Abstract
Epidemiological and experimental data suggest a close connection between inflammation and tumorigenesis. Solid tumors are typically infiltrated with immune cells and inflammation impacts most, if not all, stages of tumorigenesis. Molecular and cellular pathways, which connect inflammation and cancer, have emerged as attractive targets for prevention and therapy. In this review we discuss general mechanisms and concepts of cancer promoting inflammation.
Keywords: cancer, inflammation, immunity, cytokines
Introduction
Most, if not all, solid tumors are infiltrated with immune and inflammatory cells. This can represent an ongoing anti-tumor response or be a sign of immune system subversion by the tumor for its own benefit. The first possibility has been addressed within the frame of the “tumor immunosurveillance” concept, proposed by Old, Schreiber and coworkers [1]. The immune system can play an anti-tumorigenic role in certain cases, especially in blood, chemically and virally induced cancers by eliminating pre-malignant as well as fully transformed cells. This process largely depends on altered immunogenic epitopes expressed by cancer cells as well as on stress, necrosis and other immunostimulatory signals, which help immune system to recognize tumor antigens as non-self. On the effector side, immunosurveillance relies on CD8+ cytotoxic T cells (CTLs) and natural killer (NK) cells as well as help from antigen presenting dendritic cells (DCs) and CD4+ Th1 cells [1].
However, cancer cells possess a great ability to mutate, evolve and rapidly grow. Hence, the cancer can easily outsmart the immune system through the growth of low-immunogenic or resistant clones or by directly subverting the anti-tumor immune response and use it for tumor promotion. Often referred to as “tumor escape” [2], this situation is very well illuminated by the fact that advanced tumors always exhibit a significant immune infiltrate but are rarely rejected. Tumors also may remain dormant for a long time, reflecting an “equilibrium” between tumor growth (immune dependent or independent) and immune destruction [3]. Thus, while the host immune system may be engaged in early tumor detection and destruction, it has become increasingly evident that immune cells and inflammatory processes that either precede or are subsequent to cancer development play a pivotal pro-tumorigenic role [4,5]. Various immune cells, including T and B lymphocytes, macrophages, DC, neutrophils, NK cells, mast cells and other cell types are frequently found to be concentrated in tumors relative to the surrounding tissue [6–9]. Therefore, it appears that such cells are actively recruited in response to tumor-derived signals as a result of tumor selection and evolution. However, it is also plausible that such cells may be initially recruited into the tumor as a part of the anti-tumor response, but once present within the tumor microenvironment they are diverted towards pro-tumorigenic responses. For instance, myeloid cells, which can give rise to “M1” macrophages that produce IL-12 and other anti-tumorigenic products, differentiate within the tumor microenvironment into myeloid derived suppressor cells (MDSC) or “M2” macrophages that produce various immunosuppressive and pro-angiogenic molecules [10–12]. Similarly, various tumor promoting T cells, including Th2, Th17 and Treg cells, can be recruited or differentiated in situ within tumors, while cells important for anti-tumor responses, such as Th1 cells or CD8+ CTLs, are either underrepresented or functionally disarmed [13–16]. Importantly, there is no unequivocal correlation between the presence of a T cell infiltrate and tumor prognosis as for sporadic colon cancer it represents better prognosis [17,18], while in breast cancer an infiltrate with a high CD4+ to CD8+ ratio correlates with worse prognosis [19]. Furthermore, tumors can induce and perpetuate tumor-associated inflammation and use it to their own benefit [20].
What determines the overall contribution of inflammatory processes to tumor development? 1) First, many inflammatory mediators (for instance, cytokines) are also important growth and survival factors that stimulate the survival and proliferation of pre-malignant cells [21]. 2) Inflammatory mediators often activate oncogenic transcription factors, such as NF-κB and STAT3 [22–24], whereas oncogenes such as Ras and Myc can initiate inflammatory response [25,26]. 3) Tumor-associated inflammation can suppress anti-tumor immune response and divert tumor specific immune cells from being anti-tumorigenic to become pro-tumorigenic. 4) Inflammation can stimulate tumor angiogenesis. 5) Inflammation can stimulate tumor invasiveness and metastatic dissemination [27].
