Abstract
Pneumocystis carinii (Pc) causes severe pneumonia in immunocompromised hosts. The binding of Pc trophic forms to alveolar epithelial cells is a central feature of infection, inducing the expression and activation of PcSte20, a gene participating in mating, proliferation, and pseudohyphal growth. In related fungi, Ste20 proteins are generally activated by immediate upstream small G proteins of the Cdc42-like family. PcCdc42 has not been previously described in Pneumocystis. To address the potential role of such a G protein in Pneumocystis, PcCdc42 was cloned from a Pc cDNA library. Using the full-length 576-bp PcCdc42 cDNA sequence, a CHEF blot of genomic DNA yielded a single band, providing evidence that this gene is present as a single copy within the genome. The total length of PcCdc42 cDNA was 576 bp with an estimated molecular mass of ∼38 kDa. BLASTP analysis demonstrated greater than 80% homology with other fungal Cdc42p proteins. Northern analysis indicated equal mRNA expression in both cystic and trophic life forms. Heterologous expression of PcCdc42 in Saccharomyces cerevisiae (Sc) demonstrated that PcCdc42p was able to restore growth in an ScCdc42Δ yeast strain. Additional assays with purified PcCdc42 protein demonstrated GTP binding and intrinsic GTPase activity, which was partially but significantly suppressed by Clostridium difficile toxin B, characteristic of Cdc42 GTPases. Furthermore, PcCdc42 protein was also shown to bind to the downstream PCSte20 kinase partner in the presence (but not the absence) of GTP. These data indicate that Pc possesses a Cdc42 gene expressing an active G protein, which binds the downstream regulatory kinase PcSte20, important in Pc life cycle regulation.
Keywords: Cdc42, G protein
pneumocystis species are poorly understood ascomycetous fungi that cause severe pneumonia in immunocompromised hosts. Pneumocystis pneumonia (PcP) continues as a devastating acquired immunodeficiency syndrome (AIDS)-defining illness in patients with human immunodeficiency virus (HIV) (37). The number of patients who are receiving chronic immunosuppressive medications or who have an impaired immune system placing them at risk for PcP is rising (34). The mortality rate of PcP can range anywhere from 10 to 50%, representing a significant healthcare issue worldwide (30).
The binding of Pneumocystis carinii (Pc) trophic forms to alveolar epithelial cells (AECs) and extracellular matrix components of the host such as fibronectin and vitronectin is an important component of infection (29, 31). The attachment of Pneumocystis trophic forms to host cells induces propagation of the organism and is associated with the extension of filopodia, which interdigitate with membranes of host epithelial cells to mediate firm adherence (5, 16, 31, 32). Previous studies in our lab have demonstrated that expression of PcSte20, a gene participating in mating and pseudohyphal growth in certain fungi, is strongly upregulated following adherence of Pc to AECs (19). We have further learned that PcSte20 and the coregulated cell wall biosynthesis kinase (PcCbk1) are functionally active in mating, proliferation, and morphology changes following heterologous expression in related fungi (19). Studies in our laboratory have indicated that PcSte20 protein functions as a kinase capable of directly phosphorylating cell wall biosynthesis kinase 1 (PcCbk1), an environmentally responsive gene that functions in signaling pathways necessary for cell growth and mating (21). While phosphorylation of PcCbk1 by PcSte20 is upregulated when P. carinii trophic forms are exposed to AECs, our recent work suggests that Clostridium difficile toxin B (CDTB) suppresses this effect, strongly suggesting a role for a Cdc42-like G protein in Pneumocystis.
In fungi related to P. carinii, Ste20 proteins are generally activated by immediate upstream small G proteins of the Rho subfamily (25). Studies have demonstrated that yeast Ste20 kinase activity is stimulated by GTP-bound Cdc42 in vivo and that this effect is blocked by point mutations in the Cdc42/Rac interaction binding domain of Ste20 (25). These data suggest that the upregulation of PcSte20 kinase activity upon Pneumocystis trophic form binding to AECs may be facilitated by an upstream small G protein. Accordingly, we sought to characterize whether Pneumocystis exhibits such an upstream PcCdc42 molecule, with potential activity on the PCSte20 kinase important in regulation of the Pneumocystis life cycle.
Accordingly, in the current study, we present evidence that P. carinii contains a Cdc42-like molecule with GTP binding and intrinsic GTPase activity. The amino acid sequence of the protein is markedly homologous to related fungal species indicating that Cdc42 molecules are highly conserved across related fungal species. This PcCdc42 homolog from Pneumocystis is present at similar levels in both trophic and cyst forms under basal conditions. We also provide evidence that the P. carinii PcCdc42 is functionally active, as demonstrated by its ability to restore growth of a temperature-sensitive yeast deficient in Cdc42-related activity. Furthermore, we demonstrate that the PcCdc42 protein in the presence of GTP binds with the downstream PcSte20 regulatory kinase, a molecule important for Pneumocystis life cycle regulation.
MATERIALS AND METHODS
Materials, strains, and vectors.
P. carinii was originally derived from American Type Culture Collection (ATCC) culture collections and grown for 8 to 10 wk in immunosuppressed, corticosteroid-treated rats, as previously reported (12, 23). P. carinii cysts and trophic forms were purified from infected rat lungs via homogenization of the lungs, followed by filtration through a 10-μm filter. To exclude the presence of other infectious organisms in the P. carinii isolates, the preparations were routinely stained (Diff-Quick modified Wright-Giemsa stain; Dade Diagnostics, Aguada, Puerto Rico) to exclude samples contaminated with bacteria or other fungi. Isolates with significant contamination of other microorganisms were discarded. The isolates were examined for P. carinii nuclei with Diff-Quick-stained smears, and P. carinii trophic forms represented greater than 99% of the material on Diff-Quick-stained smears (40). For experiments requiring separation of the cysts and trophic forms, differential filtration through a 3-μm filter was performed, as we reported (21). Such 3-μm filtration resulted in 99.5% pure trophic forms and >40-fold-enriched cysts (21). Nitrocellulose membranes containing Pc chromosomes separated by contour-clamped homogenous field electrophoresis (CHEF) were gifts of Dr. M. T. Cushion, University of Cincinnati (8).
