Abstract
Elderly traumatic brain injury (TBI) patients have higher rates of mortality and worse functional outcome than non-elderly TBI patients. The mechanisms involved in poor outcomes in the elderly are not well understood. Hypoxia-inducible factor–1 alpha (HIF-1α) is a basic helix-loop-helix transcription factor that modulates expression of key genes involved in neuroprotection. In this study, we studied the expression of HIF-1α and its target survival genes, heme oxygenase–1 (HO-1), vascular endothelial growth factor (VEGF), and erythropoietin (EPO) in the brains of adult versus aged mice following controlled cortical impact (CCI) injury. Adult (5–6 months) and aged (23–24 months) C57Bl/6 mice were injured using a CCI device. At 72 h post-injury, mice were sacrificed and the injured cortex was used for mRNA and protein analysis using real-time reverse transcription—polymerase chain reaction (RT-PCR) and Western blotting protocols. Following injury, HIF-1α, HO-1, and VEGF showed upregulation in both the young and aged mice, but in the aged animals the increase in HIF-1α and VEGF in response to injury was much lower than in the adult injured animals. EPO was upregulated in the adult injured brain, but not in the aged injured brain. These results support the hypothesis that reduced expression of genes in the HIF-1α neuroprotective pathway in aging may contribute to poor prognosis in the elderly following TBI.
Key words: aging, erythropoietin, HIF-1α, heme oxygenase–1, neuroprotection, traumatic brain injury, VEGF
Introduction
Traumatic brain injury (TBI) is a common cause of death and disability throughout the world. In the United States alone, someone suffers from a TBI every 15 seconds—resulting in more than 1.2 million injuries, 50,000 deaths, and 80,000–90,000 new cases of long-term disability each year (Langlois et al., 2005). The elderly population has been observed to be at a particularly higher risk of TBI (Frankel et al., 2006; Livingston et al., 2005; Rapoport et al., 2006; Testa et al., 2005; Yatsiv et al., 2005). In the past decade, there has been a 21% increase in TBI events in individuals over the age of 65 (Adekoya et al., 2002), and the incidence of TBI doubles with every decade after the age of 65 (Galbraith 1987; Leblanc et al., 2006; Pennings et al., 1993). The aged population also has two times higher hospitalization and mortality rates from TBI and its complications than young and adult victims (Rutland-Brown et al., 2006), and the hospital stay is longest for elderly patients with moderate or severe TBI (Leblanc et al., 2006). In addition, the functional outcome and long-term recovery is worse for these patients when compared with younger patients (Mosenthal et al., 2004; Susman et al., 2002; Thompson et al., 2006).
Age has also been found to be an independent predictor of mortality and poor functional outcome in the geriatric human population with TBI (Mosenthal et al., 2002). However, nearly all preclinical studies of TBI mechanisms have focused on adults, despite the fact that the incidence is higher and outcomes are worse in the aged. Many factors can contribute to poor outcomes after TBI in the elderly. Over the last few years, studies from several laboratories, including ours, have shown that aging can substantially exaggerate inflammatory responses (Kyrkanides et al., 2001; Sandhir et al., 2004, 2008), enhance neurodegeneration and behavioral impairments (Onyszchuk et al., 2008), disrupt blood-brain barrier (BBB) function (Campbell et al., 2007; Onyszchuk et al., 2008), increase oxidative stress (Shao et al., 2006), and alter neurotrophin metabolism and signaling (Williams et al., 2006), all of which may have detrimental consequences on recovery of the nervous system. Interestingly, the role of mechanisms involved in neuroprotective responses in the aging brain after TBI has not been fully investigated.
Hypoxia and hypotension are both common findings following TBI, occurring with a frequency of up to 46% (Thomas et al., 2000). Differential blood flow in traumatized brain results in a gradient of tissue oxygenation from core to penumbra and to peri-infarct region (Belayev et al., 1997; Geddes and Whitwell, 2004; Heiss et al., 1997; Liu et al., 2004; Longhi et al., 2007; Zhao et al., 1997). Exposing cells to low oxygen triggers hypoxic pathways centered on the regulated expression of hypoxia-inducible factor–1 alpha (HIF-1α). HIF-1α is thought to be one of the most crucial signaling molecules in tissue responses to hypoxia, as it regulates many genes that are important in promoting cell survival such as erythropoietin (EPO) (Siren et al., 2001), glucose transporters (Bergeron et al., 1999), vascular endothelial growth factor (VEGF) (Jin et al., 2000), and heme oxygenase—1 (HO-1) (Lee et al., 1997). In stroke models, penumbra and peri-infarct regions have been shown to be particularly rich in HIF-1α (Bergeron et al., 1999; Marti et al., 2000). It has been observed that increasing HIF-1α expression protects neurons from hypoxic injury (Siddiq et al., 2005), while specific inactivation of neuronal HIF-1α worsens hypoxic brain injury (Baranova et al., 2007). HIF-1α and its target genes have been shown to be upregulated in spinal cord injury (Xiaowei et al., 2006), and recombinant human EPO treatment has been shown to be neuroprotective in mice following closed head injury (Yatsiv et al., 2005). This pathway is significant in TBI because pO2 monitoring has shown that hypoxia is a significant co-event in TBI patients (Geddes and Whitwell, 2004; Longhi et al., 2007). Despite these important roles of HIF-1α in neuronal survival and death in hypoxia, the mechanism of HIF-1α regulation in TBI has not been investigated, and the potential changes in this response in the elderly are unknown. The present study has been designed to test the hypothesis that aging impacts the neuroprotective molecules in the HIF-1α pathway, reducing the ability of the cells to respond to hypoxic environment and rendering them less able to recover from such an insult. Understanding the role of the HIF-1α pathway may help to establish specific therapeutic strategies to improve the outcome of elderly patients following TBI.