Types of tumor-promoting inflammation
Several types of inflammation, which differ by cause, mechanism, outcome and intensity exist [28], and all of them potentially can promote cancer development and progression. How tumor-promoting inflammation is induced? First, repetitive injury and infections can result in a chronic inflammatory response, for instance infection with Helicobacter pylori or Hepatitis C virus (HCV) cause gastritis, ulcers and hepatitis, eventually leading to gastric or liver cancer, respectively [21]. Infection with Bacteroides sp. facilitates tumor development in spontaneous intestinal cancers [29]. Chronic inflammation can also be induced by environmental exposure or dietary/metabolic factors. Particulate material and other components of tobacco smoke trigger injury and chronic lung inflammation, thereby increasing the risk of lung cancer [30]. Obesity is a risk-factor for liver cancer development and obesity-associated inflammation may serve as a critical driving force for liver cancer promotion [31–34]. Several types of autoimmunity may also contribute tumor development, for example inflammatory bowel disease increases the risk of colitis-associated cancer (CAC) and Celiac disease is a risk factor for lymphoma [35–37]. However, not all chronic inflammatory diseases increase cancer risk equally. Ulcerative colitis imposes much greater risk for CAC than Crohn’s disease, and rheumatoid arthritis does not increase cancer risk at all. Most likely, chronic inflammation needs to act synergistically with carcinogen exposure and tissue injury and repair.
Even cancers that evolve without underlying chronic inflammation exhibit tumor associated inflammation and contain inflammatory infiltrates [26]. Oncogene activation (as shown for Ras and Myc), or cell senescence induced by DNA damage or oncogene activation, can enhance the transcription of pro-inflammatory genes, coding for cytokines and chemokines [25,38–40]. The last but not least, type of inflammatory response associated with cancer is therapy-induced inflammation. Since most of the cancer cells are resistant to apoptosis, their death induced by chemo- or radiotherapy is necrotic in nature and proteins released by necrotic are potent inducers of inflammation [41]. A similar inflammatory response can be triggered by hypoxia and nutrient deprivation, which result in the necrotic death of cells at the core of large tumors. Two outcomes, which are not mutually exclusive, can be induced by this type of inflammation. First, activation of the immune system by products of necrotic cells can enhance the efficiency of tumor antigen presentation resulting in anti-tumor immunity that helps the host to eradicate the remaining tumor [42,43]. Second, inflammatory mediators released by dying cancer cells can lead to activation of tumor infiltrating macrophages that produce cytokines that eventually activate oncogenic transcription factors in remaining cancer cells that stimulate their survival and proliferation [23,44]. The balance between therapy-induced tumor eradication and regrowth is very delicate and is likely to depend on the extent of therapy induced cell death, the type of cancer being treated and the inflammatory microenvironment associated with the tumor.
Does inflammation induce tumorigenesis?
Epidemiological, pharmacological and genetic evidences provide a solid support that inflammation can increase cancer risk and can promote tumor progression [26]. However, it remains to be determined whether chronic inflammation can cause tumor-initiating genetic alterations or can only act in conjunction with carcinogen exposure. In the case CAC, it was suggested that chronic inflammation and colonic injury can directly cause DNA alterations [45,46]. However, chronic inflammation and loss of protective mucus can also increase intestinal permeability for environmental toxins and mutagens, which induce mutations in stem cells that give rise to cancer [47]. Furthermore, inflammation can stimulate the proliferation of cells that harbor oncogenic mutations induced by carcinogens rather than induce mutations themselves. Nonetheless, inflammation can result in the production of reactive oxygen and nitrogen species (ROS and RNI) by immune cells as well as immune mediated stimulation of ROS production in pre-malignant cells, induction of “mutagenic” enzymes such as activation-induced cytidine deaminase (AID) [48,49] and inactivation of DNA damage gatekeeper pathways such as the mismatch repair response (MMR) [10,45] or p53 [50,51]. Inflammation may also lead to epigenetic modifications including DNA and histone methylation that eventually lead to silencing of tumor suppressor loci [52–55]. In summary, it remains to be fully established whether inflammatory alone can result in tumor initiation (Figure 1) but as described below, there is ample evidence that inflammation is tumor promoting.