Yeast expression studies were conducted in the yeast cdc42–1 mutant strain DJTD2-16A (Mata cdc42–1 ura3–54 his4 leu2 trp1 gal2) obtained from ATCC and conducted as reported (6). For complementation studies, the yeast expression plasmid pYES2.1 TOPO, under the control of the GAL1 promoter, obtained from Invitrogen (Carlsbad, CA), which drives expression of the protein in the presence of galactose, but not in the presence of glucose, was engineered to contain the full-length PcCdc42 cDNA. The control vector used was pYES2.1 TOPO/lacZ (Invitrogen). After transformation of strain DJTD2-16A with the respective experimental and control vectors strains, yeast were plated on Synthetic Complete medium minus uracil (SC-U) with 2% glucose to select for transformants. For complementation studies, strains were then streaked on SC-U with 2% glucose or SC-U plates with 2% galactose and grown at either 23°C or 36°C.
Molecular cloning of the PcCdc42 homolog.
BlastX analysis of Pc expressed sequence tags (EST) from the Pc genome-sequencing project (http://pgp.cchmc.org/) with the full-length yeast cdc42 nucleotide sequence revealed a unique partial 281-bp EST with substantial similarity to the yeast Cdc42 protein. To obtain the full-length cDNA, 5′and 3′ “gene racing” (GeneRacer Kit, Invitrogen) with either known sense or antisense primers combined with 5′ or 3′ anchored PCR primers and methods we previously reported were used to obtain the full-length cDNA (21). The gene and nucleic acid sequences of this molecule were denoted PcCdc42 (italicized) and the related protein was designated PcCdc42 (not italicized).
Chromosomal and Southern hybridization of the PcCdc42 homolog.
To further verify the authenticity of the final full-length 576-bp reading frame, this PCR product was radiolabeled and hybridized to Pc chromosomes separated by CHEF blot as previously described (8). In addition, the 576-bp amplicon was also hybridized to Pc genomic DNA digested with the restriction enzymes BamHI, XhoI, and XbaI (Invitrogen). In parallel reactions, rat genomic DNA (Bioline, Randolph, MA) was digested with the same restriction enzymes and hybridized in a similar fashion. The 576-bp amplicon was labeled using [α-32P]dATP with the Radprime DNA labeling system (Invitrogen). Twenty micrograms of genomic DNA was digested with the restriction enzymes specified, separated on a 1% agarose gel, and transferred to nitrocellulose. Hybridization and subsequent washes were conducted according to ExpressHyb protocol (Clontech, Mountain View, CA).
Assessment of PcCdc42 mRNA expression over the life cycle of Pneumocystis.
Whole P. carinii isolates were separated into cyst and trophic form populations by differential filtration (20, 36). In brief, P. carinii cysts are retained by 3-μm pore-size Nucleopore filters and resuspended after exhaustive washing. P. carinii trophic forms pass through the device and are recovered by centrifugation. This separation procedure yielded trophic form populations containing 99.5% pure trophic forms and >40-fold-enriched cyst preparations (21). RNA was extracted from the isolated life cycle forms (TRIzol reagent, Gibco-BRL), and equal RNA (5 μg) separated by electrophoresis through 1.2% agarose in the presence of 2.2 M formaldehyde. RNA was transferred to nitrocellulose, and the blot was probed with the radiolabeled 576-bp amplicon as described above and hybridized according to ExpressHyb protocol (Clontech).
PcCdc42 and LacZ protein expression.
The full-length PcCdc42 insert was cloned into the pET 102/D-TOPO vector (Invitrogen) according to the Champion pET Directional TOPO Expression Kit protocol. The control vector used was pET102/D/lacZ, with both vectors containing COOH-terminal V5 and 6xHis fusion tags. PcCdc42 and LacZ protein was expressed utilizing the Rapid Translation System (RTS) (Roche, Indianapolis, IN) according to the RTS 100 E. coli HY Kit protocol. Protein expression was confirmed by immunoblotting with anti-V5-HRP antibody (Invitrogen). RTS expressed PcCdc42p and LacZ proteins were purified utilizing the Y-PER 6xHis Fusion Protein Purification Kit (Pierce, Rockford, IL). Purified protein was then dialyzed into GTP binding and GTPase activity assay buffers, as defined below.
Assessment of GTP binding and intrinsic GTPase activity.
[λ-35S]GTP binding measurements were performed as previously described (13). Specifically, aliquots (30 μl) were sampled in triplicate from assay mixtures containing 250 ng of soluble purified PcCdc42 protein in buffer containing 5 mM MgCl2, 50 mM Tris·HCl (pH 7.6), 250 mM (NH4)2SO4, 1 mM EDTA, and 1 mM DTT. One microliter of [λ-35S]GTP (6,000 Ci/mmol; Perkin Elmer, Waltham, MA) was added at time 0 for each time point determination. The binding assays were performed over 0–55 min at 37°C with each aliquot added to 1 ml of ice-cold wash buffer [50 mM Tris·HCl (pH 7.6), 25 mM MgCl2, 1 mM EDTA, and 0.01% BSA], then immediately applied to 0.45-μm nitrocellulose filters (Whatman, Dassel, Germany) and washed three times with 9 ml of wash buffer. Bound [λ-35S]GTP was determined by liquid scintillation counting. In additional parallel reactions, purified protein reaction samples were preincubated for 10 min with 50 ng/ml CDTB (Sigma, St. Louis, MO) to inhibit potential Cdc42-like activity (3).
GTPase activity was determined in similar fashion as previously described (11). Briefly, 1 μg of purified protein was incubated with 4 μl [λ-32P]GTP (6,000 Ci/mmol, Perkin Elmer) in 0.5 mM MgCl2, 50 mM Tris·HCl (pH 7.6), 50 mM NaCl, and 0.1 mM DTT for 10 min at 37°C. The exchange reaction was stopped by placing the reaction mix on ice and adding MgCl2 to a final concentration of 5 mM. Assays were performed for 0–15 min, and 30-μl aliquots were removed in triplicate, diluted in 1 ml of ice-cold wash buffer [50 mM Tris·HCl (pH 7.6), 0.1 mM DTT, 5 mM MgCl2], and then immediately applied to 0.45 mM nitrocellulose filters (Whatman) and washed three times with 9 ml of wash buffer. Remaining [λ-32P]GTP was determined by liquid scintillation counting. In parallel reactions, purified protein was preincubated for 10 min with 50 ng/ml CDTB (Sigma).
