Abstract
Here we report on the safety, immunogenicity, and vaccine efficacy of the naturally occurring plasmid-free attenuated Chlamydia trachomatis L2-5667R strain in a murine infection model. Intravaginal Immunization induced both chlamydial specific serum antibody and systemic CD4+ Th1 biased immune responses but failed to induce local IgA antibodies. Immunization induced no pathological changes in the urogenital tract. Protective immunity was evaluated by vaginal challenge with a natural occurring non-attenuated plasmid positive C. trachomatis urogenital strain (serovar D). Vaccinated mice were not protected from colonization/infection but exhibited a reduction in infectious burden at early time periods (1-2 weeks) post-challenge. Partial protective immunity did not protect against inflammatory disease. Thus, intravaginal vaccination with the live-attenuated L2R stain is safe, induces a systemic antibody and CD4+Th1 biased immune response, but its protective efficacy is limited to reducing chlamydial burden at early time periods post-infection.
Keywords: Live-attenuated Chlamydia vaccine, immunogenicity, safety, protective efficacy, urogenital infection model
1. Introduction
Chlamydia trachomatis infections are the most common bacterial cause of sexually transmitted disease (STD) [1]. Re-infection is common despite antibiotic therapy and often leads to severe complications such as pelvic inflammatory disease, tubal infertility, and ectopic pregnancy. Control of chlamydial STD will likely require the development of a preventive vaccine. Toward this end there has been considerable effort spent evaluating the immunogenicity and vaccine efficacy of whole inactivated organisms and subunit based immunogens that have yielded varying degrees of success; ranging from partial protection [2-6] to near sterilizing immunity [7]. There have been no reported studies on the use of a live-attenuated C. trachomatis vaccine (LACV). A LACV might in fact represent a better vaccine strategy for the prevention of chlamydial STD for the following reasons; (1) targets the natural site of infection (genital mucosa), (2) stimulates both mucosal and systemic immunity, (3) generates both antibody and cell mediated immunity, and (4) the immunogenic repertoire will consist of targets representative of both structural and secreted antigens. Conversely, a LACV must be safe and be shown not to evoke deleterious immunity following re-challenge or exposure to virulent organisms.
Peterson et al., [8] previously reported a human clinical isolate L2(25667R) that lacked the 7.5 kb cryptic C. trachomatis plasmid. In a recent report [9], our laboratory demonstrated that the plasmidless LGV strain L2(25667R) (L2R) was highly attenuated following intravaginal infection of C3H/HeJ female mice. Interestingly, attenuation was restricted to in vivo infectivity arguing that the cryptic plasmid was an important virulence factor and suggesting that the L2R strain would be an attractive first generation LACV. Here we report on the immunogenicity and protective efficacy of the L2R strain in a murine model of C. trachomatis genital tract infection.
2. Materials and Methods
2.1 Animals
Female 6-8 weeks of age C3H/HeJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and used throughout the study. The mice were given food and water ad libitum and all research involving animals was conducted in accordance with Animal Care and Use guidelines and animal protocols were approved by the Animal Care and Use Committee at RML
2.2 Bacteria
Chlamydia trachomatis serovars L2(5567R) and D/UW-3/Cx were propagated on HeLa 229 cells and EBs purified by density gradient centrifugation and stored at -80°C as previously described [10]
2.3 L2R immunization, specimen collection, and chlamydial challenge
Mice received 2.5 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, MI) subcutaneously at day 10 and 3 before vaginal immunization and prior to the challenge. The mice were immunized intravaginally (1° immunization) with 4×107 inclusion-forming units (IFU) per mouse (10 ID50) of L2R strain. Control mice were sham immunized with SPG only. Chlamydial cervico-vaginal shedding was monitored by swabbing the vaginal vault and performing cultures on monolayers of HeLa 299 cells at 3, 7, 14, 21 and 28 days post immunization (dpi). Infectious loads in cervico-vaginal swabs were determined following immunostaining of methanol fixed cells and inclusions were visualized by indirect immunofluorescence using the genus-specific anti-lipopolysaccharide MAb EVI-H1 and fluorescein isothiocyanate (FITC)-labeled goat anti mouse IgG. The mice received a second immunization (2° immunization) at 35 dpi. Mice were bleed and vaginal washes collected at the time points indicated for analysis of systemic and mucosal antibody responses following L2R immunization. Spleens were collected at the same time points for the analysis of CD4+ T cell immunity.
Sham and L2R immunized mice were intravaginally challenged with 4.3×104 IFU/mouse (10 ID50) of serovar D/UW-3/Cx following the 2° L2R immunization. Vaginal swabs from serovar D challenged mice were cultured for recoverable IFU on 3, 7, 10, 14 and 28 days post-challenge as previously described. Non-parametric Kruskal-Wallis test was used in statistical analysis. Blood and vaginal washes were collected for analysis of systemic and mucosal antibody responses as indicated above.
Five mice were euthanized by cervical dislocation at various times following a L2R immunization, C. trachomatis serovar D infection, or sham immunization (SPG). The entire genital tract was removed, fixed in 10% buffered formalin and embedded in paraffin. Longitudinal 4 μm sections were cut, stained with hematoxilin and eosin (H&E) and evaluated by a veterinary pathologist blinded to the experimental design. Sections from vagina, cervix, uterine horn, oviduct and ovary were assessed independently for the presence of acute (predominantly or increased neutrophils, edema, fibrin, hemorrhage), and chronic inflammation (predominantly or increased lymphocytes, macrophages, plasma cells, fibroblasts or fibrosis). A semi-quantitative scoring system was used to quantify the acute and or chronic inflammation as follows: 0= absent or normal, 1= minimal (one or few, small, focal aggregates comprised of very low numbers of inflammatory cells), 2= mild (one or more, focal aggregates comprised of low numbers of inflammatory cells, or more diffuse infiltrate of low numbers of inflammatory cells), 3= moderate (one or more larger aggregates comprised of many inflammatory cells, or more diffuse infiltrate of many inflammatory cells), 4= severe (one or more large aggregates comprised of numerous/myriad inflammatory cells with possible coalescing of aggregates, or more diffuse infiltrate of numerous/myriad inflammatory cells). Immunodetection of Chlamydia inclusions was performed as previously described [11] using the C. trachomatis monoclonal antibody L2I5 that is specific for MOMP.
