Abstract
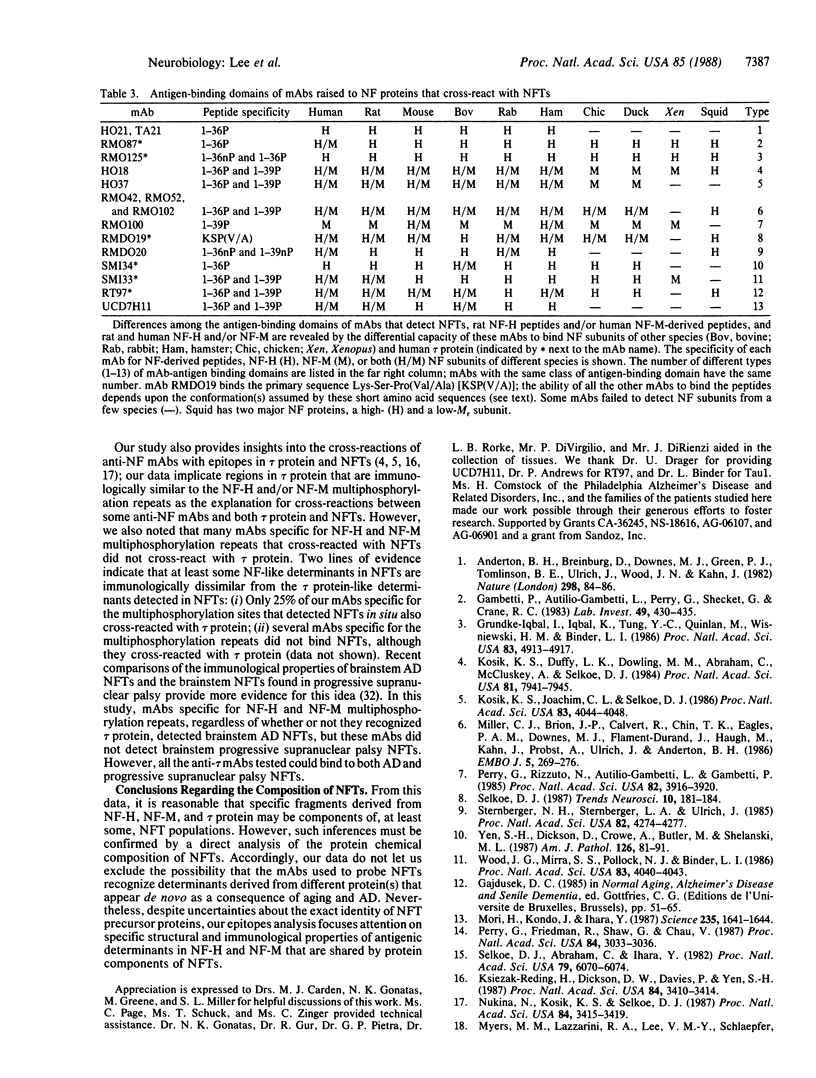
Immunological and structural analyses of neurofilament (NF) proteins with greater than 500 anti-NF monoclonal antibodies (mAbs) enumerated epitopes shared by NF proteins and Alzheimer neurofibrillary tangles. We identified the multiphosphorylation domain of the rat heaviest NF subunit--tandem repeats of Lys-Ser-Pro-Xaa (where Xaa is a small uncharged amino acid and serine is phosphorylated)--as the determinant recognized by 15 of the 16 mAbs from this collection of greater than 500 mAbs that detected neurofibrillary tangles. Most (11) of these 16 mAbs also recognized the previously characterized multiphosphorylation repeat in the human middle sized NF subunit. However, although these mAbs shared the ability to recognize NFTs, the antigen-binding domains of these 16 mAbs represented 13 separate classes based on their differential recognition of 12 synthetic peptides derived from the rat heaviest NF subunit and the human middle-sized NF subunit multiphosphorylation sites, NF subunits of 10 diverse species, and normal human tau protein. We conclude that NFTs share highly specific immunological and structural properties with specific rat heaviest NF subunit and human middle-sized NF subunit multiphosphorylation repeats.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Gambetti P., Autilio-Gambetti L., Perry G., Shecket G., Crane R. C. Antibodies to neurofibrillary tangles of Alzheimer's disease raised from human and animal neurofilament fractions. Lab Invest. 1983 Oct;49(4):430–435. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W. Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci. 1986 Aug;6(8):2179–2186. doi: 10.1523/JNEUROSCI.06-08-02179.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V., Wu H. L., Schlaepfer W. W. Monoclonal antibodies recognize individual neurofilament triplet proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6089–6092. doi: 10.1073/pnas.79.19.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Miller C. C., Brion J. P., Calvert R., Chin T. K., Eagles P. A., Downes M. J., Flament-Durand J., Haugh M., Kahn J., Probst A. Alzheimer's paired helical filaments share epitopes with neurofilament side arms. EMBO J. 1986 Feb;5(2):269–276. doi: 10.1002/j.1460-2075.1986.tb04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Lazzarini R. A., Lee V. M., Schlaepfer W. W., Nelson D. L. The human mid-size neurofilament subunit: a repeated protein sequence and the relationship of its gene to the intermediate filament gene family. EMBO J. 1987 Jun;6(6):1617–1626. doi: 10.1002/j.1460-2075.1987.tb02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987 May;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. A., Wion D., Anderton B. H. Isolation of a cDNA for the rat heavy neurofilament polypeptide (NF-H). FEBS Lett. 1986 Dec 15;209(2):203–205. doi: 10.1016/0014-5793(86)81111-5. [DOI] [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Schmidt M. L., Gur R. E., Gur R. C., Trojanowski J. Q. Intraneuronal and extracellular neurofibrillary tangles exhibit mutually exclusive cytoskeletal antigens. Ann Neurol. 1988 Feb;23(2):184–189. doi: 10.1002/ana.410230212. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C., Ihara Y. Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Dickson D. W., Crowe A., Butler M., Shelanski M. L. Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol. 1987 Jan;126(1):81–91. [PMC free article] [PubMed] [Google Scholar]