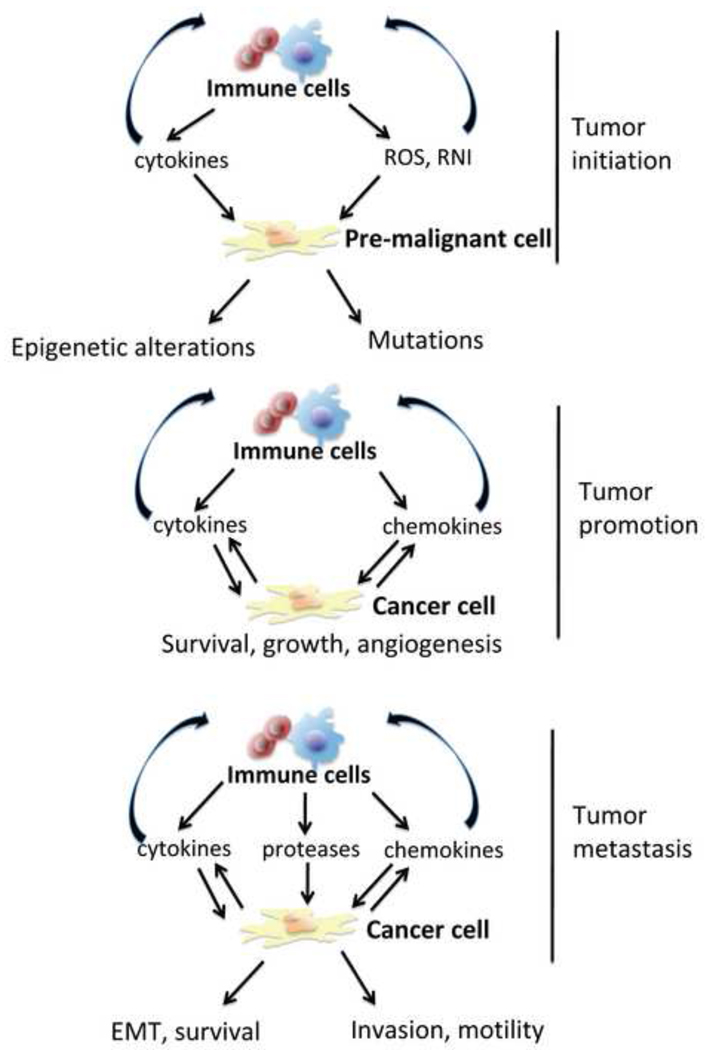
Figure 1.
Inflammation is important at various stages of tumorigenesis. Inflammation may contribute to tumor initiation by inducing DNA damage through intermediates like ROS and via activation of epigenetic mechanisms, which lead to silencing of tumor suppressor genes. During tumor promotion, immune and inflammatory cells produce cytokines and chemokines, which facilitate cancer cell survival, proliferation and promote the angiogenic switch. This results in increased tumor growth. Cytokines and chemokines also induce further recruitment and differentiation of immune cells in the tumor microenvironment. At the tumor progression and metastasis stages, immune cells further contribute by production of cytokines and chemokines to increase cell survival, motility and invasiveness, as well as to promote epithelial mesenchymal transition (EMT).
Inflammation and tumor growth
Tumor growth (often called tumor promotion) is the sum total of malignant cell proliferation vs. malignant cell death. Both processes are strongly impacted by inflammation and inflammatory cytokines produced by tumor infiltrating immune cells, such as IL-6 and TNF-α, can serve as mitogens and survival factors for pre-malignant and fully established cancer cells (Figure 1). Inflammation also contributes to the induction of angiogenesis, which is critical for supplying the growing tumor with necessary nutrients and oxygen [56,57].