2.2 Analysis of serum and secretory antibody responses
Serum and secretory chlamydial-specific antibody class (IgA and IgG) and isotype (IgG1 and IgG2a) titers were assayed by enzyme-linked immunosorbent assay (ELISA). Pre-immunization sera and vaginal secretions were used as negative controls. Dilutions of sera and vaginal secretions were assayed using formalin-inactivated C. trachomatis serovar D, or L2 EBs. Briefly, polystyrene microtiter plates (Immulon 2HB; Thermo, Milford MA) were coated with 100 μl (protein 10 μg/ml) of formalin-inactivated EB in 50mM Tris buffer pH 7.5, 0.15M NaCl overnight at 4°C. After adsorption, the wells were blocked with freshly prepared 2% BSA in 0.012 M Tris pH 7.4, 0.14 M NaCl, 3.0 mM KCl, 0.05% Tween 20 (TBST buffer) for 1.5 h at 37°C. Chlamydial-specific antibodies were detected with alkaline phosphatase-conjugated anti-mouse Ig antibodies (class and isotype specific; SouthernBiotech Associates, Birmingham AL.) and p-nitrophenyl phosphate (PNPP) as substrate. The optical density was measured at 405 nm by an ELISA plate reader (Dynex Technologies, Chantilly, VA). Antibody titers were expressed as the highest dilution giving an absorbance of at least 0.3 or 3 times that of the pre-immune sample (consistently < 0.1). C. muridarum (MoPn) convalescent sera and vaginal washes tested against MoPn EBs were used as positive controls. Vaginal secretions were collected by rinsing the vaginal vault twice with 75 μl of 10mM phosphate-buffered saline (PBS) and frozen -20°C until they were assayed.
2.3 CD4+ T cell immune response
The spleens of L2R or sham-immunized mice were collected two weeks following the resolution of the 2° L2R immunization. CD4+ T cells were enriched from spleens using magnetic beads following the manufacturers instructions (Miltenyi Biotech. Bergisch Gladbach, Germany) and plated in quadruplicate (2.5 × 106) in 48-well flat-bottomed tissue culture plates containing (1.5 × 106) gamma-irradiated (3,000 rads; 137Cs) autologous splenoctyes pulsed with UV inactivated serovar D EBs (MOI: 4). Cultures were incubated at 37°C in a 5% CO2 atmosphere for 72 h. Concentrations of secreted cytokines in the culture supernatants were determined using the Bio-Plex mouse cytokine Th1/Th2 panel (Bio-Rad Laboratories, Hercules CA).
2.4 MOMP variable domain peptide immunoassay
Peptides corresponding to the amino acid sequence of the MOMP VDI (amino acids 64-80) and VDII (amino acids 139-152) for serovars, D and L2 were synthesized at 98% purity (Genscript Corporation, Piscatway, NJ) using published sequences [12]. The peptide ELISA procedure described by Su H. et al., [13] was used. Briefly, Peptides were dissolved to 3 mg/ml in distilled water containing 0.025% sodium azide and stored in silicone-coated glass vials at 4°C. Polystyrene microtiter plates (Immulon 2HB; Thermo, Milford MA) were coated with 100 μl of 100 μg/ml of peptide (10 μg/well) in 0.05 M Tris buffer pH 7.5 containing 0.15 M NaCl. The plates were sealed and incubated overnight at 4°C. After adsorption, the plates were washed three times with PBS containing 0.05% Tween 20 (PBS-Tween) and then blocked with freshly prepared 2% BSA in PBS-Tween (blocking solution) for 2 h at 37°C. The plates were washed once in PBS-Tween and then 100 μl of immune or control mouse sample diluted in blocking solution was added and incubated for 1.5 h at 37°C. The plates were washed five times with PBS-Tween. Bound antibody was detected with goat anti-mouse IgG2a conjugated with alkaline-phosphatase (ShouthernBiotech, Birmingham AL) diluted in PBS-Tween. After washing, 100 μl of substrate (PNPP) was added and the plates incubated 40 minutes at 37°C. The enzymatic reaction was terminated by the addition of 50 μl of 3N NaOH to each well. The optical density was measured at 405 nm by an ELISA plate reader (Dynex Technologies, Chantilly, VA). A two-tailed Student's t-test was used for analyzing the differences in ELISA results.
3. Results
3.1 Immunization of C3H/HeJ mice with C. trachomatis L2R
We have described the overall experimental design for L2R immunization, evaluation of immune responses, disease and protective immunity to challenge (Materials and Methods section 2.3). Mice were infected at days 0 and 35 with L2R (Fig. 1) Figure 1A shows the recoverable IFU following the primary and secondary immunizations. The number of culture positive mice at each time point is shown in Figures 1B and 1C, respectively. The majority of mice were colonized following a primary L2R immunization (Fig. 1B) with peak recoverable IFU occurring at day 7 PI. Recoverable IFU decreased thereafter and all mice cleared Chlamydia by day 28 PI. On day 35 post-immunization mice were re-infected with L2R intravaginally. The susceptibility to infection, infectious burden, and duration of infection did not differ from the primary infected group.
Figure 1. Immunization of C3H/HeJ mice with C. trachomatis L2R.
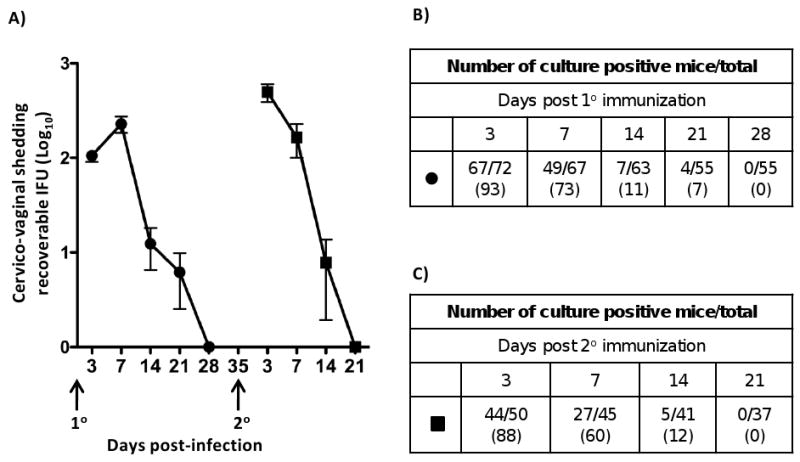
A) A group of 72 progesterone treated female C3H/HeJ mice were infected intravaginally (1° immunization) with 4×107 IFU/mouse (10 ID50). Control mice were sham infected with SPG only. Chlamydial cervico-vaginal shedding was monitored by swabbing the vaginal vault and performing cultures on monolayers of HeLa 299 cells at 3, 7, 14, 21 and 28 days post infection (dpi). Infectious loads in cervico-vaginal swabs were determined following immunostaining of methanol fixed cells and calculating inclusion forming units (IFU). Fifty mice received a second infection (2° immunization) at 35 dpi, 7 days after clearance of the 10 infection. The filled circles and squares represent the mean number of recoverable IFUs ± SEM in 10 and 20 infected mice, respectively. The number of culture-positive mice per total mice at each culture time point during 1° L2R infection (B), or 2° (C). The numbers in parenthesis represent the percent of infectivity.
