Abstract
In hippocampus, synchronous activation of CA1 pyramidal neurons causes a rapid, extracellular, population alkaline transient (PAT). It has been suggested that the plasma membrane Ca2+-ATPase (PMCA) is the source of this alkalinization, because it exchanges cytosolic Ca2+ for external H+. Evidence supporting this hypothesis, however, has thus far been inconclusive. We addressed this long-standing problem by measuring surface alkaline transients (SATs) from voltage-clamped CA1 pyramidal neurons in juvenile mouse hippocampal slices, using concentric (high-speed, low-noise) pH microelectrodes placed against the somata. In saline containing benzolamide (a poorly permeant carbonic anhydrase blocker), a 2-s step from −60 to 0 mV caused a mean SAT of 0.02 unit pH. Addition of 5 mM HEPES to the artificial cerebrospinal fluid diminished the SAT by 91%. Nifedipine reduced the SAT by 53%. Removal of Ca2+ from the saline abolished the SAT, and addition of BAPTA to the patch pipette reduced it by 79%. The inclusion of carboxyeosin (a PMCA inhibitor) in the pipette abolished the SAT, whether it was induced by a depolarizing step, or by simulated, repetitive, antidromic firing. The peak amplitude of the “antidromic” SAT of a single cell averaged 11% of the PAT elicited by comparable real antidromic activation of the CA1 neuronal population. Caloxin 2A1, an extracellular PMCA peptide inhibitor, blocked both the SAT and PAT by 42%. These results provide the first direct evidence that the PMCA can explain the extracellular alkaline shift elicited by synchronous firing.
INTRODUCTION
During synchronous neural activity, a rapid rise in extracellular pH (pHe) occurs in the hippocampus, cerebellum, and some cortical regions. The hippocampal alkaline transient can attain magnitudes as large as 0.1–0.2 unit pH (Chesler 1990, 2003) and can be detected within tens of ms after a stimulus (Gottfried and Chesler 1996; Tong et al. 2006). The alkalosis was amplified by inhibitors of carbonic anhydrase, both in vivo (Kraig et al. 1983) and in vitro (Chen and Chesler 1992b; Walz 1989). This effect was caused by decreased extracellular buffering, because of the slower rate of CO2 hydration. Thus the alkaline response was attributed mainly to the rapid removal of protons from the extracellular space, and was termed a “proton sink” (Chesler 2003; Chesler and Kaila 1992).
The cause of the proton sink has not been determined. The amplification caused by carbonic anhydrase inhibitors distinguished it from other mechanisms of activity-dependent alkalinization. For example, an alkalosis can occur because of the efflux of HCO3− across GABAA channels (Kaila and Voipio 1987); however, such responses are inhibited by carbonic anhydrase blockers (Kaila et al. 1992). Similarly, a slower alkaline shift in the caudate nucleus that was correlated with increases in blood flow (and presumed washout of CO2) was also reduced by block of carbonic anhydrase (Venton et al. 2003).
It has been proposed that the proton sink arises from the neuronal plasma membrane Ca2+-ATPase (PMCA) (Schwiening et al. 1993), a ubiquitous transporter that exchanges internal Ca2+ for external H+ (Carafoli 1991; Carafoli and Stauffer 1994). To date, the evidence supporting this long-standing hypothesis has been inconclusive. For example, the alkaline response depends on the presence of external Ca2+ (Grichtchenko and Chesler 1996; Paalasmaa and Kaila 1996), but myriad processes can be influenced by Ca2+ influx. Additionally, the entry of Ca2+ is typically associated with cytosolic acidification (Irwin et al. 1994; Meech and Thomas 1977; Trapp et al. 1996; Wang et al. 1994), which is in keeping with the operation of the PMCA. However, Ca2+ entry can acidify cells by means other than a proton influx across the plasma membrane. Generation of metabolic acid, as well as Ca2+-H+ exchange across the membranes of the endoplasmic reticulum (ER) or mitochondria, could also cause an intracellular acidification (Chesler 2003). Pharmacologic dissection of this acidosis is not possible, because both the PMCA and these organellar transporters are blocked by the commonly used inhibitors such as orthovanadate and eosin (Kosterin et al. 1996; O'Neal et al. 1979; Watson et al. 2003). Thus the suppression of a Ca2+-dependent fall in intracellular pH by these agents (Trapp et al. 1996) cannot necessarily be attributed to block of the PMCA.
To avoid such ambiguity, a PMCA-related proton influx might be detected via the rise in pH at the extracellular surface of a single neuron. Proof of principle was first shown by Schwiening et al. (1993) on giant snail neurons and later in studies of large, isolated retinal neurons from the skate (Molina et al. 2004) and catfish (Kreitzer et al. 2007). Similar experiments have not been performed on single mammalian neurons. To relate such surface measurements to the extracellular proton sink generated by synchronous neural activity, these experiments would have to be performed in situ.
Previously, such efforts would have been hampered by reliance on conventional pH microelectrodes, which have high inherent noise and poor temporal resolution. Here, we address this issue in mouse hippocampal slices, using recently designed, high-speed, low-noise, concentric pH microelectrodes (Fedirko et al. 2006). By placing the pH electrode against the soma of a voltage-clamped CA1 pyramidal neuron, we show rapid surface alkaline shifts elicited by depolarization. This alkalinization was apparent within 200 ms, required entry of Ca2+, and was inhibited by intracellular and extracellular PMCA inhibitors. The surface response of a singly activated neuron amounted to 11% of the peak alkalosis attained by antidromic activation of the pyramidal neuron population. Moreover, caloxin, an extracellular peptide inhibitor of the PMCA, curtailed the surface and population responses to the same degree. These data provide strong evidence that the extracellular alkaline shift evoked by synchronous activity is generated by the neuronal PMCA. A preliminary report has been published (Makani and Chesler 2008).
METHODS
Preparation and solutions
BRAIN SLICE PREPARATION.
Transverse hippocampal slices were prepared from P6–P14 mice of either sex. All procedures were performed with the approval of the New York University Langone School of Medicine Institutional Animal Care and Use Committee. The brain was blocked in ice-cold artificial cerebrospinal fluid (ACSF) and cut into 250-μm slices using a Vibratome. The slices were incubated in standard ACSF at room temperature for ≥1 h before use. Experiments were conducted in a submersion-style incubation chamber at 32°C. The standard ACSF contained (in mM) 124 NaCl, 3.0 KCl, 1.0 CaCl2, 1.5 MgCl2, 26 NaHCO3, 1.0 NaH2PO4, and 10 d-glucose, with pH 7.4 (equilibrated with 95% O2-5% CO2). In some experiments, we used a modified ACSF that contained 1, 5, or 20 mM HEPES acid in addition to 26 mM HCO3−. The HEPES solutions were formulated using an appropriate addition of extra NaHCO3 to maintain a pH of 7.4 in 5% CO2 and therefore a final HCO3− concentration of 26 mM (Stewart 1978). To maintain a constant Na+ concentration, the NaCl content was reduced accordingly. In several experiments, a zero Ca2+ solution was made by omitting CaCl2 from the Ringer and adding 1 mM EGTA. Where Cd2+ was added, phosphate was removed from the saline to prevent precipitation. The poorly permeant carbonic anhydrase inhibitor benzolamide (10 μM) was included (unless otherwise noted) to decrease the effective extracellular buffering capacity, thereby keeping all extracellular pH changes relatively large and independent of possible differences in extracellular carbonic anhydrase activity (Chen and Chesler 1992b). To prevent HCO3− efflux through GABAA anion channels, which could cause a confounding extracellular alkalosis (Chen and Chesler 1992a; Kaila and Voipio 1987), picrotoxin was included in all experiments.
