Abstract
Endocannabinoids are lipid derivatives that mediate paracrine and juxtacrine signaling between cells. In the hippocampal CA1 region, a retrograde endocannabinoid signal suppresses GABA release by acting on presynaptic cannabinoid receptor-1 (CB1) and can be functionally manifested as depolarization-induced suppression of inhibition (DSI). In the present study, whole cell patch-clamp recordings in hippocampal slices were made to examine DSI in rats from P7–P21. Robust DSI develops in rat hippocampus at postnatal ages greater than two weeks, but only modest DSI is observed in P7-9 rat. DSI in neonatal rats can be enhanced by activation of group I metabotropic glutamate receptors (mGluRs) or muscarinic acetylcholine receptors in those neonatal rats. The DSI is also enhanced by sustained low-frequency (1 Hz) stimulation (5 min). This stimulus-enhanced DSI was prevented in the presence of 6-methyl-2-(phenylethynyl)-pyridine (10 μM), a group I mGluR antagonist. WIN55212-2, a synthetic CB1 agonist, produced a similar level of inhibition of GABAergic synaptic transmission at different postnatal time points. Therefore postsynaptic mechanisms appear to be mainly responsible for developmental changes in DSI, although presynaptic mechanisms cannot be ruled out entirely. We have also obtained evidence that tonic endocannabinoid release suppresses GABAergic transmission in the mature but not the neonatal hippocampus. The differential DSI magnitude at different stages of maturation could alter synaptic plasticity and learning and memory during hippocampal development.
INTRODUCTION
The major psychoactive ingredient of marijuana is Δ-9-tetrahydrocannabinol (Δ9-THC) (Gaoni and Mechoulan 1971), which affects synaptic transmission in brain primarily by binding to the G-protein-coupled cannabinoid receptor-1 (CB1) (Matsuda et al. 1990). Several endogenous agonists for CB1 receptors (the so-called endocannabinoids) have been identified to date. Among these compounds, the lipid derivatives arachidonylethanolamide (anandamide) and 2-arachidonylglycerol (2-AG) appear to be the best candidates for mediation of endocannabinoid signaling (Devane et al. 1992; Sugiura et al. 1995).
Exogenous cannabinoids disrupt learning and impair short-term memory, while endogenous cannabinoids may serve to facilitate memory and synaptic plasticity (Carlson et al. 2002; Marsicano et al. 2002; Sullivan 2000). In the hippocampus, high levels of CB1 receptors are expressed on the presynaptic terminals of cholecystokinin-positive GABAergic inhibitory interneurons (Herkenham et al. 1990; Katona et al. 1999; Tsou et al. 1999). At synapses made on CA1 pyramidal neurons by these terminals, endocannabinoids function as rapid retrograde signaling molecules, as exemplified by depolarization-induced suppression of inhibition (DSI) (Wilson and Nicoll 2001). DSI is initiated by strong postsynaptic activation leading to an increase in intracellular Ca2+ (Lenz et al. 1998). This presumably leads to synthesis and release of an endocannabinoid molecule that acts on CB1 receptors on presynaptic GABAergic terminals where DSI is expressed as a decrease in release probability (Morishita and Alger 1997; Ohno-Shosaku et al. 2001; Pitler and Alger 1992; Wilson et al. 2001).
The induction and magnitude of DSI can be enhanced by the application of agonists for muscarinic acetylcholine receptors (mAChRs) (Martin and Alger 1999) and metabotropic glutamate receptors (mGluRs) (Varma et al. 2001). The mGluR-dependent DSI enhancement appears to result from activation of postsynaptic group I mGluRs that facilitate endocannabinoid production. Based on these findings, it would stand to reason that intense glutamatergic transmission, in conjunction with postsynaptic depolarization, might lead to especially strong suppression of inhibition.
We have investigated the development of DSI during maturation of rat hippocampus. We observed that DSI produced by depolarization alone becomes more robust after the first 2 wk of postnatal development. This maturation of DSI appears to involve an increase in synthesis or release of endocannabinoids in CA1 pyramidal neurons. Furthermore we have observed that in neonatal rats, mGluR-dependent DSI enhancement can be induced by sustained low-frequency stimulation without the need for agonist application. Such a mechanism may underlie the increase in synthesis or release of endocannabinoids from the postsynaptic neurons during development. In light of the fact that DSI has been proposed as a mechanism for enhancement of induction of longer-lasting forms of plasticity (e.g., long-term potentiation, LTP) (Carlson et al. 2002), the difference in DSI magnitude at different ages may contribute to developmental changes in the threshold for other forms of synaptic plasticity.