3.2 Systemic and local antibody responses in C3H/HeJ mice after primary and secondary L2R immunization
ELISA antibody titers against serovar D EBs in sera and vaginal washes are shown in Figure 2. Chlamydial antibodies were not detected in the sera or vaginal washes after a single L2R immunization; a finding consistent with the lack of protection to L2R secondary immunization (Fig. 1). In contrast, following the 2° L2R immunization all 10 mice produced serum IgG2a antibodies (mean titer of 1:136), whereas only 5/10 animals had detectable IgG1 antibodies (mean titer of 1:12). Notably, none of the L2R re-challenged mice had detectable chlamydial-specific serum or vaginal wash IgA antibodies. In contrast, mice that received and cleared a 2° L2R immunization produced a marked chlamydial specific serum antibody response (Fig. 2). Interestingly however, vaginal washes of 2° infected mice did not have detectable anti-chlamydial IgG or IgA. These results suggest that unlike the systemic antibody response elicited following a 2° L2R challenge, secondary immunization of the urogenital mucosa was not sufficient to induce a mucosal antibody response. The inability to detect chlamydial specific antibody in vaginal washes was not due to technical reasons as vaginal washes from C. muridarum and C. trachomatis serovar D infected mice were positive for both IgG and IgA (results not shown).
Figure 2. Systemic and local antibody responses in C3H/HeJ mice after primary and secondary L2R immunization.
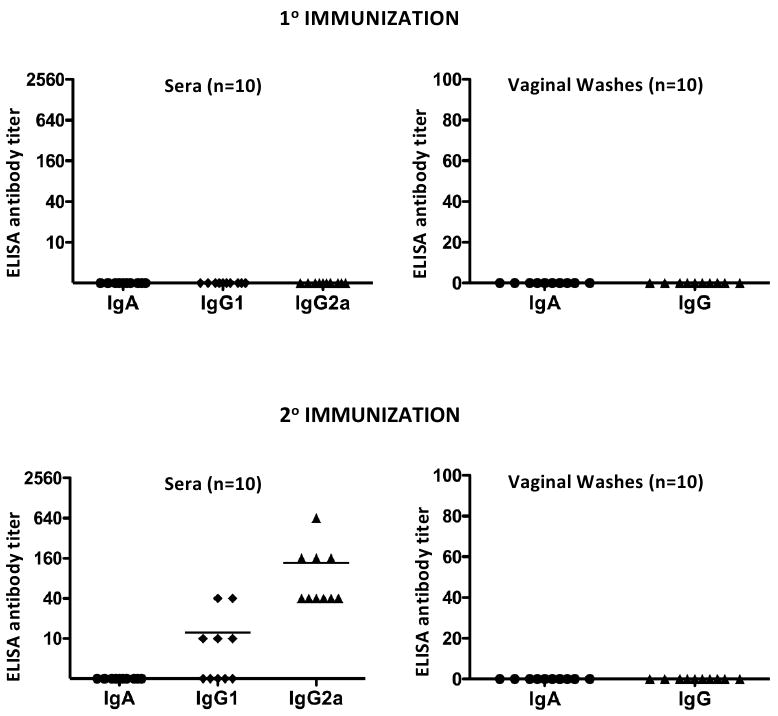
Sera and local antibody class and isotype-specific anti-chlamydial responses were assayed 30 and 25 days post 1° and 2 ° L2R immunizations, respectively. Antibody titers were expressed as the highest dilution giving an absorbance of at least 0.3 or 3 times that of the control sample (consistently < 0.1). C. muridarum (MoPn) convalescent sera and vaginal washes tested against MoPn EBs were used as a positive control (data not shown). Ten mice were tested for each immunization group and the symbols denote the response of individual mice. Bars denote the mean antibody titer. Note that mice did not generate a chlamydial specific antibody following clearance of a primary immunization. In contrast, all L2R immunized mice produced a predominant IgG2a antibody response following resolution of the secondary immunization. Chlamydial-specific IgA or IgG antibodies were not detected in the vaginal washes of L2R infected mice following resolution of either the 1° or 2° immunization.
3.3 L2R immunization induces a systemic CD4+ Th1 biased immune response
CD4+ T cell immunity was determined by assaying the cytokine secretion profiles of antigen stimulated splenic CD4+ T cells isolated from L2R and sham immunized mice following the 2° immunization (Fig. 3). CD4+ T cells from L2R but not sham-immunized mice produced significant levels of IFN-γ and IL-2. Low but detectable levels of IL-10, GM-CSF, IL-12 p40, and IL-17 were also produced by CD4+ T cells isolated from L2R immunized mice. IL-4, IL-5, and TNF-α were not detected. Collectively, these findings together with those presented in Figure 2, demonstrate that a 2° immunization with L2R elicits a significant adaptive CD4 Th1 biased immune response. These immunogenicity findings were encouraging as antibody and CD4+ Th1 mediated immune responses have been shown to be critical in the development of protective immunity against chlamydial infection in the murine urogenital model [11, 14-16].
Figure 3. Splenic CD4+ T cells from L2R immunized mice produce a dominant Th1 biased cytokine response.
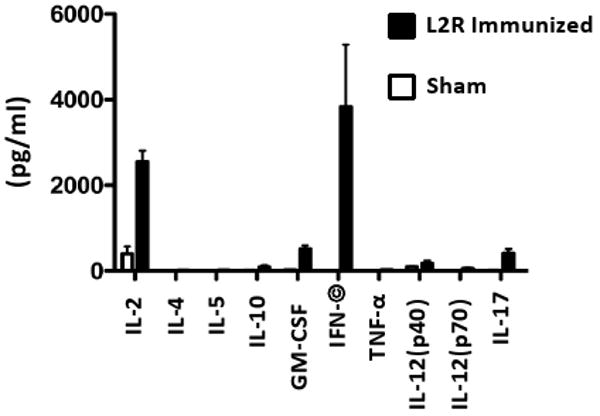
The in vitro production of cytokines by splenic CD4+ T cells from sham or L2R immunized mice was assayed two weeks following the resolution of the 2° L2R infection. The CD4+ T cells were re-stimulated in vitro with UV-inactivated serovar D EB and the supernatants were collected after 72 h of culture for determining the cytokine concentration. The black bars represent the mean ± standard deviation of four individual L2R treated mice tested in quadruplicate. The white bars represent the results of two individual sham infected mice and expressed as the mean ± standard deviation. There was a significant production of IFN-γ by chlamydial pulsed CD4+ T cells.
