Abstract
Many studies have shown that perinatal nutritional iron deficiency (ID) produces learning impairments in children. Research has also shown that catecholamines like epinephrine and norepinephrine play a pivotal role in the consolidation of memories. In this study, we sought to determine if perinatal ID impairs the following: 1) noradrenergic synaptic function in the hippocampus; and 2) several forms of hippocampus-dependent fear learning. Electrophysiological brain slice methods were used to examine noradrenergic-mediated synaptic efficacy in the CA1-hippocampus of rats that were subjected to perinatal ID or control (CN) diets. Rats were fed ID (3 mg Fe/kg) or CN (45 mg Fe/kg) diets on gestational d 14. These diets were maintained until postnatal d (P) 12 after which all rats were switched to the CN diet. Hippocampal slices were prepared between P26 and P30. The noradrenergic agonist isoproterenol (ISO) (1, 2, or 4 μmol) was used to induce modulatory increases in synaptic efficacy in the hippocampal slices. CN slices showed a long-lasting increase in synaptic efficacy as the result of ISO perfusion in the slice bath, whereas ID slices did not show increases in synaptic efficacy as the result of ISO perfusion. ID and CN groups did not differ when ISO was perfused through slices from adult rats (P61). Both young and adult ID rats showed reduced levels of hippocampus-dependent fear learning compared with the young and adult CN rats. Together, these findings suggest that ID may impair early forms of noradrenergic-mediated synaptic plasticity, which may in turn play a role in adult learning deficits.
Introduction
Iron deficiency (ID)5 is the most prevalent nutritional disorder in the world. Many studies have shown that perinatal ID produces learning impairments in infants and children (1,2). Several recent animal studies from our laboratory have demonstrated that perinatal nutritional ID impairs hippocampus-dependent forms of memory (3,4) as well as synaptic transmission in the hippocampus (5). Work from other laboratories suggests that perinatal ID in rats may result in impairments in hippocampal synaptic transmission that last well into adulthood (6,7).
Extensive research has demonstrated that the consolidation and retrieval of memories is modulated by catecholamines such as norepinephrine, epinephrine, and dopamine (8,9). Several studies have shown that norepinephrine blockers infused into the hippocampus impair contextual memory retrieval in mice (10,11). Other work has shown that systemic infusion of adrendergic agents improves memory performance (9,12,13). There is also evidence that the ability of epinephrine to modulate memory is mediated by the release of norepinephrine and activation of β-adrenoceptors in the brain (9,13,14).
Recent work suggests that noradrenergic brain function may be altered as the result of perinatal ID. Unger et al. (15) demonstrated that perinatal ID reduces levels of norepinephrine and epinephrine in frontal brain regions. This group also showed that iron chelation in PC12 cells reduces norepinephrine activity and concentrations of the norepinephrine transporter (16). The same study also showed that perinatal nutritional ID in rats reduces levels of the norepinephrine transporter in the brain. Thus, it is possible that alterations in noradrenergic synaptic function play a role in the impairments in hippocampal physiology and learning that are known to result from perinatal ID.
In this study, we used electrophysiological brain slice methods to determine if perinatal ID impairs noradrenergic-mediated synaptic efficacy in the hippocampus of rats. Noradrenergic-mediated synaptic efficacy was examined by perfusing isoproterenol (ISO) into the medium used to bathe hippocampal slices. ISO is a β-adrenergic receptor agonist and numerous studies have shown that it and other noradrenergic agonists produce a long-lasting potentiation of the field recordings and population spikes in the CA1 area of the hippocampus (e.g. 17–20). This ISO method was used in the present study to determine if perinatal ID impairs noradrenergic-mediated synaptic plasticity in the CA1 area of the hippocampus. Hippocampus-dependent trace and contextual fear conditioning were also examined in a portion of the animals in this study. Although our previous work has shown that perinatal ID impairs forms of trace conditioning (3,4), no studies have ever examined the effects of perinatal ID on contextual fear conditioning.
Methods
Subjects and diets.