METHODS
Slice preparation and electrophysiology
Transverse hippocampal slices (400 μm) were cut from Sprague-Dawley rat (Charles River or Taconic; P7–P21). Experiments were performed according to guidelines for the care and use of rodents in the intramural research program at the National Institutes of Health, and the experimental procedures were approved by the institutional animal care and use committee of the NIAAA Division of Intramural Clinical and Biological Research. The composition of the external recording buffer was (in mM): 124 NaCl, 3 KCl, 1.3 Mg2SO4, 2 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 10 glucose, 0.005 NBQX (Tocris), and 0.025 AP5 (Sigma). The external buffer was equilibrated with a 95%-5% mixture of O2-CO2 at room temperature (22–23°C). Slices were maintained in the external buffer for ≥1 h before recording. Patch pipettes had resistances of 4–6 M after filling with internal solution containing (in mM): 150 CsCl, 10 HEPES, 0.2 BAPTA, 2 MgCl2, 3 Mg-ATP, 0.3 GTP, and 5 QX314. All recordings were made at room temperature. The holding potential was −60 mV in all experiments. Approximately half-maximal monosynaptic GABAergic currents [evoked inhibitory postsynaptic currents (eIPSCs)] were evoked with bipolar electrodes (Teflon-coated platinum-iridium wire, 75 μm in diameter with the coating) placed in or near CA1 stratum pyramidale. DSI was tested every 4 min and consisted of 33 stimuli at 0.33 Hz with depolarization from −60 to 0 mV for 4 s inserted after the seventh stimulus. For DSI analysis, the means of the five eIPSCs evoked just prior to depolarization and the three eIPSCs just after depolarization were used as Ampbaseline and Amptest, respectively [DSI magnitude (%) = 100 (1− Amptest/Ampbaseline)].
Pharmacology
Carbachol (IGN Biomedicals), 6-methyl-2-(phenylethynyl)-pyridine (MPEP) and (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R)-ACPD (Tocris) were dissolved in water. The stock solutions for SR141716 (NIMH chemical synthesis program) and WIN55212-2 (Tocris) were made with DMSO (final dilution is 1/5,000 during experiments). For experiments with SR141716 and WIN55212-2, bovine serum albumin (BSA) (0.5 mg/ml), as a carrier to facilitate drug delivery, was included in the external buffer during the baseline recording and drug application. Whenever SR141716 or WIN55212-2 were used, the recording system was thoroughly cleaned with ethanol followed by BSA and distilled water.
Analysis and statistics
eIPSCs were analyzed using cursor-based measurement in Clampfit 9.0. Traces were averaged using MiniAnalysis Software (Synaptosoft, Decatur, GA). Data are expressed as mean ± SE; statistical significance was determined using one-way ANOVA, unless otherwise indicated. Criterion for significance was P < 0.05.
RESULTS
Postnatal development of depolarization-induced DSI in hippocampal neurons
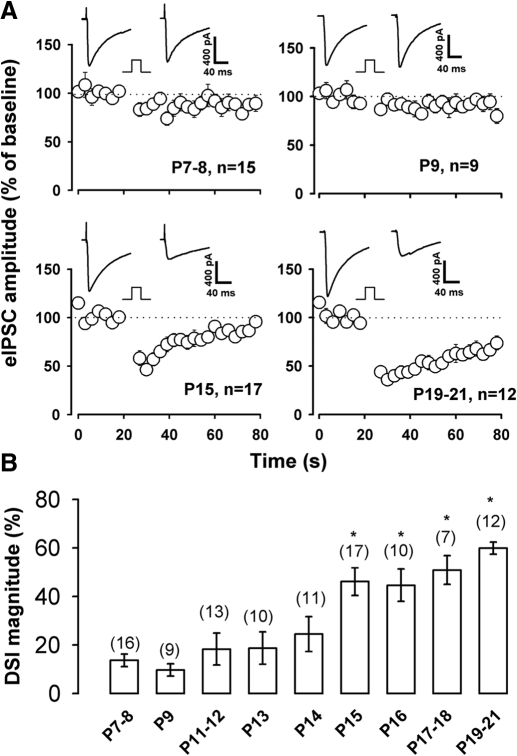
To determine if DSI magnitude or duration was altered during hippocampal postnatal development, we examined the effect of postsynaptic depolarization on eIPSCs using whole cell recording in CA1 pyramidal neurons at different ages (P7-21). Figure 1A shows that a 4-s depolarizing step results in only a modest suppression of eIPSCs in P7–P9 rat hippocampal neurons, whereas a robust DSI is produced in P15–P21 rats. For P7–P8 rat neurons, DSI magnitude averaged 13 ± 3% decrease relative to baseline eIPSC amplitude, and 46 ± 6 and 60 ± 3% for P15 and P19–21 rats, respectively. DSI persistence after a depolarizing step was much longer in P19-21 rat than in P15 rat neurons (Fig. 1A). Overall, we examined DSI in single CA1 pyramidal neurons in rats at a variety of ages as summarized in Fig. 1B. There was no significant difference in DSI magnitude in neurons from P7–P8, P9, P13, and P14 rats (P > 0.05, ANOVA). A significant increase in the magnitude of DSI was first detected at P15 (P < 0.05, compared with P7–P8 and P9, ANOVA), and even a greater increase in DSI magnitude was produced by depolarization in more mature rats (P17-18 and P19-21, Fig. 1B; P < 0.01, ANOVA relative to P7-8 and P9 groups).