Methods
Animals
Male C57BL/6 mice were obtained from Charles River (Wilmington, MA) from the contracted colony maintained by the National Institute on Aging (NIA) in a Specific Pathogen-Free (SPF) barrier facility. Two age groups of mice were used: 5–6 months (adult) and 22–24 months (aged). Body weight of the mice was 28–32 g. Animals were housed individually in the AAALAC-accredited Laboratory Animal Resources (LAR) of the University of Kansas Medical Center on a 12:12-h light/dark cycle, with free access to standard rodent diet (8604; Harlan Teklad Laboratories, Madison, WI) and water. All the procedures were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee, and adhered to the NIH Guide for the Care and Use of Laboratory Animals. A total of 44 mice were used in the study (adult, n = 20; aged, n = 24).
Traumatic brain injury
The injury device and surgical procedures used for administration of controlled cortical impact (CCI) injury were performed as previously described (Onyszchuk et al., 2007). Briefly, mice were anesthetized with 4% isoflurane in 78% nitrogen and 21% oxygen, placed in a stereotactic frame, and maintained on 1–2% isoflurane with mask ventilation and spontaneous breathing. Breathing and temperature were monitored throughout surgery. After midline incision, a craniotomy (approximately 3.5 mm in diameter) was made lateral to the mid-sagittal suture, with center coordinates: AP = 0; ML = + 2.0 rostral to bregma. The mouse was subjected to a moderate TBI by the CCI device, with tip 3.0 mm in diameter, compression depth of 1.0 mm, velocity of 1.5 m/sec, and contact time of 85 msec. The tip contact area included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas. After surgery, the scalp was reapproximated and sutured, anesthesia was discontinued, and mice were allowed to recover in a temperature-controlled environment. At 72 h postoperatively, the animals were sacrificed. Age-matched uninjured mice were used as controls, because even a small injury to the skull has been shown to cause BBB disruption (Stokely, Orr 2008). The 72-h interval allows for maximal swelling and inflammatory responses to occur (Clifton et al., 1980; McD Anderson and Opeskin, 1998; Unterberg et al., 1997). We also observed maximum microglial activation and BBB opening at 72 h after CCI injury (Onyszchuk et al., 2008; Sandhir et al., 2008).
Real-time RT-PCR
Animals were anesthetized and decapitated, brains were removed and placed in RNA later (Ambion, Austin, TX) for preservation of RNA. Ipsilateral cortex was dissected into a coronal block extending 2.5 mm rostral and 2.5 mm caudal from the injury epicenter using a mouse brain matrix (Harvard Apparatus, Holliston, MA). This block was further dissected into a 5-mm-wide sample centered on the injury epicenter. The tissue sampled by this method is comparable in adult and aged mice because they demonstrate the same lesion volume following TBI using this injury paradigm (Onyszchuk et al., 2008). Total RNA was prepared using mirVana miRNA Isolation kit (Ambion, Austin, TX). Purity and concentration of the samples were assessed using a Bioanalyzer 2100 (Agilent, Santa Clara, CA).
Real-time PCR was performed on an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). Specific quantitative assays for HIF-1α, HO-1, VEGF, and EPO were carried out using SYBR Green dye. The sequences of all the primers pairs used are given in Table 1. RNA samples were treated with DNase to eliminate potential genomic DNA contamination. Complementary DNA (cDNA) was synthesized by using 1 μg total RNA from each sample and random hexamers in a Taqman reverse transcription reaction (Applied Biosystems). 10 ng cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq-DNA polymerase, dNTPs mixture dUTP, and optimal buffer components (Applied Biosystems), and subjected to PCR amplification (one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 sec and 60°C for 1 min). The data were collected and analyzed with Sequence Detection Software 2.0 (Applied Biosystems). The resulting amplicon products were visualized on an agarose gel to verify size and specificity of RT-PCR reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene. Relative gene expression was determined using the delta-delta Ct method (Pfaffl et al., 2002).
Table 1.
Primer Sequences Used for Real-Time RT-PCR
| Gene | Primer sequence | Position | Amplicon size (bp) | |
|---|---|---|---|---|
| HIF-1 α | Sense | 5′-TCA-CCA-GAC-AGA-GCA-GGA-AA-3′ | 2292–2311 | 187 |
| NM 010431 | Anti-sense | 5′-CTT-GAA-AAA-GGG-AGC-CAT-CA-3′ | 2459–2478 | |
| VEGF | Sense | 5′-TTA-CTG-CTG-TAC-CTC-CAC-C-3′ | 1061–1079 | 189 |
| NM 001025250 | Anti-sense | 5′-ACA-GGA-CGG-CTT-GAA-GAT-G-3′ | 1231–1249 | |
| HO-1 | Sense | 5′-CGC-CTT-CCT-GCT-CAA-CAT-T-3′ | 743–761 | 62 |
| NM 010442 | Anti-sense | 5′-TGT-GTT-CCT-CTG-TCA-GCA-TCA-C-3′ | 783–804 | |
| EPO | Sense | 5′-CCT-GTC-CCT-GCT-CTC-AGA-AGC-3′ | 347–367 | 177 |
| NM 007942 | Anti-sense | 5′-GTG-GTA-TCT-GGA-GGC-GAC-ATC-3′ | 503–523 | |
| GAPDH | Sense | 5′-ATG-ACA-TCA-AGA-AGG-TGG-TG-3′ | 769–788 | 177 |
| XR003802 | Anti-sense | 5′-CAT-ACC-AGG-AAA-TGA-GCT-TG-3′ | 926–945 |
Western blot analysis
Proteins for Western blot analysis were isolated from fresh-frozen cortex, dissected by the same method described above, with T-PER extraction reagent (Pierce Biotechnology, Rockford, IL) containing mammalian protein inhibitor cocktail (P8340; Sigma Aldrich, St. Louis, MO) and 0.1 mM phenylmethylsulfonyl fluoride (Sigma Aldrich). Protein concentrations were determined using micro-bicinchoninic method (Pierce Biotechnology). For Western blot analysis, 30 μg of protein was fractionated by 12% SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA), and probed using a primary monoclonal antibody to HIF-1α (1:1000; sc-10790, Santa Cruz Biotechnology, Santa Cruz, CA), HO-1 (1:1000, AB 1284; Chemicon International Inc., Temecula, CA), VEGF (1:200, sc-152; Santa Cruz Biotechnology) or EPO (1:1000, sc-7956; Santa Cruz Biotechnology). Bands were visualized by the addition of IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA) and Alexa 680 (Invitrogen, Eugene, OR)–conjugated secondary antibodies using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Relative band intensity was determined using Odyssey software, version 2.0 (LI-COR).
