Abstract
We studied the response of dysferlin-null and control skeletal muscle to large- and small-strain injuries to the ankle dorsiflexors in mice. We measured contractile torque and counted fibers retaining 10-kDa fluorescein dextran, necrotic fibers, macrophages, and fibers with central nuclei and expressing developmental myosin heavy chain to assess contractile function, membrane resealing, necrosis, inflammation, and myogenesis. We also studied recovery after blunting myogenesis with X-irradiation. We report that dysferlin-null myofibers retain 10-kDa dextran for 3 days after large-strain injury but are lost thereafter, following necrosis and inflammation. Recovery of dysferlin-null muscle requires myogenesis, which delays the return of contractile function compared with controls, which recover from large-strain injury by repairing damaged myofibers without significant inflammation, necrosis, or myogenesis. Recovery of control and dysferlin-null muscles from small-strain injury involved inflammation and necrosis followed by myogenesis, all of which were more pronounced in the dysferlin-null muscles, which recovered more slowly. Both control and dysferlin-null muscles also retained 10-kDa dextran for 3 days after small-strain injury. We conclude that dysferlin-null myofibers can survive contraction-induced injury for at least 3 days but are subsequently eliminated by necrosis and inflammation. Myogenesis to replace lost fibers does not appear to be significantly compromised in dysferlin-null mice.
Keywords: inflammation, limb-girdle muscular dystrophy type 2B, Miyoshi myopathy, muscle injury
mutations in the gene that encodes the ∼230-kDa protein dysferlin are linked to human muscle diseases known as dysferlinopathies. There are three clinically distinct forms of dysferlinopathies: limb-girdle muscular dystrophy type 2B (LGMD2B), Miyoshi myopathy (MM), and distal anterior compartment myopathy (DACM) (5, 21, 23, 27). Although in vitro evidence suggests that dysferlin plays a role in sarcolemmal repair by vesicle fusion (2, 3, 17, 26), it is still unclear whether defects in repair are the only factors underlying pathogenesis in dysferlinopathies. For instance, many patients with dysferlinopathies are initially misdiagnosed as having an inflammatory myopathy because of the large number of inflammatory cells in their muscle biopsies (12, 16, 31, 44). Although the inflammatory response may be secondary to the necrosis of myofibers after failed membrane repair, normal macrophages in vitro are more aggressive when the expression of dysferlin is suppressed by small interfering RNA (38), suggesting that inflammation plays an important role in the pathogenesis of dysferlinopathies.
Lengthening (eccentric) muscle contractions are often used to gain insights into muscle diseases. Because lengthening contractions can disrupt the plasma membrane, or sarcolemma, of skeletal myofibers (20, 28), they can be used to learn whether a dystrophic muscle phenotype is linked to increased susceptibility to injury (3, 4, 39). Furthermore, study of the structure and function of muscles recovering from damage caused by lengthening contractions has revealed some of the mechanisms used by muscle to regain function that is lost after physiological injuries (30, 40, 41, 49). Dysferlin-null muscles subjected to lengthening contractions from downhill running do not show a more extensive disruption of membrane integrity than that seen in control muscles (3). Although studies of recovery after such injuries have not yet been performed, these experiments suggest that dysferlin does not affect susceptibility to injury from numerous small-strain lengthening contractions.
We have reported (30) that rat skeletal muscle injured by a single large-strain lengthening contraction recovers differently from muscle injured by 150 small-strain lengthening contractions. Recovery from the former mainly involves the repair of the sarcolemmal membrane of injured myofibers without significant levels of new fiber formation, or myogenesis, whereas recovery from the latter primarily requires myogenesis without significant levels of sarcolemmal repair. We have applied these two different types of contraction-induced injury to mice lacking dysferlin, to determine whether dysferlinopathic muscle is deficient in sarcolemmal repair or in myogenesis. Our findings from a mouse model of large-strain injury showed that wild-type muscles recovered by repairing their sarcolemmal membranes without undergoing significant levels of myogenesis, whereas dysferlin-null muscles showed delayed recovery, associated with massive infiltration of mononuclear cells and necrotic death of myofibers followed by extensive myogenesis (42).
Here we compared recovery of control and dysferlin-null mice from large-strain injury, involving 15 repetitive lengthening contractions (15R), to recovery from injury induced by 150 small-strain lengthening contractions (150R injury). By blunting the proliferation of satellite cells (SCs) with X-irradiation before injury, we also assessed the extent to which myogenesis is necessary for recovery of dysferlin-null muscle from these injuries. Our experiments were designed to test the hypotheses that myogenesis is not necessary for recovery from 15R injury in control mice but is necessary for recovery from 15R injury in dysferlin-null animals and that myogenesis is necessary for recovery from 150R injury in both control and dysferlin-null muscles. Our results support these hypotheses and indicate further that dysferlin-null muscle experiences a strong inflammatory response that contributes to its slower recovery from injury caused by lengthening contractions.
MATERIALS AND METHODS
We induced injury and studied recovery of function in the ankle dorsiflexor (DF) muscles and then examined tibialis anterior (TA) muscles, which account for most of the torque generated by this muscle group (23). Induction of injury, measurement of contractile function, and collection of tissues were performed under general anesthesia induced by 2% isoflurane inhalation (VetEquip, Pleasanton, CA).
Animals.
We studied two inbred strains of mice, A/J and A/WySnJ (male, 12–16 wk of age; Jackson Laboratory, Bar Harbor, ME). A/J mice lack dysferlin and so model human dysferlinopathies (22). A/WySnJ mice were used as control animals because of their shared genetic background with the A/J strain, albeit with normal expression of dysferlin (22). Forty animals of each strain were assigned to the following groups: 1) injury by 15 repetitive large-strain lengthening contractions (15R) without irradiation; 2) the 15-repetition injury, preceded by irradiation to prevent myogenesis (15R IR); 3) injury by 150 repetitive small-strain lengthening contractions without irradiation (150R); and 4) the 150-repetition injury after irradiation (150R IR). An additional set of eight animals from the A/WySnJ strain were studied to confirm the effectiveness of our irradiation protocol (see Cardiotoxin injection and Supplemental Fig. S1).1 An additional set of four irradiated and four unirradiated animals of the A/J strain were studied at days 0 and 28. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
Injury models and assessment of contractile function.
We studied the loss and recovery of contractile function after two different protocols to induce injury in the ankle DFs of the left hindlimb by lengthening contractions performed in vivo, as described previously (28–30, 42, 47). In both protocols, 300-ms pulse trains were used to tetanize the DFs and a lengthening contraction was initiated at 150 ms after the onset of stimulation. In the 15R protocol, the ankle was plantarflexed from 90° to 180° at an angular velocity of 1,200°/s. A 3-min rest was given between successive lengthening contractions to eliminate the effect of fatigue. In the 150R protocol, adapted from Rathbone et al. (41), the ankle was plantarflexed from 70° to 110° at an angular velocity of 1,200°/s. Lengthening contractions in the 150R protocol were performed at the rate of 5 contractions/min, over a 30-min period.
We measured maximal tetanic torque of the DFs 10 min before injury, 10 min after injury [day 0 (D0)], and then 3, 7, 14, and 21 days later (D3, D7, D14, and D21), to assess the loss and recovery of contractile function. The apparatus used to induce injury and to measure ankle DF torque has been described in detail in earlier reports (28–30, 42, 47). Isolated contractions of the ankle DFs were elicited by depolarizing the peroneal nerve transcutaneously with a bipolar electrode (BS4 50-6824, Harvard Apparatus, Holliston, MA) placed over the head of the fibula. Impulses of 0.1-ms duration were generated by an S48 square pulse stimulator (Grass Instruments, West Warwick, RI); a PSIU6 stimulation isolation unit (Grass Instruments), placed between the electrode and stimulator, restricted current amplitude to a maximum of 15 mA. Pulse amplitude was adjusted to obtain maximal twitch torque, and 300-ms trains of varying pulse frequencies were used to generate a torque-frequency curve. Maximal fused tetany occurred at 80–90 Hz. Functional data were recorded from eight animals per strain for each of the groups at each time point. Maximum tetanic torque recorded at each time point was expressed as a percentage of the maximum tetanic torque measured for each mouse before injury.
Assay of membrane integrity.