Drugs
Drugs were added to the external ACSF or to the intracellular pipette solution, as noted, in the following concentrations: d-2-amino-5-phosphonovalerate (APV; 50 μM), 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX; 10 μM) benzolamide (10 μM), picrotoxin (100 μM), lidocaine N-ethyl bromide (QX-314; 4 mM), cadmium (300 μM), nifedipine (25 μM), carboxyeosin (0.5 or 5 μM), and BAPTA (1 mM). Benzolamide was a gift from Dr. Erik Swenson (University of Washington, Seattle, WA). Caloxin 2A1 (VSNSNWPSFPSSGGG-NH2) was custom-synthesized by Bio Basic (Ontario, Canada). All other agents were obtained from Sigma-Aldrich (St. Louis, MO).
Concentric pH-sensitive microelectrodes
pH-sensitive microelectrodes with response times of a few milliseconds were fabricated as detailed by Fedirko et al. (2006). In brief, a thin-walled borosilicate glass capillary with an OD of 2.0 mm and an ID of 1.5 mm (A-M Systems 6185) was pulled to a tip size of 2–4 μm and silanized by injection of pure N,N-dimethyltrimethylsilylamine (Fluka 41720), followed by heating with a hot air gun. A pH-selective mixture (Fluka 95291) was introduced into the tip by suction. The inner micropipette was pulled from thin-walled glass (OD, 1.2 mm; ID, 0.9 mm; 6160; A-M Systems) to a tip diameter of ∼0.5 μm. This pipette was filled with phosphate-buffered 3 M KCl (pH 7.4) and inserted within the outer, ion-selective barrel and into the ion exchange column. With its tip 4– 6 μm from the end of the outer pipette, the inner pipette was secured in place with dental wax. The pH microelectrodes were calibrated in 50 mM K+/Na+ phosphate buffers of pH 6.87 and 7.42. The slope response for the concentric electrodes was 57–59 mV per decade change in H+ activity. The mean response time constant of similar concentric pH microelectrodes constructed in this laboratory was reported to be 15 ms (Fedirko et al. 2006).
Whole cell recording
The somata of CA1 pyramidal neurons were visualized under infrared differential interference contrast microscopy using a Zeiss Axioscop 2 Plus, fixed-stage microscope, fitted with a ×40, water immersion objective (0.75 numerical aperture), and an Olympus Optical 150 video camera. Patch pipettes were pulled from 1.5 mm OD × 1.12 mm ID borosilicate tubing (World Precision Instruments, Sarasota, FL) using a Narishige PP-830 two-stage puller (Japan). The intracellular filling solution contained the following (in mM): 120 K-gluconate, 20 KCl, 2.0 MgCl2, 25 Na-HEPES, and 2 Mg2+-ATP. In a few experiments, K-gluconate was replaced with Cs-gluconate and QX-314 was added to the filling solution. After adjusting the pH to 7.3 with KOH (or CsOH), the final osmolarity was 280–290 mOsm. Pipettes had resistances of 3–5 mΩ. In experiments in which carboxyeosin or BAPTA was added to the intracellular solution, pipette tips were first dipped in drug-free solution and then back-filled with the drug-containing solution. Data were acquired using an Axopatch 1D amplifier and Digidata board 1320A, controlled by Clampex 8.2, and analyzed using ClampFit (Molecular Devices, Union City, CA). Data were accepted if cells had a series resistance of <25 MΩ that did not change by >20% during the experiment.
Surface alkaline transients elicited by voltage-clamp steps
Before placement of the surface pH microelectrode, a gigohm seal was obtained on the targeted soma with a standard patch pipette. The concentric pH microelectrode was advanced until its tip made contact with the same cell body. Brief suction was applied to the patch pipette to break through into the whole cell configuration. Surface pH responses were elicited by depolarizing voltage-clamp steps from a holding potential of −60 mV. The depth of targeted cells ranged from the slice surface to ∼100 μm. The depth did not correlate with the size of surface pH responses. In these single cell experiments, the concentric pH electrode was used without an accompanying reference pipette. To determine the degree to which DC artifacts contaminated the surface pH records, separate experiments were performed in which the pH microelectrode was replaced by a similar micropipette filled with 2 M NaCl (see results). pH electrodes were fitted with Ag–AgCl junctions that fed a high-input impedance (>1013 Ω) head stage. Surface pH electrode records were elicited at 15-s intervals and filtered at 2 kHz.
Antidromic stimulation of the CA1 neuronal population
Constant current pulses of 300-μs duration were delivered to the alveus by a pair of 50-μm-diam, Teflon-insulated platinum-iridium wires in saline containing 10 μM DNQX, 50 μM APV, and 100 μM picrotoxin. These agents insured that activation did not occur via synaptic transmission. This was confirmed by monitoring of the short-latency, extracellular, antidromic population spike.
Simulated antidromic invasion
To determine whether a train of action potentials could evoke a surface pH shift, we devised a paradigm to simulate the voltage response of a neuron to repetitive antidromic activation. First, we recorded the whole cell current-clamp response of a CA1 pyramidal neuron to antidromic stimulation of the alveus at 100 Hz for 2 s (see above). This waveform served as the voltage command to evoke surface pH shifts on other cells.
Recording of population pHe responses
The concentric pH microelectrode and a separate reference micropipette were mounted on a dual micromanipulator, with a tip separation of 5–10 μm, as described by Fedirko et al. (2006). The array was advanced into the CA1 cell body layer until the antidromic population spike was maximal, which typically occurred at a depth of 100–150 μm. Capacitance neutralization was used to match the time constant of the reference and pH electrodes, as judged by the response to a 1-ms, 1-mV calibration pulse in the common ground circuit. Slow DC potentials recorded on the reference barrel were continuously subtracted from the pH recording to yield the H+ signal. Trains of stimuli were delivered every 2 min to obtain raw traces.
Data analysis and presentation
Statistics are presented as means with SE. Values of n refer to the number of neurons or slices studied, as indicated. Dual comparisons between mean values were made with a two-tailed, Student's paired t-test. Multiple comparisons were made using paired ANOVA with a Student-Newman-Keuls post hoc test. Three to five raw traces of all responses were averaged before data analysis. In all records, alkaline shifts were presented as upward deflections.
RESULTS
Surface measurements from CA1 pyramidal neurons
In all experiments, the holding potential was −60 mV. Initial studies were performed on 10 CA1 pyramidal neurons. With the pH electrode placed against the cell soma, its voltage response (VpH) to a 2-s depolarizing step to 0 mV was dominated by a rapidly rising negativity (Fig. 1, top row, left), with a peak amplitude of −1.08 ± 0.17 mV (equivalent to an alkalosis of 0.019 ± 0.003 unit pH). Termination of the voltage-clamp step was followed by a roughly exponential decline of VpH toward baseline. With inclusion of APV and DNQX in the saline (to block N-methyl-d-aspartate (NMDA) and AMPA receptors, respectively), plus addition of Cs+ and QX-314 to the patch solution (to respectively inhibit K+ and Na+ channels), similar surface responses could be elicited (n = 4 cells, data not shown).