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM) of n determinations. Comparisons among means of groups were made with one-tailed heteroscedastic Student's t-test using GraphPad InStat, version 3.06, for Windows (GraphPad Software, San Diego, CA). All p-values less than 0.05 were considered significant.
Results
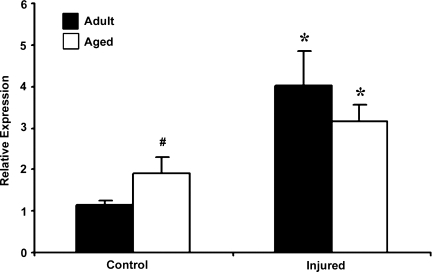
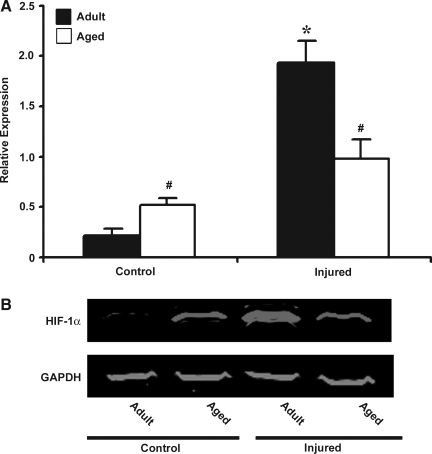
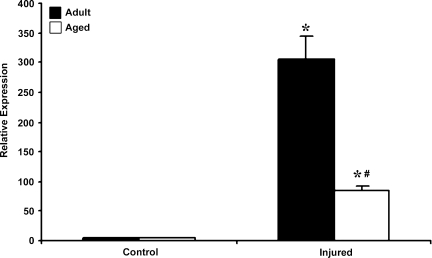
The two purposes of this study were to determine whether HIF-1α–related genes respond to TBI and whether the response changes in aged mice. Basal HIF-1α mRNA expression was significantly higher in the aged brain. In response to injury, HIF-1α mRNA expression increased in both adult and aged brain (Fig. 1). The increase in HIF-1α mRNA expression post-injury was 3.5-fold over baseline levels in the adult brain, compared to 1.67-fold in the aged brain. Immunoblot analysis was performed to observe whether these changes in transcription result in increased HIF-1α protein levels (Fig. 2). This analysis showed that basal HIF-1α protein levels were higher in the aged cortex when compared to adult cortex, but the increase in HIF-1α protein expression following injury was significantly reduced in the aged animals compared with the adults, demonstrating an impaired HIF-1α response in the aged brain.
FIG. 1.
Expression of HIF-1α. mRNA in cerebral cortex of adult and aged mice 3 days post-injury analyzed by real-time reverse transcription—polymerase chain reaction (RT-PCR). Real-time reactions were performed in duplicate for both the target gene and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as a housekeeping control. Relative expression was calculated using delta-delta Ct method. Data are presented as mean ± standard error of the mean (SEM; n = 6/group). Statistical significance, Student's t-test: *p < 0.05 injured versus respective control; #p < 0.05 aged versus adult.
FIG. 2.
Western blot analysis of HIF-1α in protein extract from cerebral cortex of adult and aged mice 3 days post-injury. (A) Relative levels of protein based on optical density normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. Data are presented as mean ± SEM (n = 3/group). (B) Representative Western blot for HIF-1α, along with GAPDH used as loading control. Statistical significance, Student's t-test: *p < 0.05 injured versus respective control; #p < 0.05 aged versus adult.
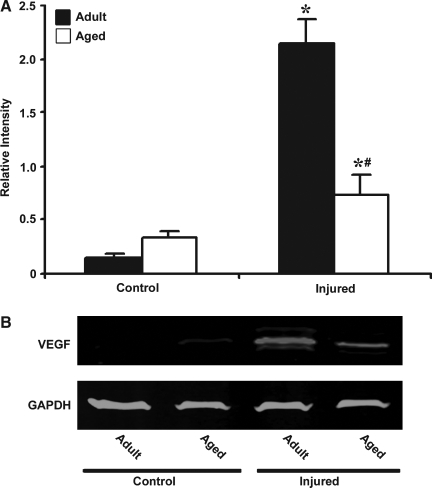
VEGF, one of the major target genes regulated by HIF-1α, is a potent angiogenic and neuroprotective growth factor that plays an important role in brain repair. We observed that basal VEGF mRNA levels were 1.5-fold higher in aged than in adult mice (Fig. 3). After injury there was a significant increase in the expression of VEGF mRNA in both adult (45-fold) and aged (15-fold) cortex. However, the response to injury was reduced in the aged brain when compared to the adult brain (p < 0.05). Immunoblot analysis confirmed reduced expression of VEGF protein following injury in the aged mice (Fig. 4).
FIG. 3.