Fluorescein dextran (FDx, 10 kDa; D1820, Invitrogen, Carlsbad, CA), injected intraperitoneally (2.5 mg/ml in PBS, 0.01 ml/g body wt) 1 h before injury, was used to assess membrane damage and resealing (34, 37). Dye-injected animals were euthanized under general anesthesia by perfusion with 2% paraformaldehyde in PBS. TA muscles were excised, weighed, pinned at resting length on Sylgard 184 (Dow Corning, Midland, MI)-coated dishes, and immersed in 2% paraformaldehyde for an additional 30 min. The excised, fixed muscles were then rinsed with 0.05 M glycine in PBS and allowed to sink in 20% sucrose in PBS at 4°C for cryoprotection. Samples were placed in cryovials, frozen slowly at −30°C for ∼12 h, and stored at −80°C. TA muscles were later cryosectioned at the midbelly of the muscle, yielding 16-μm-thick cross sections (Frigocut 2800, Reichert-Jung, Nußloch, Germany). Sections were collected on Superfrost Plus slides (Erie Scientific, Portsmouth, NH), washed three times with 0.05 M glycine in PBS (5 min/wash), and mounted under a coverslip in Vectashield (Vector Labs, Burlingame, CA). Sections were examined under confocal fluorescent optics (Zeiss LSM410, ×25 objective, pinhole size = 1 Airy unit; Carl Zeiss, Poughkeepsie, NY). Digital images of six to eight visual fields across the cross section of each TA were obtained for analysis. Two images of each visual field were captured, one after adjustment of brightness and contrast to reduce background fluorescence and the other after increasing the background signal in order to visualize all myofibers in the field. The percentage of myofibers in each field that were uniformly labeled with FDx was determined and averaged to get the percentage of FDx+ cells for each TA muscle.
Hematoxylin and eosin staining.
We performed hematoxylin and eosin (H & E) staining to assess general morphology and to visualize mononuclear cells and centrally nucleated muscle fibers (CNFs). We stained 8-μm cross sections at the midbelly of unfixed, snap-frozen TAs with Harris modified hematoxylin (HHS 16, Sigma) and aqueous eosin-Y (HT110216, Sigma). Sections were collected as above and placed in tap water for 5 min. After hydration, sections were immersed in hematoxylin for 5 min, rinsed in tap water briefly, treated for 30 s with PBS (pH = 8), and then rinsed thoroughly in tap water to remove excess hematoxylin. Sections were then dipped in eosin (<1 s), washed thoroughly in tap water to remove excess eosin, allowed to dry at room temperature in a fume hood, and mounted under a coverslip in PBS containing 5 mM sodium azide. The sections were observed under a light microscope the same day (Zeiss Axioskop, ×20 objective, Carl Zeiss), and digital images of six to eight visual fields, randomly selected across the cross section of each TA muscle, were obtained for analysis.
Assessment of myofiber necrosis.
We identified necrotic fibers on cross sections stained with H & E, as described previously (19). Fibers with pale and fragmented myoplasm, with or without invasion by mononuclear cells, were adjudged as necrotic. We calculated the percentage of necrotic fibers from a total of 18 visual fields from 2 different TA muscles at each time point.
Assessment of inflammation.
The severity of inflammation was quantified from sections stained with H & E by counting the number of mononuclear cells in the interstitial spaces and within the cytoplasm of degenerating myofibers (48). To characterize the mononuclear cells in the TA muscle at the peak of infiltration 3 days after injury, we immunolabeled 16-μm cross sections with antibodies to specific markers for fibroblasts (ER-TR7), myoblasts (myoD), neutrophils (Ly6G), T cells (CD3), B cells (CD19), NK cells (CD49b), and macrophages (CD68). Sections were routinely colabeled with antibodies to dystrophin to mark the margins of myofibers and 4′,6′-diamidino-2-phenylindole (DAPI) to label nuclei. Because the majority of mononuclear cells were CD68+, we quantified the number of CD68+ cells per square millimeter of the TA muscle in control and injured muscles from two animals per group. Counts of CD68+ macrophages from nine random visual fields (Zeiss LSM410, ×25 objective, pinhole size = 1 Airy unit; Carl Zeiss) in each TA muscle were used for statistical analyses.
Assessment of myogenesis.
We counted CNFs in images obtained from sections stained with H & E. We averaged the percentage of CNFs per visual field to get the percentage of CNFs per TA muscle. The percentage of CNFs in each TA was calculated, and data from three TA muscles at each time point were subjected to statistical analysis.
We also assessed myogenic activity from the expression of developmental isoforms of myosin heavy chain (dMHC) 5 days after injury (D5), based on earlier reports that the number of dMHC+ fibers peaks 5–7 days after injury (14, 46). We labeled 16-μm cross sections of TA muscles with antibodies to dMHC (VP-M664, Vector Laboratories, Burlingame, CA) to label regenerating myofibers, with antibodies to dystrophin (NCL-Dys2, Novocastra Laboratories, Newcastle upon Tyne, UK) to mark the margins of myofibers, and with DAPI (71-03-01, KPL, Gaithersburg, MD) to label nuclei. Because the antibodies against dMHC and dystrophin are mouse monoclonals, we used Zenon labeling technology to conjugate the antibodies to Alexa 488 and Alexa 568 dyes, respectively, before applying them to tissue sections (Z-25002 and Z-25006, Invitrogen). We followed the steps recommended by the manufacturer, selecting 1% BSA-PBS as a blocking buffer and 0.2% Triton X-100 for permeabilization, and a final antibody concentration of 5 μg/ml). We quantified the percentage of dMHC+ cells from images of nine random visual fields from two different muscles (Zeiss LSM410; see above).
X-irradiation.
We irradiated the left hindlimbs of mice with a single localized dose of ionizing radiation, as described previously (30). The total dosage was 25 Gy (2,500 rad) delivered with a Pantak-Seifert 250KpV X-Irradiator (HF 320, North Branford, CT) over a period of 10–12 min. This dosage inhibits activation of SCs and subsequent SC-mediated myogenesis, with no significant systemic side effects or effects on mature muscle (1, 19, 30, 36, 41, 43). We performed ion chamber dosimetry (PTW model 31006, Freiburg, Germany) in the path of the beam, outside the lead shielding, to ensure that the proper dosage was being delivered. Dosimetry within the shielding showed that there was no significant backscatter of X-rays.
Cardiotoxin injection.
We used cardiotoxin (CTX), a myotoxin that induces necrosis of myofibers followed by myogenic recovery (50), to test the effectiveness of our irradiation protocol in blunting SC activation and myogenesis. We injected the TA muscles of eight A/WySnJ mice with CTX (0.1 ml of 10 μM solution in PBS; C9759, Sigma) at three different sites (proximal, middle, and distal thirds of the TA). Hindlimbs of four of these animals were exposed to irradiation before injection. We harvested TA muscles from two irradiated animals and two unirradiated animals at D5 (to assess dMHC expression) and D14 (to count CNFs) after injection.
Statistical methods.
All statistical analyses were performed with SAS (Cary, NC). Data were analyzed by multifactorial ANOVA, with mouse strain, type of injury, irradiation status, and day as the independent variables. The percentage of preinjury torque, the percentage of FDx+ fibers per TA, number of inflammatory cells per field, the percentage of necrotic fibers, the number of macrophages, the percentage of CNFs per TA, and the percentage of dMHC+ fibers were the dependent variables. Data for raw torque were analyzed by a multifactorial ANOVA, as above, with repeated measures on time. Specific comparisons were made by Tukey's post hoc tests, with α set at 0.05. All data are expressed as means ± SD.
RESULTS
Effectiveness of irradiation.
We evaluated the effectiveness of our irradiation protocol in blunting myogenesis in the mouse by assessing its effects on the ability of hindlimb muscle to recover after injection of CTX, a process that requires extensive myogenesis (13). We counted CNFs 14 days after injection and irradiation and myofibers labeled for dMHC at 5 days after irradiation (Supplemental Fig. S1). Fourteen days after CTX injection, unirradiated TA muscles had 40 ± 6% CNFs, whereas irradiated TA muscles had only 4 ± 2% CNFs (P < 0.0001, 6 visual fields per cross section). Five days after CTX injection, unirradiated TA muscles had 44 ± 13% dMHC+ fibers, whereas irradiated TA muscles had only 4 ± 2% dMHC+ fibers (P < 0.0001, 9 visual fields per cross section). Thus, as in the rat (30), our irradiation protocol suppressed >90% of SC activation and myogenic activity induced by injection of CTX. We used this irradiation protocol, together with histochemical markers, to assess the role of myogenesis in recovery from injuries caused by large-strain and small-strain lengthening contractions in dysferlin-null and control mice.
Contractile function.
We found no significant differences between the torques generated by the uninjured DF group of unirradiated control A/WySnJ mice (2.28 ± 0.14 Nmm) and irradiated A/WySnJ mice (2.19 ± 0.25 Nmm) or unirradiated dysferlin-null A/J mice (2.38 ± 0.21 Nmm) and irradiated A/J mice (2.30 ± 0.24 Nmm). Thus contractile torques of A/J and A/WySnJ are not significantly different from one another, and uninjured muscles of both strains are not affected by the dose of irradiation used in this study. This is consistent with previous reports, and with evidence that doses of X-irradiation in the same range as we have used have minimal effects on myofibers and on other cell types present in muscle tissues (1, 19, 30, 41, 43).