Fig. 1.
Developmental changes in depolarization-induced suppression of inhibition (DSI) in hippocampus. A: in CA1 pyramidal neurons, a 4-s depolarizing step resulted in modest suppression of the evoked inhibitory postsynaptic current (eIPSC) at postnatal days 7–8 and 9 but produced a robust suppression at P15 and P19-21. Each inset superimposes the average of 5 consecutive eIPSCs pre-DSI and 3 trials post-DSI. Square step indicates a 4-s depolarization from −60 to 0 mV. B: DSI magnitude was measured as the mean percentage reduction of 3 consecutive eIPSCs after the depolarizing step. Numbers above each bar indicate the number of cells. * P < 0.05 compared with P7-8 rats.
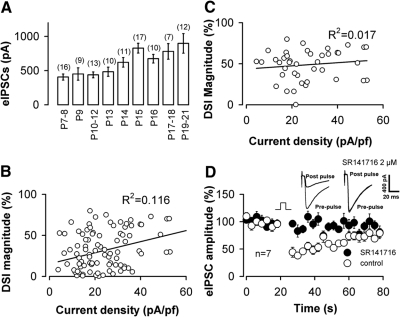
In P8-12 rat neurons, increasing the duration of the depolarizing pulse did not lead to further enhancement of DSI. The eIPSC amplitude was decreased by 17 ± 5% relative to the baseline amplitude by a 4-s depolarizing step and 15 ± 4% for an 8-s step (P < 0.05, n = 7, paired t-test). Developmental increases in neuronal size may also contribute to the increase in DSI, for example by affecting the density of currents needed for DSI induction. Indeed we observed that neuronal size increased with development as indicated by an increase in whole cell capacitance from 25 ± 1.6 pF for P7–P14 rats (n = 59) to 33 ± 1.7 pF for rats (n = 45) over P14 (P < 0.01). There was a small but significant change (P < 0.05) in the eIPSC density (21 ± 1.4 and 25 ± 2.0 pA/pF for P7-14 rats and rats over P14, respectively). However, we found that there was no significant relationship between the magnitude of DSI and the pre-DSI eIPSC current density, as shown in Fig. 2B and C. DSI in mature rat neurons can be abolished by SR141716, a CB1 antagonist (Fig. 2D). Overall, our findings indicate a developmental change in endocannabinoid-mediated DSI in CA1 neurons.
Fig. 2.
The baseline eIPSC amplitude is not correlated with DSI magnitude. A: bar plot of average amplitude of eIPSCs vs. postnatal age. The eIPSC amplitude was significantly larger in >P14 rats than in P7/8 ∼ P13 groups of rat (P < 0.05, ANOVA). There was no significant difference in eIPSC amplitude among the P7-8 ∼ P13 and P14 ∼ P19-21groups (P > 0.05, ANOVA). B: plot of prepulse eIPSC density vs. DSI magnitude from 105 cells (P7–P21). The continuous line is a linear regression to all data points. C: a subset of the data plotted in B (P15–P21). —, a linear regression to all data points, indicating a poor relationship between the eIPSC density and the magnitude of DSI even in these more mature rats (>P14). D: in the presence of SR141716 (2 μM), a 4-s depolarizing step results in little or no suppression of IPSCs (n = 7) in mature rats (P17–P20). Insets: from the same neuron in the absence and presence of SR141716.