Much of the growth stimulating cross-talk between immune and malignant cells is mediated by cytokines that activate the oncogenic transcription factors NF-κB and STAT3 (reviewed in [22]). Activation of either NF-κB or STAT3 is found in over 50% of all cancers [23,44,58] and is a pre-requisite for the expression of a variety of target genes important for tumorigenesis, including anti-apoptotic genes (c-IAP, Bcl-xL, Bcl-2, c-FLIP), proliferative genes (Cyclins, c-Myc), stress-response genes (SOD2, ferritin heavy chain, hsp70), chemokines and pro-angiogenic molecules (VEGF, bFGF, CXCL12) [22,59–61]. On the other hand, in immune cells, NF-κB and STAT3 are instrumental for the production of pro-inflammatory cytokines, which mediate NF-κB and STAT3 activation in cancer cells, including IL-1, TNF, IL-6 and IL-23.
In models of inflammation-associated colon and liver cancers, ablation of IKKβ, a protein kinase required for NF-κB activation in myeloid cells, reduces the production of various pro-inflammatory cytokines including IL-1, IL-6, TNF and IL-12/IL-23 and results in decreased tumor multiplicity and size [62,63]. The tumor promoting action of these cytokines is mostly mediated by NF-κB (e.g. TNF and IL-1) or STAT3 (e.g. IL-6, IL-11) activation in pre-malignant enterocytes and hepatocytes [64–70]. Other relevant STAT3 activators in tumor cells can include members of the IL-6 family, such as OSM, LIF, CNTF and IL-27, cytokines of the IL-10 family (IL-22, IL-19 and others), and growth factors such as EGF [71–74]. NF-κB in both immune cells and cancer cells can also be activated by signals from Toll-like or NOD-like receptors or by inflammatory cytokines such as IL-1 or TNF. It was also suggested that IL-17 can drive NF-κB activation [75] and can indirectly cause STAT3 activation by stimulating IL-6 production [15]. STAT3 itself was shown to prolong NF-κB activation and nuclear retention [68], thereby providing a basis for the cross-talk between these important oncogenic pathways driven by inflammatory signals. Importantly, however, NF-κB and STAT3 are not classical oncogenes, at least not in solid tumors, as they are not subject to direct mutational activation.
The interplay between NF-κB and STAT3 in colitis associated cancer: malignant cooperation between immune and cancer cells
The critical role of NF-κB in linking inflammation and tumorigenesis was first demonstrated in a mouse model of CAC [62]. Ablation of IKKβ in intestinal epithelial cells largely abolished the development of colonic adenomas. The pro-oncogenic role of NF-κB in CAC is most likely mediated through induction of anti-apoptotic proteins, particularly Bcl-XL [62,64,65]. NF-κB activation in epithelial cells has no effect on cell proliferation. By contrast, STAT3 in intestinal epithelial cells impacts both cell proliferation and cell survival [64,65]. Accordingly, STAT3 ablation in intestinal epithelial cells results in a dramatic decrease in both tumor induction and tumor growth, as well as reduced expression of Bcl-XL, and other pro-survival and tissue protective proteins and cell cycle regulators [64,65,74]. As mentioned above, NF-κB activation in myeloid cells also enhances tumor growth but the effect is mainly due to enhanced epithelial cell proliferation rather than survival [62], This effect of NF-κB in myeloid cells is mediated through the production of TNF [76], IL-6 [64,77] and other cytokines.