Large-strain injury.
Measurements of torque after the 15R large-strain injury and subsequently, during recovery of contractile function, are presented in Fig. 1, top. Immediately after injury, torque decreased to a similar level in all four groups [not significant (NS) at D0]. By D3, torque in unirradiated A/WySnJ improved significantly compared with the levels measured immediately after injury (P < 0.0001). Paired comparisons of raw torque before injury (2.24 ± 0.10 Nmm) and at D3 (2.13 ± 0.21 Nmm) showed no statistical difference, suggesting complete recovery by D3. These results suggest that, as in the rat (20), recovery of A/WySnJ mice from injury caused by large-strain eccentric contractions does not require myogenesis (NS at all time points).
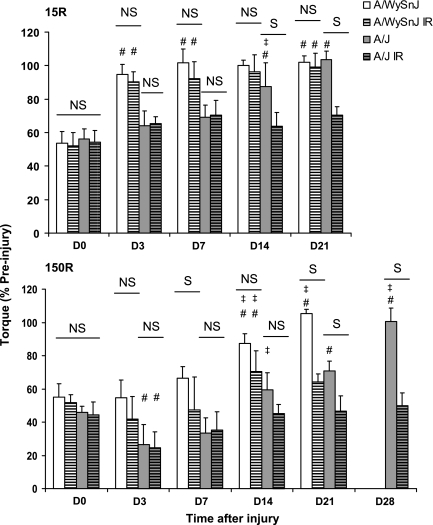
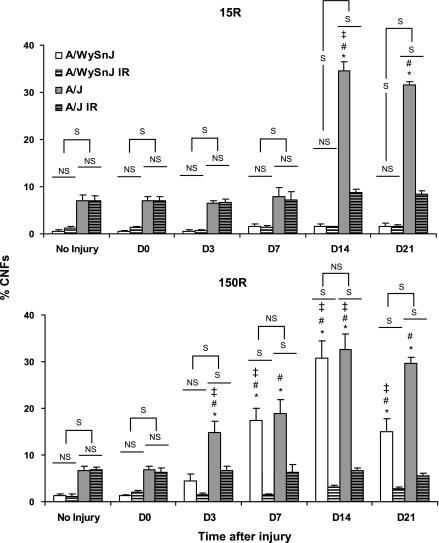
Fig. 1.
Contractile torque after the 15 (15R) and 150 (150R) repetitive lengthening contraction injury protocols. We measured contractile torque from 8 animals at each time point and expressed our results as mean ± SD % of the torque measured before injury. Raw torque data are presented in the text. NS, no significant difference between bars under the line; S, significant difference between bars under the line. #Significant difference compared with day 0 (D0); ‡significant difference from the preceding time point other than D0. Both 15R and 150R injury protocols induced the same loss of torque in both strains of mice immediately after injury at D0. After the 15R protocol, irradiated (IR) and unirradiated A/WySnJ mice recovered completely by D3, but unirradiated A/J mice recovered completely by D14. Recovery from the 15R injury was inhibited in irradiated A/J mice. After the 150R protocol, unirradiated A/WySnJ mice recovered significantly from D0 levels at D14 and recovered further to slightly exceed preinjury levels by D21. At D14 irradiated A/WySnJ mice showed a similar level of recovery as unirradiated A/J mice, but they did not recover beyond D14. Unirradiated A/J mice recovered only partially by D21 but recovered to preinjury levels by D28. After the 150R protocol, both unirradiated and irradiated A/J mice showed a significant drop in torque at D3, after which irradiated mice failed to show any improvement and unirradiated mice showed significant improvement only between D7 and D14 (P < 0.0001).
Unlike A/WySnJ mice, unirradiated A/J mice required 2 wk to recover completely from the functional deficits caused by the 15R protocol, as previously reported (42). Complete recovery at D14 was confirmed by the lack of significance in paired comparisons of raw torque before injury (2.27 ± 0.22 Nmm) and torque at D14 (2.0 ± 0.21 Nmm). We measured a significant increase in torque from D7 to D14 (P = 0.0203), but not between D0 and D3, D3 and D7, or D14 and D21, suggesting that recovery of contractile function was delayed after the injury but concerted in time. By contrast, irradiated A/J mice subjected to the 15R protocol showed no improvement in torque from D0 through D21. As a result, unirradiated A/J mice produced significantly higher torque than irradiated A/J mice at D14 and D21 (P < 0.0001).
These results suggest that the loss of muscle function immediately following large-strain lengthening contractions is not affected by the absence of dysferlin, and that recovery of muscle function after large-strain injury occurs without myogenesis in controls but requires myogenesis in dysferlin-null A/J mice.
Small-strain injury.
We next examined the effects of the dysferlin-null phenotype on recovery of unirradiated and irradiated mice subjected to injury caused by many small-strain lengthening contractions (150R protocol; Fig. 1, bottom). Immediately after injury, torque decreased to a similar level in all four groups (NS at D0). The loss of torque after the 150R protocol was not significantly different from that seen after the 15R protocol. Unirradiated A/WySnJ mice showed significant improvement between D0 and D14 (P < 0.0001). Paired comparisons of raw torque before injury (2.21 ± 0.15 Nmm) and torque at D14 (1.92 ± 0.21 Nmm) were significantly different (P = 0.0059), however, indicating that recovery in A/WySnJ mice was still not complete at D14. There was a further improvement in torque from D14 to D21 (P = 0.0207). Paired comparisons of raw torque before injury (2.25 ± 0.25 Nmm) and torque at D21 (2.38 ± 0.27 Nmm) showed a small but significant net gain in torque of ∼5% from D0 to D21 (P = 0.0043). In irradiated A/WySnJ mice, torque remained unchanged after the 150R protocol from D0 until D14, at which point it increased significantly (P < 0.0001). However, raw torque measured before injury (2.10 ± 0.23 Nmm) and torque at D14 (1.48 ± 0.31 Nmm) were significantly different. There was also no significant change in torque from D14 to D21, suggesting that irradiation inhibited complete recovery in A/WySnJ mice after the 150R protocol.
Unirradiated A/J mice did not show significant improvement after the 150R protocol until D21 (P < 0.0001, D0 compared with D21). However, raw torque at D21 (1.71 ± 0.23 Nmm) was significantly lower than torque before injury (2.40 ± 0.19 Nmm; P < 0.0001). Even the partial recovery in torque in unirradiated A/J mice at D21 was inhibited by irradiation, as is evident from a lack of improvement in torque at all time points after D0. A notable feature seen in the A/J but not the A/WySnJ mice after the 150R protocol was a decline in torque from D0 to D3, both in unirradiated (P = 0.0093) and irradiated (P = 0.0043) animals. After the drop in torque at D3, unirradiated A/J mice showed no significant improvement from D3 to D7 but did show improvement at D14 compared with D3 (P < 0.0001). A significant level of improvement at D14 also occurred in irradiated A/J mice (P < 0.0022). At D14, unirradiated A/J mice were not significantly different in torque from irradiated A/J mice, but by D21 unirradiated A/J mice produced significantly higher torque than irradiated A/J mice (P < 0.0001). At D14 and D21, irradiated A/J mice produced significantly lower torque than irradiated A/WySnJ mice (P < 0.0001 and P = 0.0293, respectively). Thus both unirradiated and irradiated muscles from A/J mice do not recover from the 150R protocol as effectively as muscles from A/WySnJ mice. We tested an additional group of four unirradiated A/J mice and another group of four irradiated A/J mice at D28 and found that unirradiated muscles recovered completely, reaching preinjury levels by this time. However, irradiated muscles from A/J animals did not show additional improvement from D21 and D28.
These results suggest that, although the loss of muscle function following multiple small-strain lengthening contractions is not immediately affected by the absence of dysferlin, dysferlin-null but not wild-type muscles lose function subsequent to this type of injury. They show further that the recovery of muscle function after small-strain injury requires myogenesis in both controls and dysferlin-null A/J mice but that the recovery of the latter is delayed compared with wild type, even when myogenesis proceeds unimpeded.
Membrane damage and resealing.
We used the uptake and retention of FDx to assay damage to myofibers as well as their ability to reseal their sarcolemmal membranes after injury (8, 34, 37). Representative images are presented in Fig. 2, and quantitative data are presented in Fig. 3.