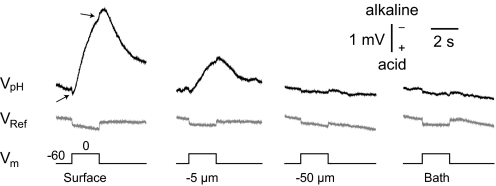
Fig. 1.
Distance profile of traces from pH-sensitive vs. saline-filled microelectrodes. Top: surface pH electrode responses to a depolarizing voltage step from −60 to 0 mV, at various distances from the cell body of a pyramidal neuron. Arrows indicate transient voltage artifacts. Middle: shows the respective profile obtained when a 2 M NaCl-filled reference pipette replaced the pH microelectrode. Note that the small, downward, rectangular deflection was common to both electrodes and present at all distances. Alkaline responses are indicated by upward deflections in this and all subsequent figures. All solutions contained benzolamide unless noted otherwise.
Brain slice experiments that use ion-sensitive electrodes typically make use of a second reference barrel, to enable subtraction of common DC voltages. Such potentials can arise from real physiological responses across the extracellular space (Somjen 1973), or from simple voltage drops across the bath and ground pathway during current passage. In this study, which used concentric ion-selective microelectrodes on the cell surface without an accompanying reference pipette, it was therefore essential to first determine whether such slow potentials significantly contaminated VpH.
At the start and end of the voltage-clamp pulse, small, brief, positive and negative deflections of VpH were consistently noted (Fig. 1, top row, arrows), suggestive of a minor DC component. To examine this further, we withdrew the pH microelectrode from the somata in steps. The large, negative component of VpH was reduced by about one half at 5 μm from the cell membrane and disappeared altogether by 10 μm. However, a small, positive, rectangular DC deflection appeared, and remained evident, even as the electrode was withdrawn from the slice into the overlying saline, millimeters away from the voltage-clamped cell (Fig. 1, top row, right). This indicated that the DC deflections represented a voltage drop across the current path from bath to ground. To further confirm this, we recorded surface potentials by replacing the pH electrode with a 2 M NaCl-filled reference micropipette (VRef). Here, the voltage step to 0 mV produced similar, small (90 ± 50 μV), positive, rectangular, DC shifts (n = 6 cells) at the cell surface, which remained unchanged during withdrawal of the micropipette through the slice, and into the bath (Fig. 1, middle row). We were therefore confident that the on-off transients on the raw VpH records were caused by the same phenomenon and unrelated to changes in pH.
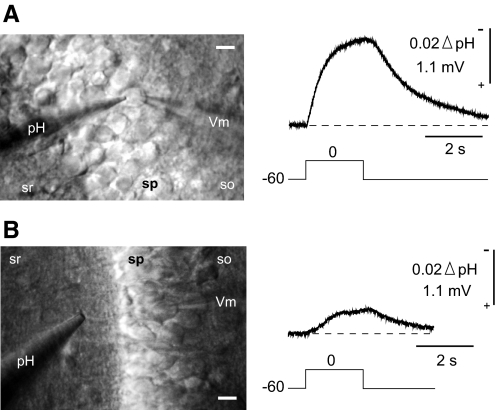
Because these DC artifacts were <10% of the VpH responses, rectangular in waveform, and consistent throughout a given experiment, we removed the transients from the VpH traces. This was done by simple upward displacement of the records during the voltage-clamp pulse. With the exception of Fig. 1, the displayed surface records were all corrected in this manner. A corrected recording is shown in Fig. 2A along with a photograph showing the placement of the pH and patch electrodes on a soma.
Fig. 2.
Surface pH electrode responses observed at soma and dendrite. The pH microelectrode was placed either opposite the patch pipette on the soma (A) or on the proximal dendrite (B). Recordings and photographs in A and B are from different slices. sr, s. radiatum; sp, s. pyramidale; so, s. oriens; Vm, patch pipette. The calibration bars represent 10 μm.
Somatic depolarization also evoked surface VpH responses from the proximal dendrite, as shown in Fig. 2B. This response, however, was smaller (−0.30 to −0.50 mV) and less reliably obtained, because it was difficult to visually match a given soma to the correct proximal dendrite. We therefore concentrated our efforts on the somatic responses.
Effect of altering extracellular buffering capacity
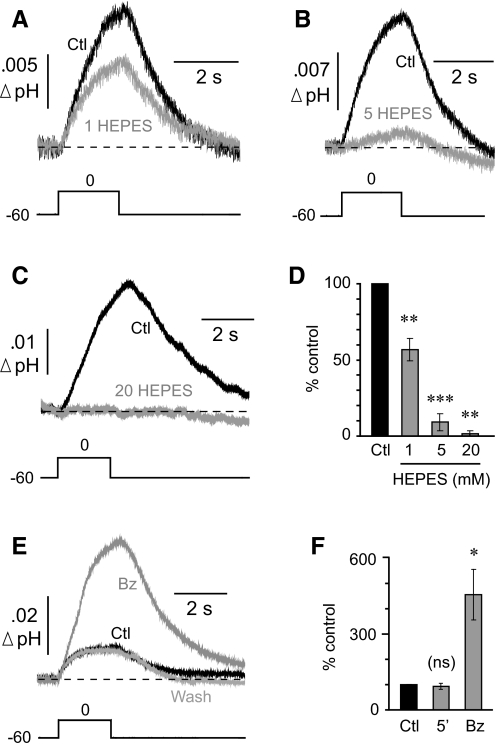
To confirm that the response of the surface pH electrodes represented a real change in pH, we manipulated the extracellular buffering capacity. Buffering was increased by the addition of HEPES to the standard bicarbonate-buffered ACSF (which contained benzolamide). In control ACSF, a voltage step to 0 mV caused a mean peak surface shift of −0.74 ± 0.16 mV that was reduced by 43 ± 7.3% after the addition of 1 mM HEPES (n = 5 cells; P < 0.01), as shown in Fig. 3A. With addition of 5 mM HEPES, the control responses (−0.65 ± 0.08 mV) were reduced by 90.9 ± 5.7% (n = 5 cells; P < 0.001; Fig. 3B). Finally, with addition of 20 mM HEPES, the control response (−0.64 ± 0.12 mV) was reduced by 98.8 ± 1.2% (n = 7 cells; P < 0.01; Fig. 3C). The effects of the HEPES solutions are summarized in Fig. 3D.
Fig. 3.
pH electrode response is a surface alkaline transient (SAT). A: addition of 1 mM HEPES to the bicarbonate-buffered media significantly reduced the SAT. B: addition of 5 mM HEPES suppressed most of the SAT. C: addition of 20 mM HEPES completely eliminated the SAT. D: summary of HEPES data. All statistical comparisons were paired to respective controls. E: comparison of SAT before (Ctl) and after superfusion of 10 μM benzolamide (Bz). F: mean effect of a 5-min delay or application of Bz compared against respective controls.
