Abstract
Single-voxel proton magnetic resonance imaging (1H-MRS) and proton MR spectroscopic imaging (1H-MRSI) were used to compare brain metabolite levels in semi-acute mild traumatic brain injury (mTBI) patients (n = 10) and matched healthy controls (n = 9). The 1H-MRS voxel was positioned in the splenium, a region known to be susceptible to axonal injury in TBI, and a single 1H-MRSI slice was positioned above the lateral ventricles. To increase sensitivity to the glutamate (Glu) and the combined glutamate-glutamine (Glx) signal, an inter-pulse echo time shown to emphasize the major Glu signals was used along with an analysis method that reduces partial volume errors by using water as a concentration standard. Our preliminary findings indicate significantly lower levels of gray matter Glx and higher levels of white matter creatine-phosphocreatine (Cr) in mTBI subjects relative to healthy controls. Furthermore, Cr levels were predictive of executive function and emotional distress in the combined groups. These results suggest that perturbations in Cr, a critical component of the brain's energy metabolism, and Glu, the brain's major neurotransmitter, may occur following mTBI. Moreover, the different pattern of results for gray and white matter suggests tissue-specific metabolic responses to mTBI.
Key words: cognitive, creatine, glutamate, mild traumatic brain injury, spectroscopy
Introduction
Mild traumatic brain injury (mTBI) is by far the most prevalent form of brain injury, accounting for over 70% of hospital visits related to head injury, with many cases likely to go unreported (Arciniegas et al., 2005; Jennett, 1998). Although most patients recover fully, mTBI produces acute cognitive and emotional symptoms by mechanisms that are still not understood. Furthermore, the effects of repeated mTBI may be cumulative or enhance susceptibility to injury upon a subsequent insult (DeFord et al., 2002; Matser et al., 1998; Vagnozzi et al., 2008; Vagnozzi et al., 2007). These issues raise unique questions in the medical management of mTBI, such as when to advise otherwise healthy-appearing patients to return to work, to the playing field, or, as has become more relevant in recent years, the battlefield (Hoge et al., 2008). Moreover, as standard clinical imaging modalities produce negative results in the majority of mTBI cases, there is a great need for the development of clinical measures that are more sensitive to the subtle alterations in brain morphology, physiology, and function that may underlie mTBI (Belanger et al., 2007). Among the more promising of these measures is proton magnetic resonance spectroscopy (1H-MRS), which measures the levels of several key metabolites in the brain (Belanger et al., 2007; Brooks et al., 2001; Shutter et al., 2006).
To date, the majority of mTBI studies using 1H-MRS have been aimed at detecting group differences in N-acetylaspartate (NAA) or in the ratio of the total NAA signal to the signal from other metabolites in the 1H-MRS spectrum, such as choline (Cho) or creatine-phosphocreatine (Cr) (Babikian et al., 2006; Cimatti, 2006; Cohen et al., 2007; Govindaraju et al., 2004; Kirov et al., 2007; Son et al., 2000; Tavazzi et al., 2007; Vagnozzi et al., 2005; Vagnozzi et al., 2008; Vagnozzi et al., 2007). Most often the combined NAA and N-acetylaspartylglutamate (NAAG) signal is measured, since the latter is relatively small and difficult to resolve at clinical magnetic field strengths. As the NAA, Cho, and Cr methyl “singlet” peaks are the most prominent signals in the 1H-MRS spectrum, their detection is relatively reliable. Typical acquisition parameters (i.e., long echo times) emphasize these peaks while discriminating against other metabolite and macromolecule signals in the otherwise crowded and overlapping 1H-MRS spectrum. Lower levels of NAA have generally been interpreted to reflect either neuronal loss, metabolic dysfunction, or myelin repair, owing respectively, to its predominant location in neurons, its synthesis in neuronal mitochondria, and its possible role as an acetyl group donor in lipid synthesis (Moffett et al., 2007). Additionally, NAA has been proposed to act as an osmolyte tightly coupled to synaptic transmission (Baslow et al., 2007). Changes in the Cho signal, primarily from membrane lipid metabolites phosphorylcholine and glycerol phosphorylcholine, have been interpreted to reflect membrane damage and/or repair in TBI (Garnett et al., 2000a; Garnett et al., 2000b; Shutter et al., 2004; Yeo et al., 2006). Creatine and phosphocreatine, in equilibrium with adenosine tri- and diphosphate (ATP and ADP), maintain the cell's ATP levels. Since their individual 1H-MRS signals are generally not resolvable at clinical scanner field strengths, their combined signal (Cr) is often assumed to be constant, regardless of cellular energy status. However, this assumption has been challenged by recent studies showing the Cr signal to be higher or lower relative to healthy controls in different disease states (Hattingen et al., 2008; Inglese et al., 2003; Munoz Maniega et al., 2008).
Owing to the increased challenges in reliably measuring more complex “multiplet” 1H-MRS signals, far fewer studies have been directed toward measuring the levels of glutamate (Glu), glutamine (Gln), or γ-aminobutyric acid (GABA) after TBI, even though these neurotransmitters or neurotransmitter metabolites (i.e., Gln) are prime candidates for disruption after TBI, and their importance for normal brain function is much better understood than that of NAA. Glu, the brain's major neurotransmitter, is taken up in the synaptic cleft by astrocytes and converted to Gln, which is then shuttled back to the presynaptic neuron and converted to Glu. However, there are a number of alternative metabolic pathways for both Glu and Gln, including energy metabolism and the production of glutathione. The flux through any particular pathway depends on local demands for maintaining homeostasis during neuronal firing (McKenna, 2007). Several studies using microdialysis probes have detected a disruption in this homeostasis, specifically, elevated Glu levels that persist for several hours following severe TBI in humans (Alves et al., 2005; Koura et al., 1998; Vespa et al., 1998; Zauner et al., 1996), and in animal models of moderate to severe TBI (Di et al., 1999; Globus et al., 1995; Hartley et al., 2008; Nilsson et al., 1990; Palmer et al., 1993). In studies using 1H-MRS, an elevation in the sum of the glutamate and glutamine signals (Glx) and Cho was related to poorer outcome on the Glasgow Outcome Scale from 6 months to 1 year later in severely injured adults (Shutter et al., 2004). The 1H-MRS Glx signal has also been shown to be elevated in moderate to severe pediatric TBI (Ashwal et al., 2004), and Glx levels measured within 2–10 days of TBI have been found to correlate negatively with cognitive function in children 1–4 years after injury (Babikian et al., 2006). To our knowledge, a study examining Glu or Glx changes in a well-characterized cohort of mTBI patients has not been performed to date.
