Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases affecting aging men. Clinicians commonly use the term BPH to describe a clinical syndrome consisting of three components: lower urinary tract symptoms, benign prostatic enlargement, and bladder outlet obstruction (1). Clinical manifestations range widely from minimally bothersome symptoms to urinary retention and renal failure. BPH is histologically defined as overgrowth of the epithelial and stromal cells of the transition zone and periuretral area. Although the influence of androgens and estrogens on the development of BPH has been demonstrated, hormonal factors alone may not fully explain hyperplasic development of the prostate gland. So far, a variety of growth factors associated with epithelial/stromal interaction have been described in the pathophysiology of BPH (2) but the cellular and molecular processes underlying the pathogenesis and development of BPH remain poorly understood.
The goal of the current study was to analyze the gene expression profiles of BPH and to find association with phenotypic correlates to reveal candidate genes involved in the pathophysiology of BPH and to aid in the development of novel therapeutic targets.
Materials and Methods
BPH Samples
Thirty seven BPH specimens were used in this study. Prostate tissue was obtained after written consent from each patient and was approved by the local ethics committee. Samples were obtained from 12 patients undergoing open prostatectomy and 22 patients undergoing transurethral resection of the prostate for symptomatic BPH. For prostatectomy specimens, the tissue samples were consistently taken from the transitional zone area. In addition, BPH transition zone lobules from 3 other patients operated for bladder cancer by cysto-prostatectomy with bladder outlet obstruction were analyzed. Histologic review of the prostate tissue ensured the absence of bladder cancer in these 3 patients and absence of prostate cancer in all other samples, respectively. All samples were immediately processed at the time of surgery. The prostate tissue was flash frozen in liquid nitrogen and stored at −80°C until RNA extraction in the Tissue Bank of Henri Mondor Hospital.
Patient characteristics are presented in table 1. Mean prostate volume calculated on trans-rectal ultrasonography was 86ml. BPH treatment was taken in account in the study when given to patient prior surgery for a period of at least one month. The international prostate symptom score (IPSS) was used to determine symptoms intensity. All 37 patients had obstructive symptoms and 5 of them had also major irritative symptoms. On pathological examination, the mean percentage of glandular epithelium was 25% (range 5–60). Inflammation intensity was also assessed on microscopic examination: two cases had no prostatitis, 23 had low prostatitis, 11 had moderate prostatitis, and one had marked prostatitis.
Table 1.
Characteristics of 37 patients with BPH, and assessment of the association between samples clustering and clinical parameters.
| All patients (SD) | Group 1 n=18 (SD) | Group 2 n=19 (SD) | Univariate analysis p value | Multivariate analysis p value | |
|---|---|---|---|---|---|
| Age | 68 yo (11) range 47–89 | 67.7 yo (10) | 68.4 yo (12) | Mann-Whitney p=0.7 | |
| Preoperative prostate volume | 86ml | 54ml(25) | 119ml (35) | Mann-Whitney p<0.00001 | 0.034 |
| Preoperative PSA | 9.1 ng/ml (13) range 1–66 | 3.4ng/ml (2.5) | 14.8ng/ml (16.3) | Mann-Whitney p<0.001 | 0.27 |
| History of AUR | 21/37 | 13/18 | 8/19 | Chi-square p=0.19 | |
| Major irritative symptoms | 5/37 | 5/18 | 0/19 | Chi-square p=0.048 | 0.53 |
| IPSS | 15 (7) | 16 (7) | 15 (7) | Mann-Whitney p=0.6 | |
| Q max | 8.5 ml/s (5) | 8.7ml/s (5) | 8.3ml/s(6) | Mann-Whitney p=0.7 | |
| Type of surgery: TURP/PR/CP | 22/12/3 | 14/2/2 | 8/10/1 | Chi-square p=0.03 | 0.2 |
| Absence/low/moderate/marked prostatitis | 2/23/11/1 | 2/11/5/0 | 0/11/7/1 | Chi-square p=0.37 | |
| % of epithelium on pathology | 25 (13) range 5–60 | 21 (11) | 27 (14) | Mann-Whitney p=0.07 | |
| Treatment by Alpha blocker | 19/37 | 13/18 | 6/19 | Chi-square p=0.03 | 0.45 |
| Treatment by Finasteride | 10/37 | 5/18 | 5/19 | Chi-square p=0.9 |
AUR, acute urinary retention. Q max, Maximum urinary flow. SD, standard deviation. yo, years old. IPSS, international prostate symptom score. TURP, Transuretral resection of the prostate. PR, prostatectomy. CP, cysto-prostatectomy.