Inflammation and metastasis
Ninety percent of cancer deaths are due to metastatic growth. Immune cells are present in all advanced tumors and specifically at the invasive front of the tumor and are involved in various forms of direct and indirect interactions with metastasizing cells and micrometastases [20,27]. Indeed, the inflammatory microenvironment was found to influence several key stages of metastatic process [78] (Figure 1). The process of epithelial-mesenchymal transition (EMT), which is critical for metastasis, can be triggered by several cytokines, including TGFβ, IL-1, TNF-α and IL-6 [79–81] and may be a consequence of NF-κB and STAT3 activation [23] through induction of EMT regulators such as Snail, ZEB and Twist [80,82]. Inflammatory signals also regulate the production and activity of various proteases, which degrade extracellular matrix and facilitate invasion and extravasation of cancer cells [23,83]. Chemokines can directly stimulate the migration of malignant cells towards blood vessels [23,84,85], whereas cytokines such as TNF can increase vascular permeability [86]. Furthermore, cytokines are important for the survival, recruitment, colonization and regrowth of the metastatic seeds through the same mechanisms that affect the growth and survival of primary tumors [87–89].
Conclusions and perspective
The connection between inflammatory immune responses and tumorigenesis has been extensively investigated during the past decade and some of the underlying mechanisms have been elucidated. As a result, our view of the role played by the immune system in tumorigenesis has shifted from a strict anti-tumorigenic function to a more balanced view according to which the immune system, while having some negative effects on tumor growth at early stages of the tumorigenic process, has an overall tumor promoting effect. Chronic inflammation caused by infection, autoimmune disease and exposure to irritants as well as tumor associated inflammation, contribute to tumor promotion, progression and metastatic spread. Whereas in inflammation-associated cancer, inflammation can be viewed as a causative agent affecting either tumor initiation or early promotion, tumor-elicited inflammation acts as a late tumor promoter to enhance progression and metastasis. Many of the tumor promoting effects of inflammation depend on production of chemokines and cytokines and activation of the oncogenic transcription factors NF-κB and STAT3. Interference with the production, secretion and receptor binding by chemokines and cytokines, which have been confirmed to have pro-tumorigenic activities, represent new therapeutic approaches whose efficacy should be evaluated both as single agents and as adjuvants for chemo- and radio-therapy. Keeping in mind the reciprocal relationship between the anti-tumor (immunosurveillance) and pro-tumor (cancer promoting inflammation) arms of the immune system, it is also of importance to evaluate the therapeutic efficacy of agents that interfere with activation of pro-tumorigenic pathways in combination with agents or treatment that enhance anti-tumor immunity.
Acknowledgements
This work was supported by Research Fellowship Award from Crohn`s and Colitis Foundation of America (CCFA #1762) to S.G. and the National Institutes of Health and the American Association for Cancer Research to M.K., who is an American Cancer Society Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no competing financial interests.
References
- 1. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. (** Seminal review which discusses in details tumor immunosurveillance and tumor immune escape)
- 2.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 3. Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. (** This paper provides unequivocal evidence for the existence of the equilibrium between tumor and immune system in vivo)
- 4.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 7.Johansson M, Tan T, de Visser KE, Coussens LM. Immune cells as anti-cancer therapeutic targets and tools. J Cell Biochem. 2007;101:918–926. doi: 10.1002/jcb.21230. [DOI] [PubMed] [Google Scholar]
- 8. de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. (* This paper demonstrates previously unexpected tumor-promoting role of B lymphocytes in cancer)
- 9.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 10.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 11.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts SJ, Ng BY, Filler RB, Lewis J, Glusac EJ, Hayday AC, Tigelaar RE, Girardi M. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–6775. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. (* establishes the pro-metastatic role of CD4+ T cells in breast cancer and shows that CD4+ T cells educate tumor-associated macrophages