Postsynaptic mechanisms responsible for developmental enhancement of DSI
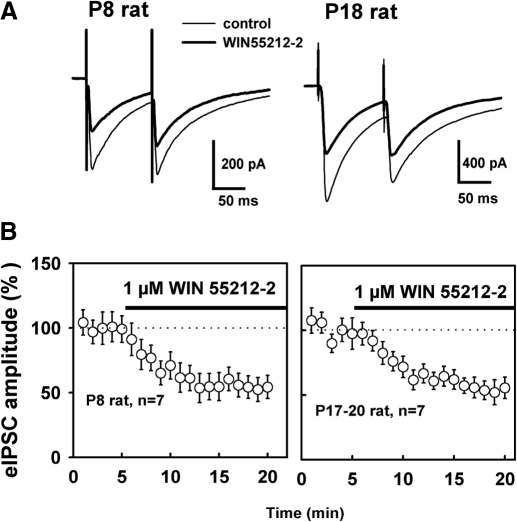
It is well known that DSI involves both pre- and postsynaptic mechanisms. DSI requires Ca2+ influx in the postsynaptic neuron which is initialized by depolarization (Ohno-Shosaku et al. 2001; Wilson and Nicoll 2001). DSI expression is most likely presynaptic, consistent with high levels of G-protein-coupled CB1 receptors expressed on the presynaptic terminals of a subset of GABAergic neurons (Katona et al. 1999; Tsou et al. 1999). In searching for synaptic mechanisms of this developmental change in CB1 signaling, we first asked whether the developmental increase in DSI results from an increase in presynaptic CB1 receptor actions on GABAergic inhibitory terminals. Alternatively these young animals may lack the subset of GABAergic neurons that express CB1 receptors. We thus examined the effect of WIN55212-2, a synthetic CB1 agonist, on eIPSC amplitude in the youngest and most mature animals used in this study. As shown in Fig. 3B, bath application of WIN55212-2 (1 μM) produced a 52 ± 7% reduction in the eIPSC amplitude in P8 rat slices and a 46 ± 6% reduction in mature rat (P17-20). Thus the magnitude of WIN55212-2 inhibition of eIPSC is similar across the range of postnatal ages examined at present (P = 0.51, t-test). WIN55212-2 also increased the ratio of amplitudes of two closely paired eIPSCs (i.e., paired pulse ratio, PPR; Fig. 3A) by 17 ± 4% (n = 8) and 15 ± 5% (n = 6), respectively, for P8 and P17-20 groups; this generally agrees with a decrease in the probability of GABA release from the axon terminals during CB1-mediated synaptic depression. In addition, a low concentration of WIN55212-2 (50 nM) produced 25 ± 6% reduction in eIPSC amplitude in P8–P9 rats from control levels of 208 ± 40 pA (P < 0.05, n = 4). These findings indicate that differences in the level of presynaptic CB1 receptor inhibition or lack of a CB1-expressing subset of interneurons do not contribute to the changes in DSI magnitude during development.
Fig. 3.
Presynaptic modulation by cannabinoid receptor-1 (CB1) receptors does not change during development. A: representative experiments from P8 or P18 rat show that the synthetic CB1 agonist WIN55212-2 elicited a robust suppression of eIPSCs and an increase in the amplitude ratio of 2 closely paired eIPSCs. Traces are average eIPSCs from 20 trials before WIN55212-2 and 10 min after drug application. B: WIN55212-2 produced a 52 ± 7% reduction in eIPSC amplitude in P8 rat and a 46 ± 6% reduction in mature rat (P17-20). There was no significant difference between WIN55212-2 effects in young and mature rats (P = 0.51).
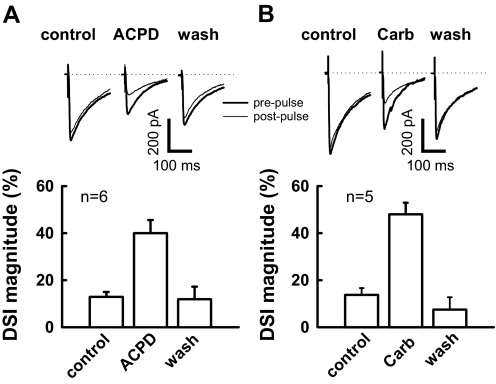
If the development of DSI does not involve changes in presynaptic CB1 receptors, it might involve an increase in endocannabinoid release. Group I mGluRs are mainly located on postsynaptic structures in the CA1 region (Lujan et al. 1997), and activation of these mGluRs enhances DSI in this brain region (Varma et al. 2001). We examined whether mGluR agonists could facilitate DSI in neonatal rat. As shown in Fig. 4, in the same neuron from a P8 rat, a depolarizing step only produces a modest DSI, but results in a robust DSI (40 ± 6% reduction in eIPSC amplitude) in the presence of the mGluR agonist (1S,3R)-ACPD (10 μM). We obtained similar results using the mAChR agonist carbachol, which has been demonstrated to postsynaptically enhance DSI via a G-protein-dependent mechanism (Kim et al. 2002). Indeed preincubation with carbachol (2 μM) for 4–8 min markedly enhanced DSI in P8 rat neurons (Fig. 4). The eIPSC amplitude was decreased by 12 ± 2% relative to baseline values by depolarization before drug application, and this decrease was enhanced to 48 ± 5% in the presence of carbachol. Both ACPD and carbachol effects on DSI were reversible (Fig. 4). Consistent with agonist effects observed in previous studies (Kim et al. 2002; Varma et al. 2001), ACPD and carbachol caused 39 ± 3% (P < 0.01, n = 6) and 24 ± 7% reduction of the eIPSC amplitude (P < 0.05, n = 5), respectively.