In the present study, we used both single-voxel 1H-MRS and proton MR spectroscopic imaging (1H-MRSI) to compare brain metabolite levels, including those of Glu, in a group of subjects within 3 weeks of mTBI to metabolite levels in a group of normal healthy control subjects matched for age, gender, and education. The single 1H-MRS voxel was positioned in the splenium to observe any metabolic changes in a region known to be susceptible to axonal injury upon TBI (Thierry et al., 2004), and a single 1H-MRSI slice was positioned above the lateral ventricles in tissue comprised of both gray matter (GM) and white matter (WM).
Methods
Participants
Ten (6 female, 4 male) patients with mTBI and nine healthy uninjured (5 female, 4 male) gender-, age-, and education-matched controls were recruited for the current study (see Table 1 for demographic data). One mTBI patient did not complete the neuropsychological assessment, but did receive the full MRS protocol. All mTBI patients experienced a closed head injury resulting in an alteration in mental status. They were evaluated clinically (mean assessment at 10.89 days post-injury; range 4–19 days), and with the imaging protocol (mean assessment at 10.67 days post-injury; range 3–19 days) as soon after injury as was practical, given scheduling issues. The etiologies of the brain injuries included two motor vehicle accidents, six falls, one assault, and one patient who was struck by a falling object. Inclusion criteria for mTBI patients were based on the American Congress of Rehabilitation Medicine recommendations, and included a Glasgow Coma Scale score of 13–15 (at presentation in the emergency department), and an alteration in mental status (e.g., confusion, loss of consciousness, or post-traumatic amnesia) at the time of injury. Loss of consciousness (if present) was limited to 30 min in duration, and post-traumatic amnesia was limited to a 24-h period. Mild TBI participants and controls were excluded if there was a previous history of neurological disease, major psychiatric disturbance, additional closed-head injuries with more than 5 min of loss of consciousness, learning disorder, ADHD, or history of substance or alcohol abuse. At the time of assessment, two of the mTBI subjects were being prescribed medications for pain. Informed consent was obtained from subjects according to institutional review board guidelines of the University of New Mexico.
Table 1.
Demographic Data for the Mild TBI and Healthy Control Participants
| |
Mild TBI |
Healthy controls |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age | 29.00 | 9.71 | 21–49 | 27.56 | 9.07 | 21–49 |
| Education | 12.60 | 2.68 | 6–16 | 13.67 | 2.12 | 9–16 |
| WTAR | 47.33 | 9.17 | 33–59 | 51.44 | 9.06 | 40–63 |
| Handedness quotient | 89.49 | 29.55 | 18–100 | 62.87 | 48.87 | –43–100 |
The Wechsler Test of Adult Reading (WTAR) provides an estimate of premorbid intellectual functioning.
Image and 1H-MRSI acquisition
MRI and 1H-MRSI experiments were performed on a Seimens 3T scanner. T1-weighted images were acquired in a sagittal plane perpendicular to the inter-hemispheric fissure with a 3-D multi-echo MPRAGE sequence [echo time (TE) = 1.64 msec, repetition time (TR) = 2.53 sec, 7° flip angle, number of excitations (NEX) = 1, slice thickness = 1 mm, FOV (field of view) = 256 × 256 mm, matrix = 256 × 256, voxel size = 1 × 1 × 1 mm3]. T2-weighted images [TE = 77.0 msec, TR =1.55 sec, NEX = 1, slice thickness = 1.5 mm, FOV = 220 mm, matrix = 192 × 192, voxel size = 1.15 × 1.15 × 1.5 mm3] were acquired with a 3-D variable flip-angle turbo-spin-echo sequence in an oblique axial plane perpendicular to the inter-hemispheric fissure and parallel to an axis defined by the inferior borders of the splenium and the genu in the T1-weighted image.
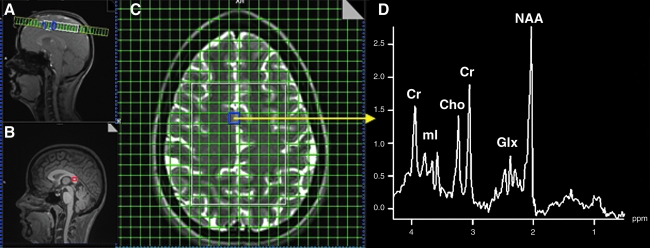
1H-MRSI was performed with a phase-encoded version of a point-resolved spectroscopy sequence (PRESS) with and without water presaturation (TE = 40 msec, TR = 1500, slice thickness = 15 mm, FOV = 220 × 220 mm, circular k-space sampling [radius = 24], total scan time = 582 sec). A TE of 40 msec was chosen to enhance Glu detection (Mullins et al., 2008). The nominal voxel size was 6.25 × 6.25 × 15 mm3 after zero-filling in k-space to 32 × 32 samples. The 1H-MRSI volume of interest (VOI) was selected with strong saturation bands to reduce chemical shift artifacts and was prescribed with the TSE image to lie 1 cm above the lateral ventricles and parallel to the AC-PC axis. This location was chosen to include the superior aspects of the anterior cingulate gyrus, the inferior aspects of the medial frontal gyrus, and the superior longitudinal fasciculus. To further minimize the chemical shift artifact, the transmitter was set to the frequency of the NAA methyl peak during the acquisition of the metabolite spectra, and to the frequency of the water peak during the acquisition of the unsuppressed water spectra. Additionally, the outermost rows and columns of the VOI were excluded from analysis. Figure 1 (panels A, C, and D) shows the location of a representative 1H-MRSI VOI and spectrum.
FIG. 1.