Effective buffering capacity was decreased by the addition of benzolamide to the ACSF (Tong et al. 2006). These were the only experiments in which the control solution did not contain the carbonic anhydrase inhibitor. In the control ACSF, a step depolarization to 0 mV caused a peak surface shift of −0.53 ± 0.11 mV (n = 5 cells), which corresponds to a pH shift of roughly 0.01. The addition of benzolamide reversibly increased the peak response by 356 ± 100% (range, 137–703%; P < 0.05; Fig. 3E). The effect of benzolamide is summarized in Fig. 3F.
The changes noted with manipulation of buffering power were not caused by run-down or run-up of the responses, because simple delays of 5 min, corresponding to wash in time, had no significant effect on the amplitude of the surface alkalinizations (n = 6 cells; P = 0.56; Fig. 3F). Thus these data collectively indicated that the pH electrode responses were indeed surface alkaline transients (SATs).
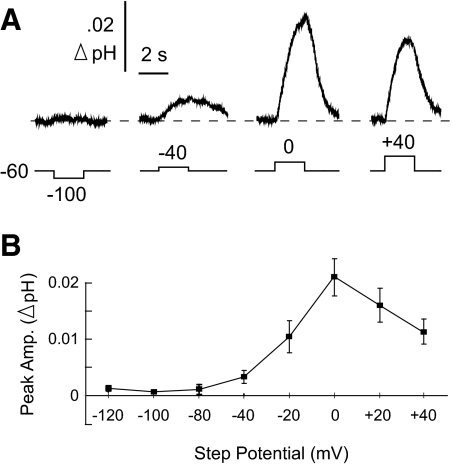
Effect of membrane potential on the SAT
The peak magnitude of the SAT was studied over a range of voltage-clamp steps from −120 to +40 mV, in 20-mV increments. Representative traces are shown in Fig. 4A. Hyperpolarizing steps produced no observed surface pH change (Fig. 4, A and B). The SAT was greatest at 0 mV and declined for steps to +20 and +40 mV (Fig. 4B; n = 10 cells; repeated-measures ANOVA with Student-Newman-Keuls post hoc test). These data are consistent with the SAT being caused by a transporter. If it was mediated instead by a conductive pathway, one would predict the alkalosis to reverse into an acidosis at voltages more positive to the equilibrium potential for H+ (EH+) (Chesler 1990; Roos and Boron 1980). Assuming a pHe of 7.2 in the mouse hippocampal slice (Tong et al. 2000), and a pHi of 7.3, EH+ would have been approximately +6 mV. Because the SAT was maximal at 0 mV, and remained alkaline at +40 mV, the response could not have arisen because of a flux of H+, or one of its acid-base equivalents, through a channel.
Fig. 4.
Surface alkaline transient varied with voltage. A: selected SATs from 1 cell at various step potentials. B: mean SAT peak amplitudes in response to step potentials ranging from −120 to +40 mV. Mean response at 0 mV was significantly greater than that at all other potentials (repeated-measures ANOVA with Student-Newman-Keuls post hoc test). Note the surface alkalinization never reversed into an acid shift.
Dependence of the SAT on extracellular Ca2+
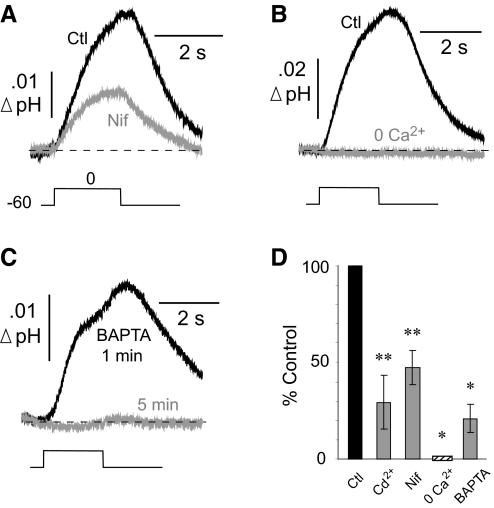
The PMCA is activated after entry of Ca2+. With elevation of cytosolic Ca2+, calmodulin binds to the PMCA, relieving the suppressive effect of an autoinhibitory domain located on the C terminus of the transporter (Falchetto et al. 1991, 1992; James et al. 1988). If the SAT of hippocampal neurons was caused by the PMCA, one would expect a diminished response if the influx of Ca2+ was inhibited. In experiments on six neurons, the addition of 300 μM Cd2+ to the extracellular fluid reduced the control SAT (0.014 ± 0.002 unit pH) by 71 ± 14.0% (P < 0.01; data not shown). Because Cd2+ can independently inhibit the PMCA (Akerman et al. 1985; Verbost et al. 1988), we also tested nifedipine, a more specific blocker of high-threshold L-type Ca2+ channels. Nifedipine (25 μM) reduced the control SAT (0.019 ± 0.004 unit pH) by 53 ± 9.0% (n = 6 cells; P < 0.01; Fig. 5A). In saline with zero added Ca2+ (plus 1 mM EGTA), the control SAT (0.032 ± 0.010 unit pH) was completely abolished (n = 4 cells; P < 0.05; Fig. 5B). Thus generation of the SAT required the entry of Ca2+ ions.
Fig. 5.
Surface alkaline transient requires entry of Ca2+. A: comparison of superimposed SATs before and after the addition of nifedipine (25 μM). B: comparison of SATs in control solution (Ctl) and after Ca2+ was removed from the artificial cerebrospinal fluid (ACSF). C: BAPTA (1 mM) included in the patch pipette greatly reduced the SATs. The SAT responses were compared at 1 and 5 min after breakthrough into whole cell mode. D: mean effects of manipulating the entry of Ca2+ or its cytosolic rise. All statistical comparisons were paired against respective controls.
To suppress a rise in intracellular Ca2+, we included the Ca2+ chelator BAPTA (1 mM) in the patch pipette and recorded surface responses 1 and 5 min after breakthrough into whole cell mode. At 1 min, the SAT measured 0.009 ± 0.002 unit pH and was reduced by 79 ± 7.2% after 5 min (n = 5 cells; P < 0.05). An experiment in which BAPTA abolished the SAT is shown in Fig. 5C. These data indicated that the generation of the SAT was dependent on a rise in cytosolic Ca2+. The effects on the SAT caused by inhibition of Ca2+ entry, or suppression of the cytosolic Ca2+ rise, are summarized in Fig. 5D.
Effect of PMCA inhibitors on the SAT
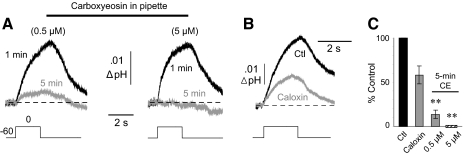
Carboxyeosin (CE) is a relatively potent blocker of the PMCA that acts on the cytoplasmic side of the transporter, with a reported KI of 20 nM (Gatto and Milanick 1993). Because of the charged nature and poor membrane permeability of this compound, we included it in the patch pipette to determine the contribution of the PMCA to the SAT. Although eosin derivatives are known to also block Ca2+/H+ exchange across the ER and mitochondria (Kosterin et al. 1996; O'Neal et al. 1979; Watson et al. 2003), inhibition of these mechanisms would not decrease a surface alkalosis. With 0.5 μM CE in the filling solution, the mean SAT at 1 min measured 0.007 ± 0.001 unit pH and was reduced by 86 ± 4.8% after 5 min (n = 8 cells; P < 0.01; Fig. 6A, left). With 5 μM CE in the pipette, the SAT measured 0.010 ± 0.003 unit pH at 1 min and was completely abolished after 5 min (n = 10 cells; P < 0.01; Fig. 6A, right). After 5 min of dialyzing the cells with 5 μM CE, neither the input resistance nor the holding current was significantly changed from their values at 1 min. Thus the suppression of the SAT was not caused by a nonspecific loss of cell viability.
