Abstract
Studying the evolutionary mechanisms of feline immunodeficiency virus in the domestic cat (Felis catus), FIVFca, provides a good comparison to other lentiviruses, such as HIV and FIVPco in the cougar (Puma concolor). We review the current epidemiological and evolutionary findings of FIVFca,. In addition to the five accepted FIVFca, subtypes, several recent phylogenetic studies have found strains that form separate clades, indicative of novel subtypes. In New Zealand cats, these strains of unknown subtype have been found to be involved in complex patterns of intergenic recombination, and whole genome sequences are required to resolve these. Evidence of recombination events has been documented with the highest levels in the env gene, the region involved in host cell receptor recognition. Several cases of FIVFca, multiple infection, both inter- and intra-subtype, have been reported. The findings of both unknown subtypes and relatively high levels of recombination suggest the need for further testing of the current vaccine. Limited studies on the evolutionary rate of FIVFca, document a value twice to three times that of FIV in the cougar, a result suggesting the different levels of co-adaptation between the viruses and their respective hosts. We studied the tissue distribution of FIVFca, in feral domestic cats, finding the first case of FIV compartmentalisation, a phenomenon well-documented in HIV-1 patients.
Keywords: Feline Immunodeficiency Virus, domestic cat, Felis catus, subtype, evolution, recombination
Introduction
Feline Immunodeficiency Virus (FIV) is a lentivirus that infects members of the Felidae and Hyaenidae (Troyer et al., 2005), and each host species usually has its own FIV strain (Brown et al., 1994; Carpenter et al., 1996; Carpenter et al., 1998; Troyer et al., 2005; Franklin et al., 2007; Pecon-Slattery et al., 2008). The strain that circulates in populations of the domestic cat (Felis catus), FIVFca,, is pathogenic and can lead to feline AIDS, with symptoms similar to that produced by Human Immunodeficiency Virus (HIV) in humans (Pedersen et al., 1989; Bendinelli et al., 1995; VandeWoude and Apetrei, 2006). Furthermore, shared characteristics of HIV-1 and FIVFca,, such as the worldwide distribution, the occurrence of recombinants, and high viral RNA loads in plasma suggest that FIVFca, is a good model for HIV-1 (Carpenter et al., 1998; Yamamoto et al., 2002; Yamamoto et al., 2007).
In other host species, such as the African lion (Panthero leo) and the North American cougar (Puma concolor), FIV is apparently less pathogenic than in the domestic cat (Carpenter and O’Brien, 1995; Carpenter et al., 1996; Bull et al., 2003; Brennan et al., 2006; Roelke et al., 2006). The difference in the disease status of these hosts suggests that FIV has persisted in the lion and cougar much longer than FIV in the domestic cat, such that a period of host adaptation has occurred (Carpenter et al., 1996). This hypothesis is also supported by a higher prevalence and greater genetic variation of FIV in lion and cougar populations compared to the domestic cat (Brown et al., 1994; Carpenter and O’Brien, 1995; Biek et al., 2003). The similarity between FIVFca, and HIV-1, and the difference between FIVFca, and, for example, FIVPco in the cougar, provide two important reasons for studying FIVFca,.
Here we provide a general overview of the prevalence, subtypes and recombination of FIVFca, and include some recent findings on the evolutionary rate and tissue distribution of FIV in the domestic cat. For this review, we define three categories of domestic cat populations: companion, feral, and stray. Companion cats are pets; owned by and reliant on humans. Feral cats are free-ranging, inhabit rural areas like forest and scrubland, and have minimal or no human contact. Stray cats are also free-ranging but inhabit urban areas and have some human contact.
1. Prevalence of FIVFca, in F. catus populations
Companion cat prevalence
Generally, the prevalence of FIVFca, in companion cat populations worldwide is about 4–12 % (Courchamp and Pontier, 1994). Companion cats in the USA and Canada have FIV prevalence at the lower end of the range, between 1 % and 7 % in low-risk and high-risk cats respectively (Shelton et al., 1989; Yamamoto et al., 1989; O’Connor et al., 1991). In contrast, companion cats in Japan have a higher prevalence value, of up to 44 % in clinically ill cats, which is suggested to be the result of relatively higher cat density (Ishida et al., 1989). The worldwide distribution of FIVFca, in domestic cats is thought to be a result of low virulence levels and low rates of transmission of the virus (Fromont et al., 1997).
Feral cat prevalence
Worldwide studies of feral and free-ranging cats have found FIVFca, prevalence of about 8–19 % (Baneth et al., 1999; Winkler et al., 1999; Ostrowski et al., 2003; Danner et al., 2007; Hayward, 2009) but see Carpenter et al. (1998) and Yamaguchi et al. (1996). The higher FIVFca, prevalence observed in feral cats compared to companion cats may be explained by differences in behavioural patterns and the main route of FIV transmission. Feral cats tend to be free-ranging and more aggressive in their interactions with other cats, and as such, have a higher frequency of biting encounters (Courchamp et al., 1998). For this same reason, male, sexually mature cats are at the highest risk of FIVFca, infection (Hosie et al., 1989; Courchamp et al., 1998; Levy et al., 2006).