Gene expression analysis
RNA from BPH samples was extracted using Qiagen RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA). RNA sample quality was determined by electrophoresis through agarose gels, staining with ethidium bromide, and visualization of the 180 and 280 RNA bands under ultraviolet light. Then, the 37 BPH samples RNA were hybridized on Human Genome U133 2.0 plus Affymetrix chip containing 54’000 oligonucleotide probes. Hybridization process was done according to the standard protocol of the Dana Farber Cancer Institute (http://chip.dfci.harvard.edu/).
Statistical analysis
The data was normalized, log transformed, filtered for bad spots and median centered. Then, data was hierarchically clustered initially in an unsupervised analysis. Clinical parameters were compared between the groups defined in hierarchical analysis. Chi-square test was used to compare qualitative data between groups. For comparison of quantitative data, a Mann and Whitney test was used. Then, a supervised analysis was performed using Significance Analysis of Microarrays (SAM) software (3) to look for an association between gene expression profile and two classes’ qualitative and quantitative data. Cluster analysis (Stanford University, Palo Alto, CA) and generation of figures with TreeView were done using software developed by Eisen et al. (4). For genes with scores greater than an adjustable threshold, SAM uses permutations of the repeated measurements to estimate the percentage of genes identified by chance referred to as the false discovery rate. Cluster, Treeview, and SAM softwares can be obtained at http://www.dnachip.org (Stanford University). Data related to genes contained on expression arrays were downloaded from Affymetrix website providing the gene annotation subsequently associated with specific pathways supposed to be involved in BPH including growth factors genes, cell cycle genes, apoptose genes, inflammation genes, genes of hyperplasia, and androgen regulated genes.
Additional statistical analysis was done using SPSS software (SPSS inc., Chicago, IL). A p-value < 0.05 was considered as significant, and all p-values were two-tailed.
Results
Unsupervised analysis
Hierarchical cluster analysis separated the BPH samples into two main groups of 18 and 19 samples, respectively. The figure 1 represents clustering as visualized with Tree view software.
Figure 1.
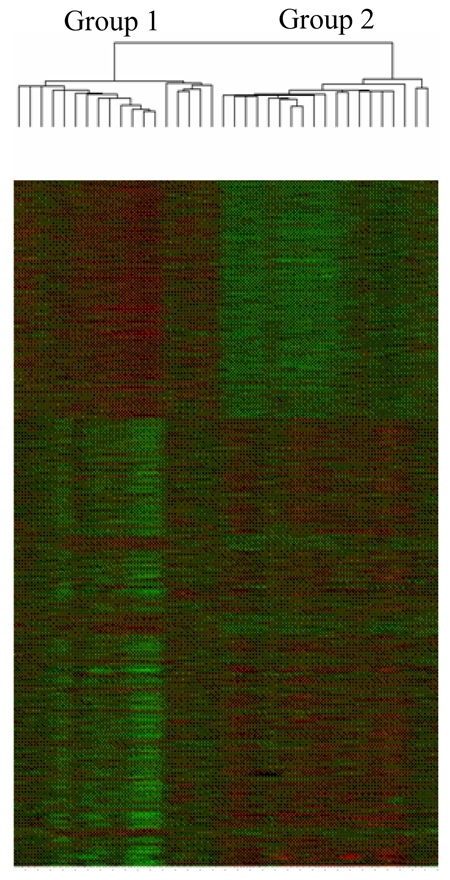
Hierarchical cluster analysis of BPH samples as visualized with Tree view software: two main groups of 18 and 19 samples, respectively, are observed.