3.4 Histopathological evaluation of genital tract tissues from C3H/HeJ mice immunized with L2R
L2R immunized mice exhibited no evidence of an acute or chronic inflammatory response following 1° or 2° immunization (Fig. 4A and B). Histopathologically, the tissues from L2R immunized mice were comparable to sham infected controls (score= 0-1, normal or absent, Fig. 4E and F). Conversely, infection with C. trachomatis serovar D (challenge strain) resulted in a mild to moderate sub-mucosal chronic inflammatory response (score 2 to 3). The inflammation was restricted to the submucosa of the cervix (Fig 4C and D). Thus, the L2R strain is infectious, induces a strong systemic immune response, but does not induce potentially damaging inflammatory changes in infected urogenital tissues following either a 1° or 2° immunization.
Figure 4. Histopathological evaluation of genital tract tissues from C3H/HeJ mice immunized with L2R.
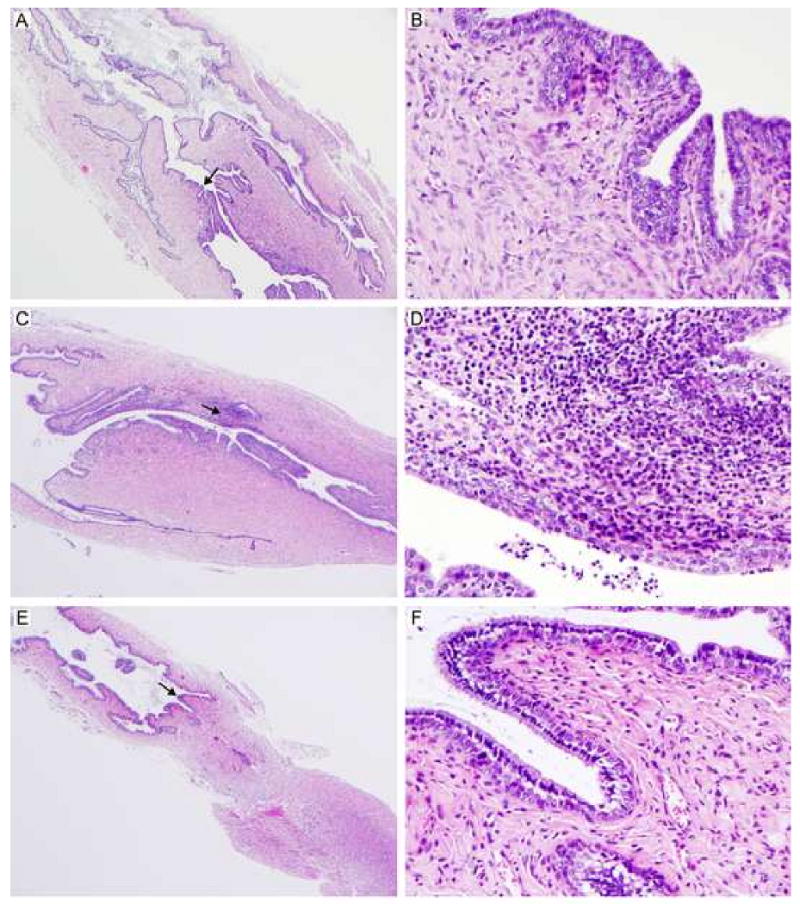
Five mice were euthanized 14 days following a 2° L2R infection, C. trachomatis serovar D infection, or sham infection (SPG). The entire genital tract was removed for histopathological evaluation. Micrographs of histological sections of the cervical region from L2R immunized mice (A, B); Serovar D infected (C, D) and sham infected (E, F) are shown. Magnification 40×: (A, C, E) and 400× (B, D, F). Note that there were no pathological changes in L2R or sham infected mice. In contrast, infection with C. trachomatis serovar D produced a moderate sub-mucosal infiltrate presented as multifocal aggregates composed of lymphocytes, macrophages and plasma cells. Occasionally, low numbers of these cellular aggregates were present within the epithelium lining the lumen. Similarly, smaller and less densely arranged inflammatory aggregates were noted in the muscular layers.
3.5 Immunostaining of C3H/HeJ female mice genital tracts following immunization or infection with C. trachomatis serovar L2R or D
Immunoperoxidase staining was used to evaluate and localize chlamydial infection in genital tract tissues (Figure 5). L2R staining was only found in the underlying submucosa of the cervix and uterine horns, (Fig. 5A and B). L2R inclusions tended to be present as focal aggregates in the tissues. Inclusions were compact and occupied the majority of the infected cell cytosol. We were not able to identify the phenotype of the L2R immunostaining cells. Conversely, C. trachomatis serovar D inclusions were seen as large intra-epithelial structures that were distributed over the cervix and uterine-horn epithelial surface (Fig. 5C and D). These findings support the conclusion that both serovar L2R and D infect the mouse genital tract but the type and location of infected cells differs between the two strains.
Figure 5. Immunostaining of genital tract tissue following immunization or infection with chlamydiae.