2. FIV-Fca subtypes
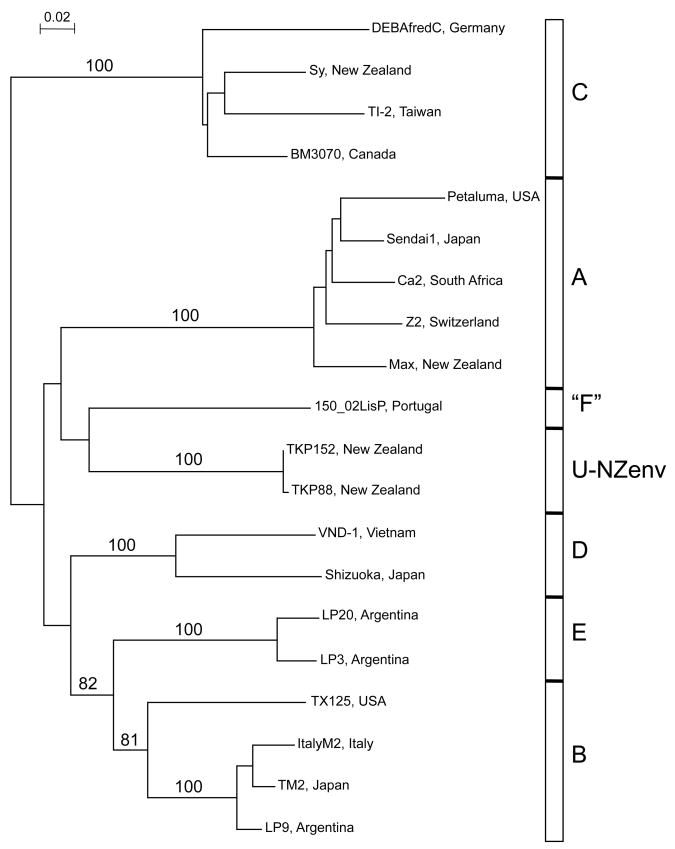
Five FIVFca, subtypes, A to E, (Fig. 1) have been established based on phylogenetic analyses of sequences from the env V3-V5 region (Sodora et al., 1994; Kakinuma et al., 1995; Pecoraro et al., 1996). Recent papers have also used the gag gene to confirm the env clades (Kakinuma et al., 1995; Duarte et al., 2002; Steinrigl and Klein, 2003; Reggeti and Bienzle, 2004; Weaver et al., 2004; Hayward and Rodrigo, 2008). Subtypes A, B and C are most widespread worldwide, with subtype D only found in Japan and Vietnam (Kakinuma et al., 1995; Nakamura et al., 2003) and subtype E only found in Argentina (Pecoraro et al., 1996). The four subtypes A to D are found in cat populations from Japan (Kakinuma et al., 1995; Nishimura et al., 1998) but see Yamamoto et al. (2007) for worldwide FIV prevalence and subtype distribution details. There appears to be subtype-specific differences in disease, for example, subtype A infection has been reported to be associated with neurological disease (Nishimura et al., 1996; de Rozieres et al., 2008) while subtype B is less likely to be symptomatic (Bachmann et al., 1997).
Figure 1.
Neighbour Joining phylogenetic tree of env V3-V5 sequences. Tree was constructed using a general time reversible model incorporating invariant sites (0.4086) and a gamma distribution of mutation rates (shape = 1.6859), as determined by Modeltest3.7 (Posada and Crandall, 1998). Numbers shown are bootstrap values, based on 1000 iterations. Subtypes A to E, “F” (Duarte and Tavares, 2006), and U-NZenv (Hayward et al., 2007; Hayward and Rodrigo 2008) are shown along the right side of the tree. Strains are DEBAfredC (U57020), Sy (GQ357641), TI-2 (AB016026), BM3070 (AF474246), Petaluma (M25381), Sendai1 (D37813), Ca2 (DQ873714), Z2 (X57001), Max (GQ357642), 150_02LisP (DQ072566), TKP152 (GQ357640), TKP88 (EF153977), VND-1 (AB083502), Shizuoka (D37811), LP20 (D84498), LP3 (D84496), TX125 (AY139094), ItalyM2 (X69501), TM2 (M59418), LP9 (D84497).
In addition, sequences of unknown subtype have been documented. Eleven FIVFca, isolates from Texas were tentatively designated subtype F (Weaver et al., 2004). More recently, these Texas sequences were assigned as a subclade within subtype B and the Portuguese sequences were proposed as subtype F (Fig. 1) (Duarte and Tavares, 2006). Furthermore, eighteen env sequences from New Zealand (NZ) cats have been identified as distinct from any previously-described subtype, designated U-NZenv (Fig. 1) (Hayward et al., 2007; Hayward and Rodrigo, 2008). Phylogenetic analyses of gag and pol sequences from NZ cats also showed evidence of one and two unknown subtypes respectively, although the samples included in these unknown clades were not identical across the three genes (Hayward and Rodrigo, 2008). The finding that the NZ env unknown subtype group is not monophyletic across the three main genes leads us to question the suitability of FIVFca, subtyping using the env V3-V5 region only. In light of recent sequencing technologies and the HIV-1 nomenclature recommendations that at least two full-length genomes from epidemiologically-unrelated hosts are required to name a new subtype (Robertson et al., 1999), a precautionary approach in designating new subtypes is suggested.