Matched pairs of ID and control (CN) diet rat pups were used to obtain measures of hippocampus dependent fear learning and hippocampal synaptic efficacy. All procedures used in this study were approved by The Pennsylvania State University Animal Care and Use Committee and were in accordance with the guidelines specified by NIH Guide for the Care and Use of Laboratory Animals. All rat pups were obtained from timed pregnant dams ordered from Charles River Laboratories. The model of nutritional ID used in this study is identical to the model of ID used in our previous work, which has been shown to produce reliable deficits on hippocampus-dependent learning tests (4). Pregnant dams were fed an ID diet or a CN diet on gestational d 14. Each pregnant ID dam was yoked to a CN dam so that all experimental groups received simultaneous timing of pregnancy, dietary regimen, rearing manipulations, behavioral testing, and slice preparation. The ID and CN diets were purchased from Research Diets. Two tests conducted by Research Diets showed that the iron content of the ID diet was 3 mg Fe/kg and the CN diet was 45 mg Fe/kg. All other constituents of the diet were equated exactly according to the AIN 93-G diet (21). The same diets were maintained after birth. Litters were culled to 8 on postnatal day (P) 2 retaining as many males as possible. Only male rats were used for testing. Each experimental testing group was formed from a minimum of 3 different litters of rats. All ID pups were switched to the CN diet on P12. All pups were weaned on P21 and continued to be fed the CN diet.
Behavioral fear conditioning procedures.
Prior to the preparation of hippocampal slices, some rats were tested for behavioral fear conditioning. During fear conditioning, yoked pairs of ID and CN pups were tested side by side in identical testing chambers. Each rat was always tested in the same chamber, but the placement of the ID and CN rats in the right and left chambers was switched for each subsequent pair of rats. Fear conditioning included an acclimation day followed 24 h later by a training day, and a memory testing day that followed the training day by 72 h. Rats received these fear conditioning tests at either a young age or an adult age, whereby acclimation began on P19 for the young group and P59 for the adult group. No adult rats were trained and tested at the young time period. A holding cage was used to transport rats (in groups of 4–8) from their home cage to the laboratory on each of the days of training. On the acclimation day, each rat was handled for 2 min in the training room and immediately returned to the holding cage. On the training day, each rat was placed in a 31 × 21 × 48 cm acrylic training chamber with a shock grid floor with 1-cm bar spacing. The training chamber was located in a sound-attenuating chamber with a 60-W white incandescent bulb. Rats received 3 trace fear conditioning trials on the day of training, consisting of a 10-s auditory conditioned stimulus (CS; 6000 Hz; 75 dB) followed by a 30-s empty trace interval, then a 0.8-s shock unconditioned stimulus (scrambled 1 mA produced by a Coulbourn constant current shocker).
Seventy-two hours later, rats received an auditory-cue memory test followed 3 h later by a context memory test. During the auditory-cue test rats were placed in a novel clear acrylic chamber 50 × 22 × 30 cm located within the same sound-attenuating chamber used during the training phase. To increase the novelty of the auditory cue test, a red 40-W incandescent house light was used instead of the white light used during the training phase, and the floor of the acrylic chamber was filled with corn cob bedding. Freezing-fear responses were assessed during a 30-s baseline period followed by a 2-min auditory-CS and a 30-s post period. Freezing-inactivity was measured using a Columbus Opto-Varimex infrared activity monitor. Following the auditory-cue test, rats were returned to the holding cage where they remained for 3 h before receiving a context memory test. During the context memory test, each rat was placed in the original shock chamber that was used during the training phase with the white house light illuminated. Freezing-fear responses were measured for a 3-min period using the activity monitors. A computer controlled the delivery of all stimuli and the collection of all activity data. Changes in freezing to the auditory CS were used as an indicator of trace fear memories. Thus, animals exhibiting >90% freezing during the pre-CS period were excluded from the trace fear conditioning analyses.
A digital video camera (Xirlink; IBM) was attached to the top of the sound-attenuating chamber and was used to track the rats' location and distance moved per second. The digital video was time stamped and stored on a computer prior to the delivery of the trace fear conditioning trials. Path length ambulation was calculated in centimeters using a frame by frame analysis of the digital video. Path length ambulation in the novel chamber prior to the delivery of any stimuli served as a measure of open field anxiety. These methods were adapted from several other studies of rodent anxiety (3,22).
Hippocampal slice preparation.