- 15.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 18. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. (* References 17 and 18. T cell infiltration in sporadic colon cancer tumors can be used for the prediction of the outcome of disease and seem to have anti-tumorigenic role)
- 19.Kohrt HE, Nouri N, Nowels K, Johnson D, Holmes S, Lee PP. Profile of immune cells in axillary lymph nodes predicts disease-free survival in breast cancer. PLoS Med. 2005;2:e284. doi: 10.1371/journal.pmed.0020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grivennikov S, Greten FR, Karin M. Immunity, inflammation and cancer: the good, the bad and the ugly. Cell. 2010 doi: 10.1016/j.cell.2010.01.025. in press. (* This review describes in details types for tumor-promoting inflammation, discusses the role of inflammation at different stages of tumorigenesis and outlines common specific mechanisms for tumorigenesis driven by inflammation)
- 21.Greten FR, Karin M. NF-κB: Linking Inflammation and Immunity to Cancer Development and Progression. Nature Reviews Immunology. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikov S, Karin M. Dangerous liasons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010 doi: 10.1016/j.cytogfr.2009.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 24.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 25.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. (* Recent review, which summarizes our current knowledge about cancer related inflammation represented by anti-tumor immunity and cancer-promoting inflammation)
- 27.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 28. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. (** Recent review which describes the causes, types and outcomes of inflammatory reactions)
- 29. Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009 doi: 10.1038/nm.2015. (** This paper describes a role of specific strain of commensal bacteria, which can trigger intestinal injury, colitis and promote tumor growth in a classical sporadic colon cancer model. It also establishes the role of Th17 T cells and STAT3 in tumor promotion)
- 30.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamasaki K, Hayashi Y, Okamoto S, Osanai M, Lee GH. Insulin-independent promotion of chemically induced hepatocellular tumor development in genetically diabetic mice. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill-Baskin AE, Markiewski MM, Buchner DA, Shao H, DeSantis D, Hsiao G, Subramaniam S, Berger NA, Croniger C, Lambris JD, et al. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park EJ, Lee JH, Yu GY, Ali SR, Holzer R, Osterreicher C, Takahashi H, Karin M. Dietary and genetic obesity promote liver carcinogenesis by enhancing IL-6 expression. Cell. 2010 doi: 10.1016/j.cell.2009.12.052. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Semin Immunopathol. 2009;31:249–256. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- 36.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Kristinsson SY, Goldin LR, Bjorkholm M, Caporaso NE, Landgren O. Increased risk for non-Hodgkin lymphoma in individuals with celiac disease and a potential familial association. Gastroenterology. 2009;136:91–98. doi: 10.1053/j.gastro.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 42.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 43. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009 doi: 10.1038/nm.2028. (* This paper shows that chemotherapeutic intervention leads to the activation of inflammasome and production of IL-1b, which drives anti-tumor immune responses)
- 44.Karin M. NF-κB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- 45.Hofseth LJ, Khan MA, Ambrose M, Nikolayeva O, Xu-Welliver M, Kartalou M, Hussain SP, Roth RB, Zhou X, Mechanic LE, et al. The adaptive imbalance in base excision-repair enzymes generates microsatellite instability in chronic inflammation. J Clin Invest. 2003;112:1887–1894. doi: 10.1172/JCI19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. (** This paper establishes the role of chronic inflammation in tumor initiation. In a model of chronic chemically-induced colitis authors found that chronic inflammation directly induces DNA damage and mutations and causes colitis associated cancer without administration of environmental mutagen)