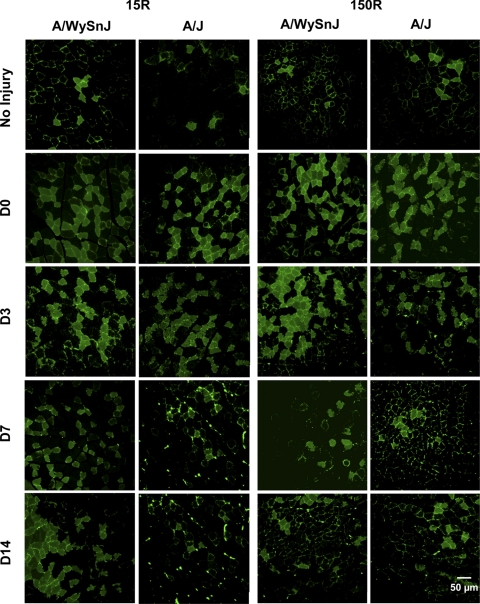
Fig. 2.
Membrane damage and resealing after injury. Representative images are shown of tibialis anterior (TA) muscle cross sections obtained from muscles exposed to fluorescein dextran (FDx) before injury and collected at different times thereafter. After 15R injury, the same percentage of myofibers in TA muscles of A/WySnJ and A/J mice took up FDx. These FDx-positive (FDx+) fibers were retained through D14 in A/WySnJ mice but were lost in A/J mice. After 150R injury, the same percentage of myofibers in TA muscles of A/WySnJ and A/J mice took up the dye. By D14, however, these fibers were lost in both mouse strains and the values for FDx-labeled myofibers returned to preinjury levels.
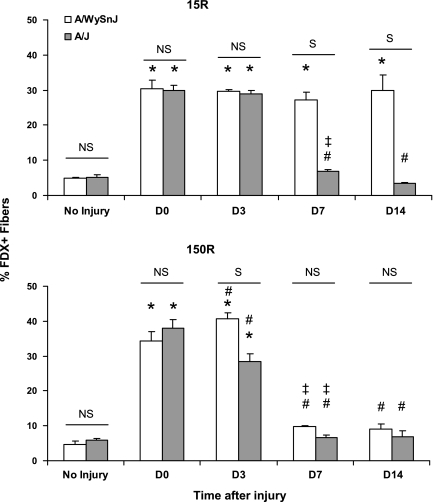
Fig. 3.
Quantitation of FDx-labeled muscle fibers. FDx data obtained from 3 TA muscles at each time point, for both the 15R and 150R protocols, are presented as mean ± SD % of labeled fibers. After the 15R protocol, an equal percentage of myofibers took up FDx in A/WySnJ and A/J TAs. The same percentage of FDx+ fibers persisted at D14 in TA muscles from A/WySnJ but not from A/J mice. After the 150R protocol, an equal percentage of myofibers took up FDx in A/WySnJ and A/J TA muscles. At D3, A/WySnJ TAs showed a slight increase in FDx+ fibers from D0 levels, while A/J TAs showed a drop in percentage of FDx+ fibers. By D7, both A/WySnJ and A/J TAs showed levels of FDx labeling comparable to those seen before injury. *Significant difference compared with preinjury levels. Other symbols are as described in Fig. 1.
After the 15R protocol (Fig. 2; Fig. 3, top), an equal percentage of fibers took up FDx (FDx+) in the TA muscles of A/WySnJ and A/J mice (NS at D0). The same percentage of myofibers that had taken up FDx at D0 retained the dye until D3 in both strains. Notably, the number of A/J myofibers at D3 that retained FDx was much higher than preinjury levels (P < 0.0001). However, the percentage of FDx+ myofibers in TA muscles from A/J mice on D7 decreased significantly from D0 (P < 0.0001) and D3 (P < 0.0001) levels. These levels decreased further by D14 (P < 0.0001 compared with A/J samples at D0; P < 0.0001 compared with A/J samples at D7), at which time the percentage of FDx+ fibers was similar to preinjury levels. These results are consistent with the progressive loss of FDx+ myofibers in the TA muscles of A/J mice after D3 following the 15R protocol. In contrast, TA muscles from A/WySnJ mice retained the same percentage of FDx+ fibers from D0 through D14, suggesting that, as reported for the rat (30), control (A/WySnJ) myofibers damaged by large-strain lengthening contractions resealed their sarcolemmal membranes and survived the 15R injury protocol. Notably, although the intensity of labeling of individual fibers by FDx can vary, the number of fibers labeled with FDx at D3 was reproducible and did not differ significantly between A/WySnJ and A/J muscles. After the 150R protocol (Fig. 2; Fig. 3, bottom), an equal percentage of myofibers took up FDx in both strains of mice (NS at D0). By D3, the percentage of FDx+ fibers increased in A/WySnJ mice (P = 0.0082 compared with A/WySnJ samples at D0) but decreased in A/J mice (P < 0.0001 compared with A/J samples at D0). Despite this decrease, the number of FDx+ A/J myofibers at D3 remained much higher than preinjury levels (P < 0.0001). At D7, both strains of mice lost a significant number of FDx+ fibers compared with D3 levels (P < 0.0001 for A/WySnJ and A/J samples) and were not different from each other (NS at D7). At D7, the percentage of FDx+ fibers reached preinjury levels in the TA muscles of both strains of mice. No significant changes were observed between D7 and D14. The data suggest that myofibers that were labeled with FDx after the 150R protocol were eliminated between D3 and D7 from TA muscles in both A/WySnJ and A/J mice.
Comparisons of FDx labeling after the 15R and 150R injury protocols showed that, in A/J samples but not A/WySnJ samples, more fibers were labeled with FDx immediately after the 150R protocol than after the 15R protocol (P = 0.0003). This suggests that more myofibers in A/J mice sustain membrane damage after repeated small-strain injury than after large-strain injury. The fact that A/J fibers, subjected to either large- or small-strain lengthening contractions, retain FDx for 3 days suggests that, like control muscles, they are capable of taking up or retaining this 10-kDa marker long after the initial injury.
Macrophage infiltration.
We assessed the inflammatory response after injury by counting the number of infiltrating mononuclear cells in several microscopic fields labeled with H & E. Our results (Fig. 4) indicated that, although mononuclear cells did not appear in large numbers in A/WySnJ muscles after the 15R protocol, they were quite prominent at D3 after injury in A/WySnJ muscles after the 150R protocol and in A/J muscles after either injury protocol. Preliminary characterization of these cells by immunofluorescence labeling showed the majority to be CD68+, which identified them as macrophages (not shown). This population was especially apparent in muscles when mononuclear cell infiltration peaked at D3 (Figs. 4, 5, 6). The combined counts of fibroblasts (ER-TR7+), myoblasts (myoD+), neutrophils (Ly6G), T cells (CD3), B cells (CD19), and NK cells (CD49b) constituted <40% of the mononuclear cells (DAPI+) at D3 (not shown).
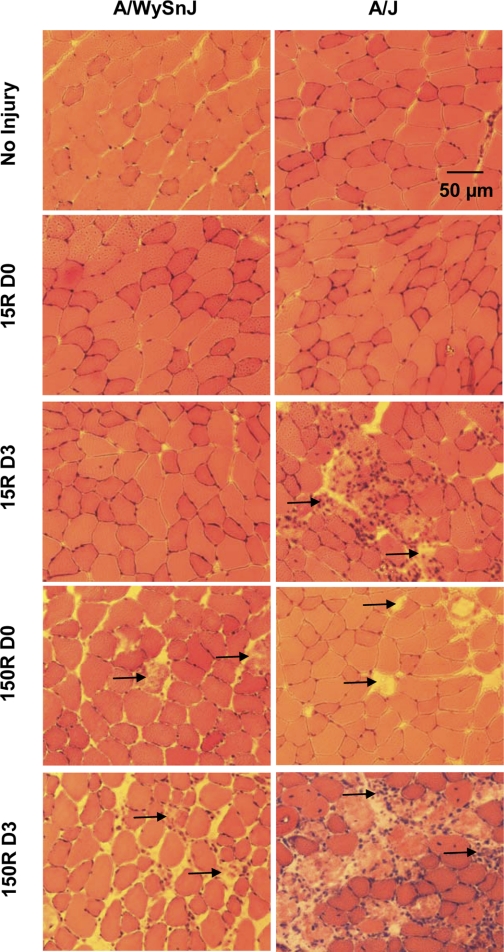
Fig. 4.
Mononuclear cell infiltration and myofiber necrosis after injury. Cross sections of TA muscles were stained with hematoxylin and eosin (H & E) before injury, immediately after injury, and 3 days later. Immediately after the 15R protocol, neither A/WySnJ nor A/J muscles showed significant necrosis or mononuclear infiltration. Three days after the 15R protocol, however, necrosis and mononuclear cell infiltration increased in TA muscles of A/J but not A/WySnJ mice. Immediately after the 150R protocol, there was a modest increase in necrotic fibers but not inflammatory cells in TA muscles from both A/WySnJ and A/J mice. However, 3 days after 150R injury, there was a severalfold increase in the number of necrotic fibers concomitant with significant mononuclear infiltration in TA muscles of A/WySnJ and A/J mice, with the latter being more severe. Arrows point to examples of necrotic fibers.