Spectroscopic regions of interest locations and example spectrum. (A) Location of supraventricular axial proton magnetic resonance spectroscopy imaging (1H-MRSI) slice in sagittal plane. (B) Location of 1-cm3 single-proton magnetic resonance imaging (1H-MRS) voxel in splenium (red box). (C) Location of 1H-MRSI slice in axial plane (region of interest is within the white box). (D) Spectrum from the cingulate region (from voxel in purple box in C) (Cr, creatine-phosphocreatine; Cho, choline; ml, myoinositol; Glx, glutamate-glutamine; NAA, N-acetylaspartate).
Single-voxel 1H-MRS data from a 1 × 1 × 1-cm voxel positioned within the splenium (Fig. 1B) was obtained with a PRESS sequence with and without water suppression (TR/TE = 1500/40 msec, 192 averages with water suppression, 16 averages without water suppression). The transmitter frequency was set to 2.3 ppm for the acquisition of the metabolite spectrum, and to 4.7 ppm (water resonance) for the acquisition of the water spectrum, to minimize the chemical shift artifact.
1H-MRS data processing
After zero-filling to 32 × 32 points in k-space, applying a Hamming filter with a 50% window width, and 2-D spatial Fourier transformation (FT), the time domain 1H-MRSI data were analyzed using the LCModel (Provencher, 1993) using tissue water as a concentration reference. We used the LCModel output statistic on the Cramer-Rao lower bounds of the fit to the peak of interest as a criterion to exclude data of poor quality from the final analysis. If this statistic was >20% for the major peaks of interest, the spectrum was excluded. Since the major signals from NAAG are not resolved from those of NAA at 3T, and moreover are expected to be much less intense than those of NAA (Pouwels and Frahm, 1997), we report the combined NAA and NAAG concentration in this work (which we refer to as NAA). The results from the LCModel were corrected for CSF, WM, and GM content (partial volume effects) as previously reported (Gasparovic et al., 2006). Briefly, tissue segmentation was performed on the T1-weighted image using SPM5 (Ashburner and Friston, 2005). The individual GM, WM, and CSF maps were then registered to the spectroscopic region of interest and convolved with the theoretical 1H-MRSI point spread function (PSF) to smooth the maps to the resolution of the 1H-MRSI data. Before the convolution step, the map pixels outside of the 1H-MRSI VOI are set to zero, since protons in this region are assumed to contribute negligibly to the signal intensities within the VOI. To convolve the maps with the 1H-MRSI PSF, an inverse FT was applied to the map. This k-space matrix is multiplied by a matrix that is the inverse FT of the 1H-MRSI PSF. The latter matrix is constructed as a Hamming function (used as the spatial filter for the 1H-MRSI data) with radius 24, normalized to a peak amplitude of 1, and centered in a matrix of zeros with the resolution of the map (i.e., 1922 or 2562). This product is Fourier transformed, resulting in a map effectively smoothed to the resolution of the 1H-MRSI data. To obtain the fractional GM, WM, and CSF in each 1H-MRSI voxel, the pixel values of the smoothed maps are summed and normalized over the volume of each 1H-MRSI voxel for each tissue class. The resolution of the water density and relaxation-corrected water density maps are similarly reduced to that of the 1H-MRSI data by summing and normalizing the data over the volume spanned by each 1H-MRSI pixel. Finally, the LCModel results are corrected for partial volume effects in each voxel according to the equation:
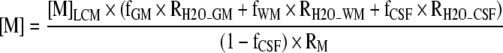 |
[1] |
where [M]LCM is the concentration in mmol per kg of MR-visible water (mmolal) as determined by the LCmodel, based on using tissue water as a concentration reference; fGM, fWM, and fCSF are the water density fractions for GM, WM, and CSF, respectively; and the RH2O terms are the relaxation attenuation factors for the water signal in each tissue class, based on reported values for T1 and T2 and the equation RH2O_y = exp[-TE/T2_H2O_y](1-exp[-TR/T1_H2O_y]), where T1_H2O_y and T2_H2O_y are the T1 and T2 relaxation times of water in compartment y, TE is the sequence echo time, and TR is the repetition time. Similarly, RM is the relaxation attenuation factor for the metabolite signal, assumed to be similar in both GM and WM.
The fractional water densities appearing in Eq. [1] are related to the tissue volume fractions obtained by tissue segmentation by taking into account the relative water densities (WD) in each volume fraction:
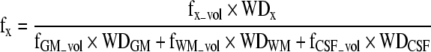 |
[2] |
where the various terms refer to the volume fractions and associated water densities of each tissue or CSF (i.e., x = GM, WM, or CSF). In this study, we used a CSF T1 value of 4 sec based on a recent report (Rooney et al., 2007) and a CSF T2 estimate of 2.47 sec based on a previous measurement at our site. Otherwise, the previously reported T1, T2, and WD values used were as follows: GM: T1 = 1.304 sec, T2 = 0.093 sec (Vymazal et al., 1999), WD = 0.78 (Kreis et al., 1993); WM: T1 = 0.660 sec, T2 = 0.073 sec (Vymazal et al., 1999); WD = 0.65 (Kreis et al., 1993); CSF: WD = 0.97 (Ashburner and Friston, 2005; Kreis et al., 1993). Estimates of metabolite T1 and T2 values at 3T were drawn from Mlynarik and associates (Mlynarik et al., 2001). The Gln T1 and T2 values were assumed to be equal to the Glu values.
After estimating metabolite concentrations in each spectroscopic voxel by the methods above, the concentrations in pure GM and WM for each subject were estimated by linear regression of the metabolite concentration against the normalized GM fraction (fGM_vol/(fGM_vol + fWM_vol) in each voxel. Extrapolation of the regression line to 0 or 1 provides an estimate of the metabolite content of pure WM or pure GM, respectively. All of the steps outlined above were performed with a program developed in MATLAB® 7.2.
Single-voxel 1H-MRS data were also corrected for partial volume effects as above, with the exception of reducing the resolution of the segmentation maps or performing linear regression. Owing to the small size of the voxel, and consequently low signal-to-noise ratio of the data, Cramer-Rao lower bounds of <20% were achieved consistently only for NAA, Cho, and Cr in this data set.