Expression of vascular endothelial growth factor (VEGF) mRNA in cerebral cortex of adult and aged mice 3 days post-injury analyzed by real-time reverse transcription—polymerase chain reaction (RT-PCR). Real-time reactions were performed in duplicate for both the target gene and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as a housekeeping control. Relative expression was calculated using delta-delta Ct method. Data are presented as mean ± standard error of the mean (SEM; n = 6/group). Statistical significance, Student's t-test: *p < 0.05 injured versus respective control; #p < 0.05 aged versus adult.
FIG. 4.
Western blot analysis of vascular endothelial growth factor (VEGF) in protein extract from cerebral cortex of adult and aged mice 3 days post-injury. (A) Relative levels of protein based on optical density normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. Data are presented as mean ± standard error of the mean (SEM; n = 3/group). (B) Representative Western blot for VEGF along with GAPDH used as loading control. Statistical significance, Student's t-test: *p < 0.05 injured versus respective control; #p < 0.05 aged versus adult.
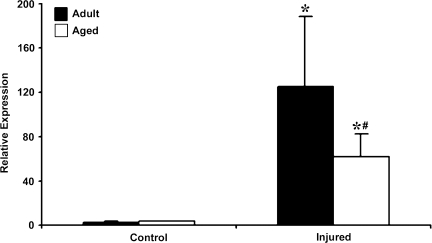
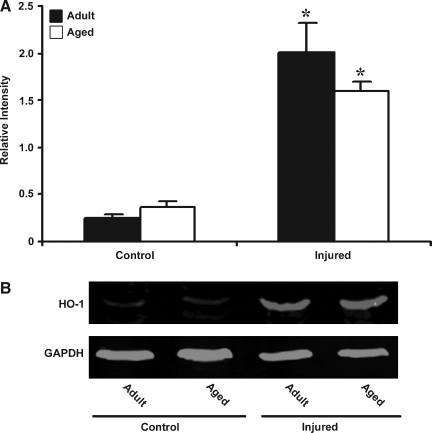
HO-1 is another target gene in the HIF-1α pathway that plays a crucial role in protecting tissues from hypoxic stress. In addition, it also has potent anti-inflammatory properties that have been reported in various models of tissue injury. No difference was observed in the basal expression of HO-1 mRNA in the adult and aged brain (Fig. 5). However, HO-1 expression was upregulated in both adult (82.8-fold) and aged brain (18-fold) following injury. As is the case with HIF-1α, the magnitude of increase in HO-1 mRNA was significantly lower in the aged injured cortex than in the adult. However, HO-1 protein levels analyzed by Western blot analysis were not significantly different in aged cortex than adult cortex following injury (Fig. 6).
FIG. 5.
Expression of heme oxygenase–1 (HO-1) mRNA in cerebral cortex of adult and aged mice 3 days post-injury analyzed by real-time reverse transcription—polymerase chain reaction (RT-PCR). Real-time reactions were performed in duplicate for both the target gene and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used as a housekeeping control. Relative expression was calculated using delta-delta Ct method. Data are presented as mean ± standard error of the mean (SEM; n = 6/group). Statistical significance, Student's t-test: *p < 0.05 injured versus respective control; #p < 0.05 aged versus adult.
FIG. 6.
Western blot analysis of heme oxygenase–1 (HO-1) in protein extract from cerebral cortex of adult and aged mice 3 days post-injury. (A) Relative levels of protein based on optical density normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. Data are presented as mean ± standard error of the mean (SEM; n = 3/group). (B) Representative Western blot for HO-1 along with GAPDH used as loading control. Statistical significance, Student's t-test: *p < 0.05 injured versus respective control.
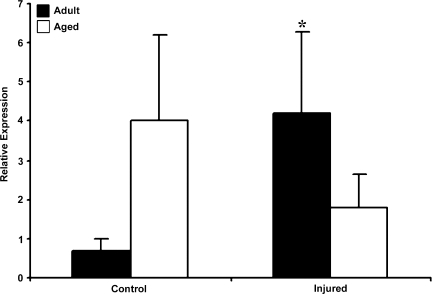
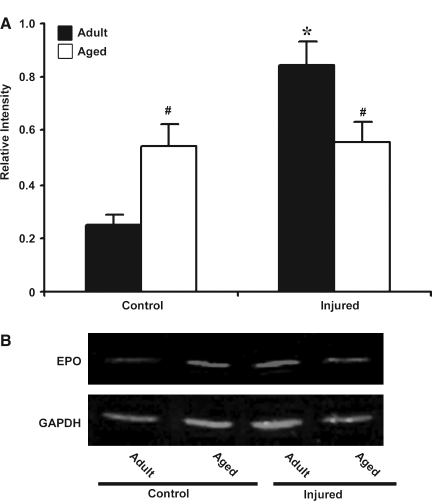
EPO, a hemotopoietic growth factor, has neuroprotective actions and is also regulated by HIF-1α. Real-time RT-PCR for EPO mRNA was performed in adult and aged cortex following TBI (Fig. 7). There was 5.71-fold increase in basal expression of EPO mRNA in the aged brain relative to adult brain. In response to injury, the adult brain showed a 6-fold increase in EPO mRNA. However, in the aged animals there was a decline in EPO mRNA expression compared to basal and mRNA levels were 0.45-fold lower than in the aged control. The immunoblot analysis of EPO correlated with the mRNA data showing an impaired EPO response in the aged brain following TBI (Fig. 8). These results suggest that a failure to induce EPO following injury in the aged might contribute to the reduced neuroprotective response.
FIG. 7.
Expression of erythropoietin (EPO) mRNA in cerebral cortex of adult and aged mice 3 days post-injury analyzed by real-time reverse transcription—polymerase chain reaction (RT-PCR). Real-time reactions were performed in duplicate for both the target gene and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), used as a housekeeping control. The relative expression was calculated using delta-delta Ct method. Data are presented as mean ± standard error of the mean (SEM; n = 6/group). Statistical significance, Student's t-test: *p < 0.05 injured versus respective control.