Slices were prepared from each yoked pair of ID and CN pups on the same day of electrophysiological recording. Slice preparation occurred at a young age (P26–30) or an adult age (P61–65). Rat pups were deeply anesthetized using halothane (4% in 100% O2). Rats were then decapitated. The brain was quickly removed (∼25 s) and placed in ice-cold, pre-aerated (95% O2, 5% CO2) dissecting solution consisting of 88.55 mmol/L NaCl, 1.42 mmol/L NaH2PO4, 2.55 mmol/L KCl, 25.77 mmol/L NaHCO3, 25.76 mmol/L glucose, 1.55 mmol/L MgCl2, 77.42 mmol/L sucrose, and 2.07 mmol/L CaCl2, at pH 7.4. Each hippocampus was dissected from the brain and transverse slices (400–500 μm) were prepared with a vibrating slicer. Slices were placed in aerated artificial cerebrospinal fluid (ACSF) at room temperature for at least 1 h prior to recording. The ACSF was composed of 125.25 mmol/L NaCl, 1.46 mmol/L NaH2PO4, 5.03 mmol/L KCl, 26.19 mmol/L NaHCO3, 10.49 mmol/L glucose, 1.52 mmol/L MgCl2, and 2.52 mmol/L CaCl2, at pH 7.4.
Extracellular population spike recordings.
During electrophysiological recordings, hippocampal slices were submerged in a recording chamber and continuously perfused at 3 mL/min with aerated ACSF at room temperature (∼23°C). During recordings, the experimenter was unaware of the ID and CN group designation. A computer-controlled analogue stimulus isolation unit (A-M Systems 2200) was used to deliver constant current bipolar pulses (0.1 ms/phase) through 2 Teflon-insulated tungsten wires (75-μm diameter each) bonded together. Stimulation was delivered to the Schaffer collaterals to evoke field potentials in the CA1 region. Somatic field potentials were recorded extracellularly with a glass pipette (tip diameter 15 μm) filled with 9% NaCl. Field potentials were amplified (1000×; A-M Systems Model 1800), filtered between 10 Hz and 1 kHz, and sampled by a DT3010 data acquisition board (Data Translation) at 20 kHz.
Measures of synaptic efficacy were based on the population spike amplitude of the CA1 somatic field potential. The amplitude of the population spike was defined as the difference between the peak negative voltage and the subsequent positive peak voltage. Population spikes were allowed to stabilize for 10 min before an input-output measurement was obtained. The input-output curves were measured by delivering a range of current intensities between 100 and 600 μA in 20-μA steps (30 s between). Following the IO curve, measurement slices were examined for responses to the β-adrenergic receptor agonist ISO [R(-)-isoproterenol(+)-bitartrate, Sigma]. ISO was mixed with ACSF using a 1-, 2-, or 4-μmol/L concentration. Individual slices were subjected to only a single concentration of ISO. Stimulation pulses were delivered at an intensity of 50% of the maximum population spike amplitude observed during the IO curve measurement. Pulses were delivered every 30 s during a 10-min baseline period, followed by a 10-min ISO perfusion period, then a 30-min washout period with ACSF alone in the bath.
Statistical and follow-up analyses.
Measures of synaptic efficacy were averaged into a baseline period preceding ISO perfusion, an ISO perfusion period, and the period following ISO perfusion. Repeated-measures ANOVA were used to examine measures of synaptic efficacy using a 3-level repeated-measure factor (baseline, ISO, and post ISO) and a between-groups factor with 2 levels (ID and CN). Measures of fear conditioning were also subjected to repeated-measures ANOVA using a repeated measure factor with 5 levels (5 30-s bins) and a between-groups factor with 2 levels (ID and CN). Significant interactions were followed up with Newman-Keuls post hot tests. Comparison of individual group measures of anxiety and hematocrits were accomplished using an independent t test. An α level of 0.05 was used to determine significant effects. Statview software v5.0 was used for all analyses.
A hematocrit RBC count was obtained from randomly selected rats from multiple litters to quantify levels of ID anemia. The rats that received behavioral or synaptic efficacy testing were not examined for hematocrits. We chose this strategy so that behaviorally tested rats did not receive unnecessary handling or blood loss due to a tail prick procedure. Hematocrit sampling was accomplished using methods described elsewhere (23). Briefly, blood was collected from each rat in 2 heparinized capillary tubes and centrifuged for 5 min. The percentage of RBC was measured under a 2× magnification glass using a sliding micrometer. The mean percentage was calculated from the 2 capillary tubes. An independent t test was used to compare the hematocrits between groups. Values in the text are mean ± SEM.