Assessment of the ability of yeast expressed PcCdc42 to interact with PcSte20 isolated from Pneumocystis organisms.
We next assessed the ability of recombinantly expressed PcCdc42 protein to bind to PcSte20 kinase in Pneumocystis extracts. To accomplish this, heterologous expression of PcCdc42p was performed in yeast. S. cerevisiae containing PcCdc42 in pYES2.1/V5-His-TOPO were grown in Ura(−) minimal media containing 2% galactose (inducing conditions) or 2% glucose (noninducing conditions). Yeast cells were lysed using a French press with the Y-PER extraction reagent (Pierce Chemical, Rockford, IL) in the presence of complete mini-protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). PcCdc42 V5-tagged protein was captured with anti-V5-agarose (Sigma) and washed five times with 1× TBS to remove unbound proteins. Next, ∼500 μg of protein extract from freshly isolated P. carinii organisms lysed also by French press was added to the PcCdc42/V5 complexes and allowed to incubate for 1 h at room temperature. Subsequently, these complexes were washed with 1× TBS five times and resuspended in Laemmli buffer, separated by 4–15% PAGE gel, and proteins transferred to nitrocellulose. Membranes were cut and Western blotted separately with synthetic peptide antibodies recognizing either PcSte20 or PcCdc42.
PcCdc42 and PcSte20 interactions in the presence of GTP.
GTP binding of PcCdc42 protein is often necessary for successful interactions with Ste20 kinases (27). In the prior system, it is difficult to control for the presence or absence of GTP. To specifically address the role of GTP in these interactions, both PcSte20 and PcCdc42 were subcloned into pGEX-4T-1 (GE Life Sciences, Piscataway, NJ) in frame with the NH2-terminal glutathione S-transferase tag and under control of the tac promoter. These two constructs were then transformed into E. coli BL21 (DE3) pLysS cells (Novagen). After overnight growth of one colony in 10 ml of media, the culture was added to 1 liter of media and grown until an OD 600 nm of 0.6 at 22°C. At OD 600 nm, 0.1 mM IPTG was added, and the cultures again were grown overnight at 18°C. The following day, the cells were recovered by centrifugation and washed once with cold TBS. Cells were then resuspended in 20 ml of lysis buffer (TBS containing 1% Triton X-100, 1 mM DTT, and 1 mM PMSF) and applied to a French press for lysis. The cells were then sonicated for 3 × 15 s on ice and collected by centrifugation at 20,000 g for 30 min. Supernatants were removed and GST-tagged PcCdc42 and PcSte20 proteins were purified over glutathione-sepharose according to manufacturer's directions (GE Life Sciences). After purification of the proteins, dialysis of the proteins was conducted in TBS with 1 mM DDT and 10% glycerol for 8 h × 3 times at 4°C. For PcCdc42 and PcSte20 protein interactions, the assay was conducted as previously described (25). Briefly, 5 μg of PcSte20-GST protein was added to 5 μg of PcCdc42-GST protein for 20 min at room temperature. Before this, PcCdc42-GST was either preloaded with GTPγS by incubating PcCdc42-GST in 30 μl of buffer (50 mM Tris·HCl buffer, pH 7.5, containing 4 mM EDTA, 0.5 mM DTT, 0.5% BSA, 0.1 mM GTPγ) or buffer without GTP for 15 min at 30°C. The reactions were stopped by adding 10 mM MgCl2 and cooling on ice. Protein complexes were then immunoprecipitated with PcSte20 antibody followed by Protein A-Sepharose to capture the antibody/protein complex. These complexes were then run on a 4–15% PAGE gel and transferred to nitrocellulose membrane and cut to Western blot separately for PcSte20 and PcCdc42, respectively.
Next, to determine whether PcCdc42 can interact equally with PcSte20 kinase in cysts and trophic forms, equal protein extracted from these forms (∼150 μg) and equally processed uninfected control lung were immunoprecipitated in ice-cold kinase lysis buffer (50 mM Tris·HCl, pH 7.5, 100 mM NaCl, 50 mM NaF, 1% Triton X-100, 5 mM EDTA, 1 mM EGTA) with protease inhibitors (Roche). Protein lysates were precleared with protein A-Sepharose at 4°C for 30 min and transferred to clean tubes. A PcSte20-specific antibody was added (1:100 by volume), and the mixture was stirred end-over-end at 4°C for 2 h. Immune complexes were precipitated with protein A-Sepharose for 60 min and collected by centrifugation. The pellet was washed four times, resuspended in Laemmli buffer, separated on 15% SDS-PAGE, and transferred to nitrocellulose. The membrane was Western blotted with a pan-specific cdc42 antibody (1 μg/ml) followed by a goat anti-mouse IgG HRP conjugate.
Finally, to determine whether PcCdc42 protein binds the downstream PcSte20p kinase in the presence of GTP, the V5-tagged PcCdc42 protein was heterologously expressed in yeast and allowed to interact with Pneumocystis protein extract. PcCdc42 bound complexes were immunoprecipitated with anti-V5 agarose. Precipitated proteins were separated by SDS-PAGE and transferred, and the membranes were cut in half. The presence of PcCdc42 and PcSte20 proteins was detected by Western blotting with synthetic peptide antibodies recognizing each of these proteins.
RESULTS
P. carinii contains a Cdc42-like molecule with an amino acid sequence homologous to related fungi.
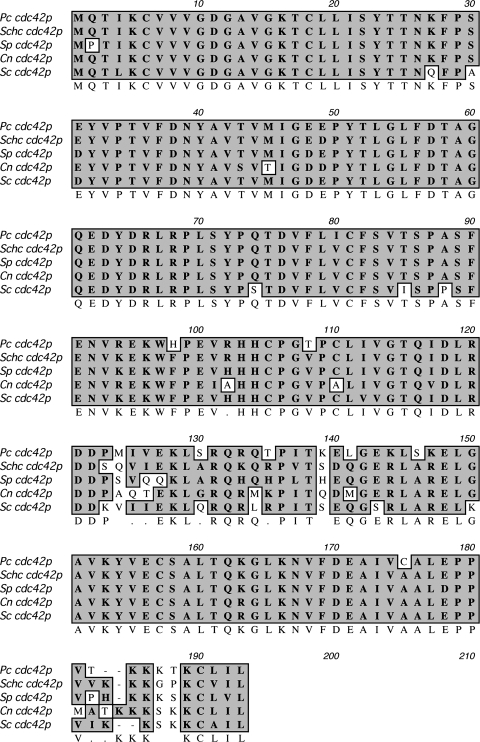
PcCdc42 was cloned from a P. carinii cDNA library utilizing a partial 281-bp EST originally identified within the partially completed Pc genomic database project. The full-length 576-bp cDNA was subsequently obtained utilizing a PCR-based sequence extension gene-racing approach (GeneRacer System, Invitrogen) with known gene-specific sense or antisense primers combined with 5′ or 3′ anchored PCR primers. The nucleotide sequence for PcCdc42 was deposited in the NCBI genome database under accession no. AY542314. Multiple sequence alignment of the Cdc42 family-related GTPases revealed significant sequence conservation across related fungal species (Fig. 1). Protein sequence homology as assessed by BLASTP analysis indicated that predicted PcCdc42 protein exhibits greatest homology to the cognate GTPases of Schizophyllum commune (87%) followed by Schizosaccharomyces pombe (85%) and Cryptococcus neoformans (84%).