The Fel-O-Vax vaccine (Fort Dodge), which is commercially available in a number of countries including USA, Australia, Japan and NZ, confers protection against subtypes A, B and D but has not yet been tested on subtype C, despite the wide distribution of this subtype (Yamamoto et al., 2002; Kusuhara et al., 2005). Furthermore, the findings of FIV strains of unknown subtypes suggest further testing of the vaccine in cat populations is warranted.
3. Recombination in FIV
Fca, Retroviruses have been documented to have relatively rapid rates of recombination due to the presence of a diploid genome (that is, two identical copies of ssRNA) and the occurrence of multiple infection (Hu and Temin, 1990). Naturally-occurring multiple FIVFca, infection, either as a result of co-infection or superinfection, has been identified in cats from Australia, USA and NZ (Kyaw-Tanner and Robinson, 1996; Bachmann et al., 1997; Kann et al., 2007; Hayward and Rodrigo, 2008). Several of these cases involve two strains of the same subtype (Kyaw-Tanner and Robinson, 1996; Hayward and Rodrigo, 2008). Given that multiple infection is a prerequisite for recombination, it is expected that the level of multiple infection would be similar to the level of unique recombinants circulating in cat populations. However, intra-subtype recombination is difficult to document, because of the similarity of the parent strains involved (Hayward and Rodrigo, 2008). It is important to note that recombination is only detected when the two parent strains are different but recombination does also occur in cells infected with virus whose genome is homodimeric, that is, when the two copies of the viral RNA are identical.
For clarity, here we distinguish between two types of recombinant sequences. Intragenic recombinant sequences include a crossover event within a single gene region while intergenic recombinants include a crossover between two gene regions. Intragenic recombinant FIVFca, sequences from the env region have been isolated from cats in Canada (A/B), Japan (A/B, B/D), NZ (A/C) and USA (A/B, A/C, A/B/C) (Bachmann et al., 1997; Carpenter et al., 1998; Reggeti and Bienzle, 2004; Hayward and Rodrigo, 2008). Gag intragenic recombinant sequences have been found in samples from Canada (A/B, A/C) and NZ (A/C) (Reggeti and Bienzle, 2004; Hayward and Rodrigo, 2008).
Across all studies, the env gene has the highest detected level of recombination. Indeed, about 6 % (n = 156) of NZ env sequences were found to be putative recombinants compared to 2 % (n = 48) of the gag sequences (Hayward and Rodrigo, 2008). Immunologically, this is an expected result because env encodes the surface proteins, which are important in recognition of viruses by the host immune system, and therefore subject to positive selection (Flynn et al., 1995; Seibert et al., 1995). High levels of variation, caused by a high evolutionary rate, are observed in this gene allowing the virus to escape the immune response. Recombination is one factor that aids in increasing the evolutionary rate of a virus (Coffin et al., 1997). However, it is also possible that the reason for the greater number of recombination events observed in env is simply a consequence of our ability to better detect recombination in highly diverse regions.
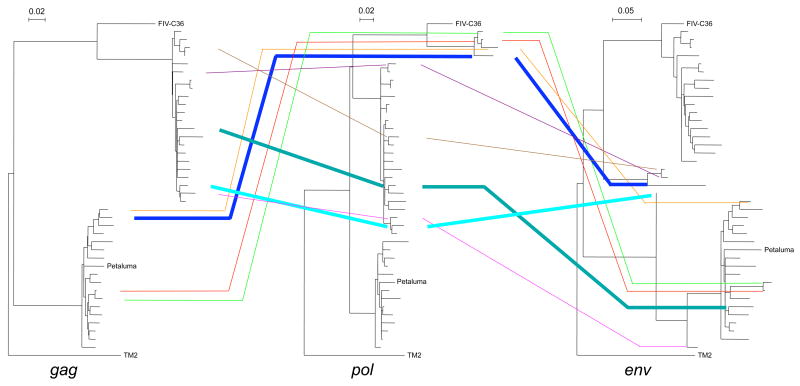
Complex intergenic recombination patterns, between subtypes A and undefined parent strains, have been identified from FIVFca, sequences isolated from NZ cats (Fig. 2) (Hayward and Rodrigo, 2008). For example, one cat was found with subtype A FIV in the env region, “unknown” subtype (most closely related to subtype C) FIV in the pol region, and subtype A FIV in the gag region. Sequences encompassing the whole FIV genome are needed to obtain a complete picture of the recombination events that have occurred between the different gene regions.
Figure 2.
Complex intergenic recombination patterns between fragments of the env, gag and pol genes (modified from Hayward and Rodrigo, 2008). Lines link sequences isolated from the same host, to highlight incongruities in subtype assignment between the different gene regions. The three thickened lines show the significant intergenic recombinant sequences, as determined by the SH test (Shimodaira and Hasegawa, 1999) (see Hayward and Rodrigo, 2008). Reference sequences are FIV-C36 (subtype C; AY600517), Petaluma (subtype A; M25381), TM2 (subtype B; M59418).
4. Evolutionary rate of FIVFca
Only one published study has reported an evolutionary rate for FIV-Fca, of 3.4 × 10−3 substitutions per site per year from the V1-V2 env region (Greene et al., 1993). and was calculated from a single sequence from each of three serial samples taken one year apart from a naturally-infected cat. Recently, we estimated the evolutionary rate of the env V3-V6 region using a Bayesian coalescent method as implemented in the program BEAST (Drummond and Rambaut, 2007). We used endpoint sequences generated from serial blood samples taken six to eleven months apart, from three FIV-infected NZ companion cats. A strict molecular clock model was used and the MCMC chain was run for 50,000,000 iterations. We used a prior to constrain the root of each tree and this was set to the age of each cat. Our estimates range from 3.1 × 10−3 to 6.6 × 10−3 substitutions per site per year (Hayward, 2009), thus confirming Greene et al.’s earlier estimate (Table 1).