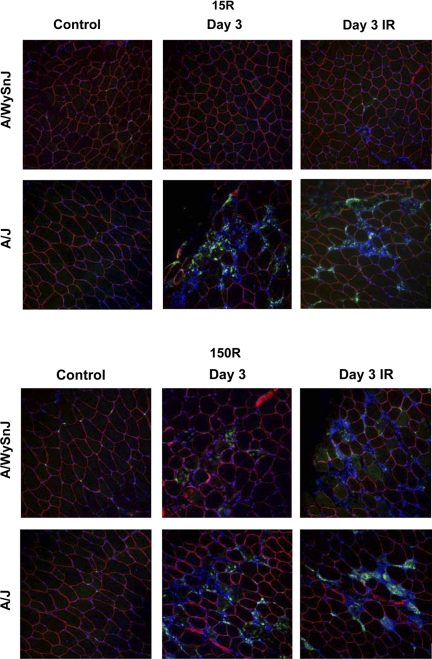
Fig. 5.
Macrophage infiltration after injury assessed by CD68 labeling. Cross sections of TA muscles of A/J and A/WySnJ mice harvested 3 days after 15R and 150R protocols, when mononuclear cell infiltration was at its peak, were labeled with antibodies to CD68, a marker for macrophages (green). Sections were colabeled with antibodies to dystrophin to mark myofiber boundaries (red) and 4′,6′-diamidino-2-phenylindole (DAPI) to mark nuclei (blue dots). Data show that the majority of mononuclear cells in the TA muscle of A/J mice 3 days after either large-strain or small-strain injury, and of A/WySnJ mice 3 days after small strain injury, are macrophages.
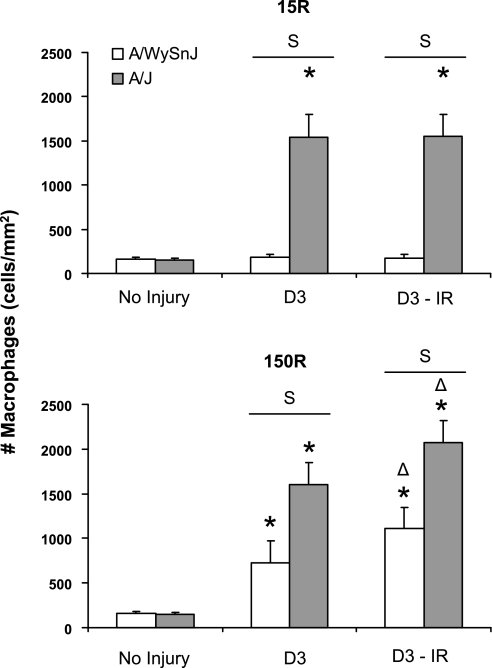
Fig. 6.
Quantitation of macrophage infiltration. Macrophages were counted from images like those in Fig. 8. Data are presented as means ± SD. TA muscles of A/J mice showed a significant increase in macrophage infiltration after both 15R and 150R injuries. TA muscles of A/WySnJ mice showed no significant macrophage infiltration after 15R injury but did show significant infiltration after 150R injury. Macrophage infiltration in TA muscles of A/J mice after both the 15R and 150R injuries was significantly greater than in A/WySnJ muscles after the 150R injury (P < 0.0001). Irradiation had no effect on inflammation after the 15R protocol, but it mildly enhanced the inflammatory response after the 150R protocol (see text). *Significant difference compared with preinjury levels; Δsignificant difference between irradiated and unirradiated muscles. Other symbols are as described in Fig. 1.
We quantitated the macrophage population in control and A/J muscles before and after large-strain and small-strain injuries (Figs. 5 and 6). Consistent with the results of our H & E staining, we did not observe a significant increase in macrophages in the A/WySnJ strain 3 days after the 15R protocol. In contrast, 3 days after 15R injury macrophages in A/J muscles increased ∼11-fold compared with uninjured strain-matched TA muscles (P < 0.0001). Three days after the 150R injury protocol, CD68+ cells increased significantly from respective strain-matched control levels in both the A/WySnJ and A/J mice. However, there were approximately twice as many CD68+ cells in A/J than in A/WySnJ in both unirradiated and irradiated muscles (P < 0.0001), consistent with the further loss of specific torque seen in A/J muscles, but not controls, between D0 and D3. X-irradiation before injury did not reduce the number of infiltrating macrophages, suggesting that the inhibition of myogenesis by X-irradiation is not accompanied by inhibition of macrophage infiltration. Although irradiation may alter microcirculation in the muscle (25), it is not sufficient to induce inflammation, because muscles subjected to irradiation followed by the 15R injury showed no change in inflammatory infiltrates. Irradiation was associated with an increase in the number of macrophages in both A/WySnJ and A/J muscles injured by the 150R protocol, however, perhaps because inflammation is enhanced when muscle regeneration is suppressed.
Myofiber necrosis after injury.
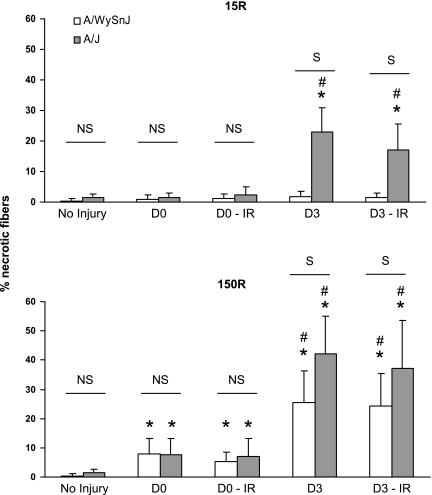
We also examined sections of muscle labeled with H & E to assess the extent of necrosis immediately after injury and at D3, when mononuclear infiltration peaked (Figs. 4, 7). We found that the 15R protocol did not immediately induce significant necrosis in either strain of mice. There was, however, a significant increase in necrotic fibers 3 days later in TA muscles of A/J mice (P < 0.0001 compared with D0) but not A/WySnJ mice. The 150R protocol induced comparably low levels of necrosis in both mouse strains immediately after injury. After 3 days, however, A/J muscles had many more necrotic fibers than A/WySnJ muscles (P < 0.0001), consistent with the decrease in torque that we observed (Fig. 1). There was no difference in necrosis between irradiated and unirradiated muscles.
Fig. 7.
Quantitation of necrotic fibers. The number of necrotic fibers was counted and expressed as % of the total number of myofibers in each visual field. Immediately after the 15R injury, there was no significant increase in the number of necrotic fibers in TA muscles of A/J and A/WySnJ mice, compared with preinjury levels. Three days after the 15R injury the number of necrotic fibers increased significantly in A/J but not A/WySnJ muscles. Immediately after the 150R protocol, there was a similar small increase in necrotic fibers in A/WySnJ and A/J muscles. Three days later, the number of necrotic fibers increased in both mouse strains compared with their respective D0 levels, with A/J muscles having significantly more necrotic fibers than A/WySnJ muscles. Irradiation had no effect on the number of necrotic fibers that appeared after either injury. *Significant difference compared with preinjury levels. Other symbols are as described in Fig. 1.
Myogenic activity after injury.
We assessed the role of myogenesis in recovery from injury directly, by counting the number of myofibers with central nuclei (CNFs) or expressing dMHC. As an extra test of these measures, we also examined these parameters in irradiated muscles. We found significant increases in these myogenic markers in A/WySnJ mice after the 150R protocol and in A/J mice after either the 15R or 150R protocol, all of which were inhibited by prior irradiation.