Fig. 6.
Surface alkaline transient is blocked by plasma membrane Ca2+-ATPase (PMCA) inhibitors. A: carboxyeosin (CE) was included in the patch pipette solution at a concentration of either 0.5 (left) or 5 μM (right). SATs were measured at 1 and 5 min after breakthrough into whole cell mode. B: comparison of SATs in control solution (Ctl) and after the addition of caloxin (2 mM). C: mean effects of PMCA inhibitors on the SAT. Comparisons were paired against respective controls.
Although CE in the patch pipette was appropriate for blocking the SAT, we sought a means of inhibition via an extracellular approach, because this would be necessary for a comparison against the population response. The only available pharmacologic tools that block the PMCA at an extracellular locus are the caloxin peptides. We tested caloxin 2A1, with a reported KI of 0.4 mM (Chaudhary et al. 2001). In experiments on six pyramidal neurons, superfusion of 2 mM caloxin reduced the control SAT (0.017 ± 0.003 unit pH) by 42 ± 10.2% (P < 0.05; Fig. 6B). The addition of caloxin had no effect on the input resistance or holding current in these experiments; thus it was unlikely that a loss of cell viability was responsible for the reduction in the SAT. Effects of intracellular CE, and extracellular caloxin on the SAT are summarized in Fig. 6C.
Simulated antidromic invasion evokes an SAT
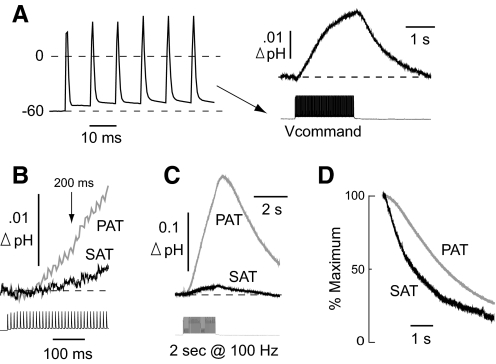
To determine whether the transient depolarizations associated with a repetitive spike train could also elicit an SAT, a series of experiments was carried out to simulate antidromic firing. Here, we used the whole cell current-clamp response to a 100-Hz, 2-s antidromic stimulus train (previously obtained from a different cell; Fig. 7A, left) as the voltage-clamp command. This protocol had advantages over the injection of constant current because it avoided irregularity in responses (e.g., caused by accommodation) and thus insured a standardized sequence of “spikes” for all cells studied. It also allowed for a meaningful comparison with a PAT elicited by real antidromic stimulation. The simulated antidromic train elicited an SAT with a peak amplitude of 0.017 ± 0.004 unit pH (n = 12 cells; Fig. 7A, right), similar to the surface responses evoked by a 2-s step depolarization to 0 mV.
Fig. 7.
Comparison of responses evoked by simulated vs. real antidromic activation. A: the whole cell current-clamp response (left) to 100-Hz (2 s) stimulation of CA1 axons was used as the voltage command to generate SATs in later experiments (right). B: overlay of initial rise of the SAT and population alkaline transient (PAT). C: overlay of an SAT generated by antidromic simulation with a PAT generated by antidromic stimulation in a different slice. Both traces were elicited by a 100-Hz, 2-s train. D: example of the normalized decays of an SAT vs. a PAT.
Simulated antidromic SAT versus antidromic population response
To compare the previously obtained SATs evoked by simulated antidromic invasion of one neuron against the pHe response from the CA1 neuronal population, we stimulated the alveus at 100 Hz for 2 s and recorded the resulting population alkaline transient (PAT) in stratum pyramidale (n = 8 slices). Thus in these PAT experiments, the stimulus duration and frequency, as well as the placement of the pH electrode, were identical to those in the SAT experiments. The only major difference between the two paradigms was the method by which neurons were activated (simulated vs. real antidromic invasion). Because accurately measuring PATs requires that pH and reference electrodes be used simultaneously, these experiments were conducted in different slices than those in which SATs were recorded.
The SAT previously elicited by a 100-Hz, 2-s train was evident at 200 ms after the start of the stimulus, at which time it measured 0.0012 ± 0.0005 unit pH. This value was 12% of the comparable magnitude of the PAT at 200 ms, which measured 0.010 ± 0.002 unit pH (Fig. 7B). These relative measures were similar at the peak of the two responses. The mean peak amplitude of the SAT had measured 0.017 unit pH, which was 11% of the mean peak amplitude of the PAT (0.155 ± 0.030 unit pH; Fig. 7C). Like the SAT, the PAT began to decline almost immediately after termination of the stimulus train; however, its decay was slower. The relaxation of the SAT had a half-time of 1,318 ± 179 ms, whereas the PAT decayed with a half-time of 2,715 ± 267 ms (Fig. 7D).
Effect of PMCA inhibitors on the simulated antidromic SAT and antidromic PAT
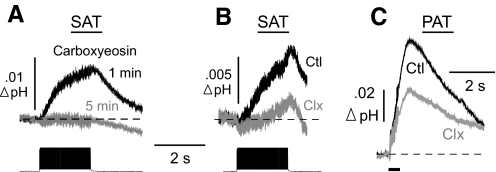
The SAT evoked by simulated antidromic firing was suppressed by inhibitors of the PMCA. With 5 μM CE in the patch pipette, the “antidromic” SAT was virtually abolished. The control response averaged 0.014 ± 0.005 unit pH at 1 min and was reduced by 99 ± 1% after 5 min of dialysis with CE (n = 5 cells; P < 0.05; Fig. 8A). A control SAT of 0.010 ± 0.002 unit pH was reduced by 43 ± 12% after addition of 2 mM caloxin (n = 5 cells; P < 0.05; Fig. 8B).
Fig. 8.
Effect of PMCA blockers on responses evoked by simulated vs. real antidromic activation. A: carboxyeosin (5 μM) was included in the patch pipette solution, and SATs were generated by antidromic simulation at 1 and 5 min after breakthrough into whole cell mode. B: comparison of a control SAT evoked by antidromic simulation and after the addition of caloxin (Clx; 2 mM) to the ACSF. C: PATs were evoked by a 20-pulse, 50-Hz real antidromic stimulus train. Overlay of control PAT (Ctl) and after the superfusion of caloxin (Clx) is shown.
To evoke a population alkaline response, CA1 axons were activated antidromically (with a 20-pulse, 50-Hz stimulus train), and pHe was recorded in stratum pyramidale, as above. The control PAT (0.068 ± 0.007 unit pH) was inhibited by 42 ± 4.7% after superfusion of 2 mM caloxin (n = 5 slices; P < 0.001; Fig. 8C). Thus the effects of caloxin on the PAT and SAT were roughly identical. Caloxin had no effect on the extracellular population spike, and in current-clamp recordings of antidromic invasion from two cells, neither the spike height nor the number of spikes was reduced (data not shown). Therefore the suppression of the PAT by caloxin could not be attributed to a reduction in excitability.