Table 1.
Estimates of evolutionary rates from FIV and HIV-1 studies
| Lentivirus and region | Rate estimate (×10−3 substitutions per site per year) | Reference |
|---|---|---|
| FIV-Fca V1-V2 env | 3.4 | (Greene et al., 1993) |
| FIV-Fca V3-V6 env | 3.1–6.6§ | (Hayward, 2009) |
| FIV-Pco V4-V5 env | 1–3 | (Biek et al., 2003) |
| HIV-1 gp160 env | 2.4 | (Korber et al., 2000) |
| HIV-1 C2-V5 | 10 | (Shankarappa et al., 1999) |
| HIV-1 V3 env | 6.7 | (Leitner and Albert, 1999) |
| HIV-1 V3 env | 8.7 | (Zhang et al., 1997) |
95 % highest posterior density (HPD) for each cat is 2.2–4.1, 5.0–8.4, and 3.3–9.2
The FIVFca, evolutionary rate estimates are twice to three times higher than that found for the env region in the cougar FIVPco strain using similar methods (Table 1) (Biek et al., 2003). A higher rate for domestic cat FIV is expected because FIVFca, is postulated to have a more recent origin than FIVPco (Carpenter et al., 1996). More recently-emerged viruses can be expected to have higher evolutionary rates than their ancestors because there has been no co-adaptation between the virus and new host species (Nelson and Holmes, 2007). Likewise, HIV-1 in humans is considered a younger virus than FIVFca,, and the HIV-1 rate estimates are generally higher than that documented for FIVFca, (Table 1). However, multiple factors can affect evolutionary rate estimation, such as the evolutionary model, the gene region and the infection stage of the host (McGrath et al., 2001).
Moreover, we found evidence of positive selection in the FIVFca, env sequences from two of the three NZ companion cats (Hayward, 2009). These two cats suffered numerous conditions typical of FIV infection and had relatively high proviral loads. We suggest that the high level of virus circulating in these two cats caused responses by the host’s immune systems, which in turn resulted in genetic changes by the virus to evade the host immune response (Yamaguchi and Gojobori, 1997). The positively-selected sites were generally identified in variable regions (Hayward, 2009), possibly indicating the importance of variation in viral escape from the immune system. The third cat, with no evidence of positive selection, was the healthiest cat with the lowest proviral load and lowest average viral diversity (Hayward, 2009). This result highlights the virus-host relationship in the early stages of infection. The lack of positive selection suggests that the immune system from this third cat is not eliciting a strong response to the presence of the virus.
5. FIVFca, distribution in tissue compartments
Compartmentalisation is the restriction of virus movement between different tissues or cell types (Nickle et al., 2003). In HIV-1 infected individuals, it is suggested that compartmentalisation is a result of differential immune system pressures in the different tissue environments (Zhang et al., 2002; Kemal et al., 2003). The genitourinary tract, CNS, lymph node and lung have all been documented as HIV-1 compartments when compared to peripheral blood in the same individuals (Ball et al., 1994; Itescu et al., 1994; Korber et al., 1994; Byrn et al., 1997; Coombs et al., 1998). To date, an equivalent restriction of movement of FIV has not been published. Recently, we investigated the distribution of FIVFca, env sequences in multiple tissues of sixteen feral cats (Hayward and Rodrigo, in prep). In general, low intrahost diversity was found e.g. the FIVFca, sequences isolated from the different tissues were very similar (Hayward and Rodrigo, in prep).
This result could be due to recent infection events in the hosts, as it has been shown that HIV-1 populations are homogeneous in the initial stages of infection (Wolfs et al., 1992; Zhang et al., 1993; Zhu et al., 1993; Delwart et al., 2002). Several cats had some diversity, however, with one cat providing the first known evidence of FIV compartmentalisation, that is, the sequences grouped in their tissue types more often than expected by chance alone (Hayward and Rodrigo, in prep).
Finally, two cases of dual infection in different tissue types were identified (Hayward and Rodrigo, in prep). One cat had same-subtype dual infection in lung, liver and lymph node tissue samples (Hayward and Rodrigo, in prep). The second cat was identified with subtypes C and “unknown” FIV in the popliteal lymph node (Hayward and Rodrigo, 2008) and lung tissue samples (Hayward and Rodrigo, in prep). These findings emphasise the freedom of virus movement between the tissue types and suggest recent infection events.
Summary
Although FIV in the domestic cat was first isolated more than twenty years ago, research is still ongoing to learn more about this lentiviral relative of HIV. Comparisons to FIV in larger cats, such as the North American cougar and the African lion, help to explain disease differences from an evolutionary perspective. One of the more significant opportunities for research involves the notion that FIV has adapted to its host in those species where it persists endemically. The biological and evolutionary mechanics of host adaptation is still an open question and FIV may be an appropriate model system to study this.
As this review highlights, further research is still required, in particular, to resolve recombination patterns and novel unknown subtypes, with further testing of the currently available vaccine.