Fig. 1.
Comparisons of the predicted PcCdc42 amino acid sequence with sequences of related fungal and yeast species. Amino acid alignments are as follows: Pc, Pneumocystis carinii; Schc, Schizophyllum commune; Sp, Schizosaccharomyces pombe; Cn, Cryptococcus neoformans; Sc, Saccharomyces cerevisiae. Multiple-sequence alignment of the Cdc42-related GTPases performed with ClustalW (MacVector 8.1.2) demonstrates significant amino acid sequence homology compared with other yeast and fungal Cdc42 molecules. The sequence data for PcCdc42 are available from GenBank under accession no. AAS48414.
Characterization of PcCdc42 genomic and cDNA sequences.
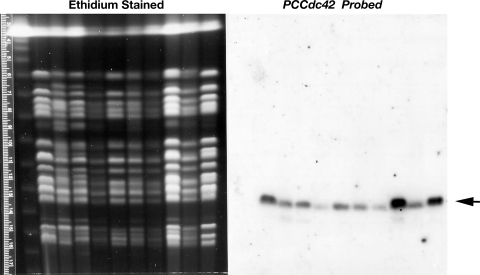
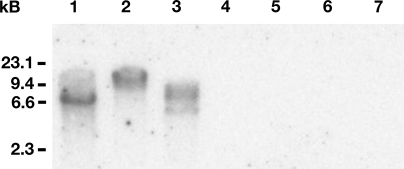
Since all Pneumocystis isolates must be derived from infected hosts, usually rodents, it is essential in such cloning strategies to confirm that derived sequences are truly of Pneumocystis origin. Thus, to verify that the isolated PcCdc42 sequences were truly derived from Pneumocystis, we assessed hybridization of these DNA sequences specifically to separate P. carinii chromosomes (Fig. 2) and restriction endonuclease digested genomic DNA (Fig. 3). The full-length 576-bp radiolabeled PcCdc42 cDNA probe hybridized specifically to a single Pc chromosome present on a CHEF blot of separated Pc chromosomes under high stringency conditions. This strongly indicated that the PcCdc42 sequences are represented within the Pneumocystis genome, being present on a single chromosome, appearing to be chromosome 13 in the organism (Fig. 2). In a separate experiment, the PcCdc42 probe hybridized to Pc genomic DNA but not rat genomic DNA cut with the same restriction enzymes (Fig. 3), further confirming that the probe was of Pc origin and suggesting that this sequence be represented as a single copy gene.
Fig. 2.
The PcCdc42 homolog is present on a single chromosome within the Pneumocystis genome. A 576-bp PcCdc42 probe hybridized under high-stringency conditions to a single Pc chromosome. Samples of Pneumocystis chromosomes resolved by contour-clamped homogeneous electrical field electrophoresis (CHEF), with each lane representing the total Pneumocystis organisms derived from a single infected rat (i.e., 10 separate rat lung samples). Left: ethidium bromide-stained CHEF gel shows separated samples of Pneumocystis chromosomes. Right: hybridization of blot of PcCdc42 probe to separated samples of Pneumocystis chromosomes transferred to nitrocellulose.
Fig. 3.
The 576-bp PcCdc42 probe hybridizes to Pneumocystis genomic DNA. Freshly isolated Pc (lanes 1–3) and rat (lanes 5–7) genomic DNA were digested with the restriction endonucleases BamHI (lanes 1 and 5), XhoI (lanes 2 and 6), and XbaI (lanes 3 and 7). The digestion products were separated by electrophoresis and transferred to nitrocellulose. The 576-bp PcCdc42 amplicon was labeled and hybridized to the membrane showing specific interaction with the Pc digestions. No hybridization was noted in the rat genomic DNA lane.
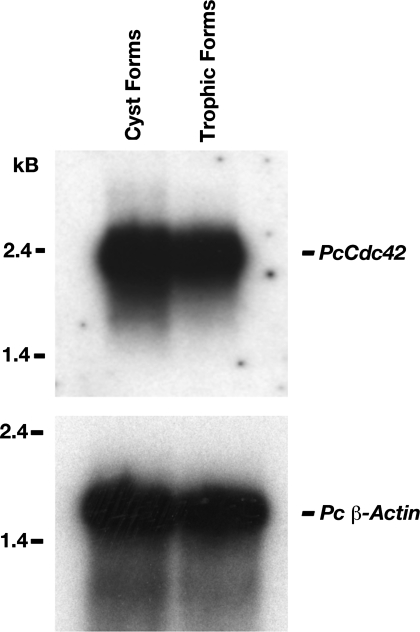
PcCdc42 mRNA appears to be expressed equally in both life cycle forms of Pneumocystis.
The life cycle of Pneumocystis alternates between diminutive trophic forms (1–2 μm in diameter) and larger cystic forms (8 μm) (5, 32). To examine whether PcCdc42 expression is differentially regulated over the life cycle of Pneumocystis, P. carinii organisms were separated into cystic and trophic populations, and total RNA was isolated from each population. RNA from the P. carinii forms was analyzed for PcCdc42 expression by Northern hybridization (Fig. 4). Steady-state PcCdc42 RNA levels in trophic and cyst forms were approximately equal, indicating that both life forms are capable of expressing PcCdc42 and that differential expression is likely not grossly different in these separate life cycle forms of the organism.
Fig. 4.