- 47.Sakaguchi T, Brand S, Reinecker HC. Mucosal barrier and immune mediators. Curr Opin Gastroenterol. 2001;17:573–577. doi: 10.1097/00001574-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Takai A, Toyoshima T, Uemura M, Kitawaki Y, Marusawa H, Hiai H, Yamada S, Okazaki IM, Honjo T, Chiba T, et al. A novel mouse model of hepatocarcinogenesis triggered by AID causing deleterious p53 mutations. Oncogene. 2009;28:469–478. doi: 10.1038/onc.2008.415. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 50.Niu G, Wright KL, Ma Y, Wright GM, Huang M, Irby R, Briggs J, Karras J, Cress WD, Pardoll D, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 52.Cooper CS, Foster CS. Concepts of epigenetics in prostate cancer development. Br J Cancer. 2009;100:240–245. doi: 10.1038/sj.bjc.6604771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn MA, Hahn T, Lee DH, Esworthy RS, Kim BW, Riggs AD, Chu FF, Pfeifer GP. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res. 2008;68:10280–10289. doi: 10.1158/0008-5472.CAN-08-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards RA, Witherspoon M, Wang K, Afrasiabi K, Pham T, Birnbaumer L, Lipkin SM. Epigenetic Repression of DNA Mismatch Repair by Inflammation and Hypoxia in Inflammatory Bowel Disease-Associated Colorectal Cancer. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jin B, Yao B, Li JL, Fields CR, Delmas AL, Liu C, Robertson KD. DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer. Cancer Res. 2009;69:7412–7421. doi: 10.1158/0008-5472.CAN-09-0116. (* References 52–55 cover important advances in how inflammation can stimulate epigenetic silencing of genes relevant to tumorigenesis and enhance tumor initiation and tumor promotion)
- 56.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 57.Zumsteg A, Christofori G. Corrupt policemen: inflammatory cells promote tumor angiogenesis. Curr Opin Oncol. 2009;21:60–70. doi: 10.1097/CCO.0b013e32831bed7e. [DOI] [PubMed] [Google Scholar]
- 58.Levy DE, Darnell JE., Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 59.Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 60.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niu G, Briggs J, Deng J, Ma Y, Lee H, Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R, et al. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha RNA expression in both tumor cells and tumor-associated myeloid cells. Mol Cancer Res. 2008;6:1099–1105. doi: 10.1158/1541-7786.MCR-07-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. (* These papers ref. 64 and 65 establish the role of transcription factor STAT3 expressed by intestinal epithelial cells and STAT3-activationg cytokines, such as IL-6 and IL-11 in the regulation of survival and proliferation of epithelial and malignant cells and development of colitis-associated cancer)
- 66.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 67.Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 68. Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. (** This intriguing paper provides an explanation and a mechanism how constitutive STAT3 activation in tumors causes prolonged NF-kB activation)
- 69. Ernst M, Najdovska M, Grail D, Lundgren-May T, Buchert M, Tye H, Matthews VB, Armes J, Bhathal PS, Hughes NR, et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J Clin Invest. 2008;118:1727–1738. doi: 10.1172/JCI34944. (*This paper shows the role of IL-11 as well as of STAT1 and STAT3 in gastric cancer which is caused by deregulated gp130-signaling)
- 70.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 72.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- 74.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. (** This paper provides first unequivocal evidence that TNF-α is not only important for IBD development but is also critical for colitis-associated cancer pathogenesis. Genetic ablation of TNFR1 or pharmacological neutralization of TNF-α significantly decreases tumor numbers and tumor size)
- 77. Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. (** This paper first implicates IL-6 trans-signaling into development of colitis-associated cancer and demonstrates that anti-tumorigenic action of TGFβ may be in part explained by its ability to negatively regulate IL-6 signaling)
- 78.Wu Y, Zhou BP. Inflammation: A driving force speeds cancer metastasis. Cell Cycle. 2009;8 doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 80. Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. (* This study demonstrates how inflammatory NF-κB dependent signaling results in stabilization of Snail. Since Snail is a key regulator of metastasis, this work shows a distinct molecular mechanism how inflammation (particularly NF-κB) can contribute to metastasis)
- 81.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 84.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 85. Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. (* This study finds a critical role of IKKα, an enzyme from alternative NF-κB pathway, in prostate cancer metastasis due to its ability to inhibit the expression of maspin. Since maspin inhibits the activity of various tissue proteases, its repression aids metastasis development)
- 86.Grivennikov SI, Kuprash DV, Liu ZG, Nedospasov SA. Intracellular signals and events activated by cytokines of the tumor necrosis factor superfamily: From simple paradigms to complex mechanisms. Int Rev Cytol. 2006;252:129–161. doi: 10.1016/S0074-7696(06)52002-9. [DOI] [PubMed] [Google Scholar]
- 87.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 88.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 89.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]