term digitalis therapy in outpatients. J Chronic Dis. 1985;38:733–739. doi: 10.1016/0021-9681(85)90115-8. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca C, Oliveira AG, Mota T, Matias F, Morais H, Costa C, Ceia F. Evaluation of the performance and concordance of clinical questionnaires for the diagnosis of heart failure in primary care. Eur J Heart Fail. 2004;6:813–820. doi: 10.1016/j.ejheart.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Bundkirchen A, Schwinger RHG. Epidemiology and economic burden of chronic heart failure. Eur Heart J. 2004;6:D57–D60. [Google Scholar]
- 15.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, Phillips MC. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 17.Brown DW, Haldeman GA, Croft JB, Giles WH, Mensah GA. Racial or ethnic differences in hospitalization for heart failure among elderly adults: Medicare, 1990 to 2000. Am Heart J. 2005;150:448–454. doi: 10.1016/j.ahj.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S, MacIntyre K, Capewell S, McMurray JJ. Heart failure and the aging population: an increasing burden in the 21st century? Heart. 2003;89:49–53. doi: 10.1136/heart.89.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(Suppl. 4A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–957. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 21.Auerbach AD, Hamel MB, Califf RM, Davis RB, Wenger NS, Desbiens N, Goldman L, Vidaillet H, Connors AF, Lynn J, Dawson NV, Phillips RS. Patient characteristics associated with care by a cardiologist among adults hospitalized with severe congestive heart failure. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Coll Cardiol. 2000;36:2119–2125. doi: 10.1016/s0735-1097(00)01005-6. [DOI] [PubMed] [Google Scholar]
- 22.Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, Lessard D, Gore JM. Twenty year trends (1975–1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol. 1999;34:1378–1387. doi: 10.1016/s0735-1097(99)00390-3. [DOI] [PubMed] [Google Scholar]
- 23.Gure TR, Kabeto MU, Blaum CS, Langa KM. Degree of disability and patterns of caregiving among older Americans with congestive heart failure. J Gen Intern Med. 2008;23:70–76. doi: 10.1007/s11606-007-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, MGarcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PWF, Kritchevsky SB. Incident Heart Failure Prediction in the Elderly: The Health ABC Heart Failure Score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 27.McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350:829–833. doi: 10.1016/S0140-6736(97)03033-X. [DOI] [PubMed] [Google Scholar]
- 28.Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 29.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145:762–769. doi: 10.1093/aje/145.8.762. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen MW, Sondergaard B, Kjoller M, Hansen EH. Agreement between self-reported data on medicine use and prescription records vary according to method of analysis and therapeutic group. J Clin Epidemiol. 2008;61:919–924. doi: 10.1016/j.jclinepi.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, Carlisle DM, Mangione CM, Kahn KL. What is the concordance between the medical record and patient self-report as data sources for ambulatory care? Med Care. 2006;44:132–140. doi: 10.1097/01.mlr.0000196952.15921.bf. [DOI] [PubMed] [Google Scholar]
- 32.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–1158. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.