Neuropsychological assessment
All participants were administered a battery of neuropsychological tests selected to provide a comprehensive assessment of attention, working memory, processing speed, executive function, memory, and emotional status. Within each cognitive domain the relevant test scores were converted to T-scores (mean = 50, SD = 10) using published age-specific norms, and then averaged to provide an overall composite score (see Table 2 for the specific tests in each composite). The Wechsler Test of Adult Reading (WTAR) provided an estimate of overall premorbid cognitive functioning. Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971).
Table 2.
Neuropsychological Tests Administered and Organization of Specific Scores into Cognitive Composites
| Domain | Test | Score |
|---|---|---|
| Attention | Trail-Making Test A | Total time |
| Paced Auditory Serial Addition Test | T-scores from 4 blocks | |
| Stroop Interference Test (Color Word) | Total read | |
| Digit Span Forward | Total points | |
| Memory | California Verbal Learning Test II | Total, trials 1–5 |
| Short-delay free recall | ||
| Long-delay free recall | ||
| Long-delay recognition | ||
| Working memory | WAIS III: Letter-Number Sequence | Scaled score |
| WAIS III: Arithmetic | Scaled score | |
| WAIS III: Digits Backward | Total points | |
| Processing speed | Grooved Pegboard | Dominant hand time |
| Non-dominant hand time | ||
| WAIS III: Digit Symbol | Scaled score | |
| Executive function | Wisconsin Card-Sorting Test | Total errors |
| Perseverative errors | ||
| Trail-Making Test B | Total time | |
| Controlled Oral Word Association Test | Total number of words | |
| Emotional distress | Beck Depression Inventory II | Total score |
| State-Trait Anxiety Scale | Total score |
WAIS III, Wechsler Adult Intelligence Scale III.
Results
There were no significant differences between the two groups (p > 0.10) on any of the major demographic variables or for hand preference (Table 1). Additionally, independent samples t-tests of each major neuropsychological domain score revealed no significant differences between the healthy controls and mTBI groups (Table 3). However, examination of group means suggested that the mTBI patients had lower scores on the executive domain and higher scores on the emotional distress domain scores. Moreover, the effect sizes for both of these variables fell in the “large” range (Cohen, 1992). For the mTBI group scores on normative measures comprising the emotional distress domain, the Beck Depression Inventory and the State-Trait Anxiety Scale, were all within the normal range, suggesting that overt psychopathology is not a compelling explanation for the neuroimaging findings described below. As evidenced in Table 3, the poorer performance by mTBI patients on tests in the executive function domain was approximately two-thirds of a standard deviation below normal, and is consistent with their common complaints of mild cognitive problems in daily life.
Table 3.
Differences in Neuropsychological Composite t-Scores for the Mild TBI and Healthy Control Participants
| |
Mild TBI |
Healthy controls |
|
||
|---|---|---|---|---|---|
| Composite | Mean | SD | Mean | SD | p Value/effect size |
| Attention | 50.89 | 7.01 | 51.44 | 6.29 | .86/0.08 |
| Memory | 51.89 | 8.64 | 49.67 | 5.43 | .52/0.31 |
| Working memory | 48.44 | 7.72 | 49.78 | 9.47 | .75/0.15 |
| Processing speed | 48.67 | 7.71 | 48.00 | 5.22 | .83/0.10 |
| Executive | 43.78 | 6.83 | 48.22 | 6.92 | .19/0.65 |
| Emotiona | 48.33 | 6.36 | 43.33 | 7.19 | .14/0.74 |
Higher t-scores on the emotion index indicate greater emotional distress.
Significance levels determined by independent samples t-tests.
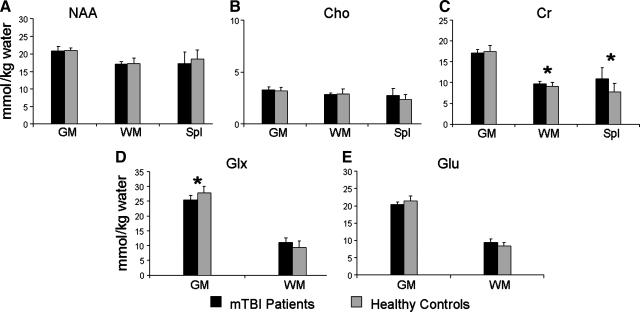
Our stringent methodological approach for neurometabolite measurements (Table 4 and Fig. 2) produced data with coefficients of variation of 3% in GM NAA and 5% in WM NAA in the healthy control subjects. Because the Glx peak includes Glu, and the concentrations of these two peaks are highly correlated (r = .78, p < 0.001 in GM, and r = 0.93, p < 0.001 in WM), we used Glx for our principal analyses to minimize the number of statistical comparisons. Specifically, group differences in neurometabolite concentrations were evaluated with a series of MANOVAs for NAA, Cho, Cr, and Glx. For the NAA, Cho, and Cr MANOVAs, the different values from GM, WM, and the splenium served as the dependent variables, with group membership being the independent variable. The splenium data were too noisy to resolve the Glx peak. Therefore only the GM and WM values were used as dependent measures for the Glx MANOVA.
Table 4.