FIG. 8.
Western blot analysis of erythropoietin (EPO) in protein extract from cerebral cortex of adult and aged mice 3 days post-injury. (A) Relative levels of protein based on optical density normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) signal. Data are presented as mean ±standard error of the mean (SEM; n = 3/group). (B) Representative Western blot for EPO along with GAPDH used as loading control. Statistical significance, Student's t-test: *p < 0.05 injured versus control; #p < 0.05 aged versus adult.
Discussion
The transcription factor HIF-1α is an important regulator of neuroprotective mechanisms following hypoxic insults (LaManna 2007). Induction of HIF-1α is an early cellular response to changes in oxygen homeostasis in brain, and HIF-1α activation promotes cell survival in hypoxic tissues (Sharp et al., 2001). We observed higher basal expression of HIF-1α mRNA and protein but an impaired injury response in the aged brain. HIF-1α has been shown to accumulate in the neuronal cytoplasm from cerebral cortex of aged rats (Rapino et al., 2005), in accordance with our findings of higher basal expression in the aged brain. We observed a blunted HIF-1α response in the aged brain at 3 days postinjury, a time point where our previous studies demonstrated maximal neuron death (Onyszchuk et al., 2008). It is possible that the time course of the HIF-1α response is altered in the aged brain, but this seems unlikely as the time course of other injury responses was not different in the aged brain (Sandhir et al., 2008). Upregulation of HIF-1α during the period of neuronal cell death has also been demonstrated in animal models of spinal cord injury and stroke. Xiaowei et al. (2006) observed maximal upregulation of HIF-1α and its target genes 3 days after spinal cord injury in adult rats. In a stroke model, Baranova et al. (2007) observed biphasic changes in HIF-1α, including an initial upregulation peaking at 6 h, a transient decline at 24 h, and a second upregulation with HIF-1α, which remained upregulated at days 2–8. They found that the initial upregulation of HIF-1α was associated with pro-death modulators, while the second phase was correlated with protective and/or regenerative responses. Other studies have confirmed upregulation of HIF-1α immunoreactivity in neurons in peri-infarct and infarct regions in stroke models in rodents and primates (Chavez and LaManna, 2002; Stowe et al., 2008), and that HIF-1α accumulation after stroke is attenuated during aging (Chavez and LaManna, 2003). Furthermore, there are additional precedents for age-related changes in baseline HIF-1α and HIF-1α responses in nonneural tissue. As we have demonstrated in the brain, basal HIF-1α activity increases with age in the lung, and the response to hypoxic stimulation is blunted (Hwang et al., 2007). In older rats, HIF-1α fails to accumulate following mild hypoxia (LaManna et al., 2004), and its function is impaired (Frenkel-Denkberg et al., 1999). The impaired HIF-1α injury response in the aged brain might contribute to the greater neurodegeneration and increased functional deficits after CCI in aged mice, which occurred in the absence of significant differences in lesion size (Onyszchuk et al., 2008). We have also observed increased astrocyte and microglial responses in the aged brain that, in combination with the impaired HIF-1α response, may contribute to poor outcomes (Sandhir et al., 2008). Taken together, these results suggest that HIF-1α is an important neuroprotective response to TBI and that the compromised HIF-1α response to injury in the aged brain may contribute to poor prognosis in the elderly.
One transcriptional target of HIF-1α is the gene encoding for VEGF, an angiogenic growth and survival factor for endothelial cells that also exhibits neurotrophic and neuroprotective effects (Sun et al., 2003; Wang et al., 2003). VEGF expression was upregulated after TBI in adult mice, but this response was significantly reduced in aged mice. VEGF is upregulated during many pathological events (Krum et al., 2008), and it is induced in astrocytes located in and surrounding edematous tissue following brain contusion (Suzuki et al., 2006). VEGF has been implicated in neovascularization that precedes brain tissue repair and nerve regeneration following brain injury and is required to re-establish metabolic support. Although VEGF is best known for its role as a growth factor for endothelial cells, its receptors are also expressed by neurons (Carmeliet and Storkebaum, 2002). It has been shown to prolong the lifespan of mesencephalic neurons in culture and rescues hippocampal and cortical neurons from serum deprivation, hypoxia, and glutamate-induced cell death (Taoufik et al., 2008). Our data suggest that the weak VEGF response following injury to the aged brain may also contribute to poor functional recovery following TBI in the elderly.
Induction of HIF-1α has been shown to promote the transcription of inducible HO-1 an important early response to enhanced oxidative stress in the brain. HO-1 is upregulated in response to oxidative stress, hypoxia, exposure to heavy metals, cytokines, and other stressors (Ferrandiz and Devesa 2008), and it is upregulated in the injured hemisphere after TBI (Chang et al., 2003). No difference was observed in the basal expression of HO-1 mRNA in the adult and aged brain, but the increase in HO-1 mRNA in response to injury was significantly lower in the aged brain. Age-related defects in HO-1 mRNA induction have been demonstrated in non-neuronal tissues (Ito et al., 2009). However, at the protein level, HO-1 expression was not significantly different in the aged injured brain. The difference between mRNA and protein levels might reflect a change in regulation of translation with aging, perhaps by one of the multiple mechanisms involved in regulation of HO-1 gene (Alam and Cook, 2007), including differential expression of microRNAs. MicroRNA-122 has been reported to control HO-1 expression at translational level in hepatocytes (Shan et al., 2007), and age-related changes in microRNAs have been reported (Maes et al., 2008). Our results suggest that neuroprotective effects of HO-1 are diminished in the aged-injured brain compared to the adult.