Comparison of these two groups revealed 4 clinical parameters that were statistically different between them: patients in group 1 had greater prostate gland volume as determined by trans-rectal ultrasonography. They also had higher prostate specific antigen (PSA) levels, were more likely to be treated by alpha blocker, to have major irritative symptoms, and to be operated by prostatectomy (table 1). Including all 5 parameters in a multivariate Cox regression analysis showed that the sole independent parameter associated with the clustering was the prostate gland volume (table 1).
Supervised analysis
Next, we further explored the role of prostate volume in BPH and using a supervised approach with a qualitative SAM analysis, we compared gene expression profiles between samples of patients with prostate glands < 60 ml (N=14, group 1) and > 60 ml (N=20, group 2). We excluded the 3 patients with cystoprostatectomy from all further analyses as their prostate volume had not been determined with transrectal ultrasonography. With a >2-fold change in gene expression level and a false discovery rate of <5%, 227 transcripts were found to be differentially expressed in both groups (table 2). Figure 2 represents our SAM plot with transcripts significantly up-regulated (N=67, red dots), and down-regulated (N=160, green dots) genes in group 1. Out of 272 transcripts, 61 were known genes (table 2).
Table 2.
List of 61 known genes significantly up- and down-regulated in > 60 ml prostates compared to <60 ml prostates (fold change ≤0.5 or >2 in SAM analysis)
| Down-regulated genes | Up-regulated genes | ||||
|---|---|---|---|---|---|
| Gene Symbol | FC | Gene Symbol | FC | Gene Symbol | FC |
| GPR34 | 0.12 | TMEM16A | 2.00 | RGS1 | 2.34 |
| HBG2 | 0.23 | LILRB2 | 2.02 | LMAN2 | 2.44 |
| HBD | 0.26 | LRAP | 2.02 | C1S | 2.46 |
| HBA2 | 0.26 | FKBP1A | 2.03 | NGFR | 2.47 |
| SERPINA9 | 0.30 | PAEP | 2.04 | TGFBR2 | 2.51 |
| PPEF2 | 0.32 | ANGPT2 | 2.04 | ZFP36 | 2.58 |
| MAGEA11 | 0.34 | TACSTD1 | 2.09 | DPP4 | 2.61 |
| HBG1 | 0.36 | CHST9 | 2.12 | GSTT2 | 2.77 |
| NPHS1 | 0.36 | MBL2 | 2.12 | NPPC | 2.90 |
| PMFBP1 | 0.36 | MEST | 2.14 | COL17A1 | 3.33 |
| HSPA1B | 0.37 | PDE4B | 2.14 | IL1F7 | 3.39 |
| ADH4 | 0.38 | TMEFF2 | 2.14 | PIP5K2A | 3.58 |
| BPIL2 | 0.43 | RTN1 | 2.14 | NLGN4Y | 3.99 |
| MAB21L2 | 0.43 | DUSP6 | 2.15 | NODAL | 3.99 |
| PCSK9 | 0.43 | PRSS8 | 2.15 | ZNF165 | 4.20 |
| ADRA1B | 0.45 | KLK11 | 2.18 | CITED1 | 4.69 |
| DYRK3 | 0.46 | AMD1 | 2.20 | CD33 | 6.38 |
| ADAM29 | 0.48 | CYP4X1 | 2.25 | ||
| PTHR2 | 0.49 | HOXB13 | 2.31 | ||
| CHRDL2 | 0.49 | CHST7 | 2.31 | ||
| FTHL17 | 0.50 | FZD1 | 2.34 | ||
| SIX5 | 0.50 | AHNAK | 2.34 | ||
FC, fold change between prostates >60ml and <60ml
Figure 2.