Acknowledgments
The authors wish to thank Dr Emma Marks and anonymous reviewers for editing of the manuscript. Jessica Hayward was supported by a University of Auckland Doctoral scholarship.
Footnotes
Conflict of interest
The authors have no financial or personal relationships with other people or organisations that could inappropriately influence or bias this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachmann MH, Mathiason-Dubard C, Learn GH, Rodrigo AG, Sodora DL, Mazzetti P, Hoover EA, Mullins JI. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball JK, Holmes EC, Whitwell H, Desselberger U. Genomic Variation of Human-Immunodeficiency-Virus Type-1 (HIV-1) - Molecular Analyses of HIV-1 in Sequential Blood-Samples and Various Organs Obtained at Autopsy. J Gen Virol. 1994;75:867–879. doi: 10.1099/0022-1317-75-4-867. [DOI] [PubMed] [Google Scholar]
- Baneth G, Kass PH, Steinfeld D, Besser M. A seroepidemiological study of Feline Coronavirus, Feline Immunodeficiency Virus and Feline Leukemia Virus among cats in Israel. Isr J Vet Med. 1999;54:39–43. [Google Scholar]
- Bendinelli M, Pistello M, Lombardi S, Poli A, Garzelli C, Matteucci D, Ceccherini-Nelli L, Malvaldi G, Tozzini F. Feline immunodeficiency virus: an interesting model for AIDS studies and an important cat pathogen. Clinical Microbiology Reviews. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek R, Rodrigo AG, Holley D, Drummond A, Anderson JCR, Ross HA, Poss M. Epidemiology, genetic diversity, and evolution of endemic feline immunodeficiency virus in a population of wild cougars. J Virol. 2003;77:9578–9589. doi: 10.1128/JVI.77.17.9578-9589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan G, Podell MD, Wack R, Kraft S, Troyer JL, Bielefeldt-Ohmann H, VandeWoude S. Neurologic disease in captive lions (Panthera leo) with low-titer lion lentivirus infection. J Clin Microbiol. 2006;44:4345–4352. doi: 10.1128/JCM.00577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EW, Yuhki N, Packer C, O’Brien SJ. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J Virol. 1994;68:5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ME, Kennedy-Stoskopf S, Levine JF, Loomis M, Gebhard DG, Tompkins WA. Evaluation of T lymphocytes in captive african lions (Panthera leo) infected with feline immunodeficiency virus. Am J Vet Res. 2003;64:1293–1300. doi: 10.2460/ajvr.2003.64.1293. [DOI] [PubMed] [Google Scholar]
- Byrn RA, Zhang DZ, Eyre R, McGowan K, Kiessling AA. HIV-1 in semen: an isolated virus reservoir. Lancet. 1997;350:1141–1141. doi: 10.1016/S0140-6736(97)24042-0. [DOI] [PubMed] [Google Scholar]
- Carpenter MA, Brown EW, Culver M, Johnson WE, Pecon-Slattery J, Brousset D, O’Brien SJ. Genetic and Phylogenetic Divergence of Feline Immunodeficiency Virus in the Puma (Puma concolor) J Virol. 1996;70:6682–6693. doi: 10.1128/jvi.70.10.6682-6693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MA, Brown EW, MacDonald DW, O’Brien SJ. Phylogeographic patterns of feline immunodeficiency virus genetic diversity in the domestic cat. Virol. 1998;251:234–243. doi: 10.1006/viro.1998.9402. [DOI] [PubMed] [Google Scholar]
- Carpenter MA, O’Brien SJ. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr Opin Gen Dev. 1995;5:739–745. doi: 10.1016/0959-437x(95)80006-q. [DOI] [PubMed] [Google Scholar]
- Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; New York: 1997. p. 843. [PubMed] [Google Scholar]
- Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, Dragavon J, Peterson G, Hooton TM, Collier AC, Corey L, Koutsky L, Krieger JN. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: Evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- Courchamp F, Pontier D. Feline immunodeficiency virus: an epidemiological review. C R Acad Sci Paris. 1994;317:1123–1134. [PubMed] [Google Scholar]
- Courchamp F, Yoccoz NG, Artois M, Pontier D. At-risk individuals in Feline Immunodeficiency Virus epidemiology: evidence from a multivariate approach in a natural population of domestic cats (Felis catus) Epidemiol Infect. 1998;121:227–236. doi: 10.1017/s0950268898008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner RM, Goltz DM, Hess SC, Banko PC. Evidence of Feline Immunodeficiency Virus, Feline Leukemia Virus, and Toxoplasma gondii in Feral Cats on Mauna Kea, Hawaii. Journal of wildlife diseases. 2007;43:315–318. doi: 10.7589/0090-3558-43.2.315. [DOI] [PubMed] [Google Scholar]