EPO is an endogenous glycoprotein hormone that has been shown to have neuroprotective properties in focal ischemia, global ischemia, neonatal hypoxia-ischemia, subarachanoid hemorrhage, and trauma (Ghezzi and Brines, 2004). There was a significant increase in basal expression of EPO in the aged brain, and the adult brain showed significant induction in EPO in response to injury. However, in the aged animals there was no significant increase in the levels of EPO following TBI. These results demonstrate a failure to induce EPO following injury in the aged brain, and that reduced response may also contribute to the reduced neuroprotective response. Studies have shown the antiapoptotic properties of EPO are related to inhibition of caspase activity, upregulation of Bcl-2 family proteins, decreased excitotoxicity (by decreased Ca2+ influx and decreased glutamate release), as well as other mechanisms (Ghezzi and Brines, 2004). EPO does not prevent necrotic cell death (Sinor and Greenberg, 2000), but it does inhibit pro-inflammatory cytokines in ischemic tissue (Villa et al., 2003). EPO therapy in human patients with focal ischemia improves neurological scores and clinical outcomes (Ehrenreich et al., 2002). Based on the present results, we hypothesize that treatments that increase EPO might be especially effective in the elderly.
Conclusion
The results from the present study demonstrate that brain responds to TBI with an increase of transcriptional factor HIF-1α and its target genes VEGF, HO-1, and EPO that help neural cells adapt to hypoxic insult and promote survival. However, the ability of the aged brain to respond to hypoxic insult is greatly diminished, and this reduced injury response may have a major impact on the course of recovery from TBI in the elderly. Our results also support the use of direct measurement of cerebral oxygenation in neurocritical care (Rose et al., 2006) and suggest that this intervention may be especially important in elderly patients. Finally, our study suggests that strategies aimed at upregulation of HIF-1α genes may prove beneficial in improving outcome following TBI in the aged brain.
Acknowledgments
We acknowledge the assistance of Eugene Gregory in carrying out the work and Eileen Roach with processing of the images. Special thanks go to Dr. Y.Y. He for his help with controlled cortical impact injury. This study was supported in part by the Steve Palermo Endowment and the National Institutes of Health (AG026482 and research core support P30 NICHD HD 02528).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adekoya N. Thurman D.J. White D.D. Webb K.W. Surveillance for traumatic brain injury deaths—United States, 1989–1998. MMWR Surveill. Summ. 2002;51:1–14. [PubMed] [Google Scholar]
- Alam J. Cook J.L. How many transcription factors does it take to turn on the heme oxygenase–1 gene? Am. J. Respir. Cell Mol. Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- Baranova O. Miranda L.F. Pichiule P. Dragatsis I. Johnson R.S. Chavez J.C. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci. 2007;27:6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belayev L. Zhao W. Busto R. Ginsberg M.D. Transient middle cerebral artery occlusion by intraluminal suture: I. Three-dimensional autoradiographic image-analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation. J. Cereb. Blood Flow Metab. 1997;17:1266–1280. doi: 10.1097/00004647-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Bergeron M. Yu A.Y. Solway K.E. Semenza G.L. Sharp F.R. Induction of hypoxia-inducible factor–1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur. J. Neurosci. 1999;11:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- Campbell S.J. Carare-Nnadi R.O. Losey P.H. Anthony D.C. Loss of the atypical inflammatory response in juvenile and aged rats. Neuropathol. Appl. Neurobiol. 2007;33:108–120. doi: 10.1111/j.1365-2990.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Storkebaum E. Vascular and neuronal effects of VEGF in the nervous system: implications for neurological disorders. Semin. Cell Dev. Biol. 2002;13:39–53. doi: 10.1006/scdb.2001.0290. [DOI] [PubMed] [Google Scholar]
- Chang E.F. Wong R.J. Vreman H.J. Igarashi T. Galo E. Sharp F.R. Stevenson D.K. Noble-Haeusslein L.J. Heme oxygenase–2 protects against lipid peroxidation-mediated cell loss and impaired motor recovery after traumatic brain injury. J. Neurosci. 2003;23:3689–3696. doi: 10.1523/JNEUROSCI.23-09-03689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez J.C. LaManna J.C. Activation of hypoxia-inducible factor–1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J. Neurosci. 2002;22:8922–8931. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez J.C. LaManna J.C. Hypoxia-inducible factor–1 alpha accumulation in the rat brain in response to hypoxia and ischemia is attenuated during aging. Arv. Exp. Med. Biol. 2003;510:337–341. doi: 10.1007/978-1-4615-0205-0_55. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Grossman R.G. Makela M.E. Miner M.E. Handel S. Sadhu V. Neurological course and correlated computerized tomography findings after severe closed head injury. J. Neurosurg. 1980;52:611–624. doi: 10.3171/jns.1980.52.5.0611. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H. Hasselblatt M. Dembowski C. Cepek L. Lewczuk P. Stiefel M. Rustenbeck H.H. Breiter N. Jacob S. Knerlich F. Bohn M. Poser W. Ruther E. Kochen M. Gefeller O. Gleiter C. Wessel T.C. De Ryck M. Itri L. Prange H. Cerami A. Brines M. Siren A.L. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz M.L. Devesa I. Inducers of heme oxygenase–1. Curr. Pharm. Des. 2008;14:473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- Frankel J.E. Marwitz J.H. Cifu D.X. Kreutzer J.S. Englander J. Rosenthal M. A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Arch. Phys. Med. Rehabil. 2006;87:57–62. doi: 10.1016/j.apmr.2005.07.309. [DOI] [PubMed] [Google Scholar]
