Abstract
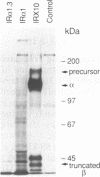
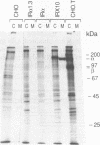
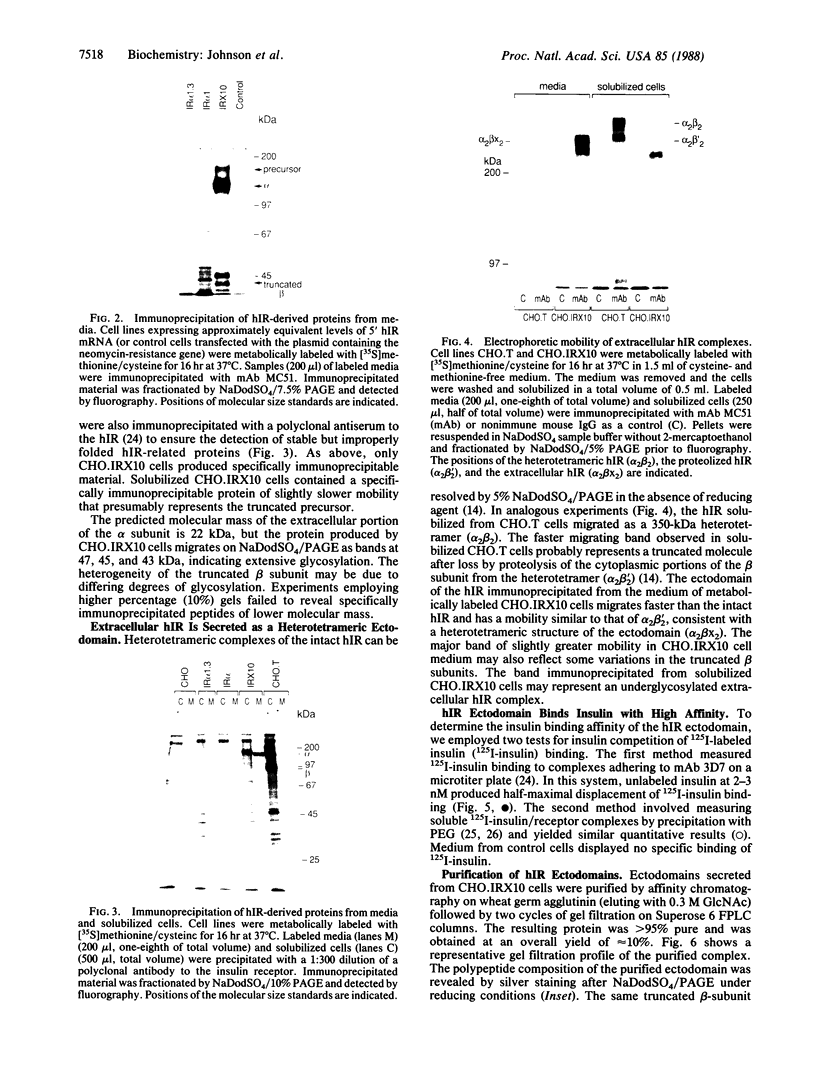
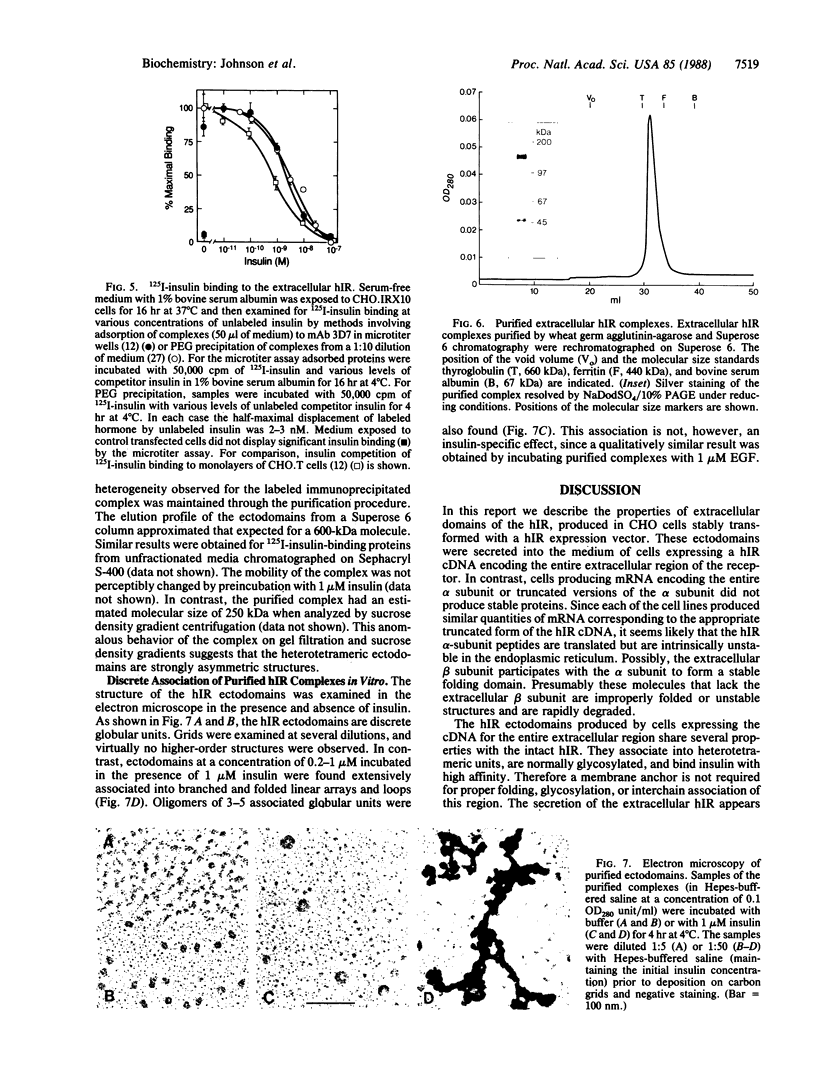
To study the properties of the extracellular insulin-binding domain of the human insulin receptor (hIR), we have expressed portions of the parent molecule in mammalian cells. Receptor cDNAs encoding the entire hIR ectodomain, the alpha subunit of the hIR alone, or a portion of the alpha subunit containing the cysteine-rich region were placed within an expression vector and in turn used to transfect CHO cells. Only cells expressing mRNA for the entire hIR ectodomain secreted hIR-related protein, suggesting that the truncated versions of this domain are unstable. The ectodomain molecules were extensively glycosylated, properly processed heterotetramers. Further, they bound insulin with an affinity similar to that of the intact hIR. In the electron microscope the secreted ectodomains appeared as discrete globular structures. After incubation with roughly equimolar quantities of insulin, the ectodomains associated to form loops or branched and folded linear macroarrays. However, these structures were not restricted to the specific ligand, insulin, since epidermal growth factor also produced the effect. Nevertheless, it seems that the receptor ectodomains can exist in two structural states. The conversion of the singular to the aggregated state may somehow be associated with transmembrane communication and activation of the biological response.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyer R. A. Structural characterization of insulin receptors. I. Hydrodynamic properties of receptors from turkey erythrocytes. J Biol Chem. 1983 Dec 25;258(24):14992–14999. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Rubin J. B., Pilch P. F. Structural requirements for the transmembrane activation of the insulin receptor kinase. J Biol Chem. 1986 Nov 15;261(32):15281–15287. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor isolated from liver and fat cell membranes. J Biol Chem. 1972 Apr 10;247(7):1980–1991. [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Edery M., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Mechanisms of receptor-mediated transmembrane communication. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):773–784. doi: 10.1101/sqb.1986.051.01.090. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Roth R. A., Rutter W. J. A membrane-anchored cytoplasmic domain of the human insulin receptor mediates a constitutively elevated insulin-independent uptake of 2-deoxyglucose. Mol Endocrinol. 1987 Jan;1(1):15–24. doi: 10.1210/mend-1-1-15. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Koshland D. E., Jr, Clauser E., Moe G. R., Bollag G., Roth R. A., Rutter W. J. Linking functional domains of the human insulin receptor with the bacterial aspartate receptor. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8137–8141. doi: 10.1073/pnas.83.21.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y. Characterization of purified insulin receptor subunits. J Biol Chem. 1984 Jan 25;259(2):1206–1211. [PubMed] [Google Scholar]
- Heffetz D., Zick Y. Receptor aggregation is necessary for activation of the soluble insulin receptor kinase. J Biol Chem. 1986 Jan 15;261(2):889–894. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Insulin receptors. Annu Rev Pharmacol Toxicol. 1983;23:461–479. doi: 10.1146/annurev.pa.23.040183.002333. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Ho L., Korn L. J., Roth R. A. Insulin action is blocked by a monoclonal antibody that inhibits the insulin receptor kinase. Proc Natl Acad Sci U S A. 1986 Jan;83(2):328–332. doi: 10.1073/pnas.83.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Mapping surface structures of the human insulin receptor with monoclonal antibodies: localization of main immunogenic regions to the receptor kinase domain. Biochemistry. 1986 Mar 25;25(6):1364–1371. doi: 10.1021/bi00354a026. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Haase B. A., Standaert M. L. Characterization of detergent-solubilized membrane proteins. Hydrodynamic and sedimentation equilibrium properties of the insulin receptor of the cultured human lymphoblastoid cell. J Biol Chem. 1981 Dec 10;256(23):12118–12126. [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Wilden P. A., Pessin J. E. Insulin-dependent intermolecular subunit communication between isolated alpha beta heterodimeric insulin receptor complexes. J Biol Chem. 1987 Dec 5;262(34):16730–16738. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. Secretion of soluble functional insulin receptors by transfected NIH3T3 cells. J Biol Chem. 1988 Mar 5;263(7):3063–3066. [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987 Mar 10;26(5):1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]