- de Rozieres S, Thompson J, Sundstrom M, Gruber J, Stump DS, de Parseval AP, VandeWoude S, Elder JH. Replication properties of clade A/C chimeric Feline Immunodeficiency Viruses and evaluation of infection kinetics in the domestic cat. Journal of Virology. 2008;82:7953–7963. doi: 10.1128/JVI.00337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E, Magierowska M, Royz M, Foley B, Peddada L, Smith R, Heldebrant C, Conrad A, Busch M. Homogeneous quasispecies in 16 out of 17 individuals during very early HIV-1 primary infection. AIDS. 2002;16:189–195. doi: 10.1097/00002030-200201250-00007. [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Marques MI, Tavares L, Fevereiro M. Phylogenetic analysis of five Portuguese strains of FIV. Arch Virol. 2002;147:1061–1070. doi: 10.1007/s00705-002-0785-7. [DOI] [PubMed] [Google Scholar]
- Duarte A, Tavares L. Phylogenetic analysis of Portuguese feline immunodeficiency virus sequences reveals high genetic diversity. Vet Microbiol. 2006;114:25–33. doi: 10.1016/j.vetmic.2005.11.056. [DOI] [PubMed] [Google Scholar]
- Flynn JN, Cannon CA, Reid G, Rigby MA, Neil JC, Jarrett O. Induction of feline immunodeficiency virus-specific cell-mediated and humoral immune-responses following immunization with a multiple antigenic peptide from the envelope V3 domain. Immunol. 1995;85:171–175. [PMC free article] [PubMed] [Google Scholar]
- Franklin SP, Troyer JL, Terwee JA, Lyren LM, Boyce WM, Riley SPD, Roelke ME, Crooks KR, VandeWoude S. Frequent transmission of immunodeficiency viruses among bobcats and pumas. Journal of Virology. 2007;81:10961–10969. doi: 10.1128/JVI.00997-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont E, Courchamp F, Artois M, Pontier D. Infection strategies of retroviruses and social grouping of domestic cats. Can J Zool. 1997;75:1994–2002. [Google Scholar]
- Greene WK, Meers J, del Fierro G, Carnegie PR, Robinson WF. Extensive sequence variation of feline immunodeficiency virus env genes in isolates from naturally infected cats. Arch Virol. 1993;133:51–62. doi: 10.1007/BF01309743. [DOI] [PubMed] [Google Scholar]
- Hayward JJ. PhD thesis. The University of Auckland; New Zealand: 2009. Molecular Epidemiology and Evolution of Feline Immunodeficiency Virus in the New Zealand Domestic Cat Felis catus. [Google Scholar]
- Hayward JJ, Rodrigo AG. Recombination in feline immunodeficiency virus from feral and companion domestic cats. Virol J. 2008;5:76. doi: 10.1186/1743-422X-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward JJ, Rodrigo AG. The distribution of feline immunodeficiency virus in systemic and tissue compartments of feral domestic cats. doi: 10.1007/s00705-010-0598-z. in prep. [DOI] [PubMed] [Google Scholar]
- Hayward JJ, Taylor J, Rodrigo AG. Phylogenetic analysis of feline immunodeficiency virus in feral and companion domestic cats of New Zealand. J Virol. 2007;81:2999–3004. doi: 10.1128/JVI.02090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie MJ, Robertson C, Jarrett O. Prevalence of Feline Leukaemia Virus and Antibodies to Feline Immunodeficiency Virus in Cats in the United Kingdom. Vet Rec. 1989;128:293–297. doi: 10.1136/vr.125.11.293. [DOI] [PubMed] [Google Scholar]
- Hu WS, Temin HM. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci USA. 1990;87:1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, Washizu T, Toriyabe K, Motoyoshi S, Tomoda I, Pedersen NC. Feline immunodeficiency virus infection in cats of Japan. J Am Vet Med Assoc. 1989;194:221–225. [PubMed] [Google Scholar]
- Itescu S, Simonelli PF, Winchester RJ, Ginsberg HS. Human-Immunodeficiency-Virus Type-1 Strains in the Lungs of Infected Individuals Evolve Independently from Those in Peripheral-Blood and Are Highly Conserved in the C-Terminal Region of the Envelope V3 Loop. Proc Natl Acad Sci USA. 1994;91:11378–11382. doi: 10.1073/pnas.91.24.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma S, Motokawa K, Hohdatsu T, Yamamoto JK, Koyama H, Hashimoto H. Nucleotide sequence of feline immunodeficiency virus: classification of Japanese isolates into two subtypes which are distinct from non-Japanese subtypes. J Virol. 1995;69:3639–3646. doi: 10.1128/jvi.69.6.3639-3646.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann R, Seddon J, Kyaw-Tanner M, Meers J. Co-infection with different subtypes of feline immunodeficiency virus can complicate subtype assignment by phylogenetic analysis. Arch Virol. 2007;152:1187–1193. doi: 10.1007/s00705-007-0940-2. [DOI] [PubMed] [Google Scholar]
- Kemal KS, Foley B, Burger H, Anastos K, Minkoff H, Kitchen C, Philpott SM, Gao W, Robison E, Holman S, Dehner C, Beck S, Meyer WA, III, Landay A, Kovacs A, Bremer J, Weiser B. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycosylation. Proc Natl Acad Sci USA. 2003;100:12972–12977. doi: 10.1073/pnas.2134064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B, Muldoon M, Theiler J, Gao F, Gupta R, Lapedes A, Hahn BH, Wolinsky S, Bhattacharya T. Timing the ancestor of the HIV-1 pandemic strains. Science. 2000;288:1789–1796. doi: 10.1126/science.288.5472.1789. [DOI] [PubMed] [Google Scholar]