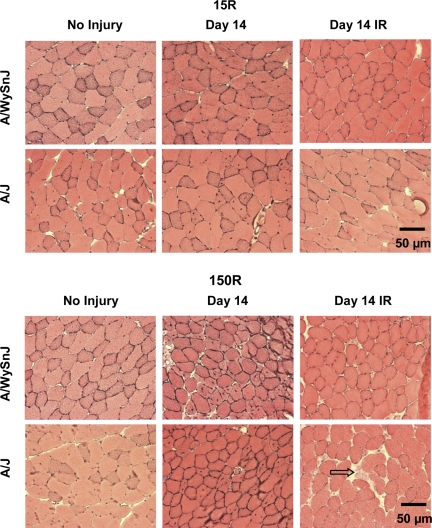
Representative images of sections stained with H & E, used to assess CNFs, are presented in Fig. 8, with quantitations in Fig. 9. Uninjured TA muscles from A/J mice had significantly higher numbers of CNFs than TA muscles from A/WySnJ mice (P < 0.0001). The 15R protocol induced a significant increase in CNFs in the TA muscles of A/J but not A/WySnJ mice (Fig. 8, top, Fig. 9, top). CNFs were not present in significantly higher numbers in A/J samples from D0 through D7, but appeared to peak by D14 after injury (P < 0.0001 compared with preinjury) and did not change significantly between D14 and D21. The 150R protocol increased CNFs significantly in the TA muscles of both A/WySnJ and A/J mice, compared with preinjury levels, with a peak at D14 (P < 0.0001; Fig. 8, bottom, Fig. 9, bottom). No significant difference was observed in the number of CNFs in TA muscles of A/J and A/WySnJ mice at D14 after this injury. Increases in CNFs between D0 and D3 were significant only in A/J mice after the 150R injury (P < 0.0001 compared with preinjury A/J), but by D7 both A/WySnJ and A/J showed large increases in CNFs that were not statistically different from each other. Between D14 and D21, the number of CNFs decreased in TA muscles from A/WySnJ mice (P < 0.0001) but not in muscles from A/J mice.
Fig. 8.
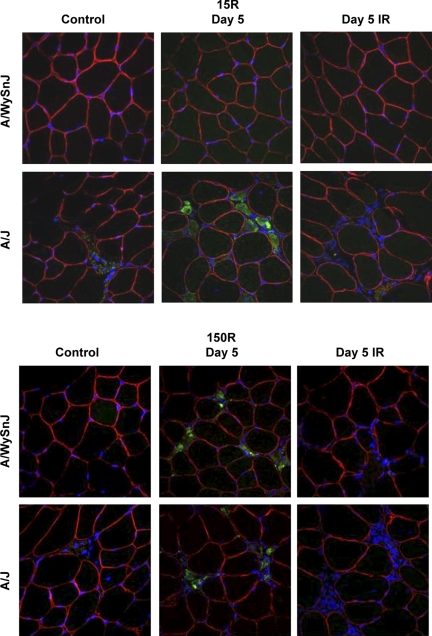
Centrally nucleated fibers (CNFs) 14 days after the 15R and 150R injury protocols. Cross sections are shown of H & E-stained TA muscles before injury and at D14, the time point at which CNFs peaked. Top: changes after the 15R protocol. Bottom: changes after the 150R protocol. Fourteen days after the 15R protocol, many CNFs were present in unirradiated A/J TA muscles but not unirradiated A/WySnJ TA muscles. Fourteen days after the 150R protocol, CNFs increased in unirradiated TA muscles of both A/WySnJ and A/J mice. Irradiated TA muscles from both mouse strains did not show an increase in the number of CNFs. Qualitatively, in irradiated A/J muscles after the 150R protocol, we observed many areas of fibrosis (e.g., arrow, bottom right).
Fig. 9.
Quantitation of CNFs. Data on CNFs obtained from 3 TA muscles at each time point, for both the 15R and 150R protocols, are presented as mean ± SD % of total myofibers observed. Uninjured TA muscles from A/J mice had more CNFs than uninjured A/WySnJ TA muscles. The 15R protocol induced an increase in CNFs in TA muscles of A/J mice but not A/WySnJ mice. This response was significantly different from strain-matched uninjured control muscles only at D14 and D21. The 150R protocol induced a significant increase in CNFs in the TA muscles of both A/WySnJ and A/J strains of mice, which peaked at D14. Despite the early increase in CNFs in A/J muscles at D3, TA muscles of both A/J mice and A/WySnJ mice showed similar levels of CNFs by D7. This similarity persisted through D14, but by D21 A/WySnJ mice had significantly fewer CNFs than strain-matched D14 TA muscles and TA muscles of A/J mice at D21. CNFs did not decrease in TA muscles from A/J mice from D14 to D21 after either the 15R or 150R protocols. *Significant difference compared with preinjury levels. Other symbols are as described in Fig. 1.
Comparisons of the results from the 15R and 150R protocols showed that there were more CNFs in A/J mice at D3 (P < 0.0001) and D7 (P < 0.0001) after the 150R protocol. However, there was no significant difference in the peak response at D14 and the subsequent persistence of CNFs through D21.
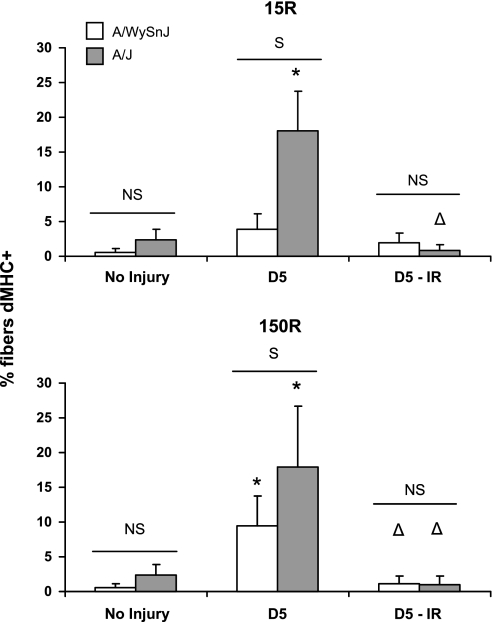
Representative images of cross sections of TA muscles labeled with antibodies to dMHC are shown in Fig. 10, with quantitations in Fig. 11. Consistent with our counts of CNFs, the 15R protocol increased the number of dMHC+ fibers in TA muscles of A/J mice approximately fivefold, but did not increase the number of dMHC+ fibers in A/WySnJ muscles. Similarly, after the 150R injury, the number of dMHC+ fibers increased significantly from preinjury levels in both strains of mice, but A/J muscles had a significantly greater number of dMHC+ fibers. Consistent with earlier reports on myogenic changes in muscle, the peak in dMHC expression preceded the peak in the number of CNFs (42).
Fig. 10.
Myofibers expressing developmental isoforms of myosin heavy chain (dMHC) 5 days after the 15R and 150R injury protocols. Cross sections of TA muscles harvested at D5 were labeled with antibodies to dMHC (green). Sections were colabeled with dystrophin to mark the margins of myofibers (green) and DAPI to mark nuclei (blue dots). A large number of small-caliber, dMHC+ fibers appear at D5 after the 15R injury in A/J muscles but not A/WySnJ muscles. After the 150R injury, dMHC+ fibers appear in both A/WySnJ and A/J muscles. Irradiation blocks the appearance of dMHC+ fibers in A/J muscle after the 15R injury and in both strains after the 150R injury.
Fig. 11.
Quantitation of dMHC-positive (dMHC+) fibers. The number of small-caliber dMHC fibers were counted and expressed as % of the total number of myofibers in each visual field. At D5, A/J muscles showed a severalfold increase in the number of dMHC+ fibers after both the 15R and 150R injuries. Although dMHC+ fibers increased in the A/WySnJ strain after the 150R injury, the increase was much more modest compared with the A/J strain after both types of injury. *Significant difference compared with preinjury levels; Δdifference between irradiated and unirradiated muscles. Other symbols are as described in Fig. 1.
Irradiation of the TA muscle before injury blunted the expression of dMHC and the appearance of CNFs in both strains of mouse, consistent with the idea that these two markers are closely linked to myogenesis during the recovery period of A/J mice after either injury and of the A/WySnJ mice after the 150R injury.
DISCUSSION
We studied the loss of contractile torque and the subsequent return of function after two different protocols that use lengthening contractions to injure the ankle DFs of the mouse, one (15R) involving 15 large-strain lengthening contractions, and the other (150R) involving 150 small-strain lengthening contractions. We assayed membrane integrity as well as markers of myogenesis, to assess the importance of these mechanisms in mediating recovery from each type of injury in control (A/WySnJ) and dysferlin-null (A/J) mice. We also manipulated the ability of muscles of each strain to undergo myogenesis by a single, localized dose of X-irradiation, a method commonly employed to ablate SCs without other adverse local or systemic side effects (1, 19, 30, 41, 43). Our results suggest that the sarcolemmal membranes of control and dysferlin-null muscles reseal equally well, as measured by the number of fibers that retain a 10-kDa form of FDx over the 3-day period following injury caused by lengthening contractions. Consistent with this, muscle fibers that lack dysferlin are no more susceptible initially to either large-strain or small-strain injury than controls, although they do recover from either injury more slowly than controls. Our results show further that, unlike controls, the muscles in dysferlin-null mice do not recover from large-strain injury if they are first irradiated, indicating that their recovery depends on myogenesis. Finally, our results indicate that the recovery of both control and dysferlin-null muscles from multiple small-strain injury is inhibited by irradiation, and therefore also depends on myogenesis.
Recovery from large-strain injury involves membrane repair but not myogenesis.