Fig. 4.
(1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R)-ACPD and carbachol facilitate DSI in neonatal rats (P8-9). A: traces showing DSI are all from the same neuron (top). In the presence of ACPD (10 μM) for 4–8 min, depolarization produced a robust suppression (40 ± 6% reduction of baseline eIPSC amplitude; n = 6; bottom). Prepulse trace is the average of the 5 eIPSCs just prior to a 4-s depolarization, and post pulse trace is the average of 3 eIPSCs after depolarization. B: carbachol (carb) enhances DSI in neonatal rat. Similar arrangement as in A, in the presence of carb (2 μM) for 4–8 min, the same depolarizing step leads to a 48 ± 5% reduction relative to baseline eIPSC amplitude (n = 5).
Low-frequency stimulation enhances DSI
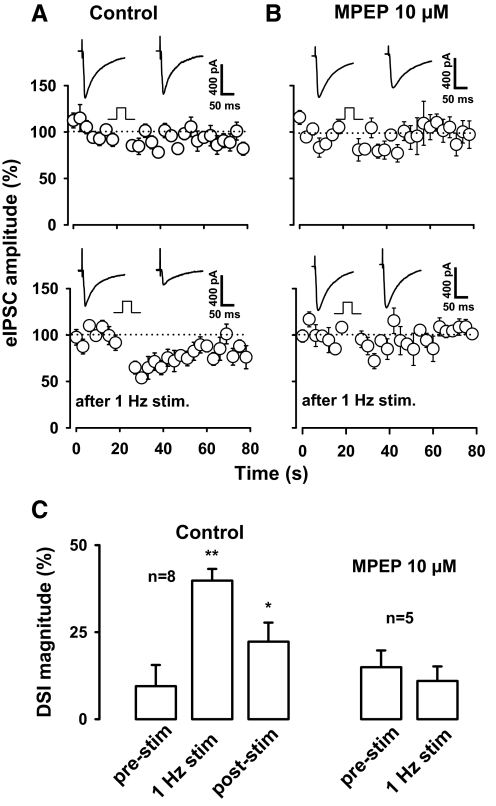
The fact that mGluR activation enhances DSI suggests that DSI should also be enhanced following strong activation of glutamatergic synaptic input. Glutamate release and formation of glutamatergic synapses increases during the first postnatal weeks of hippocampal development (Collard et al. 1993; Gasparini et al. 2000; Iwasaki and Takahashi 2001). Thus we wondered if release of glutamate leading to mGluR activation could mimic the effect of mGluR activation on DSI in the young hippocampal neurons. Specifically, we hypothesized that activation of glutamatergic afferents might produce a robust enhancement of DSI in neurons at early developmental stages when DSI is weak. We recently found that DSI in P16-P20 rat CA1 neurons can be enhanced by low frequency stimulation (1 Hz for 5 min) of afferents in the s. radiatum at the site used to evoke DSI (Zhu and Lovinger 2007). We thus examined the effect of this stimulation on DSI evoked using the standard 4-s depolarizing step in neurons from P9-11 rats. As shown in Fig. 5, DSI magnitude averaged 8 ± 6.8% before the low-frequency stimulation, 38 ± 4.3% just after the stimulation (P < 0.01, n = 8, paired t-test) and 22 ± 5.4% 8 min after the stimulation. The low-frequency stimulation also produced 27 ± 8% reduction in eIPSC amplitude from the control level of 420 ± 56 pA (P < 0.01, n = 8). In our previous study, we observed that low-frequency stimulation produces a long-lasting enhancement of DSI in neurons from more mature animals (Zhu and Lovinger 2007), and the enhancement of DSI appears to be maintained, with only partial recovery in these young animal neurons as well (Fig. 5). All experiments in this study were performed in the presence of antagonists of ionotropic glutamate receptors (see methods), and thus these receptors do not play a role in the stimulus-enhanced DSI.
Fig. 5.
Enhancement of DSI by 1-Hz stimulation. A: DSI was moderate in neonatal rats (P9-11) and was enhanced by low-frequency stimulation (1 Hz for 5 min). Inset: traces show eIPSCs from representative experiments. B: in the presence of 6-methyl-2-(phenylethynyl)-pyridine (MPEP), an mGluR5 antagonist, the same low-frequency stimulation procedure had no effect on DSI in neonatal rats (P10-11). Inset: traces are from the same neuron. C: summary of effect of 1-Hz stimulation on DSI magnitude in the absence and presence of MPEP. * P < 0.05, compared with the control in the absence of MPEP.