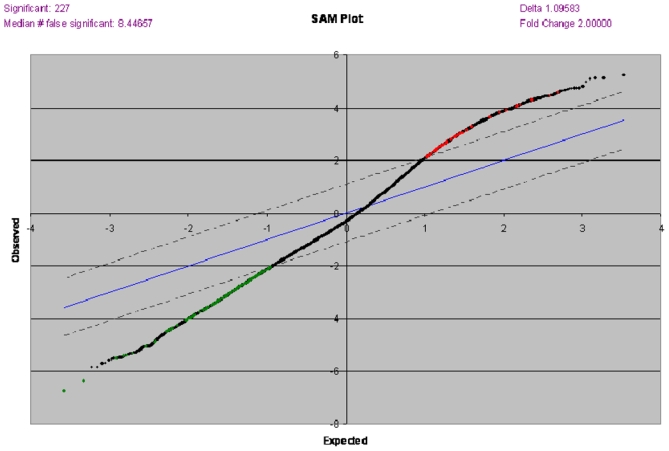
SAM plot with transcripts significantly up-regulated (N=67, red dots), and down-regulated (N=160, green dots) in 14 patients with < 60 ml prostate glands versus 20 patients with > 60 ml prostate glands.
We next looked at the annotations and reported functions of the differentially expressed genes. We delineated six specific pathways including growth factors genes, cell cycle genes, apoptose genes, inflammation genes, genes of hyperplasia, and androgen regulated genes, and explored them further using a separate qualitative SAM analysis for each Results are summarized in table 3 and table 4. In each pathway, a number of genes was dysregulated. Fifteen inflammation genes showed dysregulation. Seven of them were downregulated in large prostate glands, whereas in others pathways explored, most dysregulated genes were found to be overexpressed in large prostate glands (table 3). Gene expression profiles were compared between patients who received finasteride therapy prior surgery and those who did not. However, no statistically significant difference was observed (figure 3) in qualitative SAM analyses. Similarly, we didn’t find any significant association between BPH gene expression profiles and the percentage of epithelium or the degree of inflammation on pathology review.
Table 3.
Results of SAM analysis comparing gene expression in > 60 ml prostates and <60 ml prostates for six separately explored pathways
| Pathway | Number of genes explored in the pathway | Number of genes significantly differentially expressed | Number of genes up- regulated | Number of genes down- regulated | Median number of false significant genes |
|---|---|---|---|---|---|
| Growth factors genes | 38 | 14 | 14 | 0 | 1.3 (9%) |
| Cell cycle genes | 107 | 43 | 38 | 5 | 0.9 (2%) |
| Apoptosis genes | 99 | 40 | 40 | 0 | 0.9 (2%) |
| Inflammation genes | 49 | 15 | 8 | 7 | 0.6 (4%) |
| Genes of hypertrophy | 29 | 15 | 14 | 1 | 1 (6%) |
| Androgen regulated genes | 30 | 10 | 9 | 1 | 0.9 (9%) |
Table 4.
List of genes significantly up and down-regulated in > 60 ml prostates compared to <60 ml prostates for six separately explored pathways (list limited to genes with fold change <0.7 or >1.3 in SAM analysis).
| Gene Symbol | Fold Change (prostates >60ml vs <60ml) | Gene Symbol | Fold change (prostates >60ml vs <60ml) | Gene Symbol | Fold Change (prostates >60ml vs <60ml) | Gene Symbol | Fold Change (prostates >60ml vs <60ml) |
|---|---|---|---|---|---|---|---|
| Androgen regulated genes | Cell cycle genes | Apoptosis genes | Growth factors genes | ||||
| ARSD | 1.31 | ORC2L | 1.32 | BIRC3 | 1.31 | IGF2R | 1.34 |
| TPD52 | 1.48 | HDAC1 | 1.32 | SFRS2IP | 1.31 | ERBB3 | 1.35 |
| IQGAP2 | 1.70 | CCNH | 1.32 | CASP3 | 1.31 | KLK3 | 1.35 |
| CEBPD | 1.70 | RB1 | 1.32 | BID | 1.35 | IGFBP2 | 1.49 |
| LCP1 | 1.83 | CDK4 | 1.32 | JUN | 1.35 | VEGF | 1.55 |
| ABL1 | 1.35 | TNFRSF1A | 1.37 | ||||
| Genes of hypertrophy | E2F6 | 1.35 | TNFSF10 | 1.39 | Inflammation genes | ||
| BUB3 | 1.36 | TP53 | 1.39 | IL4 | 0.58 | ||
| IL1A | 0.67 | ORC5L | 1.38 | BCL2A1 | 1.42 | CD86 | 0.62 |
| IL1R1 | 1.34 | MAD2L2 | 1.38 | CASP4 | 1.47 | CCR5 | 0.66 |
| ADAM10 | 1.41 | SMAD4 | 1.43 | TNFRSF1B | 1.47 | IL5RA | 0.69 |
| JUND | 1.51 | PRKDC | 1.46 | NFKB1 | 1.50 | COL3A1 | 1.33 |
| VEGF | 1.55 | MCM2 | 1.50 | CASP1 | 1.53 | LCK | 1.36 |
| CYR61 | 1.61 | TFDP1 | 1.51 | RIPK1 | 1.66 | IL2RB | 1.37 |
| DUSP14 | 1.65 | PCNA | 1.53 | MAP2K4 | 1.83 | TLR2 | 1.40 |
| TCF8 | 1.86 | CDC45L | 1.72 | MYC | 2.37 | CXCR4 | 1.44 |
| ATF3 | 2.16 | ORC1L | 2.18 | TLR3 | 1.48 | ||
| TLR1 | 1.53 | ||||||
Figure 3.