Steady-state PcCdc42 mRNA appears to be expressed equally in both major life cycle forms of the organism. To examine whether PcCdc42 expression is differentially regulated over the life cycle of P. carinii, organisms were separated into cystic and trophic populations, and total RNA was isolated. Top shows hybridization of the PcCdc42 probe, whereas the bottom shows repeat hybridization of the membrane with Pneumocystis actin to confirm equal loading.
Pneumocystis PcCdc42 cDNA can function to restore proliferation of yeast strains deficient in Cdc42-related activity.
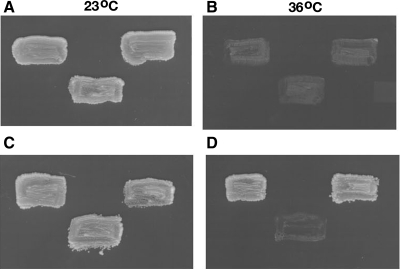
Because P. carinii cannot be cultured or transformed, it currently remains genetically intractable. Therefore, direct genetic-based testing of PcCdc42 function in P. carinii proliferation is not yet technically feasible. Instead, we evaluated the biological activity of PcCdc42 by studying its ability to complement yeast mutants exhibiting temperature-sensitive deficiencies in endogenous Cdc42 activity (Fig. 5). We have previously exploited a similar approach to identify the function of Pneumocystis PcCdc2 and PcCdc13 (24, 36). To assess activity of PcCdc42 in fungal life cycle completion and proliferation, full-length PcCdc42 cDNA was subcloned into pYES2.1 TOPO under the control of the GAL1 promoter and transformed into a ScCdc42Δ yeast strain lacking Cdc42 protein activity under nonpermissive conditions. These yeast mutants grow at the permissive temperature of 23°C but fail to proliferate at the nonpermissive temperature of 36°C (Fig. 5). After selection of transformants on selective medium with 2% glucose, single colonies were then transferred on selective media containing either 2% glucose (noninducing for protein expression) or 2% galactose (inducing for protein expression), and growth was assessed. When cultured on selective media containing 2% galactose, the vectors containing either PcCdc42 or ScCdc42 (positive control) were each able to successfully restore growth at the nonpermissive temperature, whereas the yeast transformed with empty vector alone (negative control) demonstrated no growth under these conditions (Fig. 5). Therefore, P. carinii PcCdc42 permits cell cycle completion and proliferation of these yeast mutants even under the nonpermissive temperature conditions, strongly supporting functional activity of PcCdc42-related protein in these fungi.
Fig. 5.
PcCdc42 restores growth by complementation of a temperature-sensitive cdc42Δ yeast strain. The full-length cDNA of PcCdc42 was subcloned into pYES2.1 TOPO under the control of the GAL1 promoter. After selection of transformants on selective medium with 2% glucose, single colonies were transferred on selective media containing either 2% glucose (noninducing) (A, B) or 2% galactose (C, D) to induce the GAL1 promoter. On all plates, orientation of yeast is as follows: top, left: ScCdc42Δ + pYES2.1 TOPO/ScCdc42 cDNA; top, right: ScCdc42Δ + pYES2.1 TOPO/PcCdc42 cDNA; bottom, middle: ScCdc42Δ + pYES2.1 TOPO/lacZ control vector.
P. carinii PcCdc42 protein demonstrates GTP binding and intrinsic GTPase activity, an effect that is suppressed by the pan-specific GTPase inhibitor CDTB.
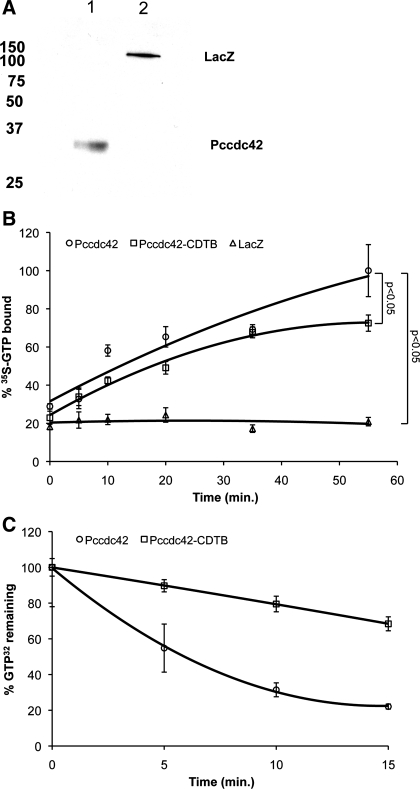
Cdc42 proteins are members of the Rho subfamily of the small GTP-binding superfamily (35). In other eukaryotic species, Cdc42 proteins demonstrate GTP binding and intrinsic GTPase activities (6). Therefore, to further determine whether PcCdc42 protein is a functional G protein, we next assessed the GTP binding and GTPase activity of this protein. To do so, PcCdc42 and the control LacZ protein were purified by nickel ion chelation chromatography after in vitro transcription/translation protein expression using the RTS system. To verify that the protein has been expressed, immunoblotting with the V5-horseradish peroxidase epitope tag antibody was undertaken demonstrating a single band at the predicted molecular mass (Fig. 6A). Next, the GTP-binding activity of the purified PcCdc42 was determined as previously described (13). These assays demonstrated that expressed PcCdc42 protein exhibited typical GTP-binding activity (Fig. 6B) with kinetics similar to that of other previously described Cdc42 molecules (15). Notably, the GTP-binding activity of PcCdc42 was significantly suppressed in the presence of CDTB (Fig. 6B), an established pan-specific inhibitor of the Rho subfamily of G proteins, further supporting that PcCdc42 truly functions as a G protein. In contrast, the control LacZ protein expressed under parallel conditions demonstrated no GTP-binding activity (Fig. 6B) indicating that the uptake of radiolabeled GTP by PcCdc42 was a specific effect.
Fig. 6.
Expression of PcCdc42 protein and its GTP binding and GTPase activity in the presence and absence of Clostridium difficile toxin B (CDTB). A: Western blotting of pET102/D-TOPO/PcCdc42 (lane 1) and LacZ (lane 2) expressed by the RTS 100 E. coli HY kit. B: the purified PcCdc42 protein was assayed for 35S-GTP binding in the presence and absence of CDTB. LacZ demonstrates absence of GTP binding of this irrelevant protein expressed by the same RTS 100 E. coli HY kit. C: intrinsic GTPase activity of PcCdc42 protein in the presence and absence of CDTB.