Results
ID impairs ISO-induced increases in synaptic efficacy.
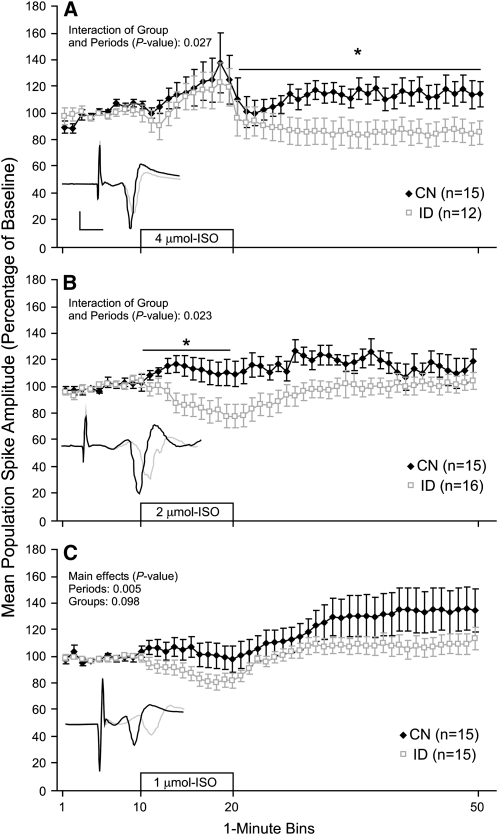
Young (P26–30) hippocampal slices were subjected to ISO perfusion to allow for the examination of noradrenergic-mediated increases in synaptic efficacy. Slices were bathed in ACSF for 10 min followed by a 10-min period of ISO + ACSF perfusion, then a 30-min washout period with ACSF alone. Each slice was subjected to only a single concentration of ISO. Perinatal ID impaired ISO-induced increases in synaptic efficacy in the CA1 hippocampus of young animals. Specifically, ISO perfusion at the 1- and 2-μmol/L concentrations resulted in an increase in population spike amplitude in the CN slices, whereas the same ISO concentrations produced a depression or no increase in spike amplitude in the ID slices (Fig. 1B,C). Analysis of the 2 μmol/L data revealed that the population spike amplitude of the CN group was greater than the ID group during the ISO perfusion period (P = 0.023). Analysis of the 1 μmol/L data revealed a group effect that approached significance (P = 0.098). The 4-μmol/L concentration resulted in an increase in population spike amplitude in the ID slices that was eliminated by the washout period, whereas the CN slices had a similar increase in population spike amplitude during 4 μmol/L ISO perfusion that was partially maintained throughout the washout period (Fig. 1A). Analysis of the 4 μmol/L data revealed that the population spike amplitude of the CN group was greater than the ID group during the washout period (P = 0.027). These data demonstrate that perinatal ID impairs ISO-induced increases in synaptic efficacy in the CA1 hippocampus of young animals.
FIGURE 1 .
Population spike amplitude recorded from the CA1 area of hippocampal slices from young p26–30 rat pups before, during, and after perfusion with 4 (A), 2 (B), or 1 (C) umol/L ISO. The 10-min ISO perfusion period is indicated by the open bar starting at the 10-min mark. Insets show exemplar population spikes immediately before and 20-min after the start of ISO perfusion (calibration bars: 5 ms, 1 mV). Each slice was subjected to only 1 ISO concentration. Values are means ± SEM with the number of slices is shown in each panel. *Different from ID at these times, P < 0.05.