Given that DSI can be enhanced by application of an exogenous mGluR agonist, it was logical to determine if the stimulus-induced enhancement of DSI might involve an mGluR. Indeed, in the presence of the mGluR5 antagonist, MPEP (10 μM), 5 min of 1-Hz stimulation had no effect on DSI (P > 0.05, n = 5). In the absence of MPEP, the low-frequency stimulus protocol reduced the amplitude of eIPSCs, and this effect was also prevented by the mGluR antagonist. eIPSC amplitude was decreased by 29 ± 8% from the prestimulus value of 416 ± 51 pA in control solution but only 16 ± 10% (P > 0.1) from a prestimulus value 415 ± 84 pA in the presence of MPEP. Furthermore, stimulus-enhanced DSI was abolished by the CB1 antagonist SR141716. After a 10-min incubation of slices with 2 μM SR141716, DSI magnitude was 11 ± 5% , and DSI magnitude was 2 ± 3% after 5 min of 1-Hz stimulation (n = 4). SR141716 also abolished the stimulus-induced reduction in eIPSC amplitude. The average amplitude of eIPSCs was 259 ± 60 pA before and 235 ± 67 after 5 min of 1-Hz stimulation in the presence of SR141916. These findings indicate that DSI can be enhanced by sustained glutamatergic transmission leading to activation of a group I mGluR in neonatal hippocampus. This pattern of sustained afferent stimulation also appears to produce an mGluR-dependent, long-lasting decrease in eIPSC amplitude (Zhu and Lovinger 2007) that resembles the iLTD recently described by Chevaleyre and Castillo (2003).
Ongoing release of endocannabinoids modulates GABAergic transmission at mature synapses
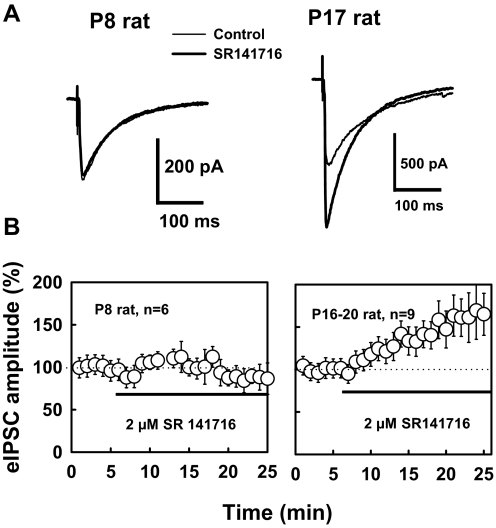
We further asked whether postnatal development of endocannabinoid formation could lead to a tonic ongoing influence on inhibitory transmission in the mature hippocampus. We thus examined the effect of the CB1 antagonist SR141716 on GABAergic transmission in CA1 neurons from rats of different ages. As demonstrated in Fig. 6, bath application of SR141716 had no significant effect on the baseline eIPSC amplitude in P8 rat (only a 7 ± 22% increase) but increased the baseline eIPSC amplitude by 43 ± 8% (P < 0.05, n = 9) in the mature rat (P16–P20). There was a significant difference (P < 0.01, t-test) in PPR between P8 and more mature rats (P16-20), and PPR averaged 0.82 ± 0.03 for P8 rat (n = 15) and 0.65 ± 0.06 for P16-20 rats (n = 12). SR141716 decreased the PPR by 14 ± 3% in mature rat (n = 6, P < 0.02, paired-t-test), but only 4 ± 7% in P8 rat (n = 7). This finding is consistent with an increase in the probability of GABA release from the axon terminals after the blockade of CB1 receptors in mature hippocampus. Thus endogenous cannabinoids appear to produce a tonic modulation of GABAergic transmission in the mature rat hippocampus but not in the hippocampus of the neonatal rat.
Fig. 6.
Ongoing release of endocannabinoids modulates GABAergic transmission in mature rat. A: representative traces are averages of responses before and after 10 min application of SR141716. SR141716 enhanced eIPSCs in mature rat, but not in P8 rat. B: in mature rat, SR141716 (2 μM) caused a 43 ± 9% increase in eIPSC amplitude (P < 0.001), but had no significant effect on the eIPSC (only 7 ± 22% increase, P = 0.71) in P8 rat.