Magnetic Resonance Spectroscopy Variables in Gray Matter (GM), White Matter (WM), and Splenium for Mild TBI Patients and Controls
| |
|
Mild TBI |
Healthy controls |
|
||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Location | Mean | SD | Range | Mean | SD | Range | p Value |
| NAA | GM | 20.84 | 1.27 | 19.63–23.61 | 20.96 | .84 | 19.54–22.14 | 0.78 |
| WM | 17.17 | .72 | 15.84–18.28 | 17.22 | 1.61 | 14.33–19.60 | 0.64 | |
| Splenium | 17.21 | 3.41 | 8.20–20.15 | 18.48 | 2.65 | 14.65–22.18 | 0.40 | |
| Cho | GM | 3.27 | .30 | 2.74–3.68 | 3.16 | .37 | 2.64–3.88 | 0.52 |
| WM | 2.84 | .13 | 2.66–3.02 | 2.90 | .46 | 2.26–3.58 | 0.99 | |
| Splenium | 2.72 | .73 | 1.96–3.98 | 2.33 | .47 | 1.63–3.03 | 0.21 | |
| Cr | GM | 17.09 | .78 | 16.20–18.85 | 17.40 | 1.51 | 16.14–21.30 | 0.68 |
| WM | 9.73 | .59 | 9.03–10.78 | 9.05 | .93 | 7.91–10.54 | 0.02 | |
| Splenium | 10.90 | 2.65 | 7.79–15.71 | 7.78 | 2.03 | 5.03–11.88 | 0.02 | |
| Glx | GM | 25.52 | 1.51 | 23.69–27.43 | 27.73 | 2.32 | 22.96–31.40 | 0.02 |
| WM | 11.00 | 1.67 | 9.16–13.69 | 9.34 | 2.23 | 5.47–13.29 | 0.08 | |
| Glu | GM | 20.36 | .73 | 18.91–21.37 | 21.44 | 1.51 | 19.04–23.61 | 0.06 |
| WM | 9.33 | 1.11 | 8.09–11.27 | 8.39 | 1.04 | 6.40–10.22 | 0.08 | |
Univariate tests were only conducted after finding significant MANOVA effects, however all values are reported (mmol/kg water).
FIG. 2.
Graphical representation of mean metabolite levels for patients with mTBI (black bars) compared to healthy controls (gray bars). Error bars indicate standard deviations. Data are presented for N-acetylaspartate (NAA), choline (Cho), creatine-phosphocreatine (Cr), glutamine (Glx), and glutamate (Glu) in gray matter (GM), white matter (WM), and splenium (Spl) (*Denotes statistically significant result).
The multivariate effect of group was not significant for MANOVAs examining NAA (p > 0.10) or Cho (p > 0.10). In contrast, the multivariate effect of group was significant for the Cr analysis (F1,15 = 5.21, p < 0.05), and a strong trend was observed for Glx (F1,17 = 3.61, p = 0.051). Univariate analyses indicated that Cr concentrations in both WM (F1,15 = 6.28, p < 0.05) and the splenium (F1,15 = 7.31, p < 0.05) were significantly greater in the mTBI group compared to the controls. Univariate tests also indicated that GM Glx concentrations were significantly lower in the mTBI group (F1,17 = 6.13, p < 0.05). However, a non-significant trend indicated that the direction of this effect was reversed for WM Glx, as patients showed greater concentrations (F1,17 = 3.42; p = 0.08). Provided with these findings, exploratory t-tests were performed to investigate whether similar results would be obtained for Glu. Consistent with the Glx results, the mTBI group displayed non-significant trends for lower GM Glu (t17 = 2.04, p = 0.058), and higher WM Glu (t17 = 1.89, p = 0.076).
Our next set of analyses evaluated whether Cr and Glx concentrations would predict the executive and emotional distress domain scores (i.e., domains with large effect sizes) collapsed across both groups of subjects. Four multiple regression analyses were conducted. The three Cr measures (GM, WM, and splenium) were used to predict executive function in one analysis and emotional distress in another. Both Cr analyses were significant. For executive function, the total model (F3,12 = 3.72, p < 0.05) was significant, with Cr from the splenium being retained as the only significant individual predictor (standardized beta = −.71, p < 0.01). For emotional distress, the total model was significant (F3,12 =3.92, p < 0.05), with Cr from the white matter being retained as the only significant individual predictor (standardized beta = .62, p < 0.05). Identical analyses were conducted with the two Glx variables; however, none of these approached statistical significance.
Finally, Vagnozzi and colleagues (2008) recently reported recovery of NAA/Cr 30 days after injury in a small sample of individuals who had suffered a concussion. Though we had limited power to examine correlations within our TBI group, and limited range in terms of chronicity, we found that overall gray plus white matter NAA/Cr was positively correlated with days post-injury at a trend level (r = 0.60, p = 0.07).
Discussion
This study of mTBI revealed perturbations in Cr, Glx, and Glu, but not NAA and Cho. Since lower white matter NAA and higher Cho have been interpreted to reflect axonal injury in past studies of TBI, an interpretation of the current findings consistent with this view would be that alterations in cerebral energy and neurotransmitter metabolism are a more fundamental abnormality in mTBI than alterations in axonal integrity. Specifically, compared to well-matched, healthy controls, mTBI patients showed elevated Cr in supraventricular WM and in the splenium, as well as reduced GM Glx and Glu. In contrast, non-significant differences were observed between the groups for both clinical and cognitive measures, consistent with the general clinical observation of mild and transient cognitive deficits following mTBI (Belanger et al., 2005; Bigler, 2008). However, large effect sizes were observed for functional deficits in executive skills and emotional distress in the mTBI patients. Hence, the current results provide confirmatory evidence that the cognitive deficits seen during the semi-acute stage (from days to 3 weeks post-injury) of mTBI are mild in nature, and these results provide preliminary evidence suggesting that 1H-MRS may be more sensitive than neuropsychological findings in predicting group differences. Moreover, Cr concentrations were associated with both executive functioning and emotional disturbances, suggesting that alterations in this metabolite may serve as a bio-marker for the mild cognitive and emotional disturbances that characterize mTBI in the semi-acute stage.
The majority of previous studies of moderately- to severely-injured patients have reported that absolute concentrations of NAA are lower and those of Cho are higher relative to control subjects (e.g., Friedman et al., 1998), findings that have been interpreted as reflecting diffuse axonal injury. Similar findings have been reported by two other MRS studies of mTBI, which focused on metabolite ratios rather than absolute concentration levels. Govindaraju and colleagues (2004) assessed ratio scores (NAA:Cr and NAA:Cho) from 25 single predominantly gray- or white-matter voxels, and reported that both ratios were reduced in mTBI patients for two of the white matter voxels. Similarly, Vagnozzi and associates (2008) recently reported reduced NAA:Cr for mTBI patients in anterior periventricular white matter. In contrast to these findings, our results indicated higher WM Cr for mTBI patients, with no statistically significant differences in WM NAA. These results for absolute metabolite concentrations suggest that previous reports of a lower NAA:Cr ratio in mTBI may be attributable to higher Cr rather than lower NAA. Vagnozzi and co-workers (2008) also observed that NAA:Cr ratios are higher in patients with additional time after injury. We observed a similar trend in our sample, with larger NAA:Cr ratios occurring with greater time after injury. Taken together, these observations highlight the dynamic nature of metabolite changes after mTBI.