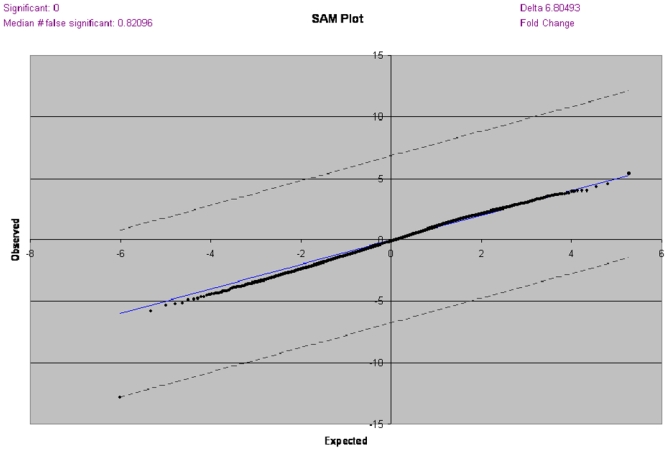
Comparison of gene expression profiles using SAM analysis between patients who received finasteride therapy prior surgery and those who did not.
Discussion
In the present analysis, gene expression signatures in BPH were found to be closely associated to prostate gland volume. In hierarchical analysis, two distinct groups were identified and the sole independent parameter associated with this dichotomous clustering was prostate volume. In a subsequent supervised analysis, prostates larger than 60 ml showed a number of genes which were significantly dysregulated compared to smaller prostate glands.
The preferred medical treatment for many men with symptomatic BPH is either an alpha-adrenergic–receptor antagonist (alpha- blocker), which reduces smooth-muscle tone in the prostate and bladder neck, or a 5 alpha –reductase inhibitor, which reduces prostate volume by inducing epithelial atrophy (5,6).
Strong evidence exists showing that baseline prostate size (and its surrogate baseline PSA level) predicts future prostate growth (1). In addition, baseline prostate volume is a powerful predictor of treatment outcome with finasteride (1). Randomized placebo-controlled finasteride trials have shown that men with larger prostate volumes and higher PSA levels experience a clinically significant response to therapy compared with those with smaller prostate volumes and lower PSA levels. It has also been demonstrated that men with larger prostate glands and higher PSA levels are at increased risk for acute urinary retention and BPH-related surgery but that finasteride reduces these risks (1). Although clinical trials have shown that prostate size plays an important role in the management of BPH, so far, no molecular explanation of that observation was reported. In the present study, we attempt to assess the molecular significance of BPH volume. We identified a list of genes which expression was significantly associated to prostate volume. Several of these genes were previously reported to be expressed in the prostate. For example, TEMFF2, a gene known to be expressed in normal prostate (7) was found to be upregulated in large prostate glands. KLK11, which was previously reported to be upregulated in prostate cancer compared to BPH (8), was shown to be overexpressed in large prostate glands in our analysis. Androgen receptor signals play a decisive role in regulating growth and differentiation of both normal and cancerous prostate cells. HOXB13 has a highly prostate specific expression and induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling (9). Interestingly, in our analysis, HOXB13 was found to be upregulated in large prostate glands. NGFR (10), TGFBR2 (11) and GSTT2 (12) which are known to play a role in prostate cancer were found to be upregulated in large prostate glands. Similarly, DPP4 which activity was reported to be higher in transition zone than peripheral zone of the prostate (13) was shown in our analysis to be upregulated in large prostate glands. HBD1 was previously reported to be expressed in normal prostate and to have a low expression in prostate cancer (14). In our analysis, HBD1 was downregulated in large prostate glands compared to smaller ones. PCSK9 which is known to be expressed in prostate tissue (15) was also downregulated in large prostate gland in our analysis.