- Frenkel-Denkberg G. Gershon D. Levy A.P. The function of hypoxia-inducible factor–1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- Galbraith S. Head injuries in the elderly. Br. Med. J. 1987;294:325. doi: 10.1136/bmj.294.6568.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J.F. Whitwell H.L. Inflicted head injury in infants. Forensic Sci. Int. 2004;146:83–88. doi: 10.1016/S0379-0738(03)00283-4. [DOI] [PubMed] [Google Scholar]
- Ghezzi P. Brines M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 2004;11(Suppl 1):S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- Heiss W.D. Graf R. Lottgen J. Ohta K. Fujita T. Wagner R. Grond M. Weinhard K. Repeat positron emission tomographic studies in transient middle cerebral artery occlusion in cats: residual perfusion and efficacy of postischemic reperfusion. J. Cereb. Blood Flow Metab. 1997;17:388–400. doi: 10.1097/00004647-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Hwang I.S. Fung M.L. Liong E.C. Tipoe G.L. Tang F. Age-related changes in adrenomedullin expression and hypoxia-inducible factor–1 activity in the rat lung and their responses to hypoxia. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:41–49. doi: 10.1093/gerona/62.1.41. [DOI] [PubMed] [Google Scholar]
- Ito Y. Betsuyaku T. Moriyama C. Nasuhara Y. Nishimura M. Aging affects lipopolysaccharide-induced upregulation of heme oxygenase–1 in the lungs and alveolar macrophages. Biogerontology. 2009;10:173–180. doi: 10.1007/s10522-008-9164-4. [DOI] [PubMed] [Google Scholar]
- Jin K.L. Mao X.O. Greenberg D.A. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc. Natl. Acad. Sci. USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krum J.M. Mani N. Rosenstein J.M. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Exp. Neurol. 2008;212:108–117. doi: 10.1016/j.expneurol.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkanides S. O'Banion M.K. Whiteley P.E. Daeschner J.C. Olschowka J.A. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J. Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- LaManna J.C. Chavez J.C. Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J. Exp. Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- LaManna J.C. Hypoxia in the central nervous system. Essays Biochem. 2007;43:139–151. doi: 10.1042/BSE0430139. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Rutland-Brown W. Thomas K.E. The incidence of traumatic brain injury among children in the United States: differences by race. J. Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Leblanc N. Chen S. Swank P.R. Levin H. Schachar R. Impairment and recovery in inhibitory control after traumatic brain injury in children: effect of age at injury, injury severity and lesion location. Brain Cogn. 2006;60:208–209. [PubMed] [Google Scholar]
- Lee P.J. Jiang B.-H. Chin B.Y. Iyer N.V. Alam J. Semenza G.L. Choi A.M.K. Hypoxia-inducible factor–1 mediates transcriptional activation of the heme oxygenase–1 gene in response to hypoxia. J. Biol. Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- Liu S. Shi H. Liu W. Furuichi T. Timmins G.S. Liu K.J. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Livingston D.H. Lavery R.F. Mosenthal A.C. Knudson M.M. Lee S. Morabito D. Manley G.T. Nathens A. Jurkovich G. Hoyt D.B. Coimbra R. Recovery at one year following isolated traumatic brain injury: a Western Trauma Association prospective multicenter trial. J. Trauma. 2005;59:1298–1304. doi: 10.1097/01.ta.0000196002.03681.18. [DOI] [PubMed] [Google Scholar]
- Longhi L. Pagan F. Valeriani V. Magnoni S. Zanier E.R. Conte V. Branca V. Stocchetti N. Monitoring brain tissue oxygen tension in brain-injured patients reveals hypoxic episodes in normal-appearing and in peri-focal tissue. Intensive Care Med. 2007;33:2136–2142. doi: 10.1007/s00134-007-0845-2. [DOI] [PubMed] [Google Scholar]
- Maes O.C. An J. Sarojini H. Wang E. Murine microRNAs implicated in liver functions and aging process. Mech. Ageing Dev. 2008;129:534–541. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Marti H.J. Bernaudin M. Bellail A. Schoch H. Euler M. Petit E. Risau W. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am. J. Pathol. 2000;156:965–976. doi: 10.1016/S0002-9440(10)64964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McD Anderson R. Opeskin K. Timing of early changes in brain trauma. Am. J. Forensic Med. Pathol. 1998;19:1–9. doi: 10.1097/00000433-199803000-00001. [DOI] [PubMed] [Google Scholar]
- Mosenthal A.C. Livingston D.H. Lavery R.F. Knudson M.M. Lee S. Morabito D. Manley G.T. Nathens A. Jurkovich G. Hoyt D.B. Coimbra R. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J. Trauma. 2004;56:1042–1048. doi: 10.1097/01.ta.0000127767.83267.33. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G. Al-Hafez B. He Y.Y. Bilgen M. Berman N.E. Brooks W.M. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J. Neurosci. Methods. 2007;160:187–196. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G. He Y.Y. Berman N.E. Brooks W.M. Detrimental effects of aging on outcome from traumatic brain injury: a behavioral, magnetic resonance imaging, and histological study in mice. J. Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Pennings J.L. Bachulis B.L. Simons C.T. Slazinski T. Survival after severe brain injury in the aged. Arch. Surg. 1993;128:784–793. doi: 10.1001/archsurg.1993.01420190083011. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. Horgan G.W. Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real–time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapino C. Bianchi G. Di Giulio C. Centurione L. Cacchio M. Antonucci A. Cataldi A. HIF-1α cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell. 2005;4:177–185. doi: 10.1111/j.1474-9726.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- Rapoport M.J. Herrmann N. Shammi P. Kiss A. Phillips A. Feinstein A. Outcome after traumatic brain injury sustained in older adulthood: a one-year longitudinal study. Am. J. Geriatr. Psychiatry. 2006;14:456–465. doi: 10.1097/01.JGP.0000199339.79689.8a. [DOI] [PubMed] [Google Scholar]