Our observation that WM Cr is higher in the semi-acute phase of recovery from mTBI stands in contrast to findings that Cr is unchanged in the chronic phase of more severe injury (Friedman et al., 1998). The most likely interpretation of this differing pattern of results is that our patients were assessed at a transient state of metabolic alteration, before eventual normalization. The Cr buffer system plays a critical role in maintaining cellular ATP stores in cells with high and fluctuating energy demands, such as neurons. Anti-apoptotic, antioxidant, and osmoregulatory roles have also been proposed for Cr, and Cr dietary supplementation has been shown to improve brain function in healthy subjects and in several brain disorders (Andres et al., 2008), including TBI (Sakellaris et al., 2006; Sakellaris et al., 2008; Sullivan et al., 2000). The group differences in Cr levels observed in the present study may be related to an elevated demand for energy production after trauma, since Cr and phosphocreatine are essential for maintaining adequate ATP stores in the brain, and have been shown to be depressed in animal models of mTBI (Vagnozzi et al., 2005). However, a greater demand for Cr in its non-energy-related roles following brain injury may account for the observed elevation.
While elevated Glx or Glu has been reported following more severe TBI injury, potentially indicating excitotoxicity (Alves et al., 2005; Ashwal et al., 2004; Babikian et al., 2006; Koura et al., 1998; Shutter et al., 2004; Vespa et al., 1998; Zauner et al., 1996), the present study revealed that Glx was significantly lower in GM and slightly higher (at a trend level) in WM after mTBI. A similar type of effect was also observed for Glu in supplementary analyses. Low Glx or Glu levels have been reported in some chronic pathologies, including multiple sclerosis (Chard et al., 2002), schizophrenia (Tayoshi et al., 2008), and major depression (Capizzano et al., 2007), but to our knowledge, not in otherwise healthy-appearing brain tissue following mTBI. In one recent study of rodent models of mild and severe brain injury induced by graded microinjection of amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, a decrease in the activity of glutaminase, the enzyme that converts Gln to Glu in the presynaptic neuron, and an increase in the activity of glutamine synthetase, the enzyme that converts Gln to Glu in astrocytes, was observed (Ramonet et al., 2004). The authors hypothesized that this alteration may be a protective adaptation against glutamate excitotoxicity. However, neither Glu nor Gln levels were measured in that study, and it is not clear that the present results can be interpreted similarly. Nonetheless, given the complexity and delicate regulation of neural Glu-Gln metabolism in normally-functioning tissue (Ramonet et al., 2004), downregulating or diverting Glu production to alternative pathways after mild injury, such as toward glutathione or energy production, might represent an adaptive response to avoid excitotoxicity following mTBI. The finding of lower GM Glx and Glu might also be consistent with a temporary diversion of Glu from dendritic regions for protective or repair purposes.
One important limitation of the current study was the sample size. Though similar to the sample sizes in previously published 1H-MRS studies on mTBI, this sample was small, and thus our findings need to be replicated in a larger independent sample. The effects of our small sample size were most notable in our neuropsychological results, where effect sizes for both emotional and executive functions were in the large range (Cohen et al., 1992), and would have likely reached conventional levels of statistical significance with a larger sample size. Likewise our observation that NAA:Cr ratios correlated with time post-injury might also reach conventional levels of statistical significance with the addition of a few more patients to the sample. Nonetheless, in spite of the sample size, significant differences were observed between mTBI patients and matched controls for several 1H-MRS metabolites, indicating the sensitivity of 1H-MRS to metabolite alterations that might serve as biomarkers of mTBI. To maximize the sensitivity of our methods, an effort was made to correct the metabolite and water reference signals for partial volume effects, with greater attention given to varying tissue water density and relaxation times than has been given previously (Gasparovic et al., 2006). It is worth noting that the molal concentrations (mol/kg of tissue water) reported in this study will be inherently higher than the molar values (moles/volume of tissue) reported by others, and will depend on assumptions concerning the tissue water density and relaxation times (Gasparovic et al., 2006). However, the use of tissue water as a concentration standard may provide a truer estimate of the effective concentration of the metabolite, and obviates many of the sources of error inherent to the use of external references. This approach allowed us to observe group differences between Glx and Glu levels that were in the range of 5–12%, while NAA levels differed by 1% or less.
In summary, the present study provides preliminary evidence for the sensitivity of 1H-MRS to detect subtle perturbations in neurometabolism following mTBI, and that the direction of these effects may differ in gray and white matter. The observation of lower gray-matter Glx and Glu, with a trend for higher white-matter Glx and Glu, may be consistent with a temporary diversion of Glu from dendritic regions for protective or repair purposes following mild injury. However, this hypothesis is highly speculative and will require further testing in animal models of injury. Our findings of higher Cr in mTBI subjects may reflect greater energy demands after injury, and furthermore suggest that interpretations of NAA:Cr must be made with the appropriate caveats in this population. Future studies should utilize prospective imaging protocols to determine if these metabolite levels normalize as a function of the spontaneous recovery process that is typical in mTBI.