In similar fashion, a GTPase activity assay was further performed as previously described (11). P. carinii-specific PcCdc42 protein again demonstrated GTPase activity (Fig. 6C), an effect that was again significantly abrogated in the presence of CDTB (Fig. 6C). Together, these assays confirm that PcCdc42 protein exhibits biochemical activity consistent with a GTPase.
PcCdc42 protein can bind its target substrate PcSte20 kinase in the presence of GTP.
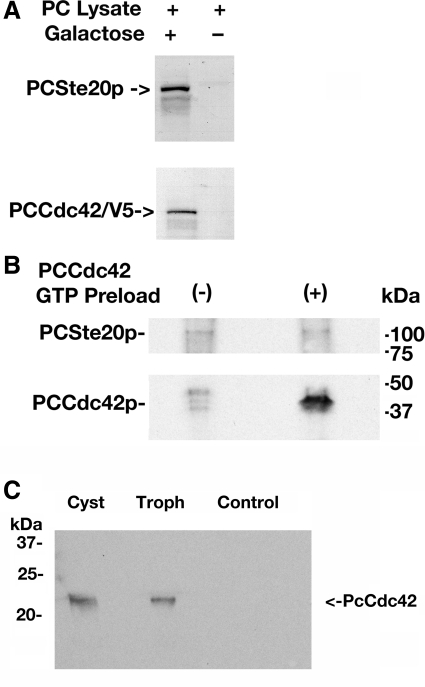
Cdc42proteins are known to bind the downstream Ste20 protein kinases in the presence of GTP, a process necessary for activation of this pathway (27). In Pneumocystis, activity of PcSte20 exerts effects in stimulating fungal mating and pseudohyphal growth (19). Accordingly, we next determined whether PcCdc42 protein could bind to PcSte20 (Fig. 7). First, we determined that PcCdc42, heterologously expressed in yeast, was able to bind to native PcSte20 present in protein extracts from freshly isolated P. carinii organisms (Fig. 7A). This initial experimental approach did not permit us to specifically assess the role of GTP, because it is highly likely that the Pneumocystis extract contained this ubiquitous nucleotide derivative. Therefore, to address the role of GTP, we next heterologously expressed both PcCdc42 and PcSte20 proteins in E. coli and assessed binding following preloading the PcCdc42 with GTP (Fig. 7B). Successful binding of PcCdc42 to PcSte20 occurred in the presence, but not the absence, of GTP. Finally, to determine whether PcCdc42 can interact equally with PcSte20 kinase in cysts and trophic forms, total proteins from these forms were immunoprecipitated with anti-PcSte20-specific antibody (Fig. 7C). Following SDS-PAGE, the membrane was Western blotted with a pan-specific cdc42 antibody revealing that PcCdc42 present in cysts and trophic forms interact equally with PcSte20. It should be noted that in Fig. 7B, the recombinantly expressed PcCdc42 was derived in E. coli with the NH2-terminal his-patch thioredoxin tag, which adds ∼13 kDa to the protein and the COOH-terminal V5/6XHis protein that adds an additional 4 kDa to the protein, yielding a theoretical molecular mass of roughly 38.3 kDa. However, in Fig. 7C, the Western blot is performed against native PcCdc42 protein itself, with a molecular mass prediction of ∼21.3 kDa. Together, our data indicated the PcCdc42 binds the downstream PcSte20 life cycle regulatory kinase in the presence of GTP.
Fig. 7.
PcCdc42 protein binds the downstream PcSte20p kinase in the presence of GTP. A: V5-tagged PcCdc42 protein was heterologously expressed in yeast and allowed to interact with Pneumocystis protein extract. PcCdc42 bound complexes were immunoprecipitated with anti-V5 agarose. Precipitated proteins were separated by SDS-PAGE and transferred, and the membranes were cut in half. The presence of PcCdc42 and PcSte20 proteins was detected by Western blotting with synthetic peptide antibodies recognizing each of these proteins. B: to specifically assess the role of GTP in these interactions, both PcCdc42 and PcSte20 were expressed in E. coli and purified. Binding was assessed following preloading (or no preloading) the PcCdc42 with GTP, complexes were immunoprecipitated with PcSte20, and the presence of both PcCdc42 and PcSte20 was assessed by immunoblotting specifically for each of these molecules. C: finally, to determine whether PcCdc42 protein can interact equally with PcSte20 kinase derived from cysts and trophic forms, total protein from isolated cyst and trophic forms was extracted, and equal amounts of protein were immunoprecipitated with an anti-PcSte20-specific antibody, separated by SDS-PAGE, and transferred to nitrocellulose. The membrane was then Western blotted with a pan-specific cdc42 antibody revealing that PcCdc42 found in cysts and trophic forms interact equally with PcSte20 in these separate Pneumocystis populations.
DISCUSSION
In the current study, we demonstrate that Pneumocystis contains a Cdc42-like molecule with characteristic GTP binding and intrinsic GTPase activity. The amino acid sequence of this protein is markedly homologous to Cdc42 molecules in related fungi, indicating that this family of regulatory GTPases is highly conserved across fungal species. Pneumocystis PcCdc42 mRNA expression is present at similar levels in both trophic and cyst forms under basal conditions. We provide further evidence that Pneumocystis PcCdc42 protein is functionally active, as demonstrated by its ability restore growth of temperature-sensitive yeast deficient in Cdc42-related activity. In addition, we also verified PcCdc42 protein activity was partially but significantly suppressed with CDTB. CDTB is a cation-dependent UDP-glucose glucosyltransferase that functions as a cellular toxin capable of inactivating the Rho subfamily of G proteins (1, 4, 7). Its mechanism of inactivating this subgroup of G proteins is by monoglucosylation of a specific amino acid residue on Rho proteins using UDP-glucose as a cosubstrate (1, 4, 7).