- Rose J.C. Neill T.A. Hemphill J.C., 3rd. Continuous monitoring of the microcirculation in neurocritical care: an update on brain tissue oxygenation. Curr. Opin. Crit. Care. 2006;12:97–102. doi: 10.1097/01.ccx.0000216574.26686.e9. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W. Langlois J.A. Thomas K.E. Xi Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Sandhir R. Puri V. Klein R.M. Berman N.E. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci. Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Sandhir R. Onyszchuk G. Berman N.E.J. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y. Zheng J. Lambrecht R.W. Bonkovsky H.L. Reciprocal effects of micro-RNA-122 on expression of heme oxygenase–1 and hepatitis C virus genes in human hepatocytes. Gastroenterology. 2007;133:1166–1174. doi: 10.1053/j.gastro.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C. Roberts K.N. Markesbery W.R. Scheff S.W. Lovell M.A. Oxidative stress in head trauma in aging. Free Radic. Biol. Med. 2006;41:77–85. doi: 10.1016/j.freeradbiomed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Sharp F.R. Bergeron M. Bernaudin M. Hypoxia-inducible factor in brain. Adv. Exp. Med. Biol. 2001;502:273–291. doi: 10.1007/978-1-4757-3401-0_18. [DOI] [PubMed] [Google Scholar]
- Siddiq A. Ayoub I.A. Chavez J.C. Aminova L. Shah S. LaManna J.C. Patton S.M. Connor J.R. Cherny R.A. Volitakis I. Bush A.I. Langsetmo I. Seeley T. Gunzler V. Ratan R.R. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J. Biol. Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor A.D. Greenberg D.A. Erythropoietin protects cultured cortical neurons, but not astroglia, from hypoxia and AMPA toxicity. Neurosci. Lett. 2000;290:213–215. doi: 10.1016/s0304-3940(00)01361-6. [DOI] [PubMed] [Google Scholar]
- Siren A.L. Fratelli M. Brines M. Goemans C. Casagrande S. Lewczuk P. Keenan S. Gleiter C. Pasquali C. Capobianco A. Mennini T. Heumann R. Cerami A. Ehrenreich H. Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokely M.E. Orr E.L. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J. Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- Stowe A.M. Plautz E.J. Nguyen P. Frost S.B. Eisner-Janowicz I. Barbay S. Dancause N. Sensarma A. Taylor M.D. Zoubina E.V. Nudo R.J. Neuronal HIF-1α protein and VEGFR-2 immunoreactivity in functionally related motor areas following a focal M1 infarct. J. Cereb. Blood Flow Metab. 2008;28:612–620. doi: 10.1038/sj.jcbfm.9600560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Jin K. Xie L. Childs J. Mao X.O. Logvinova A. Greenberg D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman M. DiRusso S.M. Sullivan T. Risucci D. Nealon P. Cuff S. Haider A. Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma. 2002;53:214–223. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Suzuki R. Okuda M. Asai J. Nagashima G. Itokawa H. Matsunaga A. Fujimoto T. Suzuki T. Astrocytes co-express aquaporin-1, -4, and vascular endothelial growth factor in brain edema tissue associated with brain contusion. Acta Neurochir. Suppl. 2006;96:398–401. doi: 10.1007/3-211-30714-1_82. [DOI] [PubMed] [Google Scholar]
- Taoufik E. Petit E. Divoux D. Tseveleki V. Mengozzi M. Roberts M.L. Valable S. Ghezzi P. Quackenbush J. Brines M. Cerami A. Probert L. TNF receptor I sensitizes neurons to erythropoietin- and VEGF-mediated neuroprotection after ischemic and excitotoxic injury. Proc. Natl. Acad. Sci. USA. 2008;105:6185–6190. doi: 10.1073/pnas.0801447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa J.A. Malec J.F. Moessner A.M. Brown A.W. Outcome after traumatic brain injury: effects of aging on recovery. Arch. Phys. Med. Rehabil. 2005;86:1815–1823. doi: 10.1016/j.apmr.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Thomas S. Tabibnia F. Schuhmann M.U. Brinker T. Samii M. Influences of secondary injury following traumatic brain injury in developing versus adult rats. Acta Neurochir. Suppl. 2000;76:397–399. doi: 10.1007/978-3-7091-6346-7_82. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. McCormick W.C. Kagan S.H. Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006;54:1590–1595. doi: 10.1111/j.1532-5415.2006.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberg A.W. Stroop R. Thomale U.W. Kiening K.L. Pauser S. Vollmann W. Characterisation of brain edema following “controlled cortical impact injury” in rats. Acta Neurochir. Suppl. 1997;70:106–108. doi: 10.1007/978-3-7091-6837-0_33. [DOI] [PubMed] [Google Scholar]
- Villa P. Bigini P. Mennini T. Agnello D. Laragione T. Cagnotto A. Viviani B. Marinovich M. Cerami A. Coleman T.R. Brines M. Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Zheng W. Christensen L.P. Tomanek R.J. DITPA stimulates bFGF, VEGF, angiopoietin, and Tie-2 and facilitates coronary arteriolar growth. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H613–H618. doi: 10.1152/ajpheart.00449.2002. [DOI] [PubMed] [Google Scholar]
- Williams B.J. Bimonte-Nelson H.A. Granholm-Bentley A.C. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl.) 2006;188:605–618. doi: 10.1007/s00213-006-0477-1. [DOI] [PubMed] [Google Scholar]
- Xiaowei H. Ninghui Z. Wei X. Yiping T. Linfeng X. The experimental study of hypoxia-inducible factor–1 alpha and its target genes in spinal cord injury. Spinal Cord. 2006;44:35–43. doi: 10.1038/sj.sc.3101813. [DOI] [PubMed] [Google Scholar]
- Yatsiv I. Grigoriadis N. Simeonidou C. Stahel P.F. Schmidt O.I. Alexandrovitch A.G. Tsenter J. Shohami E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- Zhao W. Belayev L. Ginsberg M.D. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J. Cereb. Blood Flow Metab. 1997;17:1281–1290. doi: 10.1097/00004647-199712000-00003. [DOI] [PubMed] [Google Scholar]