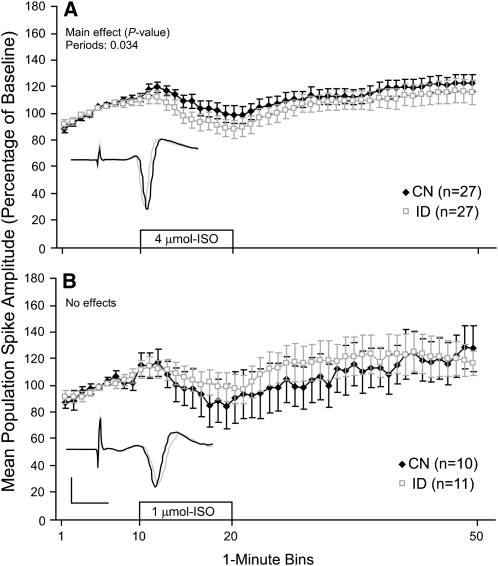
Adult (P61–65) hippocampal slices were subjected to ISO perfusion using the same protocol that was used with young slices. Slices obtained from ID and CN adult rats showed similar modest increases in population spike amplitude following ISO perfusion. Moreover, there were no significant group effects or interactions at the 1- or 4-μmol/L concentrations (Fig. 2A,B). These findings suggest that the impairments in noradrenergic-mediated synaptic plasticity that were observed in young ID slices are no longer present during the adult stages of development. No adult slices were subjected to the 2-μmol/L ISO concentration, because group effects were not revealed at the higher and lower concentrations (i.e. 1 and 4 μmol/L).
FIGURE 2 .
Population spike amplitude recorded from the CA1 area of adult P61–65 slices before, during, and after perfusion of 2 concentrations of ISO (A and B). The 10-min ISO perfusion period is indicated by the open bar starting at the 10-min mark. Insets show exemplar population spikes immediately before and 20-min after the start of ISO perfusion. Values are means ± SEM with the number of slices shown in each panel. Each slice was subjected to only a single ISO concentration.
ID produces long-lasting impairments in hippocampus-dependent fear learning.
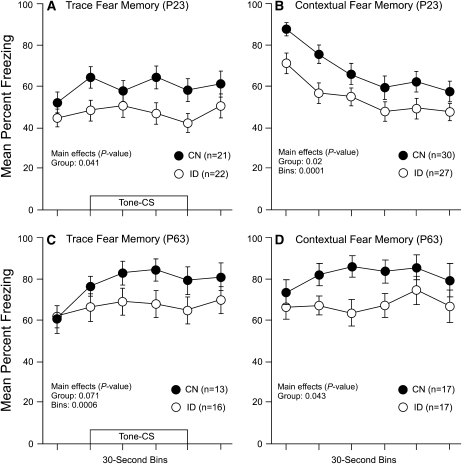
Prior to the preparation of hippocampal slices some rats were tested for hippocampus-dependent forms of fear learning. Rats received trace fear conditioning on P20 for the young group and P60 for the adult group. Memory tests for hippocampus-dependent trace and contextual fear conditioning were performed on P23 for the young group and P63 for the adult group. These tests examined memory strength for the auditory stimulus and contextual stimulus, respectively. Young ID rats showed reduced freezing fear responses to the auditory cue-CS (Fig. 3A) and the contextual cues (Fig. 3B) compared with the CN group. Analysis of the young trace and contextual fear conditioning data revealed group differences (P = 0.041 and P = 0.020, respectively). Similarly, the adult ID rats had reduced freezing fear responses to the auditory cue-CS (Fig. 3C) and the contextual cues (Fig. 3D) compared with the adult CN group. Analysis of the adult trace fear conditioning data revealed a group effect that approached significance (P = 0.071). Analysis of the adult contextual fear conditioning data revealed a significant effect of group (P = 0.043). Together, these analyses show that early perinatal nutritional ID produces impairments in hippocampus-dependent forms of fear memory that persist into adulthood.
FIGURE 3 .
Freezing-fear responses for trace and contextual fear memory tests for the young (A and B) and adult groups (C and D). The presentation of the tone-CS is shown by the open bar in A and C. Values are means ± SEM with the number of slices shown in each panel.
Learning impairments in ID rats were not due to increases in anxiety.