When focusing on specific pathways supposed to be involved in BPH, we identified some genes differentially expressed in large prostate glands compared to smaller ones. Several of these genes were previously reported to be expressed in normal prostate, BPH, or prostate cancer. Among the cell cycle genes, PCNA, which was previously reported to be significantly lower expressed in BPH than that in prostate cancer (16), was found upregulated in large prostate glands. Similarly, we found RB1 and SMAD 4 which are known to be expressed in BPH (17,18) to be upregulated in large prostate glands compared to smaller ones. ADAM10, a gene involved in hypertrophy and known to plays a role in prostate cancer (19), was shown to be upregulated in large prostate glands. It is interesting that many genes that were over-expressed in the large BPH prostates are also dysregulated in cancer. This might be explained by a possible common mechanistic link between these two proliferative diseases. Another gene of hypertrophy, CYR61, which is known to be involved in BPH: and to act as a secreted autocrine and paracrine mediator in stromale and epithelial hyperplasia (20), was found upregulated in large prostate glands in our analysis. JUN, a gene involved in apoptosis and known to be expressed in BPH (21), was found upregulated in large prostate glands. CASP3 is another apoptosis gene which expression is known to be higher in BPH than in normal prostate (22). In addition, it was reported that finasteride treatment might activate CASP3 to induce an apoptotic effect in BPH (23). In our analysis, CASP3 was shown to have a higher expression level in large prostate glands compared to smaller ones. We found several growth factors to be upregulated in large prostate glands, such as KLK3 which is known to be highly selective of prostate tissue (24), and VEGF, previously reported as downregulated in BPH (25).
We hypothesize that amongst the large prostate signature genes we identified, a subset of genes may be potential novel in vivo targets for medical therapy. Future studies will be aimed at resolving the role of genes identified in this study.
Prostate volume is strongly correlated with serum PSA level in men with BPH and no evidence of prostate cancer, and this relationship is dependent on age (26). Therefore, we were not surprised to observe in our analysis that patients with larger glands had higher PSA levels. Similarly, patients with large prostate glands are more likely to be operated by prostatectomy, whereas those with smaller prostate glands are preferentially operated through a transurethral approach. This might explain that the operative technique was associated with clustering in univariate analysis, but was not in multivariate analysis.
As finasteride therapy is more likely to be given to patients with large prostate glands (1), we hypothesized that finasteride therapy could interfere in the association between prostate volume and gene expression profiles. Finasteride therapy was found not to modify gene expression, supporting that gene expression was independently associated to prostate size. The absence of gene expression modifications related to finasteride therapy could be explained by the fact that patients included in our cohort required surgical treatment, and therefore, might not be good responders to medical therapy.
One of the parameters that was different in the two groups obtained by hierarchical cluster analysis was treatment with an alpha-blocker. Alpha-1 antagonists affect the tone in the smooth muscle cells of the prostatic stroma, and there is also evidence that they induce apoptosis in benign and malignant epithelial cells (27). Therefore, alpha-blocker therapy could affect the gene expression. However, alpha-blocker therapy was not an independent parameter associated with clustering.