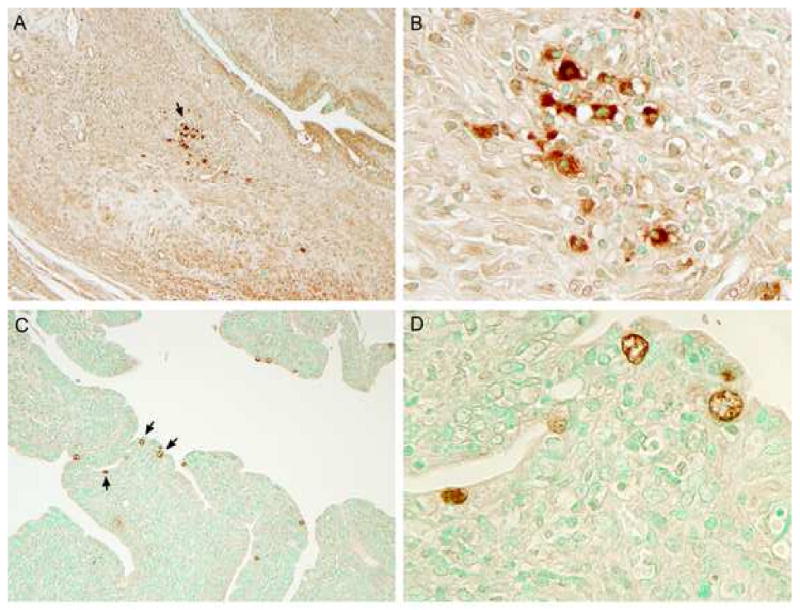
Chlamydial inclusions in genital tissues immunized with L2R (A and B) or infected with serovar D (C and D). (A) L2R inclusions appeared as focal aggregates in the submucosa of the cervix; (B) 1000× magnification from panel A. In contrast, serovar D inclusions were found exclusively within epithelial cells of the distal cervix or uterine horn (C); (D) 1000× magnification from panel C. Mock infected mice tissues were negative by immunostaining. No immunostaining was observed in tissues with a negative control anti-MOMP monoclonal antibody 33b specific to C. muridarum.
3.6 Protective efficacy of L2R immunized mice against C. trachomatis serovar D challenge
L2R and sham immunized mice were challenged intravaginally with 10 ID(50) of the plasmid positive non-attenuated serovar D strain. Serovar D was used for the challenge studies as it closely related to L2 antigenically [17], is one of the most common strains associated with human STI, and infection results in a marked chronic inflammatory response (Fig. 4). The results of a primary and secondary serovar D challenge of L2R and sham immunized mice is shown in Figure 6. All mice were colonized and infected following primary serovar D challenge and the percentage of infected mice in each group was similar throughout the culture period (Fig. 6A and 6B). There was a significant difference in chlamydial shedding at days 3 and 7 post-challenge between L2R and sham immunized mice (Fig. 6A). At days 3 and 7 post-challenge approximately 10 fold less organisms were isolated from the genital tracts of L2R immunized compared to control animals (p<0.0001). However, at later time points post-primary challenge there was no difference in recoverable IFU between L2R immunized and sham controls. Moreover, the duration of infection (28 days) was similar between the two groups. L2R and sham immunized mice were euthanized at every culture time point (3, 7 14, 21 and 28 days PI), their entire genital tract removed, fixed, embedded, and longitudinal sections scored for inflammatory cellular infiltrates following H&E staining. There were no differences in urogenital tract pathology between L2R and sham infected mice (results not shown).
Figure 6. Protective efficacy of L2R immunized mice against C. trachomatis serovar D challenge.
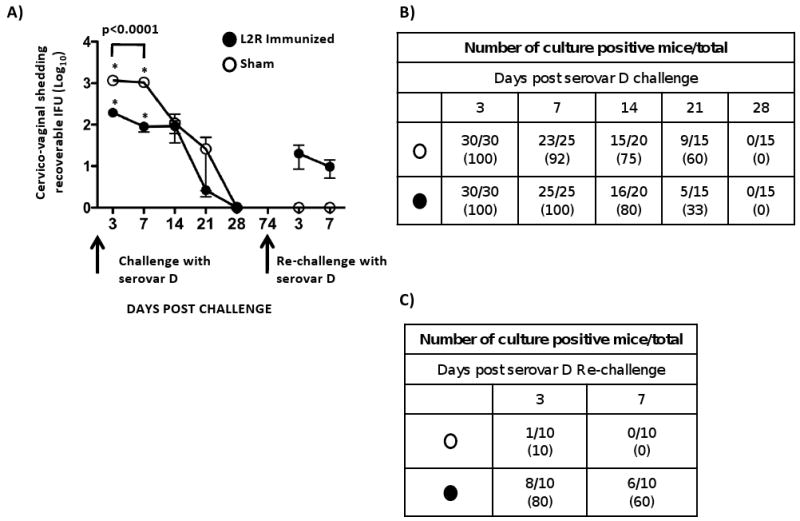
Thirty sham (O) and 30 L2R immunized mice (●) were intravaginally challenged with 4.3×104 IFU/mouse (10 ID50) of serovar D. We chose serovar D as the challenge strain because it is one of the most prevalent serovars associated with human urogenital infection and it is antigenically related to L2. (A) Vaginal swabs from serovar D challenged mice were cultured for recoverable IFU on 3, 7, 10, 14 and 28 dpi. Forty-six days after mice cleared their primary D infection they were re-challenged a second time with serovar D. Each symbol represents the mean number of recoverable IFUs ± SEM. Note that L2R immunized mice were not protected from serovar D colonization. There was a significant difference between L2R and sham immunized mice in the burden of D shedding at days 3 and 7 post-challenge (p<0.0001). However, the burden and duration of infection did not differ between the groups at day 14-28. Interestingly, sham immunized mice that had been infected and then re-challenge with serovar D exhibited near sterilizing immunity. In contrast, L2R immunized and serovar D challenged mice were less protected. All mice in this group were infected following challenge. The numbers of culture positive mice for each group of re-challenged mice are shown in (B) serovar D primary challenge, and (C) serovar D secondary challenge.
We then re-challenged L2R and sham immunized mice with serovar D following clearance of their serovar D primary infection. The results are shown in Figure 6A and 6C. Both groups of mice exhibited very significant levels of protection compared to the primary challenge evidenced by a marked reduction in infectious burden (1-2 log10) and much shorter infection duration (3-7 days). Of particular interest was the near sterilizing immunity observed for the sham-immunized D challenged mice compared to L2R immunized animals. Only 1 of 10 sham-immunized mice was infected post-challenge. The single infected animal was culture negative at day 7. In contrast, 8 of 10 L2R immunized D challenged mice were infected (Fig. 6C). Thus, although both groups exhibited significant protective immunity to serovar D re-challenge the virtual sterilizing immunity of the sham immunized group was intriguing as it suggested that local antibody might have blocked chlamydial mucosal receptor interactions thereby preventing chlamydial colonization. We felt it was important to better understand the immunological basis for these differences in immunity between groups as they could be important to future vaccine efforts.