A previous study from our laboratory showed that perinatal ID can result in increases in anxiety as measured in a novel open field test (3). Our previous study used a more severe model of ID compared with the present study. We sought to determine whether the moderate form of ID used in the present study also resulted in increases in anxiety and to determine whether anxiety interfered with the freezing-fear measures used to quantify learning in the present study. Open field anxiety measures of ambulation (i.e. pathlength of exploration) were obtained in the conditioning chamber for 15 s prior to the delivery of any stimuli. Anxiety measures obtained at P20 revealed no reduction in open field path length in the ID group (186.5 cm ± 24.0) compared with the CN group (177.5 cm ± 21.5) (P = 0.78). Anxiety measures obtained at P60 also revealed no reduction in open field path length in the ID group (183.5 cm ± 22.0) compared with the CN group (210.5 cm ± 25.5) (P = 0.43). These data suggest that the deficits in learning in the ID rats in this study were not due to increases in anxiety.
ID and anemia.
Measurements of ID anemia were obtained during several stages of development using hematocrits (i.e. percent RBC in blood). The ID and CN diets of all rats were started on gestational d12. Hematocrits were obtained from ID (n = 7) and CN (n = 7) test pups at P13. The P13 time point reflects the level of anemia on the day that ID rats were switched back to the CN diet. The hematocrits obtained at P13 revealed that there was a significant level of anemia in the ID test pups (16.69% ± 1.1) compared with the CN test pups (29.05% ± 1.6) (P = 0.0001). Hematocrits were also obtained from ID (n = 18) and CN (n = 17) pups at P27. The P27 time point reflects the level of anemia 15 d after ID diets were switched to a normal-iron CN diet. The P27 mark also reflects the level of anemia on the approximate day that hippocampal slices were prepared. Hematocrits obtained at P27 revealed that the ID pups (32.83% ± 0.47) were no longer anemic compared with the CN pups (33.61% ± 0.82) (P = 0.41).
Discussion
In this study, we applied ISO to hippocampal slices to determine if perinatal ID affects noradrenergic-mediated increases in synaptic efficacy. Perinatal nutritional ID reduced noradrenergic-mediated increases in synaptic efficacy in the CA1 hippocampus of young rats. The impairments in noradrenergic-mediated synaptic plasticity in young ID slices were no longer present during the adult stages of development. This suggests that the early impairments in noradrenergic-mediated synaptic transmission were corrected by the process of normal developmental nutrition. On the other hand, behavioral analyses of the same rats demonstrated that perinatal nutritional ID produces impairments in hippocampus-dependent forms of fear learning and these impairments lasted into adulthood. This may suggest that the early impairments in noradrenergic-mediated synaptic function were part of a physiological mechanism that permanently impairs normal hippocampal function.
The impairments in noradrenergic-mediated synaptic transmission observed in this study could be due to a number of mechanisms, some of which may include impairments in the adrenergic receptors or altered neurotransmitter release. Adrenergic receptors are G-protein coupled receptors that can be activated by epinephrine or norepinephrine. Three β-adrenergic receptor subtypes have been identified and are present in the central nervous system (24). Brain application of norepinephrine or β-adrenergic receptor agonists has been shown to enhance memory consolidation, and brain application of β-adrenergic receptor antagonists has been shown to impair memory performance (9,25–27). It is possible that one or more of the β-adrenergic receptor subtypes has been altered or the number of receptors has been altered as the result of perinatal ID. Up- and downregulation of adrenergic receptors has been shown in other studies. For example, repeated administration of tricyclic antidepressants and monamine oxidase inhibitors produces downregulation of β-adrenergic receptors (28–30). Similarly, norepinephrine transporter-deficient mice have been shown to exhibit an upregulation in α-adrenergic receptors (31). Furthermore, the results of the present study could also be explained by an alteration in the availability or release of norepinephrine neurotransmitter. For example, several studies have demonstrated that perinatal ID alters brain levels of the norepinephrine transporter (16,32) and this in turn could alter the availability of norepinephrine at the synapse.
Previous studies provide additional evidence that noradrenergic brain mechanisms may be impaired by perinatal ID. Unger et al. (15) demonstrated that perinatal ID reduces levels of norepinephrine and epinephrine in frontal brain regions of rats. This same group also showed that perinatal nutritional ID reduces levels of the norepinephrine transporter in rat brain (16). These studies along with the present study may suggest that a single noradrenergic mechanism is sensitive to early periods of nutritional ID.