Cdc42 proteins, small GTPases of the Rho subfamily, regulate signaling pathways controlling cellular processes as diverse as including cell cycle progression, morphology, migration, and endocytosis in other fungi. For instance, in S. cerevisiae, pheromone-responsive signaling of mating events requires activation of Ste20, a member of the p21-activated kinase (PAK kinase) family. Activation of Ste20p in yeast is stimulated by GTP-bound Cdc42 molecules, in a process that does not require regulation by the pheromone itself (25). Thus, Cdc42 is essential for conversion of the Ste20 kinase into an active form that subsequently regulates the mating pathway (25). Interestingly, we observed in a parallel fashion that PcCdc42 binds the downstream PcSte20 in the presence of GTP. Our prior work has established a role for PcSte20 kinase in fungal mating, proliferation, and pseudohyphal growth (19).
In S. cerevisiae, Cdc42 protein is necessary for bud site selection and hence regulates polarized growth (2, 14, 35). To our knowledge, budding does not occur within Pneumocystis species. Hence, the exact activity of PcCdc42 in the growth of Pneumocystis remains to be determined. Our inability to culture Pc in axenic systems and our inability to manipulate such proteins in the organism itself limits our ability to address such important questions at this time.
Careful review of the literature on Cdc42 molecules, in particular Saccharomyces Cdc42 (the best-studied molecule of this class), has begun to address the specific significance of particular amino acid variations within these molecules. For instance, it has been shown that mutations in Saccharomyces Cdc42 at the V36 and I182 amino acid residues confer resistance to pheromone-induced cell cycle arrest (14). Furthermore, in yeast, point mutations of the C188 residue of Cdc42 results in varying degrees of binding to yeast Ste20 (2). Of note, PcCdc42 also has cysteine (C) residue at this position. Finally, despite variability in the lysine residues at positions 185–186 of both Saccharomyces cdc42 and Pneumocystis PcCdc42, the significance of these variations is not yet known.
Electron microscopy reveals that motile Pneumocystis trophic forms attach to alveolar cells by closely approximating their cell membranes with those of lung AECs (5, 29, 32). This apposition occurs with Pneumocystis trophic forms in all mammalian hosts and appears to be a prominent component of their life cycle (28, 31). Further studies indicate that binding of Pneumocystis to alveolar cells promotes proliferation of the organism (28, 31). Thus, the binding of Pneumocystis trophic forms to host AECs is an integral part of the organism's life cycle.
Recently, studies have been undertaken to identify novel Pneumocystis molecules that mediate interactions of the trophic forms with extracellular matrix proteins and AECs (18). In light of the central role of Pneumocystis adherence in its life cycle, additional studies have been undertaken to define the early signaling systems expressed in the organism following interaction with AECs and matrix proteins. Interestingly, the mRNA expression of PcSte20 as well as PcCbk1 and a specific MAPK mediating mating were each upregulated following binding of the organism to lung epithelial cells and the ECM proteins fibronectin, vitronectin, and collagen (22). This is significant because, in other fungi, Ste20 members of the p21-associated kinase family exert key regulatory activities in proliferation and mating, invasive growth, as well as in cell wall and morphology changes (10, 17, 26). We subsequently demonstrated, through heterologous expression studies in mutant yeast lacking endogenous Ste20, that PcSte20p exerts activity in restoring fungal mating and pseudohyphal growth (19).
In contrast, our current results demonstrate the presence of equal PcCdc42 mRNA in both cysts and trophic forms under basal conditions. In addition, we did not observe upregulation of PcCdc42 mRNA expression following adherence of the organism to either matrix or lung epithelial cells (data not shown). Thus, we believe that the level of control of total PcSte20 kinase activity in Pneumocystis, and hence the regulation of downstream life cycle events, likely occurs, in part, at the level of PcSte20 mRNA expression rather than PcCdc42 mRNA expression. Further studies to evaluate the precise mechanisms of PcCdc42 and PcSte20 protein interactions, and particular events that regulate the availability of GTP-bound PcCdc42, will be required to fully define this interesting signaling cascade in Pneumocystis life cycle control.
Additionally, in related fungi, the cell wall biosynthesis kinase (Cbk) acts as a signaling intermediate downstream of Ste20 in cell cycle regulatory pathways (9, 33, 38, 39). We have previously isolated PcCbk1 encoding sequences by differential display PCR (21). In a fashion parallel to PcSte20, PcCbk1 also exhibits enhanced expression following contact with lung epithelial cells and extracellular matrix (21). Through heterologous expression, we further observed that transformation of Cbk1Δ S. cerevisiae cells with PcCBK1 restored defective cell wall morphology changes during proliferation (21).
Thus, our data strongly support the presence of a conserved kinase-signaling cascade in Pneumocystis with evidence for upstream interactions of PcCdc42 with PcSte20 protein in the presence of GTP and potential for downstream control of PcCbk1 activity. This important pathway appears active in regulation of organism mating and morphology transitions (19, 21). Unique in Pneumocystis, compared with other fungi, is the prominent role of Pneumocystis adhesion to AEC and extracellular matrix proteins in regulating the presence of PcSte20 and PcCbk1 expression in this important life cycle control mechanism. The studies presented herein provide evidence for the potential role of the small PcCdc42 G protein in this signaling cascade.
GRANTS
These studies were funded by the Mayo Foundation and National Heart, Lung, and Blood Institute Grants R01-HL-62150 and R01-HL-55934 to A. H. Limper. B. J. Krajicek was supported by Institutional Training Grant T32-HL-07897.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank Zvezdana Vuk-Pavlovic and Joshua Burgess for many helpful discussions. We further acknowledge the efforts of Deanne Hebrink and Joseph Standing in the generation of the P. carinii organisms used in these studies and thank Dr. Melanie T. Cushion, Univ. of Cincinnati, for her generous gift of the CHEF blots used in these studies.
Footnotes
Sequence data are available from GenBank (http://www.ncbi.nlm.nih.gov/Genbank/) under accession no. AAS48414.