3.7 Systemic and local antibody responses in mice after primary challenge with serovar D
We asked whether differences in either antibody isotype or titer might explain differences in immune status of L2R and sham immunized D challenged mice. The titers of anti-serovar D IgG and IgA in vaginal washes and sera for each group are shown in Figure 7. The profiles of IgA and IgG in washes and sera were very similar for both groups. Eight of ten L2R immunized D challenged mice had anti-serovar D IgA in vaginal washes; ranging in titer from 1:10 (5 mice) to 1:80 (3 mice) whereas only 3 of 10 mice had detectable IgG antibody (mean titer 1:10) (Fig. 7A). Similar results were observed in vaginal washes from sham immunized D challenged mice; eight mice were positive for IgA ranging in titer 1:10 – 1:80, and only 2/10 had detectable IgG with mean titer of 1:2 (Fig. 7B). The IgA and IgG antibody titers in sera were very different from those found in vaginal washes. Serum IgA was detected in 2 L2R immunized mice and none of the sham immunized animals. All mice in both groups had high titered anti serovar D IgG2a antibodies. Thus, differences in protective immunity observed between the two groups did not correlate with either the antibody isotype or titer in vaginal washes or sera. The consistent presence of IgA in vaginal washes likely reflects the induction of an adaptive mucosal immune response, not serum transudation, as the serum antibody profiles are inconsistent with this mechanism.
Figure 7. Systemic and local antibody responses in sham and L2R immunized mice after challenge with serovar D.
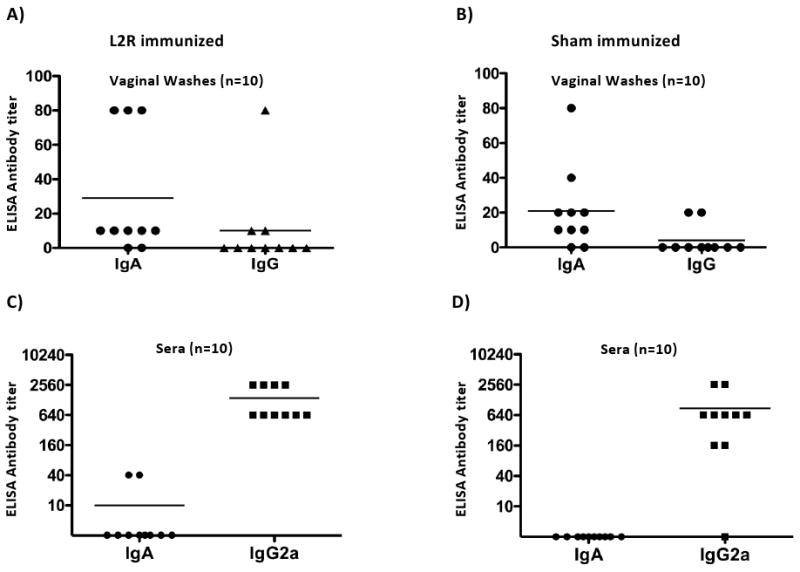
Blood and vaginal washes were collected from ten sham and L2R immunized and serovar D challenged mice 28 days after intravaginal challenge with serovar D (Figure 6). Serum and vaginal IgA and IgG antibody responses against serovar D EBs were determined by ELISA. The symbols denote the antibody response of individual mice. Bars denote the mean antibody titer.
3.8 Specificity of the recall antibody response after a sequential infection with L2R and serovar D
We next sought to determine whether antibody specificity might account for the observed differences in protective immunity. Serovar D and L2 EBs were tested by ELISA using sera from L2R and sham immunized serovar D challenged mice. We were not able to similarly test vaginal washes because of insufficient amounts of material. No differences in titer or specificity of the IgG2a response against L2 or D EB antigens were found (Fig. 8).
Figure 8. C. trachomatis specificity of the antibody response after sequential infection with L2R and serovar D.
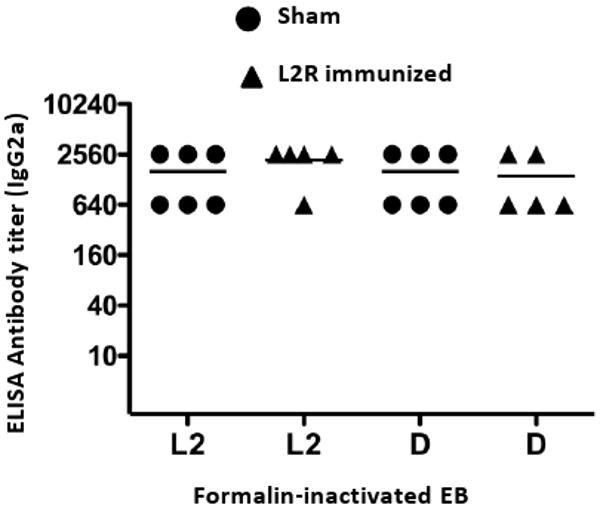
Individual sera from L2R or sham-immunized and serovar D challenged mice were assayed by ELISA against L2 and D EB antigens. Chlamydial-specific IgG2a antibody was analyzed against these antigens and the titers were expressed as the reciprocal of sera dilutions giving an absorbance of 0.3 or greater. Absorbance values for pre-immune sera were 0.1 or less. Note that sera titers were similar against L2 and D EBs.
Chlamydial EBs are antigenically complex and therefore might not be capable of differentiating subtle but functionally important differences in antibody specificity. The MOMP is the primary serotyping antigen and immunodominant epitopes that confer type-specific neutralizing antibody responses are located in four surface exposed regions or loops of the protein known as variable domains (VDI-IV). Type-specific neutralizing monoclonal antibodies map to contiguous epitopes located VDI and VDII of the D and L2 MOMPs [18]. The contiguous amino acid sequences of these VDII epitopes differs by only 7 residues between L2 and D. We therefore hypothesized that an explanation for our findings might be due to the well known original antigenic sin phenomenon [19]. In this scenario, B memory cells generated against the priming L2 MOMP VDII antigenic determinant(s) would be preferentially re-called following serovar D challenge. Because the L2 VDII antibodies would be theoretically less efficacious in neutralizing D infection they would be less protective in vivo; a finding consistent with the results shown in Figure 6A.
To experimentally test this hypothesis we analyzed the molecular specificity of serum antibodies (IgG2a) from L2R and sham immunized D challenged mice against synthetic peptides corresponding to VDI and VDII of serovars D and L2 by ELISA (Fig, 9). Consistent with previous epitope mapping results none of the sera recognized serovar D or L2 VDI synthetic peptides. In contrast, measurable levels of IgG2a antibody specific for L2 VDII were observed in two of five L2R immunized D challenged mice (Fig. 9). However, the mean titer of the L2R immunized mice against D VDII or L2 VDII was not statistically different (p=0.063). None of the sera reacted with serovar D MOMP VDII. These findings present reasonable evidence that the memory B cells in the L2R primed mice were preferentially recalled against L2 immunodominant VDII epitopes following D challenge.