Acknowledgments
This research was supported by grants from The Mind Research Network (DOE grant no. DE-FG02-99ER62764), and from the National Institutes of Health (grant R24HD050836 and R21-NS064464-01A1). Special thanks to Diana South and Cathy Smith for assistance with data collection.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Alves O.L. Bullock R. Clausen T. Reinert M. Reeves T.M. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: An experimental and clinical study. J. Neurotrauma. 2005;22:733–749. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Andres R.H. Ducray A.D. Schlattner U. Wallimann T. Widmer H.R. Functions and Effects of creatine in the central nervous system. Brain Res. Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Arciniegas D.B. Anderson C.A. Topkoff J. McAllister T.W. Mild traumatic brain injury: A neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashwal S. Holshouser B. Tong K. Serna T. Osterdock R. Gross M. Kido D. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J. Neurotrauma. 2004;21:1539–1552. doi: 10.1089/neu.2004.21.1539. [DOI] [PubMed] [Google Scholar]
- Babikian T. Freier M.C. Ashwal S. Riggs M.L. Burley T. Holshouser B.A. MR spectroscopy: Predicting long-term neuropsychological outcome following pediatric TBI. J. Magn. Reson. Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Baslow M.H. Hrabe J. Guilfoyle D.N. Dynamic relationship between neurostimulation and N-acetylaspartate metabolism in the human visual cortex: Evidence that NAA functions as a molecular water pump during visual stimulation. J. Mol. Neurosci. 2007;32:235–245. doi: 10.1007/s12031-007-0049-9. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Curtiss G. Demery J.A. Lebowitz B.K. Vanderploeg R.D. Factors moderating neuropsychological outcomes following mild traumatic brain injury: A meta-analysis. J. Int. Neuropsychol. Soc. 2005;11:215–227. doi: 10.1017/S1355617705050277. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J. Int. Neuropsychol. Soc. 2008;14:1–22. doi: 10.1017/S135561770808017X. [DOI] [PubMed] [Google Scholar]
- Brooks W.M. Friedman S.D. Gasparovic C. Magnetic resonance spectroscopy in traumatic brain injury. J. Head Trauma Rehabil. 2001;16:149–164. doi: 10.1097/00001199-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Capizzano A.A. Jorge R.E. Acion L.C. Robinson R.G. In vivo proton magnetic resonance spectroscopy in patients with mood disorders: A technically oriented review. J. Magn. Reson. Imaging. 2007;26:1378–1389. doi: 10.1002/jmri.21144. [DOI] [PubMed] [Google Scholar]
- Chard D.T. Griffin C.M. McLean M.A. Kapeller P. Kapoor R. Thompson A.J. Miller D.H. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:2342–2352. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- Cimatti M. Assessment of metabolic cerebral damage using proton magnetic resonance spectroscopy in mild traumatic brain injury. J. Neurosurg. Sci. 2006;50:83–88. [PubMed] [Google Scholar]
- Cohen B.A. Inglese M. Rusinek H. Babb J.S. Grossman R.I. Gonen O. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 2007;28:907–913. [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- DeFord S.M. Wilson M.S. Rice A.C. Clausen T. Rice L.K. Barabnova A. Bullock R. Hamm R.J. Repeated mild brain injuries result in cognitive impairment in B6c3f1 Mice. J. Neurotrauma. 2002;19:427–438. doi: 10.1089/08977150252932389. [DOI] [PubMed] [Google Scholar]
- Di X. Gordon J. Bullock R. Fluid percussion brain injury exacerbates glutamate-induced focal damage in the rat. J. Neurotrauma. 1999;16:195–201. doi: 10.1089/neu.1999.16.195. [DOI] [PubMed] [Google Scholar]
- Friedman S.D. Brooks W.M. Jung R.E. Hart B.L. Yeo R.A. Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 1998;19:1879–1885. [PMC free article] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Corkill R.G. Cadoux-Hudson T.A. Rajagopalan B. Styles P. Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain. 2000a;123(Pt. 10):2046–2054. doi: 10.1093/brain/123.10.2046. [DOI] [PubMed] [Google Scholar]
- Garnett M.R. Blamire A.M. Rajagopalan B. Styles P. Cadoux-Hudson T.A. Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: A magnetic resonance spectroscopy study. Brain. 2000b;123(Pt. 7):1403–1409. doi: 10.1093/brain/123.7.1403. [DOI] [PubMed] [Google Scholar]
- Gasparovic C. Song T. Devier D. Bockholt H.J. Caprihan A. Mullins P.G. Posse S. Jung R.E. Morrison L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Globus M.Y. Alonso O. Dietrich W.D. Busto R. Ginsberg M.D. Glutamate release and free radical production following brain injury: Effects of posttraumatic hypothermia. J. Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Govindaraju V. Gauger G.E. Manley G.T. Ebel A. Meeker M. Maudsley A.A. Volumetric proton spectroscopic imaging of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 2004;25:730–737. [PMC free article] [PubMed] [Google Scholar]
- Hartley C.E. Varma M. Fischer J.P. Riccardi R. Strauss J.A. Shah S. Zhang S. Yang Z.J. Neuroprotective effects of erythropoietin on acute metabolic and pathological changes in experimentally induced neurotrauma. J. Neurosurg. 2008;109:708–714. doi: 10.3171/JNS/2008/109/10/0708. [DOI] [PubMed] [Google Scholar]
- Hattingen E. Raab P. Franz K. Lanfermann H. Setzer M. Gerlach R. Zanella F.E. Pilatus U. Prognostic value of choline and creatine in who grade II gliomas. Neuroradiology. 2008;50:759–767. doi: 10.1007/s00234-008-0409-3. [DOI] [PubMed] [Google Scholar]
- Hoge C.W. McGurk D. Thomas J.L. Cox A.L. Engel C.C. Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Inglese M. Li B.S. Rusinek H. Babb J.S. Grossman R.I. Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn. Reson. Med. 2003;50:190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- Jennett B. Epidemiology of head injury. Arch. Dis. Child. 1998;78:403–406. doi: 10.1136/adc.78.5.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov I. Fleysher L. Babb J.S. Silver J.M. Grossman R.I. Gonen O. Characterizing “mild” in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007;21:1147–1154. doi: 10.1080/02699050701630383. [DOI] [PubMed] [Google Scholar]