DISCUSSION
In the present study, we have described the postnatal development of DSI in rat hippocampus. Our findings suggest that postsynaptic mechanisms are responsible for developmental changes in DSI. This is evidenced by the fact that DSI in early neonatal rats can be enhanced by activation of postsynaptic mGluR or mAChR receptors by enhancing endocannabinoid production. Furthermore, DSI can be strongly enhanced by sustained glutamatergic transmission in neonatal rat neurons. In contrast, we did not observe any developmental difference in inhibition produced by explicit CB1 activation in agreement with previous observations indicating a similar level of CB1 receptor expression during early postnatal and adult life in rat hippocampus (Morozov and Freund 2003). Also the postnatal development of DSI does not result from differences in the basal strength of inhibitory synaptic transmission at the different ages because DSI magnitude was not correlated with eIPSC current density. The bulk of evidence we have collected to date indicates that postsynaptic endocannabinoid production is enhanced with development and that this is the major mechanism underlying the postnatal increase in the ease of DSI induction.
Although the postnatal development of DSI is probably postsynaptic in origin, it may arise from changes in presynaptic glutamatergic input. DSI was enhanced by either mGluR activation or 1-Hz stimulation, and these treatments also reduced baseline eIPSC amplitude (which further supports the notion that DSI magnitude is not correlated with eIPSC amplitude). The blockade of group I mGluR receptors with MPEP (10 μM) abolished the stimulus-induced enhancement of DSI (Figs. 4 and 6). Thus activation of mGluRs by increased presynaptic release of glutamate enhances DSI in neonatal rats. In the early development of rat hippocampus, glutamatergic synaptic activity is low, and GABAergic transmission plays an excitatory role (Leinekugel et al. 1999). It is attractive to think that a low level of glutamate release early in development (Collard et al. 1993; Gasparini et al. 2000) is responsible for moderate DSI in neonatal rats. However, we cannot exclude the possibility that postnatal increases in mGluRs could account for developmental increases in DSI (Defagot et al. 2002).
Our findings, together with previous studies, indicate important roles for the activation of mGluRs in the enhancement of DSI in both immature and mature rat hippocampus (Ohno-Shosaku et al. 2002; Varma et al. 2001). However, DSI produced by postsynaptic depolarization alone is not dependent on mGluR activation in the mature animal (Ohno-Shosaku et al. 2002; Varma et al. 2001). We hypothesize that activation of metabotropic receptors is a necessary step for DSI early in development. This is likely due to the fact that endocannabinoid production is low during the early neonatal period, and thus a stimulus external to the postsynaptic neuron may be needed to enhance production at these time points. At later developmental time points, DSI is independent of mGluRs although still able to be enhanced by these receptors, most likely due to upregulation of the endocannabinoid production system. It is possible that mGluR activation is an important step in the transition from metabotropic receptor-dependent to metabotropic receptor-independent DSI, but this hypothesis has not yet been tested.
The observation that CB1 antagonism increases eIPSC amplitude in mature but not younger hippocampus is consistent with the idea that increased production and ongoing release of endocannabinoids occurs during postnatal development. Endocannabinoid release is generally thought to occur “on demand” (Stella and Piomelli 2001). Neuronal firing and a low level of ongoing glutamate release (Dumas and Foster 1995; Fuenzailida et al. 2009; Wasling et al. 2004) could contribute to the tonic endocannabinoid signaling in the present study. Several studies have indicated that ongoing endocannabinoid release occurs in brain slice preparations (Hentges et al. 2005; Yanovsky et al. 2003). Moreover, a recent study showing tonic endocannabinoid depression of GABA release at these synapses suggests that this modulation results from ongoing postsynaptic endocannabinoid production rather than inverse agonist drug effects (Neu et al. 2007). It should be noted that evidence of tonic endocannabinoid signaling has not always been observed in the hippocampal CA1 region (e.g., Wilson and Nicoll 2001). We do not know of any differences between our study and these other reports that could explain this discrepancy. However, not all hippocampal inhibitory synapses express CB1 receptors, and thus the ability to detect tonic endocannabinoid signaling may depend on what proportion of the sampled synapses are CB1 receptor-expressing. There are no data yet available that indicate if tonic regulation of GABAergic transmission occurs in vivo, and thus we do not know if the tonic effect only occurs in the slice preparation. Further investigation is needed to determine the significance of such ongoing endocannabinoid release.