- Korber BTM, Kunstman KJ, Patterson BK, Furtado M, McEvilly MM, Levy R, Wolinsky SM. Genetic Differences between Blood- and Brain-Derived Viral Sequences from Human Immunodeficiency Virus Type 1-Infected Patients: Evidence of Conserved Elements in the V3 Region of the Envelope Protein of Brain-Derived Sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara H, Hohdatsu T, Okumara M, Sato K, Suzuki Y, Motokawa K, Gemma T, Watanabe R, Huang C, Arai S, Koyama H. Dual-subtype vaccine (Fel-O-Vax FIV) protects cats against contact challenge with heterologous subtype B FIV infected cats. Vet Microbiol. 2005;108:155–165. doi: 10.1016/j.vetmic.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Kyaw-Tanner MT, Robinson WF. Quasispecies and naturally occurring superinfection in feline immunodeficiency virus infection. Arch Virol. 1996;141:1703–1713. doi: 10.1007/BF01718293. [DOI] [PubMed] [Google Scholar]
- Leitner T, Albert J. The molecular clock of HIV-1 unveiled through analysis of a known transmission history. Proc Natl Acad Sci USA. 1999;96:10752–10757. doi: 10.1073/pnas.96.19.10752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JK, Scott HM, Lachtara JL, Crawford PC. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc. 2006;228:371–376. doi: 10.2460/javma.228.3.371. [DOI] [PubMed] [Google Scholar]
- McGrath KM, Hoffman NG, Resch W, Nelson JAE, Swanstrom R. Using HIV-1 sequence variability to explore virus biology. Virus Res. 2001;76:137–160. doi: 10.1016/s0168-1702(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Suzuki Y, Ikeo K, Ikeda Y, Sato E, Nguyen NTP, Gojobori T, Mikami T, Miyazawa T. Phylogenetic analysis of Vietnamese isolates of feline immunodeficiency virus: genetic diversity of subtype C. Archives of Virology. 2003;148:783–791. doi: 10.1007/s00705-002-0954-8. [DOI] [PubMed] [Google Scholar]
- Nelson MI, Holmes EC. The evolution of epidemic influenza. Nature Reviews Genetics. 2007;8:196–205. doi: 10.1038/nrg2053. [DOI] [PubMed] [Google Scholar]
- Nickle DC, Jensen MA, Shriner D, Brodie SJ, Frenkel LM, Mittler JE, Mullins JI. Evolutionary Indicators of Human Immunodeficiency Virus Type 1 Reservoirs and Compartments. J Virol. 2003;77:5540–5546. doi: 10.1128/JVI.77.9.5540-5546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Goto Y, Pang H, Endo Y, Mizuno T, Momoi Y, Watari T, Hajime T, Hasegawa A. Genetic heterogeneity of env gene of feline immunodeficiency virus obtained from multiple districts in Japan. Virus Research. 1998;57:101–112. doi: 10.1016/s0168-1702(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Nakamura S, Goto N, Hasegawa T, Pang H, Goto Y, Kato H, Youn HY, Endo Y, Mizuno T, Momoi Y, Ohno K, Watari T, Tsujimoto H, Hasegawa A. Molecular characterization of feline immunodeficiency virus genome obtained directly from organs of a naturally infected cat with marked neurological symptoms and encephalitis. Arch Virol. 1996;141:1933–1948. doi: 10.1007/BF01718205. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Tonelli QJ, Scarlett JM. Report of the National FeLV/FIV Awareness Project. J Am Vet Med Assoc. 1991;199:1348–1352. [PubMed] [Google Scholar]
- Ostrowski S, Van Vuuren M, Lenain DM, Durand A. A serologic survey of wild felids from central west Saudi Arabia. Journal of wildlife diseases. 2003;39:696–701. doi: 10.7589/0090-3558-39.3.696. [DOI] [PubMed] [Google Scholar]
- Pecon-Slattery J, Troyer JL, Johnson WE, O’Brien SJ. Evolution of feline immunodeficiency virus in Felidae: implications for human health and wildlife ecology. Vet Immunol Immunopathol. 2008;123:32–44. doi: 10.1016/j.vetimm.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro MR, Tomonaga K, Miyazawa T, Kawaguchi Y, Sugita S, Tohya Y, Kai C, Etcheverrigaray ME, Mikami T. Genetic diversity of Argentine isolates of feline immunodeficiency virus. J Gen Virol. 1996;77:2031–2035. doi: 10.1099/0022-1317-77-9-2031. [DOI] [PubMed] [Google Scholar]
- Pedersen NC, Yamamoto JK, Ishida T, Hansen H. Feline immunodeficiency virus infection. Vet Immunol Immunopathol. 1989;21:111–129. doi: 10.1016/0165-2427(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Reggeti F, Bienzle D. Feline immunodeficiency virus subtypes A, B and C and intersubtype recombinants in Ontario, Canada. J Gen Virol. 2004;85:1843–1852. doi: 10.1099/vir.0.19743-0. [DOI] [PubMed] [Google Scholar]
- Robertson DL, Anderson JP, Bradac JA, Carr JK, Foley B, Funkhouser RK, Gao F, Hahn BH, Kalish ML, Kuiken C, Learn GH, Leitner T, McCutchan F, Osmanov S, Peeters M, Pieniazek D, Salminen M, Sharp PM, Wolinsky S, Korber B. HIV-1 Nomenclature Proposal. A Reference Guide to HIV-1 Classification. In: Kuiken CL, Foley B, Hahn B, Marx PA, McCutchan F, Mellors JW, Mullins JI, Wolinsky S, Korber B, editors. Human Retroviruses and AIDS: A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; Los Alamos, NM: 1999. [Google Scholar]