Perinatal ID has been shown to impair a number of catecholamine mechanisms. Recent work has demonstrated that early ID can produce long-term alterations in dopamine and norepinephrine levels (33). Numerous other animal studies show that perinatal ID disrupts brain levels of dopamine, tyrosine hydroxylase, the norepinephrine transporter, dopamine receptors, and the dopamine transporter (15,34–35). Studies have also shown that perinatal ID affects levels of other related monoamine transporters such as the serotonin transporter (32). Future studies will hopefully determine whether perinatal ID affects multiple catecholamine mechanisms or a single mechanism that in turn affects all of the catecholamines. For example, recent work suggests that perinatal ID may result in the altered synthesis and functioning of the dopamine transporter, which may in turn alter the synthesis and functioning of norepinephrine metabolism (36). Moreover, this may suggest that the noradrenergic results of the present study are due to the impact of perinatal ID on a single set of catecholamine-related mechanisms in the brain.
The results of this study demonstrate that perinatal nutritional ID reduces noradrenergic-mediated increases in synaptic efficacy in the CA1 hippocampus of young rats. It is important to mention that the model of nutritional ID used in the present rodent study may be longer or more severe than some of the uncomplicated forms of ID observed in clinical populations. The ID rats in this study were fed an ID diet (3 mg Fe/kg) starting on gestational d 14, and this diet was maintained until P12 after which rats were fed the CN diet (45 mg Fe/kg). The timing and duration of this ID diet was intended to capture a critical period of perinatal neural development; however, few studies have systematically examined the critical periods of iron nutrition and neural development in rats. Future work will hopefully determine critical levels and durations of nutritional ID during rodent neural development and be able to relate these variables to the degree and duration of anemia commonly experienced in human populations.
Acknowledgments
M.D.M designed research, wrote the paper, and has primary responsibility for final content. M.D.M. and D.N.A. analyzed statistical data, C.J.G conducted the electrophysiological slice research, and D.N.A maintained diets and rat development, and conducted behavioral research. All authors read and approved the final version of the manuscript.
Supported by NIH Grant R01 HD 050423 (M.D.M).
Author disclosures: M. D. McEchron, C. J. Goletiani, and D. N. Alexander, no conflicts of interest.
Abbreviations used: ACSF, artificial cerebral spinal fluid; CN, control diet; CS, conditioned stimulus; ID, iron deficient; ISO, isoproterenol; P, postnatal d.
References
- 1.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B, Wolf AW, Jimenez E. Iron-deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996;129:382–9. [DOI] [PubMed] [Google Scholar]
- 3.McEchron MD, Cheng AY, Liu H, Connor JR, Gilmartin MR. Perinatal nutritional iron deficiency permanently impairs hippocampus-dependent fear learning. Nutr Neurosci. 2005;8:195–206. [DOI] [PubMed] [Google Scholar]
- 4.McEchron MD, Alexander DN, Gilmartin MR, Paronish MD. Perinatal nutritional iron deficiency impairs hippocampus-dependent trace eyeblink conditioning in rats. Dev Neurosci. 2008;30:243–54. [DOI] [PubMed] [Google Scholar]
- 5.McEchron MD, Paronish MP. Perinatal nutritional iron deficiency reduces hippocampal synaptic transmission but does not impair short- or long-term synaptic plasticity. Nutr Neurosci. 2005;8:277–85. [DOI] [PubMed] [Google Scholar]
- 6.Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev Neurosci. 2003;25:412–20. [DOI] [PubMed] [Google Scholar]
- 7.Jorgenson LA, Sun M, O'Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–102. [DOI] [PubMed] [Google Scholar]
- 8.Lalumiere RT, Nguyen LT, McGaugh JL. Post-training intrabasolateral amygdala infusions of dopamine modulate consolidation of inhibitory avoidance memory: involvement of noradrenergic and cholinergic systems. Eur J Neurosci. 2004;20:2804–10. [DOI] [PubMed] [Google Scholar]
- 9.Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–33. [DOI] [PubMed] [Google Scholar]