- Koura S.S. Doppenberg E.M. Marmarou A. Choi S. Young H.F. Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir. Suppl. 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- Kreis R. Ernst T. Ross B.D. Absolute quantitation of water and metabolites in the human brain. II. Metabolite concentrations. J. Magn. Reson. B. 1993;102:9–19. [Google Scholar]
- Matser J.T. Kessels A.G. Jordan B.D. Lezak M.D. Troost J. Chronic traumatic brain injury in professional soccer players. Neurology. 1998;51:791–796. doi: 10.1212/wnl.51.3.791. [DOI] [PubMed] [Google Scholar]
- McKenna M.C. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Mlynarik V. Gruber S. Moser E. Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. N.M.R. Biomed. 2001;14:325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- Moffett J.R. Ross B. Arun P. Madhavarao C.N. Namboodiri A.M. N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins P.G. Chen H. Xu J. Caprihan A. Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn. Reson. Med. 2008;60:964–969. doi: 10.1002/mrm.21696. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S. Cvoro V. Armitage P.A. Marshall I. Bastin M.E. Wardlaw J.M. Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke. 2008;39:2467–2469. doi: 10.1161/STROKEAHA.107.507020. [DOI] [PubMed] [Google Scholar]
- Nilsson P. Hillered L. Ponten U. Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J. Cereb. Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palmer A.M. Marion D.W. Botscheller M.L. Swedlow P.E. Styren S.D. DeKosky S. T. Traumatic brain injury-induced excitotoxicity assessed in a controlled cortical impact model. J. Neurochem. 1993;61:2015–2024. doi: 10.1111/j.1471-4159.1993.tb07437.x. [DOI] [PubMed] [Google Scholar]
- Pouwels P.J. Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. N.M.R. Biomed. 1997;10:73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Ramonet D. Rodriguez M.J. Fredriksson K. Bernal F. Mahy N. In vivo neuroprotective adaptation of the glutamate/glutamine cycle to neuronal death. Hippocampus. 2004;14:586–594. doi: 10.1002/hipo.10188. [DOI] [PubMed] [Google Scholar]
- Rooney W.D. Johnson G. Li X. Cohen E.R. Kim S.G. Ugurbil K. Springer C.S., Jr. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 2007;57:308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- Sakellaris G. Kotsiou M. Tamiolaki M. Kalostos G. Tsapaki E. Spanaki M. Spilioti M. Charissis G. Evangeliou A. Prevention of complications related to traumatic brain injury in children and adolescents with creatine administration: An open label randomized pilot study. J. Trauma. 2006;61:322–329. doi: 10.1097/01.ta.0000230269.46108.d5. [DOI] [PubMed] [Google Scholar]
- Sakellaris G. Nasis G. Kotsiou M. Tamiolaki M. Charissis G. Evangeliou A. Prevention of traumatic headache, dizziness and fatigue with creatine administration. A pilot study. Acta Paediatr. 2008;97:31–34. doi: 10.1111/j.1651-2227.2007.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutter L. Tong K.A. Holshouser B.A. Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. J. Neurotrauma. 2004;21:1693–1705. doi: 10.1089/neu.2004.21.1693. [DOI] [PubMed] [Google Scholar]
- Shutter L. Tong K.A. Lee A. Holshouser B.A. prognostic role of proton magnetic resonance spectroscopy in acute traumatic brain injury. J. Head Trauma Rehabil. 2006;21:334–349. doi: 10.1097/00001199-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Son B.C. Park C.K. Choi B.G. Kim E.N. Choe B.Y. Lee K.S. Kim M.C. Kang J.K. Metabolic changes in pericontusional oedematous areas in mild head injury evaluated by 1h MRS. Acta Neurochir. Suppl. 2000;76:13–16. doi: 10.1007/978-3-7091-6346-7_3. [DOI] [PubMed] [Google Scholar]
- Sullivan P.G. Geiger J.D. Mattson M.P. Scheff S.W. Dietary supplement creatine protects against traumatic brain injury. Ann Neurol. 2000;48:723–729. [PubMed] [Google Scholar]
- Tavazzi B. Vagnozzi R. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: Oxidative and nitrosative stresses—part II. Neurosurgery. 2007;61:390–395. doi: 10.1227/01.neu.0000255525.34956.3f. discussion 395–396. [DOI] [PubMed] [Google Scholar]
- Tayoshi S. Sumitani S. Taniguchi K. Shibuya-Tayoshi S. Numata S. Iga J.I. Nakataki M. Ueno S.I. Harada M. Ohmori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy ((1)H-MRS) Schizophr Res. 2008;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Thierry A.G.M. Huisman T.A. Schwamm L.H. Schaefer P.W. Koroshetz W.J. Shetty-Alva N. Ozsunar Y. Wu O. Sorensen A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. A.J.N.R. Am. J. Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Cimatti M. Amorini A.M. Donzelli S. Delfini R. Lazzarino G. Hypothesis of the postconcussive vulnerable brain: Experimental evidence of its metabolic occurrence. Neurosurgery. 2005;57:164–171. doi: 10.1227/01.neu.0000163413.90259.85. discussion 164–171. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Floris R. Ludovici A. Marziali S. Tarascio G. Amorini A.M. Di Pietro V. Delfini R. Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: A pilot 1h-magnetic resonance spectroscopic study in concussed athletes—Part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. discussion 1295–1286. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Tavazzi B. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: Mitochondrial-related impairment—Part I. Neurosurgery. 2007;61:379–388. doi: 10.1227/01.NEU.0000280002.41696.D8. discussion 388–379. [DOI] [PubMed] [Google Scholar]
- Vespa P. Prins M. Ronne-Engstrom E. Caron M. Shalmon E. Hovda D.A. Martin N.A. Becker D.P. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. J. Neurosurg. 1998;89:971–982. doi: 10.3171/jns.1998.89.6.0971. [DOI] [PubMed] [Google Scholar]
- Vymazal J. Righini A. Brooks R.A. Canesi M. Mariani C. Leonardi M. Pezzoli G. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: Relation to iron content. Radiology. 1999;211:489–495. doi: 10.1148/radiology.211.2.r99ma53489. [DOI] [PubMed] [Google Scholar]
- Yeo R.A. Phillips J.P. Jung R.E. Brown A.J. Campbell R.C. Brooks W.M. Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. J. Neurotrauma. 2006;23:1427–1435. doi: 10.1089/neu.2006.23.1427. [DOI] [PubMed] [Google Scholar]
- Zauner A. Bullock R. Kuta A.J. Woodward J. Young H.F. Glutamate release and cerebral blood flow after severe human head injury. Acta Neurochir Suppl. 1996;67:40–44. doi: 10.1007/978-3-7091-6894-3_9. [DOI] [PubMed] [Google Scholar]