The results of experiments in which we measured torque suggest that recovery from the 15R protocol is nearly complete within 3 days after injury in A/WySnJ mice and so involves a relatively rapid mechanism. Because it takes ∼5–6 days for SCs to be activated and form myotubes in vivo (10), myogenesis alone cannot account for this rapid recovery. Our data from irradiated A/WySnJ mice support this conclusion, because they recovered as quickly and as completely as unirradiated controls. Consistent with this, neither an increase in CNFs nor an increase in myofibers expressing dMHC was apparent at any time after large-strain injury in either irradiated or unirradiated A/WySnJ mice, suggesting that myogenesis did not occur in either group to a significant extent. Our evidence obtained with FDx labeling supports an alternative mechanism of recovery that involves membrane resealing followed by recovery of injured myofibers, confirming our earlier reports (30, 42) that the TA muscle of healthy rodents recovers from large-strain injury through processes that include membrane resealing but not myogenesis.
Our studies of A/J mice injured by large-strain lengthening contractions indicate that dysferlin does not influence the extent of initial injury, but that it is needed for rapid recovery that is independent of myogenesis. A/J mice lack dysferlin (22), a protein thought to be involved in membrane repair after injury (2, 3), and require 2 wk to recover completely from injury by the 15R protocol (42), consistent with the time course required for myogenesis (41, 42). Our observations that A/J mice show an increased number of dMHC+ fibers and CNFs and that they fail to recover from the 15R protocol if they are irradiated before injury all indicate that recovery from such an injury requires myogenesis when dysferlin is absent. Consistent with this interpretation of our results, irradiation of A/J muscles before the 15R protocol severely blunted recovery of function and the appearance of CNFs and fibers expressing dMHC. Necrotic myofibers appear in TA muscles of the A/J mouse within a few days after injury by large-strain lengthening contractions (see also Ref. 42), consistent with the idea that myofibers in the dysferlin-null A/J mouse do not survive large-strain injury for more than a few days and are ultimately replaced by myogenesis.
As expected from previous reports (30, 41), recovery from the injury induced by multiple small-strain lengthening contractions in the 150R protocol also required several weeks and in both A/WySnJ and A/J mice was blunted by X-irradiation. Together with the increase in CNFs and in the number of myofibers labeled for dMHC, these results indicate that, regardless of the presence or absence of dysferlin, myofibers subjected to the 150R protocol required myogenesis to recover contractile function. Surprisingly, however, the recovery of these two strains of mice from the injury caused by the 150R protocol was not identical. Without irradiation, control A/WySnJ mice recovered their contractile activity completely 1 wk sooner than the dysferlin-null A/J mice.
Although this difference could be due to defective or delayed myogenesis in dysferlin-null muscles, A/J muscles showed more dMHC+ fibers 5 days after the 150R injury than controls, suggesting that early myogenic activity is not compromised in dysferlin-null mice. Indeed, the presence of equivalent numbers of CNFs in both A/J and A/WySnJ mice 14 days after the 150R injury suggests that dysferlin-null muscles use myogenic mechanisms effectively to recover from contraction-induced injury, when local repair is not sufficient. Consistent with this, we have found that the rate of recovery in 1-yr-old A/J muscles repeatedly subjected to large-strain injury is not different from that in young animals (unpublished results).
The present results differ from those obtained by Chiu et al. (11), which suggest that myogenesis is delayed in dysferlin-null muscle after damage induced by myotoxin injection. The differences in their results may be attributed to their use of a myotoxin to injure muscle rather than lengthening contractions, which are likely to be less damaging overall. Our results therefore suggest that myogenesis is not significantly impaired in A/J muscles, and that other factors are likely to account for the delayed recovery of dysferlin-null muscle from contraction-induced injury. Two such factors are the larger inflammatory response in dysferlin-null muscle and the greater susceptibility of dysferlin-null myofibers to necrotic cell death.
Inflammation and myofiber necrosis after 15R and 150R injury protocols.
The 15R protocol did not induce significant inflammation and necrosis in the TA muscles of A/WySnJ mice, and even the 150R protocol caused less inflammation and necrosis than in A/J mice. By contrast, the 15R protocol caused a large increase in macrophages and necrotic fibers in the TA muscles of A/J mice. The 150R protocol led to an even greater inflammation and necrosis in A/J mice than either in the control A/WySnJ mice or in A/J mice after the 15R protocol. Because X-irradiation before injury did not reduce the number of infiltrating macrophages 3 days later, the macrophages that appear at the peak of the inflammatory response are not descended from a population of cells resident within the muscle and nearby tissues but instead are derived from the circulation.
Notably, the inflammatory changes in A/J mice were more widespread throughout the TA muscles after the 150R protocol compared with A/WySnJ mice, in which the inflammation appeared to be more focal in nature. TA muscles in the A/J mouse studied on D3 after the 15R protocol also appeared to have more focal areas of inflammation than muscles examined at the same time after the 150R protocol.
The increased susceptibility of A/J muscle to inflammation following injury caused by lengthening contractions remains unexplained. The fact that immune cells express dysferlin suggests that they, like muscle fibers, may be altered when this protein is absent (38). Indeed, inflammation is a common feature of muscle from patients lacking dysferlin (12, 16, 31, 44), and recent evidence suggests that dysferlin-null monocytes are more phagocytic than controls (38). Studies of transgenic mice that lack dysferlin only in skeletal muscle or only in myeloid cells could help determine whether inflammation is downstream to a muscle defect or vice versa. Recent work by Millay et al. (35) suggests that expressing dysferlin in skeletal muscle alone is sufficient to mitigate the dystrophic phenotype, thus supporting the notion that a muscle defect is upstream of immune dysfunction.
Membrane damage and resealing.
Our experiments using 10-kDa FDx as an indicator of membrane damage and repair suggest that wild-type or dysferlin-null muscle fibers sustain damage to their sarcolemmal membranes during the 15R and 150R protocols and that they are capable of repairing this damage for 3 days or more. This interpretation of our experiments relies on the ability of myofibers to exclude FDx when they are intact, to take it up when their sarcolemmal membranes tear, and to retain it when the membranes reseal. In vivo evidence from many types of cells (6, 15, 33, 45) supports the idea that cells that retain an exogenous membrane damage marker such as peroxidase or FDx have resealed and survived membrane injury. The added advantage of using 10-kDa FDx is that it is completely cleared from the circulation by the kidneys within a few hours, and is therefore available for uptake by muscle fibers only during this time (9). Our data show that although A/J muscle is slower to recover compared with controls after either the 15R or the 150R injury, the number of myofibers that retain FDx for 3 days after injury is no different than in controls. The simplest interpretation of these results is that after the sarcolemmal membranes open briefly to allow FDx to enter the myoplasm at the time of injury, they reseal and retain this 10-kDa marker for the same period of time even when dysferlin is absent. Furthermore, very few necrotic fibers appear shortly after injury by either the 15R or 150R protocols in A/J muscle, suggesting that the absence of dysferlin does not kill myofibers within a few seconds, as reported in studies of membrane wounding in vitro (2). Fiber necrosis at the time of injury therefore does not explain the large increase in necrotic fibers and macrophage infiltration that we see 3 days after injury. Other proteins, as well as lipids (18), participate in resealing physically damaged plasma membranes (7, 26, 32), so perhaps dysferlin is neither necessary nor sufficient for resealing to occur after in vivo physiological injuries, such as those caused by lengthening contractions. However, it is likely that the process of resealing that occurs in the absence of dysferlin does not fully restore the structure and integrity of the sarcolemma over a time course of several days, rendering it susceptible to further damage. It is also likely that some mechanisms mediating membrane resealing, which are upregulated in the absence of dysferlin, can also act as chemoattractants to immune cells (24). If indeed dysferlin-null muscle fibers can survive injurious contractions but are eliminated days later because of macrophage infiltration and necrosis, modulation of this secondary response to injury might be a potential target for therapies.
An important caveat to our findings is that we do not know whether the myofibers that retain FDx 3 days after injury are still alive or fully functional, although an impermeable plasma membrane that retains macromolecular markers is usually an indication of a healthy cell (see references cited above). Additional limitations arise from the inability of our methods to quantitate the amount of damage to particular myofibers and, because we only get a “snapshot” of the cross section of the TA muscle, to reveal whether a fiber that is unlabeled by 10-kDa FDx in one optical section is uninjured at other, distant sites. Even in cross sections, however, we detected large increases in the numbers of FDx+ fibers immediately after injury in both dysferlin-null and control muscle, suggesting that our assay reliably detects changes in sarcolemmal permeability due to injury. Our findings that both dysferlin-null and control muscles show a similar loss of torque, and a similar number of FDx-labeled fibers and necrotic fibers after either injury (15R or 150R), argue against the possibility that only a subset of A/J fibers reseal and therefore retain FDx after injury, leaving the majority of the fibers, that have not resealed, unlabeled with FDx.