DISCUSSION
A major result of this study was the demonstration of an alkalosis at the surface of singly activated CA1 pyramidal neurons, elicited by both depolarizing voltage-clamp steps and simulated, repetitive firing. Our data indicated that the PMCA was the sole generator of this SAT and was most likely the underlying mechanism of the extracellular alkaline shift evoked by synchronous activation of this neuronal population. It has been previously shown that the PAT requires entry of extracellular Ca2+ (Grichtchenko and Chesler 1996; Paalasmaa and Kaila 1996). Similarly, the SAT required the presence of external Ca2+, as well as its entry and elevation in the cytosol, and the reduction of the SAT at positive holding potentials was consistent with a lower driving force for Ca2+ entry. In addition, the fractional magnitude of the response from a singly activated neuron relative to that of the population response was striking and provided additional evidence that PMCA-mediated surface responses were the likely basis for the PAT.
Inhibition studies further supported the PMCA hypothesis. These experiments were necessarily limited to pharmacological approaches. Because there are four principal isoforms of the PMCA, with numerous splice variants (Strehler and Zacharias 2001), knockdown or knockout strategies could not be readily implemented. Indeed, isoforms 2, 3, and 4 are all expressed on hippocampal pyramidal neurons (Kip et al. 2006). Available pharmacological tools to block the PMCA have certain drawbacks that warrant discussion, however.
In addition to blocking the PMCA, CE also inhibits organellar Ca2+-ATPases (Kosterin et al. 1996; O'Neal et al. 1979; Watson et al. 2003). Activity of these transporters would normally acidify the cytosol. The PMCA, however, is the only known mechanism that would be expected to generate a surface alkalosis that was reduced by internal CE. Thus the activity and inhibition of the PMCA was the most obvious and parsimonious explanation for the surface pH data.
Similarly, the reduction of the SAT by the bath application of the peptide caloxin cannot be explained by an intracellular action. Although the KI of caloxin is relatively high (0.4 mM), it is currently the most effective extracellular PMCA blocker. Importantly, caloxin reduced the SAT and PAT to a similar degree (42%). This partial block was expected, given that application of 2 mM caloxin did not completely inhibit the PMCA response in the peptide's original description (Chaudhary et al. 2001). To avoid potential nonspecific effects, we did not add higher concentrations of this agent. Because caloxin did not affect antidromic spike invasion, field potentials, or input resistance, its effect on the PAT is best explained by inhibition of the PMCA.
In giant snail neurons, Schwiening et al. (1993) reported a rise of surface pH attributable to the PMCA, and first suggested that a similar response might be the basis for the PATs that had been reported in certain regions of mammalian brain. This attractive hypothesis was supported by reports of the Ca2+ dependence of the PAT (Grichtchenko and Chesler 1996; Paalasmaa and Kaila 1996). However, unlike the PAT in hippocampal slices, which was evident within tens of milliseconds (Tong et al. 2006), the SAT of snail neurons was barely detectable after 2 s of depolarization (see Fig. 4 of Schwiening et al. 1993). The onset kinetics of SATs were also obscured in later studies of skate (Molina et al. 2004) and catfish (Kreitzer et al. 2007) horizontal cells. Thus to address the relationship between a neuronal SAT and the PAT of mammalian brain slices, improved temporal resolution was required.
In this study, use of concentric pH microelectrodes enabled detection of an SAT on single hippocampal pyramidal neurons in situ and allowed for a comparison with the antidromic PAT as early as 200 ms after onset of stimulation. Using a 2-s, 100-Hz, simulated, antidromic stimulus, the average surface response of a single pyramidal cell amounted to one-ninth the response elicited when the neuronal population was similarly activated. Under normal buffering conditions (i.e., in the absence of benzolamide), the SAT was also a sizable fraction of a PAT. Although the SAT averaged just 0.01 unit pH under these conditions, the comparable antidromic PAT in the absence of benzolamide is typically in the range of 0.05–0.10 unit pH (Chen and Chesler 1992a; Grichtchenko and Chesler 1996; Shah et al. 2005).
The principal issue raised by these results is whether the PMCA responses from single neurons can combine to produce a larger PAT. It can be intuitively argued that the greater the number of cells activated, the greater will be the amount of net acid removed from the local extracellular volume. Because H+ influx via the PMCA generates extracellular base instantly from water, it may equivalently be called a base source. With activation of a single neuron, the base source would become highly diluted and diminished within the gross extracellular space, as was evident in the experiment of Fig. 1, where the pH electrode was gradually withdrawn from the cell surface. In contrast, with activation of the population, more total base would be added, while diffusion away from the surface of each cell would be curtailed (because of a less steep concentration gradient). Accordingly, the essential differences between an SAT and a PAT would arise because of the contrasting effects of diffusion when activating single versus multiple point sources.
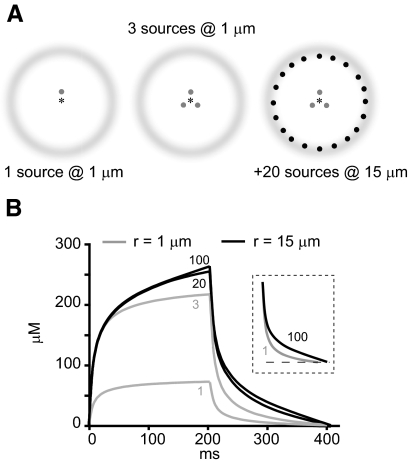
This concept can be conveyed using a highly simplified model of concentration changes at one locus, in the context of single or multiple point sources. Here we consider one versus three sources located 1 μm from a point of measurement (Fig. 9A, left 2 panels, respectively) and then add an additional 20 or more sources located 15 μm distant (Fig. 9A, right). For an arbitrary diffusible ion generated by a source Q of fixed amplitude (in mol/s), the concentration (C) at time t and distance r is given by
where Q is the source in moles per second, D is the diffusion coefficient in square centimeters per second, and erfc is the complementary error function (Nicholson and Phillips 1981). The concentration rise at 1 μm from a source turned on for 204 ms is shown by the gray bottom trace in Fig. 9B. If three such sources are placed 1 μm from the point of measurement, the responses sum, producing a faster rate of rise and a threefold larger peak. With an additional 20 sources placed 15 μm from the point of measurement, the response again grows in magnitude, but only modestly. Addition of a total of 100 sources at 15 μm causes little further increase in the magnitude.
Fig. 9.
Concentration changes for a monovalent ion originating from single vs. multiple point sources. A: left: single point source with point of measurement (*) at 1 μm. Middle: 3 point sources at 1 μm. Right: addition of another 20 point sources at 15 μm. B: concentration changes for ion resulting from 1 point source at 1 μm (bottom gray), 3 point sources at 1 μm (top gray), additional 20 sources at 15 mm (bottom black), or an additional 100 sources at 15 μm. Numerals under traces denote number of sources. Inset: normalized decay of 2 traces indicated by corresponding numerals. Curves were calculated using D = 10−6·cm2·s−1 and a step point current source of 10 μA turned on for 204 ms.