In the global analysis, more genes were significantly down-regulated than up-regulated in the large prostate glands group compared to the small glands one (160 versus 67). We have no explanation for this observation. In contrary, for the six specific pathways we analyzed, much more genes showed over-expression than down-regulation in the large prostate glands compared to smaller ones. These results are consistent with those of Fromont et al (28) who compared gene expression using multiple PCR assays in 30 BPH samples (Adenoma weight was less than 60 grams in 15 patients and more than 60 grams in the remainder) and 15 normal prostate from radical prostatectomy for prostate cancer. A total of 23 genes showed increased expression in the two BPH groups with a fold change of at least 2.5 compared to normal tissue. All except 2 of these genes had average expression values that were superior in the more than 60 grams BPH group compared to the less than 60 grams BPH group. These data supports the hypothesis that over expressed genes could be directly associated with increased prostate volume. In addition, the striking overrepresentation of up-regulated genes in BPH samples, especially of growth factors genes, cell cycle genes, apoptose genes, genes of hyperplasia, and androgen regulated genes, can thus be partially explained by the enrichment of growth-regulating elements within the BPH stroma (29).
There is emerging evidence that prostatic inflammation may contribute to prostate growth in BPH (30). Interestingly, comparing inflammation genes expression in the two samples groups (<60 ml versus > 60 ml), the numbers of up-regulated and down-regulated genes were almost similar (8 versus 7). This observation supports the fact that chronic inflammation may play a significant role in BPH progression independently of prostate size. In another microarrays gene expression analysis, a strong correlation was found between inflammation and symptomatic BPH, but the impact of prostate volume on gene expression was not assessed (31).
Acknowledgments
Virginie Fataccioli, Aurore Manceau for technical assistance (hospital Tissue Bank)
The following grants supported this work: – NIH Grant # 137962
– 2004 grant from the ARTP (Association pour la Recherche sur les Tumeurs Prostatiques)
– Pierre Fabre Médicament Grant
References
- 1.Nickel JC. Benign prostatic hyperplasia: does prostate size matter? Rev Urol. 2003;5 (Suppl 4):12–17. [PMC free article] [PubMed] [Google Scholar]
- 2.Wong YC, Wang YZ. Growth factors and epithelial-stromal interactions in prostate cancer development. Int Rev Cytol. 2000;199:65–116. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- 3.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lepor H. Role of alpha-adrenergic blockers in the treatment of benign prostatic hyperplasia. Prostate. 1990;(Suppl 3):75–84. doi: 10.1002/pros.2990170508. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan ED. Long-Term Experience With 5-alpha-Reductase Inhibitors. Rev Urol. 2003;5 (Suppl 5):22–27. [PMC free article] [PubMed] [Google Scholar]
- 7.Quayle SN, Sadar MD. A truncated isoform of TMEFF2 encodes a secreted protein in prostate cancer cells. Genomics. 2006;87:633–637. doi: 10.1016/j.ygeno.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Scorilas A, Gregorakis AK. mRNA expression analysis of human kallikrein 11 (KLK11) may be useful in the discrimination of benign prostatic hyperplasia from prostate cancer after needle prostate biopsy. Biol Chem. 2006;387:789–793. doi: 10.1515/BC.2006.099. [DOI] [PubMed] [Google Scholar]
- 9.Jung C, Kim RS, Zhang HJ, et al. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004;15(64):9185–192. doi: 10.1158/0008-5472.CAN-04-1330. [DOI] [PubMed] [Google Scholar]
- 10.Festuccia C, Muzi P, Gravina GL, et al. Tyrosine kinase inhibitor CEP-701 blocks the NTRK1/NGF receptor and limits the invasive capability of prostate cancer cells in vitro. Int J Oncol. 2007;30:193–200. [PubMed] [Google Scholar]
- 11.Sun X, Chen C, Vessella RL, et al. Microsatellite instability and mismatch repair target gene mutations in cell lines and xenografts of prostate cancer. Prostate. 2006;66:660–666. doi: 10.1002/pros.20390. [DOI] [PubMed] [Google Scholar]