REFERENCES
- 1.Aktories K. Bacterial toxins that target Rho proteins. J Clin Invest 99: 827–829, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ash J, Wu C, Larocque R, Jamal M, Stevens W, Osborne M, Thomas DY, Whiteway M. Genetic analysis of the interface between Cdc42p and the CRIB domain of Ste20p in Saccharomyces cerevisiae. Genetics 163: 9–20, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond M, Wu YJ, Sala-Newby GB, Newby AC. Rho GTPase, Rac1, regulates Skp2 levels, vascular smooth muscle cell proliferation, and intima formation in vitro and in vivo. Cardiovasc Res 80: 290–298, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Brors D, Aletsee C, Dazert S, Huverstuhl J, Ryan AF, Bodmer D. Clostridium difficile toxin B, an inhibitor of the small GTPases Rho, Rac and Cdc42, influences spiral ganglion neurite outgrowth. Acta Otolaryngol 123: 20–25, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Campbell WG., Jr Ultrastructure of Pneumocystis in human lung. Life cycle in human pneumocystosis. Arch Pathol 93: 312–324, 1972 [PubMed] [Google Scholar]
- 6.Chen W, Lim HH, Lim L. The CDC42 homologue from Caenorhabditis elegans. Complementation of yeast mutation. J Biol Chem 268: 13280–13285, 1993 [PubMed] [Google Scholar]
- 7.Ciesla WP, Jr, Bobak DA. Clostridium difficile toxins A and B are cation-dependent UDP-glucose hydrolases with differing catalytic activities. J Biol Chem 273: 16021–16026, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Cushion MT, Kaselis M, Stringer SL, Stringer JR. Genetic stability and diversity of Pneumocystis carinii infecting rat colonies. Infect Immun 61: 4801–4813, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du LL, Novick P. Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol Biol Cell 13: 503–514, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujita A, Tonouchi A, Hiroko T, Inose F, Nagashima T, Satoh R, Tanaka S. Hsl7p, a negative regulator of Ste20p protein kinase in the Saccharomyces cerevisiae filamentous growth-signaling pathway. Proc Natl Acad Sci USA 96: 8522–8527, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett MD, Self AJ, van Oers C, Hall A. Identification of distinct cytoplasmic targets for ras/R-ras and rho regulatory proteins. J Biol Chem 264: 10–13, 1989 [PubMed] [Google Scholar]
- 12.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem 278: 2043–2050, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Hart MJ, Polakis PG, Evans T, Cerione RA. The identification and characterization of an epidermal growth factor-stimulated phosphorylation of a specific low molecular weight GTP-binding protein in a reconstituted phospholipid vesicle system. J Biol Chem 265: 5990–6001, 1990 [PubMed] [Google Scholar]
- 14.Heinrich M, Kohler T, Mosch HU. Role of Cdc42-Cla4 interaction in the pheromone response of Saccharomyces cerevisiae. Eukaryot Cell 6: 317–327, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemsath L, Ahmadian MR. Fluorescence approaches for monitoring interactions of Rho GTPases with nucleotides, regulators, and effectors. Methods 37: 173–182, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Itatani CA. Ultrastructural morphology of intermediate forms and forms suggestive of conjugation in the life cycle of Pneumocystis carinii. J Parasitol 82: 163–171, 1996 [PubMed] [Google Scholar]
- 17.Kohler JR, Fink GR. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci USA 93: 13223–13228, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kottom TJ, Kennedy CC, Limper AH. Pneumocystis PCINT1, a molecule with integrin-like features that mediates organism adhesion to fibronectin. Mol Microbiol 67: 747–761, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kottom TJ, Kohler JR, Thomas CF, Jr, Fink GR, Limper AH. Lung epithelial cells and extracellular matrix components induce expression of Pneumocystis carinii STE20, a gene complementing the mating and pseudohyphal growth defects of STE20 mutant yeast. Infect Immun 71: 6463–6471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottom TJ, Limper AH. Cell wall assembly by Pneumocystis carinii. Evidence for a unique gsc-1 subunit mediating beta-1,3-glucan deposition. J Biol Chem 275: 40628–40634, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Kottom TJ, Limper AH. Pneumocystis carinii cell wall biosynthesis kinase gene CBK1 is an environmentally responsive gene that complements cell wall defects of cbk-deficient yeast. Infect Immun 72: 4628–4636, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kottom TJ, Limper AH. Subtractive hybridization analysis of Pneumocystis carinii gene activation induced by interaction with lung epithelial cells and matrix. Chest 121: 78S–79S, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kottom TJ, Thomas CF, Jr, Limper AH. Characterization of Pneumocystis carinii PHR1, a pH-regulated gene important for cell wall integrity. J Bacteriol 183: 6740–6745, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kottom TJ, Thomas CF, Jr, Mubarak KK, Leof EB, Limper AH. Pneumocystis carinii uses a functional cdc13 B-type cyclin complex during its life cycle. Am J Respir Cell Mol Biol 22: 722–731, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Lamson RE, Winters MJ, Pryciak PM. Cdc42 regulation of kinase activity and signaling by the yeast p21-activated kinase Ste20. Mol Cell Biol 22: 2939–2951, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J 11: 4815–4824, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall JE, Thomas DY. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J 16: 83–97, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limper AH. Parasitic adherence and host responses in the development of Pneumocystis carinii pneumonia. Semin Respir Infect 6: 19–26, 1991 [PubMed] [Google Scholar]
- 29.Limper AH, Martin WJ., 2nd Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J Clin Invest 85: 391–396, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limper AH, Offord KP, Smith TF, Martin WJ., 2nd Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 140: 1204–1209, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Limper AH, Thomas CF, Jr, Anders RA, Leof EB. Interactions of parasite and host epithelial cell cycle regulation during Pneumocystis carinii pneumonia. J Lab Clin Med 130: 132–138, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Long EG, Smith JS, Meier JL. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest 54: 609–615, 1986 [PubMed] [Google Scholar]
- 33.McNemar MD, Fonzi WA. Conserved serine/threonine kinase encoded by CBK1 regulates expression of several hypha-associated transcripts and genes encoding cell wall proteins in Candida albicans. J Bacteriol 184: 2058–2061, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis 34: 1098–1107, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153–208, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Thomas CF, Anders RA, Gustafson MP, Leof EB, Limper AH. Pneumocystis carinii contains a functional cell-division-cycle Cdc2 homologue. Am J Respir Cell Mol Biol 18: 297–306, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 350: 2487–2498, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA 95: 7526–7531, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss EL, Kurischko C, Zhang C, Shokat K, Drubin DG, Luca FC. The Saccharomyces cerevisiae Mob2p-Cbk1p kinase complex promotes polarized growth and acts with the mitotic exit network to facilitate daughter cell-specific localization of Ace2p transcription factor. J Cell Biol 158: 885–900, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong SJ, Vuk-Pavlovic Z, Standing JE, Crouch EC, Limper AH. Surfactant protein D-mediated aggregation of Pneumocystis carinii impairs phagocytosis by alveolar macrophages. Infect Immun 71: 1662–1671, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]