- Roelke ME, Pecon-Slattery J, Taylor S, Citino S, Brown E, Packer C, Vandewoude S, O’Brien SJ. T-lymphocyte profiles in FIV-infected wild lions and pumas reveal CD4 depletion. J Wildl Dis. 2006;42:234–248. doi: 10.7589/0090-3558-42.2.234. [DOI] [PubMed] [Google Scholar]
- Seibert SA, Howell CY, Hughes MK, Hughes AL. Natural Selection on the gag, pol, and env Genes of Human Immunodeficiency Virus (HIV-1) Mol Biol Evol. 1995;12:803–813. doi: 10.1093/oxfordjournals.molbev.a040257. [DOI] [PubMed] [Google Scholar]
- Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H, Gupta P, Rinaldo CR, Learn GH, He X, Huang XL, Mullins JI. Consistent Viral Evolutionary Changes Associated with the Progression of Human Immunodeficiency Virus Type 1 Infection. J Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton GH, Waltier RM, Connor SC, Grant CK. Prevalence of Feline Immunodeficiency Virus and Feline Leukemia Virus Infections in Pet Cats. J Am Anim Hosp Assoc. 1989;25:7–12. [Google Scholar]
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- Sodora DL, Shpaer EG, Kitchell BE, Dow SW, Hoover EA, Mullins JI. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J Virol. 1994;68:2230–2238. doi: 10.1128/jvi.68.4.2230-2238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrigl A, Klein D. Phylogenetic analysis of feline immunodeficiency virus in Central Europe: a prerequisite for vaccination and molecular diagnostics. J Gen Virol. 2003;84:1301–1307. doi: 10.1099/vir.0.18736-0. [DOI] [PubMed] [Google Scholar]
- Troyer JL, Pecon-Slattery J, Roelke ME, Johnson W, VandeWoude S, Vazquez-Salat N, Brown M, Frank L, Woodroffe R, Winterbach C, Winterbach H, Hemson G, Bush M, Alexander KA, Revilla E, O’Brien SJ. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J Virol. 2005;79:8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeWoude S, Apetrei C. Going wild: Lessons from naturally occurring T-lymphotropic lentiviruses. Clinical Microbiology Reviews. 2006;19:728. doi: 10.1128/CMR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver EA, Collisson EW, Slater M, Zhu G. Phylogenetic analyses of Texas isolates indicate an evolving subtype of the clade B feline immunodeficiency viruses. J Virol. 2004;78:2158–2163. doi: 10.1128/JVI.78.4.2158-2163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Löchelt M, Flower RLP. Epidemiology of feline foamy virus and feline immunodeficiency virus infections in domestic and feral cats: a seroepidemiological study. J Clin Microbiol. 1999;37:2848–2851. doi: 10.1128/jcm.37.9.2848-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs TFW, Zwart G, Bakker M, Goudsmit J. HIV-1 Genomic RNA Diversification Following Sexual and Parenteral Virus Transmission. Virol. 1992;189:103–110. doi: 10.1016/0042-6822(92)90685-i. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, MacDonald DW, Passanisi WC, Harbour DA, Hopper CD. Parasitic prevalence in free-ranging cats, Felis silvestris catus. Epidemiol Infect. 1996;116:217–223. doi: 10.1017/s0950268800052468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Gojobori T. Evolutionary mechanisms and population dynamics of the third variable envelope region of HIV within single hosts. Proc Natl Acad Sci USA. 1997;94:1264–1269. doi: 10.1073/pnas.94.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto JK, Hansen H, Ho EW, Morishita TY, Okuda T, Sawa TR, Nakamura RM, Pedersen NC. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989;194:213–220. [PubMed] [Google Scholar]
- Yamamoto JK, Pu R, Sato E, Hohdatsu T. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS. 2007;21:547–563. doi: 10.1097/QAD.0b013e328013d88a. [DOI] [PubMed] [Google Scholar]
- Yamamoto JK, Torres BA, Pu R. Development of the dual-subtype feline immunodeficiency virus vaccine. AIDScience. 2002;2 doi: 10.1097/QAD.0b013e328013d88a. http://aidscience.org/Articles/AIDScience020.asp. [DOI] [PubMed]
- Zhang L, Diaz RS, Ho DD, Mosley JW, Busch MP, Mayer A. Host-specific driving force in human immunodeficiency virus type 1 evolution in vivo. J Virol. 1997;71:2555–2561. doi: 10.1128/jvi.71.3.2555-2561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rowe L, He T, Chung C, Yu J, Yu W, Talal A, Markowitz M, Ho DD. Compartmentalization of Surface Envelope Glycoprotein of Human Immunodeficiency Virus Type 1 during Acute and Chronic Infection. J Virol. 2002;76:9465–9473. doi: 10.1128/JVI.76.18.9465-9473.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LQ, Mackenzie P, Cleland A, Holmes EC, Brown AJL, Simmonds P. Selection for Specific Sequences in the External Envelope Protein of Human-Immunodeficiency-Virus Type-1 Upon Primary Infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and Phenotypic Characterization of HIV-1 in Patients with Primary Infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]