Figure 9. Molecular specificity of the MOMP antibody response after sequential infection with L2R and serovar Din L2R and sham-immunized mice.
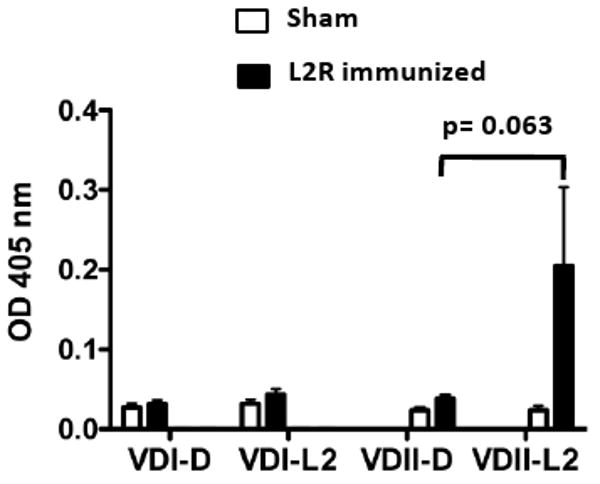
The sera of five sham or L2R immunized and serovar D challenged mice analyzed in Figure 8 was tested by ELISA against synthetic peptide antigens corresponding to MOMP VDI and VDII of serovars D and L2. Previous studies have epitope mapped serovar D and L2 specific epitopes to VDII [18]. The VDs represent exposed loop structures on intact EBs and serovar-specific epitopes located in the loops are immunodominant and targets of neutralizing antibodies (Each bar represents the OD405nm mean ± SEM from each experimental group. Absorbance values for pre-immune sera were 0.1 or less.
4. Discussion
We tested the concept of a LACV in this study using the attenuated plasmid free C. trachomatis L2R strain. In summary our findings were disappointing as we expected that the L2R vaccine would be more efficacious. Immunization with L2R provided partial protective immunity which was manifested by a significant but transient reduction in infectious burden following challenge with the plasmid positive C. trachomatis serovar D urogenital strain. However, the transient reduction in infectious burden had no effect on disease as shown by the similar intensities of sub-mucosal inflammatory infiltrates in immunized and sham immunized controls; an unexpected result because of the significantly greater infection burden in sham compared to L2R immunized mice at early time points post-challenge. The encouraging aspect of the L2R LACV was that infections of the urogenital tract were self-limiting and induced no measurable pathology following either a primary or secondary immunization. This result suggests that the L2R LACV is safe, even after repeated infection episodes, arguing against the possibility of deleterious infection mediated hypersensitivity reactions that have been associated with natural infection and inactivated whole organism chlamydial vaccines [1].
A curious finding in this study was the observation that a single urogenital tract immunization with L2R induced no detectable adaptive immune response. In contrast, a 2° L2R immunization induced a strong systemic CD4 Th1 immune response but did not induce mucosal immunity based on our inability to detect chlamydial-specific IgA in vaginal washes. These findings are perplexing but suggest that the L2R strain might not be an effective LACV for the induction of mucosal immunity. This is in contrast to infection caused by the non-attenuated serovar D strain that induced both systemic and mucosal immune responses following infection. The reason for this difference is not understood, as recoverable infection loads for the strains in the urogenital tract were comparable. A possible explanation might be because of the differences in the primary infection sites in mucosal tissues by the two strains. We found following immunostaining that L2R, a naturally invasive pathogen, was not detected in epithelial cells but localized to submucosal sites. In contrast, the plasmid positive C. trachomatis serovar D was found exclusively within the epithelium of the cervix and uterine horns. Thus, the strong mucosal immunizing properties of serovar D might be related to its infection tropism for epithelial cells. In contrast, the inability of L2R to induce mucosal immunity could be because of its transient infection or localization to columnar epithelial cells that is required to induce local immunity. Perhaps there is a relationship between the non-invasive mucosal tropic properties of a chlamydial strain that restricts grow to mucosal epithelial cells and its subsequent ability to elicit local immunity. Another possible explanation for our findings is that the L2R strain lacks the cryptic chlamydial plasmid; therefore plasmid genes might play a role in the induction of a mucosal immune response. In fact, there is experimental support for this hypothesis. O'Connell et al., [20] have described a plasmidless strain of the mouse pathogen C. muridarum (MoPn) that fails to stimulate TLR-2 dependent cytokine production in mice and infection with the strain has an attenuated disease phenotype for the upper genital tract. Thus, it is possible that plasmid genes through interaction with TLR drive innate immune responses that are critical for the induction of local mucosal immunity.
It is possible that the relatively moderate level of protection conferred by L2R immunization is directly related to plasmid function and immunogenicity. We have shown the ID50 of the L2R strain is 400-fold greater than the plasmid positive L2 strain [9] suggesting that the plasmid might function in colonization or early infection. Thus, immunity specific to plasmid expressed proteins could function in preventing colonization or limiting early infectious burden. This immunity would not be generated by infection with the L2R strain and hence immunized mice would be less protected against colonization a finding consistent with our results. The observation that infection with the plasmid positive serovar D resulted in a much greater level of immunity against infection (Fig. 6) also indirectly supports this conclusion. Lastly, immunization with the plasmid encoded protein pg3 has been shown to be partially protective supporting a role for plasmid genes in protective immunity [21].
Rather surprisingly, despite a dominant systemic CD4+ Th1 immune response, L2R immunized mice were only partially protected following intravaginal challenge with the antigenically related serovar D. There was no protective effect against chlamydial colonization and significant levels of protective immunity were delayed in onset (3-7days post-challenge). We interpret these results to mean that the protective immune response was likely systemic in nature which provided the significant, albeit delayed reduction in infectious burden post-challenge. As mentioned above, a LACV generated from mucosal tropic serovars might be more effective in inducing mucosal immunity that could provide a greater level of protection at the genitourinary mucosa.