The significance of endocannabinoid signaling in brain function is potentially far-reaching. Decreased GABAergic inhibition can enhance long-term potentiation (LTP) of glutamatergic transmission (Wigstrom and Gustafsson 1985), and a DSI-inducing protocol can facilitate the induction of LTP in the hippocampus (Carlson et al. 2002). One prominent physiological role for DSI may be to facilitate learning and memory involving the CA1 subregion of the hippocampus, as well as enhancing long-lasting changes in synaptic efficacy associated with these forms of learning and memory. In this regard, it is worth pointing out that the sustained low-frequency stimulation that enhances DSI is known to induce N-methyl-d-aspartate receptor-dependent LTD at excitatory Schaffer collateral/commissural synapses onto CA1 pyramidal neurons. It is also interesting to note the correspondence between the present findings and past studies examining postnatal development of LTP. In brain slices from the CA1 region of hippocampus, a robust LTP is observed only in rats >2 wk old (Bolshakov and Siegelbaum 1994; Harris and Teyler 1984), which is co-incident with a robust onset of DSI at this developmental time point. However, it is clear that LTP of smaller magnitude can be induced at earlier time points (Kramar and Lynch 2003). Thus developmental changes in DSI may represent a key step in the developmental progress of mechanisms involved in hippocampal-based learning and memory. Exogenous cannabinoids, like those contained in marijuana, might disturb learning and impair memory by disrupting normal feedback of information in the hippocampus especially during early development.
GRANTS
This work was supported by the National Insitute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Basic Research.
ACKNOWLEDGMENTS
Present address of P. J. Zhu: Dept. of Neuroscience, Rm. T734, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030.
REFERENCES
- Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science 264: 1148–1152, 1994 [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5: 723–724, 2002 [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38: 461–72, 2003 [DOI] [PubMed] [Google Scholar]
- Collard KJ, Edwards R, Liu Y. Changes in synaptosomal glutamate release during postnatal development in the rat hippocampus and cortex. Brain Res 71: 37–43, 1993 [DOI] [PubMed] [Google Scholar]
- Defagot MC, Villar MJ, Antonelli MC. Differential localization of metabotropic glutamate receptors during postnatal development. Dev Neurosci 24: 272–282, 2002 [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258: 1946–1949, 1992 [DOI] [PubMed] [Google Scholar]
- Dumas TC, Foster TC. Developmental increase in CA3-CA1 presynaptic function in the hippocampal slice. J Neurophysiol 73: 1821–1828, 1995 [DOI] [PubMed] [Google Scholar]
- Fuenzalida M, Aliaga E, Olivares V, Roncagliolo M, Bonansco C. Developmental increase of asynchronic glutamate release from hippocampal synapses in mutant taiep rat. Synapse 63: 502–509, 2009 [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc 93: 217–224, 1971 [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci USA 97: 9741–9746, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Developmental onset of long-term potentiation in area CA1 of the rat hippocampus. J Physiol 346: 27–48, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25: 9746–9751, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brain stem. J Physiol 534: 861–871, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10182–10191, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Lynch G. Developmental and regional differences in the consolidation of long-term potentiation. Neuroscience 118: 387–398, 2003 [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol 79: 189–201, 1999 [PubMed] [Google Scholar]
- Lenz RA, Wagner JJ, Alger BE. N- and L-type calcium channel involvement in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol 512: 61–73, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat 13: 219–241, 1997 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534, 2002 [DOI] [PubMed] [Google Scholar]
- Martin LA, Alger BE. Muscarinic facilitation of the occurrence of depolarization-induced suppression of inhibition in rat hippocampus. Neuroscience 92: 61–71, 1999 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990 [DOI] [PubMed] [Google Scholar]
- Morishita W, Alger BE. Sr2+ supports depolarization-induced suppression of inhibition and provides new evidence for a presynaptic expression mechanism in rat hippocampal slices. J Physiol 505: 307–317, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov YM, Freund TF. Post-natal development of type 1 cannabinoid receptor immunoreactivity in the rat hippocampus. Eur J Neurosci 18: 1213–1222, 2003 [DOI] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol 578: 233–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729–738, 2001 [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15: 953–961, 2002 [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci 12: 4122–4132, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol 425: 189–196, 2001 [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophysical Res Com 215: 89–97, 1995 [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem 7: 132–139, 2000 [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience 93: 969–975, 1999 [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasling P, Hanse E, Gustafsson B. Developmental changes in release properties of the CA3-CA1 glutamate synapse in rat hippocampus. J Neurophysiol 92: 2714–2724, 2004 [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta Physiol Scand 125: 159–172, 1985 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 31: 453–462, 2001 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature 410: 588–592, 2001 [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Mades S, Misgeld U. Retrograde signaling changes the venue of postsynaptic inhibition in rat substantia nigra. Neuroscience 122: 317–328, 2003 [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Persistent synaptic activity produces long-lasting enhancement of endocannabinoid modulation and alters long-term synaptic plasticity. J Neurophysiol 97: 4386–4389, 2007 [DOI] [PubMed] [Google Scholar]