Our studies of the loss of torque, the number of myofibers labeled with FDx, and, ultimately, the number of myofibers that are replaced by myogenesis are all consistent with the idea that 35–50% of A/J myofibers are damaged by lengthening contractions. None of these measures reports on the nature or the extent of the injury caused by our protocols, however. Indeed, methods to address these questions, fiber by fiber, are not yet available. As a result, we cannot be sure that, after injury, the A/J myofibers that we have shown are resealed to 10-kDa FDx are also resealed to small molecules or ions, or even to molecules larger than FDx but of a different chemical nature. Differences in the way dysferlin-null myofibers retain or exclude other molecules that are impermeant in controls may well underlie the pathological response that we have observed in A/J muscle after injury caused by large-strain lengthening contractions. Further experiments to examine damaged myofibers in A/J and control mice will be needed to address these questions. The results of these experiments, which are in progress in our laboratory, should reveal the role of dysferlin in the recovery of muscle from eccentric injuries.
GRANTS
This work was supported by the Jain Foundation (to R. J. Bloch), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (K01-AR-053235 to R. M. Lovering), the Muscular Dystrophy Association (MDA 4278 to R. M. Lovering; MDA 3771 to R. J. Bloch), and a grant from the National Football League Charities.
DISCLOSURES
The authors are not aware of financial conflict(s) with the subject matter or materials discussed in this article with any of the authors, or any of the authors' academic institutions or employers.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 283: C1182–C1195, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bansal D, Campbell KP. Dysferlin and the plasma membrane repair in muscular dystrophy. Trends Cell Biol 14: 206–213, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest 104: 375–381, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 20: 37–42, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bockman DE, Guo J, Muller MW, Friess H, Buchler MW. Cell wounding in early experimental acute pancreatitis. Lab Invest 84: 362–367, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, Ko JK, Lin P, Thornton A, Zhao X, Pan Z, Komazaki S, Brotto M, Takeshima H, Ma J. MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 11: 56–64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter GT, Kikuchi N, Horasek SJ, Walsh SA. The use of fluorescent dextrans as a marker of sarcolemmal injury. Histol Histopathol 9: 443–447, 1994 [PubMed] [Google Scholar]
- 9.Caulfield JP, Farquhar MG. The permeability of glomerular capillaries to graded dextrans. Identification of the basement membrane as the primary filtration barrier. J Cell Biol 63: 883–903, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chiu YH, Hornsey MA, Klinge L, Jorgensen LH, Laval SH, Charlton R, Barresi R, Straub V, Lochmuller H, Bushby K. Attenuated muscle regeneration is a key factor in dysferlin-deficient muscular dystrophy. Hum Mol Genet 18: 1976–1989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Confalonieri P, Oliva L, Andreetta F, Lorenzoni R, Dassi P, Mariani E, Morandi L, Mora M, Cornelio F, Mantegazza R. Muscle inflammation and MHC class I up-regulation in muscular dystrophy with lack of dysferlin: an immunopathological study. J Neuroimmunol 142: 130–136, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Couteaux R, Mira JC, d'Albis A. Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell 62: 171–182, 1988 [PubMed] [Google Scholar]
- 14.Duguez S, Bihan MC, Gouttefangeas D, Feasson L, Freyssenet D. Myogenic and nonmyogenic cells differentially express proteinases, Hsc/Hsp70, and BAG-1 during skeletal muscle regeneration. Am J Physiol Endocrinol Metab 285: E206–E215, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Farkas O, Lifshitz J, Povlishock JT. Mechanoporation induced by diffuse traumatic brain injury: an irreversible or reversible response to injury? J Neurosci 26: 3130–3140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallardo E, Rojas-Garcia R, de Luna N, Pou A, Brown RH, Jr, Illa I. Inflammation in dysferlin myopathy: immunohistochemical characterization of 13 patients. Neurology 57: 2136–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic 8: 785–794, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Serratos H, Rozycka M, Cordoba-Rodriguez R, Ortega A. Membrane healing and restoration of contractility after mechanical injury in isolated skeletal muscle fibers of the frog. Proc Natl Acad Sci USA 93: 5996–6001, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati AK. The effect of X-irradiation on skeletal muscle regeneration in the adult rat. J Neurol Sci 78: 111–120, 1987 [DOI] [PubMed] [Google Scholar]
- 20.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans blue dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200: 69–79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han R, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol 19: 409–416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho M, Post CM, Donahue LR, Lidov HG, Bronson RT, Goolsby H, Watkins SC, Cox GA, Brown RH., Jr Disruption of muscle membrane and phenotype divergence in two novel mouse models of dysferlin deficiency. Hum Mol Genet 13: 1999–2010, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-Garcia R, Palmer J, Gallano P, Baiget M, Matsuda C, Brown RH. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol 49: 130–134, 2001 [PubMed] [Google Scholar]
- 24.Kesari A, Fukuda M, Knoblach S, Bashir R, Nader GA, Rao D, Nagaraju K, Hoffman EP. Dysferlin deficiency shows compensatory induction of Rab27A/Slp2a that may contribute to inflammatory onset. Am J Pathol 173: 1476–1487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiani MF, Ansari R, Gaber MW. Oxygen delivery in irradiated normal tissue. J Radiat Res (Tokyo) 44: 15–21, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem 278: 50466–50473, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH., Jr Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 20: 31–36, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 286: C230–C238, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovering RM, Hakim M, Moorman CT, 3rd, De Deyne PG. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J Biomech 38: 1501–1507, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovering RM, Roche JA, Bloch RJ, De Deyne PG. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch Phys Med Rehabil 88: 617–625, 2007 [DOI] [PubMed] [Google Scholar]
- 31.McNally EM, Ly CT, Rosenmann H, Mitrani Rosenbaum S, Jiang W, Anderson LV, Soffer D, Argov Z. Splicing mutation in dysferlin produces limb-girdle muscular dystrophy with inflammation. Am J Med Genet 91: 305–312, 2000 [DOI] [PubMed] [Google Scholar]
- 32.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem 281: 35202–35207, 2006 [DOI] [PubMed] [Google Scholar]
- 33.McNeil PL, Ito S. Gastrointestinal cell plasma membrane wounding and resealing in vivo. Gastroenterology 96: 1238–1248, 1989 [DOI] [PubMed] [Google Scholar]
- 34.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol 19: 697–731, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Millay DP, Maillet M, Roche JA, Sargent MA, McNally EM, Bloch RJ, Molkentin JD. Genetic manipulation of dysferlin expression in skeletal muscle: novel insights into muscular dystrophy. Am J Pathol 22: 222–223, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell PO, Pavlath GK. A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol 281: C1706–C1715, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Miyake K, McNeil PL. Mechanical injury and repair of cells. Crit Care Med 31: S496–501, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Nagaraju K, Rawat R, Veszelovszky E, Thapliyal R, Kesari A, Sparks S, Raben N, Plotz P, Hoffman EP. Dysferlin deficiency enhances monocyte phagocytosis: a model for the inflammatory onset of limb-girdle muscular dystrophy 2B. Am J Pathol 172: 774–785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 562: 899–913, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathbone CR, Wenke JC, Warren GL, Armstrong RB. Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol 285: R1490–R1495, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Roche JA, Lovering RM, Bloch RJ. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport 19: 1579–1584, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenblatt JD, Parry DJ. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol 73: 2538–2543, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Rowin J, Meriggioli MN, Cochran EJ, Sanders DB. Prominent inflammatory changes on muscle biopsy in patients with Miyoshi myopathy. Neuromuscul Disord 9: 417–420, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Saito K, Miyake K, McNeil PL, Kato K, Yago K, Sugai N. Plasma membrane disruption underlies injury of the corneal endothelium by ultrasound. Exp Eye Res 68: 431–437, 1999 [DOI] [PubMed] [Google Scholar]
- 46.St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol 77: 290–297, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Stone MR, O'Neill A, Lovering RM, Strong J, Resneck WG, Reed PW, Toivola DM, Ursitti JA, Omary MB, Bloch RJ. Absence of keratin 19 in mice causes skeletal myopathy with mitochondrial and sarcolemmal reorganization. J Cell Sci 120: 3999–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki F, Nanki T, Imai T, Kikuchi H, Hirohata S, Kohsaka H, Miyasaka N. Inhibition of CX3CL1 (fractalkine) improves experimental autoimmune myositis in SJL/J mice. J Immunol 175: 6987–6996, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Takekura H, Fujinami N, Nishizawa T, Ogasawara H, Kasuga N. Eccentric exercise-induced morphological changes in the membrane systems involved in excitation-contraction coupling in rat skeletal muscle. J Physiol 533: 571–583, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.