The small effect of the additional sources can be attributed to their distance from the point of measurement. The latency of diffusion limits their contribution at early times, and their early termination occurs before their effect can fully manifest. Another property conferred by the activation of multiple point sources is the slowing of the decay, as shown in the normalized recoveries in the inset of Fig. 9B. This is attributable to a less steep concentration gradient than would occur with activation of a single point source.
The generation and spread of an alkaline shift is a more complex phenomenon. It is recorded as a pH (rather than concentration) change, which is generated by base sources distributed over the complex surface area of cells. In addition, it manifests by a process of reaction-diffusion involving mobile buffer and may be regulated by glial cells (Chesler 1990; Ransom 1992; Rose and Deitmer 1994). It would nonetheless be subject to the same principles of diffusion that pertain in a simpler system. Accordingly, the response would be expected to grow in magnitude as more local sources were activated, whereas more distant sources would contribute less to the peak but would slow the recovery phase. These concepts are consistent with both the larger amplitude of the PAT and its slower recovery compared with the SAT (Fig. 7).
The rapid PAT observed in hippocampal slices also occurs in the hippocampus in vivo (Somjen 1984; Xiong and Stringer 2000), and a similar alkalosis that was amplified by a carbonic anhydrase inhibitor (acetazolamide) was first reported in rat cerebellum (Kraig et al. 1983). However, it should be pointed out that activity-dependent alkaline responses do not all arise via this proton sink mechanism. In retina, stimulation by light caused an alkaline shift because of a fall in ongoing production of metabolic acid (Yamamoto et al. 1992). In the caudate nucleus, an activity-dependent alkalosis diminished by acetazolamide was correlated with hyperemia, consistent with increased washout of CO2 (Venton et al. 2003). In addition, a rise in pHe is not the universal response to neural activity throughout the CNS. In rat parietal cortex, synchronous activity caused a slowly developing acidosis (Chesler and Kraig 1989), and the response in adult spinal cord was dominated by a rapidly rising acid shift (Sykova and Svoboda 1990). These differences are likely caused by any number of region-specific factors, such as heterogeneity in the expression of the PMCA, voltage-gated Ca2+ channels, and various acid-base transporters on neurons and glia (Chesler 2003; Deitmer and Rose 1996).
The functional significance of the hippocampal PAT was highlighted in a recent study (Makani and Chesler 2007), in which augmentation of extracellular buffering was found to curtail postsynaptic responses mediated by NMDA receptors, which are inhibited by external protons in the physiological pH range (Tang et al. 1990; Traynelis and Cull-Candy 1990; Vyklicky et al. 1990). Similarly, an earlier report noted that epileptiform discharges of hippocampal slices were suppressed by addition of HEPES to the bathing fluid (Taira et al. 1993). These results suggest that, after synchronous excitatory synaptic activity, alkaline shifts in the microdomain of the postsynaptic membrane can rapidly attain a magnitude sufficient to relieve the proton block of NMDA receptors. In view of the NMDA receptor titration curve (Tang et al. 1990; Traynelis and Cull-Candy 1990; Vyklicky et al. 1990), these studies suggested that the magnitude of the alkaline shift at the subsynaptic membrane was considerably larger then the pH shifts recorded from the larger volume of the extracellular space by microelectrodes.
This inferred effect of the PMCA on subsynaptic extracellular pH is consistent with reports of PMCA localization. Although this transporter is ubiquitous, particularly dense staining for the PMCA was associated with the postsynaptic densities of dendritic spines in cerebellar Purkinje neurons (Hillman et al. 1996). In addition, the PMCA2b splice variant was found to colocalize with synapse associated protein 90/PSD95 at individual dendritic spines of CA1 pyramidal neurons (DeMarco and Strehler 2001). These postsynaptic proteins have been reported to also interact with NMDA receptors (Niethammer et al. 1996), suggesting that PMCA and NMDA receptors are in close proximity. Accordingly, the PMCA may be able to generate large alkaline shifts in the immediate extracellular microdomain of NMDA receptors.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-032123 to M. Chesler and National Research Service Award F31 NS-058152 to S. Makani and the Attilio and Olympia Ricciardi Fund.
ACKNOWLEDGMENTS
We thank Drs. Charles Nicholson and Margaret Rice for useful discussions about diffusion in the brain extracellular space.
REFERENCES
- Akerman KE, Honkaniemi J, Scott IG, Andersson LC. Interaction of Cd2+ with the calmodulin-activated (Ca2+ + Mg2+)-ATPase activity of human erythrocyte ghosts. Biochim Biophys Acta 845: 48–53, 1985 [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev 71: 129–153, 1991 [DOI] [PubMed] [Google Scholar]
- Carafoli E, Stauffer T. The plasma membrane calcium pump: functional domains, regulation of the activity, and tissue specificity of isoform expression. J Neurobiol 25: 312–324, 1994 [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Walia M, Matharu J, Escher E, Grover AK. Caloxin: a novel plasma membrane Ca2+ pump inhibitor. Am J Physiol Cell Physiol 280: C1027–C1030, 2001 [DOI] [PubMed] [Google Scholar]
- Chen JC, Chesler M. Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol 67: 29–36, 1992a [DOI] [PubMed] [Google Scholar]
- Chen JC, Chesler M. pH transients evoked by excitatory synaptic transmission are increased by inhibition of extracellular carbonic anhydrase. Proc Natl Acad Sci USA 89: 7786–7790, 1992b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol 34: 401–427, 1990 [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci 15: 396–402, 1992 [DOI] [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH transients of mammalian astrocytes. J Neurosci 9: 2011–2019, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Rose CR. pH regulation and proton signaling by glial cells. Prog Neurobiol 48: 73–103, 1996 [DOI] [PubMed] [Google Scholar]
- DeMarco SJ, Strehler EE. Plasma membrane Ca2+-atpase isoforms 2b and 4b interact promiscuously and selectively with members of the membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1) domain-containing proteins. J Biol Chem 276: 21594–21600, 2001 [DOI] [PubMed] [Google Scholar]
- Falchetto R, Vorherr T, Brunner J, Carafoli E. The plasma membrane Ca2+ pump contains a site that interacts with its calmodulin-binding domain. J Biol Chem 266: 2930–2936, 1991 [PubMed] [Google Scholar]
- Falchetto R, Vorherr T, Carafoli E. The calmodulin-binding site of the plasma membrane Ca2+ pump interacts with the transduction domain of the enzyme. Protein Sci 1: 1613–1621, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirko N, Svichar N, Chesler M. Fabrication and use of high-speed, concentric H+- and Ca2+-selective microelectrodes suitable for in vitro extracellular recording. J Neurophysiol 96: 919, 2006 [DOI] [PubMed] [Google Scholar]