The observation that L2R immunized mice were less protected following intravaginal secondary D challenge than sham controls (2° serovar D challenged) was unexpected and suggested to us that the reason for this difference might be due to a original antigenic sin phenomenon [19]. Stephens et al [22] first described this phenomenon in antibody recall responses for chlamydiae following immunization of mice with serologically related C. trachomatis EBs. It is known that serotyping antigenic determinants are located on surface exposed variable domains (VD) of MOMP and these epitopes are the targets of neutralizing antibodies [18]. Thus, memory B cells generated during L2 immunization may have responded to L2 specific VD epitopes following secondary challenge with serovar D. We tested this hypothesis by analyzing the molecular specificity of the antibody from the sera of L2R immunized D challenged and sham-D challenged mice. The sera from both groups of mice was tested by ELISA against synthetic peptides corresponding to VDI and VDII amino acid sequences from the MOMP of serovars L2 and D. Two of the five sera were strongly reactive with the L2 VDII consistent with the conclusion that the D challenged mice had generated a preferential memory B cell antibody response to the priming L2 VDII determinant(s) rather than the D VDII antigenic sites. Native MOMP forms a trimer and this structure is important in eliciting serovar-specific antibody responses [23]. Moreover, these conformation-dependent antigenic sites are thought to depend on the juxtapositioning of VD I and II loops on the face of the trimer [24]. Thus, it is possible antibodies recognizing conformational determinants composed of VDI and VDII structures may also have been preferentially recalled against L2 similar following D challenge infection. If so, this could explain our inability to detect higher levels of L2 VDII specific antibodies in 3 of the 5 challenged mice. Notably, this is the first demonstration that memory B cell immunity in chlamydiae exhibits an antigenic sin phenomenon following a natural infection of a mucosal site. Thus, it is possible that a similar heterotypic antibody response occurs in infected humans against the numerous C. trachomatis serovars and sub-types that cause trachoma and STI. As type specific antibody is thought to be protective in humans this phenomenon could represent a hereto-unappreciated mechanism of natural immune avoidance by chlamydiae in the human host. Lastly, a similar phenomenon has been described for cytotoxic T cells [25]. We therefore cannot exclude a similar preferential T cell memory response against L2 antigens in secondary serovar D challenged mice as an explanation for our findings.
We have described in this study a live-attenuated C. trachomatis vaccine (LACV) for chlamydial STI in a murine model. L2R immunization of the genital tract was poorly efficacious against serovar D challenge. This finding was unexpected as we hypothesized that infection of the urogenital tissues by the plasmid-negative L2R strain would evoke a cross-protective mucosal immunity effective against the antigenically similar serovar D strain. Mucosal infection with the L2R strain elicited both a systemic antibody and cellular immune response but importantly did not result in detectable mucosal immunity. We speculate that the prevalence of L2R infection in sub-mucosal, but not mucosal epithelial cells, might explain these findings. Our findings are consistent with the conclusion that the moderate to significant levels of protective immunity elicited by the epitheliatropic plasmid positive D strain might be closely linked to plasmid function and immunogenicity. In this regard, we speculate that the use of a plasmidless C. trachomatis serovar D strain, or other mucosotropic strains, might indeed result in similar ineffectiveness as a LACV for the prevention of chlamydial STI.
Acknowledgments
We thank Dr. Kim Hasenkrug, Dr. Laszlo Kari and Dr. Jeff Shannon, for critical reading of the manuscript; Dr. Chiung-Yu Huang from Biostatistics Research Branch/NIAID and Rebecca Rosenke from Veterinary Branch/RML/NIAID for her technical assistance; Gary Hettrick and Kelly Matteson, for editorial assistance. This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;(2):149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 2.Eko FO, He Q, Brown T, McMillan L, Ifere GO, Ananaba GA, et al. A novel recombinant multisubunit vaccine against Chlamydia. J Immunol. 2004;(5):3375–82. doi: 10.4049/jimmunol.173.5.3375. [DOI] [PubMed] [Google Scholar]
- 3.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007;(2):666–76. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su H, Parnell M, Caldwell HD. Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection. Vaccine. 1995;(11):1023–32. doi: 10.1016/0264-410x(95)00017-u. [DOI] [PubMed] [Google Scholar]
- 5.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham RC. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;(6):3074–8. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, Jiang X, Shen C, Karunakaran KP, Brunham RC. Novel Chlamydia muridarum T cell antigens induce protective immunity against lung and genital tract infection in murine models. J Immunol. 2009;(3):1602–8. doi: 10.4049/jimmunol.182.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005;(12):8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson EM, Markoff BA, Schachter J, de la Maza LM. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990;(2):144–8. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- 9.Carlson JH, Whitmire WM, Crane DD, Wicke L, Virtaneva K, Sturdevant DE, et al. The Chlamydia trachomatis plasmid is a transcriptional regulator of chromosomal genes and a virulence factor. Infect Immun. 2008;(6):2273–83. doi: 10.1128/IAI.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;(3):1161–76. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison RP, Feilzer K, Tumas DB. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;(12):4661–8. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y, Zhang YX, Watkins NG, Caldwell HD. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989;(4):1040–9. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Morrison RP, Watkins NG, Caldwell HD. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990;(1):203–12. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks E, Verolin M, Stensson A, Lycke N. Differential CD28 and inducible costimulatory molecule signaling requirements for protective CD4+ T-cell-mediated immunity against genital tract Chlamydia trachomatis infection. Infect Immun. 2007;(9):4638–47. doi: 10.1128/IAI.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su H, Caldwell HD. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;(9):3302–8. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;(12):6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SP, Grayston JT. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J Infect Dis. 1991;(2):403–5. doi: 10.1093/infdis/163.2.403. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YX, Stewart SJ, Caldwell HD. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;(2):636–8. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas F. On the Doctrine of Original Antigenic sin. Proceedings of the American Philosophical Society. 1960;104:572–8. [Google Scholar]
- 20.O'Connell CM, Ingalls RR, Andrews CW, Jr, Scurlock AM, Darville T. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol. 2007;179(6):4027–34. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Wang S, Wu Y, Zhong G, Chen D. Immunization with chlamydial plasmid protein pORF5 DNA vaccine induces protective immunity against genital chlamydial infection in mice. Sci China C Life Sci. 2008;(11):973–80. doi: 10.1007/s11427-008-0130-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Q, Lammel CJ, Lindquist EA, Stephens RS. Recall of original serologic response after challenge with homologous and heterologous Chlamydia trachomatis serovars. J Infect Dis. 1996;(3):609–18. doi: 10.1093/infdis/173.3.609. [DOI] [PubMed] [Google Scholar]
- 23.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009;182(12):8063–70. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun G, Pal S, Sarcon AK, Kim S, Sugawara E, Nikaido H, et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J Bacteriol. 2007;189(17):6222–35. doi: 10.1128/JB.00552-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael AJ. The original sin of killer T cells. Nature. 1998;394(6692):421–2. doi: 10.1038/28738. [DOI] [PubMed] [Google Scholar]