- 10.Cammarota M, Bevilaqua LR, Rossato JI, Lima RH, Medina JH, Izquierdo I. Parallel memory processing by the CA1 region of the dorsal hippocampus and the basolateral amygdala. Proc Natl Acad Sci USA. 2008;105:10279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43. [DOI] [PubMed] [Google Scholar]
- 12.McGaugh JL. Hormonal influences on memory. Annu Rev Psychol. 1983;34:297–323. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interaction with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–10. [DOI] [PubMed] [Google Scholar]
- 15.Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr. 2007;137:118–24. [DOI] [PubMed] [Google Scholar]
- 16.Beard JL, Wiesinger JA, Jones BC. Cellular iron concentrations directly affect the expression levels of norepinephrine transporter in PC12 cells and rat brain tissue. Brain Res. 2006;1092:47–58. [DOI] [PubMed] [Google Scholar]
- 17.Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–20. [DOI] [PubMed] [Google Scholar]
- 18.Nejtek V, Dahl D. Pathway specificity of l–isoproterenol indicates beta-adrenergic modulation of the Schaffer collateral pathway in field CA1 in the rat hippocampal slice. Neuroreport. 1997;8:745–9. [DOI] [PubMed] [Google Scholar]
- 19.Heginbotham LR, Dunwiddie TV. Long-term increases in the evoked population spike in the CA1 region of rat hippocampus induced by beta-adrenergic receptor activation. J Neurosci. 1991;11:2519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelinas JN, Nguyen PV. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 22.Carli M, Prontera C, Samanin R. Effect of 5–HT1A agonists on stress-induced deficit in open field locomotor activity of rats: evidence that this model identifies anxiolytic-like activity. Neuropharmacology. 1989;28:471–6. [DOI] [PubMed] [Google Scholar]
- 23.Andreasson B, Wahlstrom E, Jacobsson S, Bjorkholm M, Samuelsson J, Birgegard G, Wadenvik H, Kutti J. The measurement of venous haematocrit in patients with polycythaemia vera. J Intern Med. 1999;246:293–7. [DOI] [PubMed] [Google Scholar]
- 24.Pupo AS, Minneman KP. Adrenergic pharmacology: focus on the central nervous system. CNS Spectr. 2001;6:656–62. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–5. [DOI] [PubMed] [Google Scholar]
- 26.LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci. 2003;23:6754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Introini-Collison IB, Miyazaki B, McGaugh JL. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology (Berl). 1991;104:541–4. [DOI] [PubMed] [Google Scholar]
- 28.Matrisciano F, Scaccianoce S, Del Bianco P, Panaccione I, Canudas AM, Battaglia G, Riozzi B, Ngomba RT, Molinaro G, et al. Metabotropic glutamate receptors and neuroadaptation to antidepressants: imipramine-induced down-regulation of beta-adrenergic receptors in mice treated with metabotropic glutamate 2/3 receptor ligands. J Neurochem. 2005;93:1345–52. [DOI] [PubMed] [Google Scholar]
- 29.Racagni G, Mocchetti I, Calderini G, Battistella A, Brunello N. Temporal sequence of changes in central noradrenergic system of rat after prolonged antidepressant treatment: receptor desensitization and neurotrasmitter interactions. Neuropharmacology. 1983;22:415–24. [DOI] [PubMed] [Google Scholar]
- 30.Weiss B, Heydorn W, Frazer A. Modulation of the betaadrenergic receptor–adenylate cyclase system following acute and repeated treatment with antidepressants. Adv Biochem Psychopharmacol. 1982;31:37–53. [PubMed] [Google Scholar]
- 31.Gilsbach R, Faron-Górecka A, Rogóz Z, Brüss M, Caron MG, Dziedzicka-Wasylewska M, Bönisch H. Norepinephrine transporter knockout-induced up-regulation of brain alpha2A/C-adrenergic receptors. J Neurochem. 2006;96:1111–20. [DOI] [PubMed] [Google Scholar]
- 32.Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–8. [DOI] [PubMed] [Google Scholar]
- 33.Coe CL, Lubach GR, Bianco L, Beard JL. A history of iron deficiency anemia during infancy alters brain monoamine activity later in juvenile monkeys. Dev Psychobiol. 2009;51:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianco LE, Unger EL, Earley CJ, Beard JL. Iron deficiency alters the day-night variation in monoamine levels in mice. Chronobiol Int. 2009;26:447–63. [DOI] [PubMed] [Google Scholar]
- 35.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–18. [DOI] [PubMed] [Google Scholar]
- 36.Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–15. [DOI] [PubMed] [Google Scholar]