- Gatto C, Milanick MA. Inhibition of the red blood cell calcium pump by eosin and other fluorescein analogues. Am J Physiol 264: C1577–C1586, 1993 [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Chesler M. Temporal resolution of activity-dependent pH shifts in rat hippocampal slices. J Neurophysiol 76: 2804–2807, 1996 [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Calcium- and barium-dependent extracellular alkaline shifts evoked by electrical activity in rat hippocampal slices. Neuroscience 75: 1117–1126, 1996 [DOI] [PubMed] [Google Scholar]
- Hillman DE, Chen S, Bing R, Penniston JT, Llinas R. Ultrastructural localization of the plasmalemmal calcium pump in cerebellar neurons. Neuroscience 72: 315–324, 1996 [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Long RT, Paul SM. N-methyl-D-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci 14: 1352–1357, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Maeda M, Fischer R, Verma AK, Krebs J, Penniston JT, Carafoli E. Identification and primary structure of a calmodulin binding domain of the Ca2+ pump of human erythrocytes. J Biol Chem 263: 2905–2910, 1988 [PubMed] [Google Scholar]
- Kaila K, Paalasmaa P, Taira T, Voipio J. pH transients due to monosynaptic activation of GABA-A receptors in rat hippocampal slices. Neuroreport 3: 105–108, 1992 [DOI] [PubMed] [Google Scholar]
- Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330: 163–165, 1987 [DOI] [PubMed] [Google Scholar]
- Kip SN, Gray NW, Burette A, Canbay A, Weinberg RJ, Strehler EE. Changes in the expression of plasma membrane calcium extrusion systems during the maturation of hippocampal neurons. Hippocampus 16: 20–34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterin SA, Bratkova NF, Babich LG, Shinlova OP, Slinchenko NN, Shlykov SG, Zimina BP, Rovenets NA, Velkich TA. Effect of inhibitors of energy-dependent Ca2+ -transporting systems on calcium pumps of a smooth muscle cell. Ukr Biokhim Zh 68: 50–61, 1996 [PubMed] [Google Scholar]
- Kraig RP, Ferreira-Filho CR, Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol 49: 831–850, 1983 [DOI] [PubMed] [Google Scholar]
- Kreitzer MA, Collis LP, Molina AJ, Smith PJ, Malchow RP. Modulation of extracellular proton fluxes from retinal horizontal cells of the catfish by depolarization and glutamate. J Gen Physiol 130: 169–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Chesler M. Endogenous alkaline transients boost postsynaptic NMDA receptor responses in hippocampal CA1 pyramidal neurons. J Neurosci 27: 7438–7446, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani S, Chesler M. A Ca2+ -ATPase-mediated alkaline transient on the surface of individual CA1 neurons in mouse hippocampal slices. Soc Neurosci 2008 [Google Scholar]
- Meech RW, Thomas RC. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol 265: 867–879, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina AJ, Verzi MP, Birnbaum AD, Yamoah EN, Hammar K, Smith PJ, Malchow RP. Neurotransmitter modulation of extracellular H+ fluxes from isolated retinal horizontal cells of the skate. J Physiol 560: 639–657, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol 321: 225–257, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neuroscience 16: 2157–2163, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal SG, Rhoads DB, Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun 89: 845–850, 1979 [DOI] [PubMed] [Google Scholar]
- Paalasmaa P, Kaila K. Role of voltage-gated calcium channels in the generation of activity-induced extracellular pH transients in the rat hippocampal slice. J Neurophysiol 75: 2354–2360, 1996 [DOI] [PubMed] [Google Scholar]
- Ransom BR. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis. Prog Brain Res 94: 37–46, 1992 [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. The buffer value of weak acids and bases: origin of the concept, and first mathematical derivation and application to physico-chemical systems—the work of M. Koppel and K. Spiro (1914). Respir Physiol 40: 1–32, 1980 [DOI] [PubMed] [Google Scholar]
- Rose CR, Deitmer JW. Evidence that glial cells modulate extracellular pH transients induced by neuronal activity in the leech central nervous system. J Physiol 481: 1–5, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening CJ, Kennedy HJ, Thomas RC. Calcium-hydrogen exchange by the plasma membrane Ca-ATPase of voltage-clamped snail neurons. Proc R Soc Lond B Biol Sci 253: 285–289, 1993 [DOI] [PubMed] [Google Scholar]
- Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, Sly WS. Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci USA 102: 16771–16776, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG. Electrogenesis of sustained potentials. Prog Neurobiol 1: 201–237, 1973 [PubMed] [Google Scholar]
- Somjen GG. Acidification of interstitial fluid in hippocampal formation caused by seizures and by spreading depression. Brain Res 311: 186–188, 1984 [DOI] [PubMed] [Google Scholar]
- Stewart PA. Independent and dependent variables of acid-base control. Respir Physiol 33: 9–26, 1978 [DOI] [PubMed] [Google Scholar]
- Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev 81: 21–50, 2001 [DOI] [PubMed] [Google Scholar]
- Sykova E, Svoboda J. Extracellular alkaline-acid-alkaline transients in the rat spinal cord evoked by peripheral stimulation. Brain Res 512: 181–189, 1990 [DOI] [PubMed] [Google Scholar]
- Taira T, Smirnov S, Voipio J, Kaila K. Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport 4: 93–96, 1993 [DOI] [PubMed] [Google Scholar]
- Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci USA 87: 6445–6449, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Chen K, Chesler M. Kinetics of activity-evoked pH transients and extracellular pH buffering in rat hippocampal slices. J Neurophysiol 95: 3686–3697, 2006 [DOI] [PubMed] [Google Scholar]
- Tong CK, Cammer W, Chesler M. Activity-dependent pH shifts in hippocampal slices from normal and carbonic anhydrase II-deficient mice. Glia 31: 125–130, 2000 [DOI] [PubMed] [Google Scholar]
- Trapp S, Luckermann M, Kaila K, Ballanyi K. Acidosis of hippocampal neurones mediated by a plasmalemmal Ca2+ / H+ pump. Neuroreport 7: 2000–2004, 1996 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345: 347–350, 1990 [DOI] [PubMed] [Google Scholar]
- Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem 84: 373–381, 2003 [DOI] [PubMed] [Google Scholar]
- Verbost PM, Flik G, Lock RA, Wendelaar Bonga SE. Cadmium inhibits plasma membrane calcium transport. J Membr Biol 102: 97–104, 1988 [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Jr, Vlachova V, Krusek J. The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol 430: 497–517, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. pH shifts evoked by neuronal stimulation in slices of rat hippocampus. Can J Physiol Pharmacol 67: 577–581, 1989 [DOI] [PubMed] [Google Scholar]
- Wang GJ, Randall RD, Thayer SA. Glutamate-induced intracellular acidification of cultured hippocampal neurons demonstrates altered energy metabolism resulting from Ca2+ loads. J Neurophysiol 72: 2563–2569, 1994 [DOI] [PubMed] [Google Scholar]
- Watson WD, Facchina SL, Grimaldi M, Verma A. Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors identify a novel calcium pool in the central nervous system. J Neurochem 87: 30–43, 2003 [DOI] [PubMed] [Google Scholar]
- Xiong ZQ, Stringer JL. Extracellular pH responses in CA1 and the dentate gyrus during electrical stimulation, seizure discharges, and spreading depression. J Neurophysiol 83: 3519–3524, 2000 [DOI] [PubMed] [Google Scholar]
- Yamamoto F, Borgula GA, Steinberg RH. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res 54: 685–697, 1992 [DOI] [PubMed] [Google Scholar]