- 12.Jariwala U, Prescott J, Jia L, et al. Identification of novel androgen receptor target genes in prostate cancer. Mol Cancer. 2007;6:39. doi: 10.1186/1476-4598-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MJ, Haller R, Li SY, et al. Elevation of dipeptidylpeptidase iv activities in the prostate peripheral zone and prostatic secretions of men with prostate cancer: possible prostate cancer disease marker. J Urol. 2005;174:1124–1128. doi: 10.1097/01.ju.0000168621.84017.5c. [DOI] [PubMed] [Google Scholar]
- 14.Bullard RS, Gibson W, Bose SK, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: New insight into its potential role in cancer. Mol Immunol. 2008;45:839–848. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt RJ, Zhang Y, Zhao Y, et al. A Novel Splicing Variant of Proprotein Convertase Subtilisin/Kexin Type 9. DNA Cell Biol. doi: 10.1089/dna.2007.0667. In press. [DOI] [PubMed] [Google Scholar]
- 16.Harper ME, Glynne-Jones E, Goddard L, et al. Relationship of proliferating cell nuclear antigen (PCNA) in prostatic carcinomas to various clinical parameters. Prostate. 1992;20:243–253. doi: 10.1002/pros.2990200309. [DOI] [PubMed] [Google Scholar]
- 17.Phillips SM, Barton CM, Lee SJ, et al. Loss of the retinoblastoma susceptibility gene (RB1) is a frequent and early event in prostatic tumorigenesis. Br J Cancer. 1994;70:1252–1257. doi: 10.1038/bjc.1994.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng L, Rowland RG, Lele SM, et al. Apoptosis incidence and protein expression of p53, TGF-beta receptor II, p27Kip1, and Smad4 in benign, premalignant, and malignant human prostate. Hum Pathol. 2004;35:290–297. doi: 10.1016/j.humpath.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 19.McCulloch DR, Akl P, Samaratunga H, et al. Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res. 2004;10:314–323. doi: 10.1158/1078-0432.ccr-0846-3. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto S, Yokoyama M, Prakash K, et al. Development of quantitative detection assays for CYR61 as a new marker for benign prostatic hyperplasia. J Biomol Screen. 2003;8:701–711. doi: 10.1177/1087057103259159. [DOI] [PubMed] [Google Scholar]
- 21.Tiniakos DG, Mitropoulos D, Kyroudi-Voulgari A, et al. Expression of c-jun oncogene in hyperplastic and carcinomatous human prostate. Urology. 2006;67:204–208. doi: 10.1016/j.urology.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 22.Shariat SF, Ashfaq R, Roehrborn CG, et al. Expression of survivin and apoptotic biomarkers in benign prostatic hyperplasia. J Urol. 2005;174:2046–2050. doi: 10.1097/01.ju.0000176459.79180.d1. [DOI] [PubMed] [Google Scholar]
- 23.Bozec A, Ruffion A, Decaussin M, et al. Activation of caspases-3, -6, and -9 during finasteride treatment of benign prostatic hyperplasia. J Clin Endocrinol Metab. 2005;90:17–25. doi: 10.1210/jcem.90.8.9993. [DOI] [PubMed] [Google Scholar]
- 24.Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem. 2007;53:1423–1432. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- 25.Soulitzis N, Karyotis I, Delakas D, et al. Expression analysis of peptide growth factors VEGF, FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol. 2006;29:305–314. [PubMed] [Google Scholar]
- 26.Roehrborn CG. 5-alpha-Reductase Inhibitors Prevent the Progression of Benign Prostatic Hyperplasia. Rev Urol. 2003;5 (Suppl 5):12–21. [PMC free article] [PubMed] [Google Scholar]
- 27.Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–4555. [PubMed] [Google Scholar]
- 28.Fromont G, Chene L, Latil A, et al. Molecular profiling of benign prostatic hyperplasia using a large scale real-time reverse transcriptase-polymerase chain reaction approach. J Urol. 2004;172:1382–1385. doi: 10.1097/01.ju.0000137819.92305.46. [DOI] [PubMed] [Google Scholar]
- 29.Luo J, Dunn T, Ewing C, et al. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate. 2002;51:189–200. doi: 10.1002/pros.10087. [DOI] [PubMed] [Google Scholar]
- 30.Kramer G, Mitteregger D. Marberger Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Prakash K, Pirozzi G, Elashoff M, et al. Symptomatic and asymptomatic benign prostatic hyperplasia: molecular differentiation by using microarrays. Proc Natl Acad Sci U S A. 2002;99:7598–7603. doi: 10.1073/pnas.112191399. [DOI] [PMC free article] [PubMed] [Google Scholar]


