Abstract
Increased matrix metalloproteinase (MMP) activity has been implicated in the pathogenesis of lymphangioleiomyomatosis (LAM). The objective of this study was to investigate how tuberous sclerosis complex (TSC) 1 or TSC2 deficiency alters MMP expression and regulation. We studied immortalized cells that lack TSC2 derived from an angiomyolipoma of a patient with LAM, a TSC2 addback derivative, and murine embryonic fibroblast cells that lack Tsc1 or -2 and respective controls. Global gene expression analysis was performed in the angiomyolipoma and derivative cell lines. MMP levels in the conditioned media from these cells were analyzed by zymography and ELISA. We found increased MMP-2 expression in cells lacking TSC1/TSC2 compared with their respective controls by zymography. MMP-2 overproduction by these cells was not affected by rapamycin treatment. Gene expression analysis confirmed increased MMP-2 gene expression that was not affected by rapamycin. Furthermore, multiple other genes were found to be overexpressed in rapamycin-treated TSC2-deficient cells compared with TSC2+ cells. We conclude that TSC1/TSC2 deficiency leads to MMP-2 overproduction that is rapamycin-insensitive, and that several genes exhibit similar patterns, suggesting that TSC1/TSC2–dependent, but mammalian target of rapamycin–independent, pathways may be involved in the pathogenesis of LAM.
Keywords: interstitial collagenase, neoplasms, sirolimus
CLINICAL RELEVANCE.
This research provides data linking increase matrix metalloproteinase expressions in tuberous sclerosis complex (TSC)–related diseases. This report also suggests that rapamycin-insensitive pathways may be involved in TSC-related diseases.
Pulmonary lymphangioleiomyomatosis (LAM) is a progressive interstitial lung disease that has no proven effective treatment. Clinically, the disease can present with insidious onset of dyspnea, or sudden onset of dypsnea and/or chest pain due to spontaneous pneumothorax (1). Pathologically, LAM is characterized by both destruction of lung parenchyma with development of thin-walled cysts, and occurrence of numerous deposits of distinctive spindle-shaped and epithelioid cells, often in clusters termed LAM nodules (2). LAM occurs in roughly 30% of adult women with the genetic disorder tuberous sclerosis complex (TSC), but is more commonly seen in women with no history of TSC—so-called sporadic LAM (1). In both TSC-associated and sporadic LAM, kidney involvement by a related neoplasm, angiomyolipoma (AML), occurs frequently (1, 2).
Biallelic inactivating mutations in TSC2 are common in both TSC-associated and sporadic LAM (3, 4). In addition, kidney AML and LAM cells from patients with sporadic LAM have the same mutation in TSC2 (in each individual patient), suggesting that both lesions are derived from the same cell lineage, and may spread from one site to another through a metastatic mechanism (3). These genetic data are consistent with pathologic studies demonstrating that there is widespread involvement of the lymphatic system, both within the thorax and infradiaphragmatically, by proliferating cells of appearance highly similar to those seen in LAM nodules (2, 5).
The TSC1 and TSC2 proteins (also called hamartin and tuberin, respectively) form a complex that has a critical function in regulating the state of activation of the mammalian target of rapamycin (mTOR) kinase through Rheb-GTP (2, 6, 7). Thus, phospho-S6 (downstream of mTOR complex [mTORC] 1 activation) is elevated in the AML and LAM cells of patients with TSC. This observation, with support from positive results in mouse and rat models of TSC, has led to clinical trials testing the efficacy of rapamycin, an mTORC1 inhibitor, in LAM (1, 8, 9).
Matrix metalloproteinases (MMPs) are a group of enzymes able to degrade components of the extracellular matrix (ECM). MMPs have been implicated in the pathogenesis of chronic lung diseases (10), as well as tumor growth and metastasis (11). Recent studies have provided evidence that MMPs, especially MMP-2, are increased in expression in LAM nodules by immunohistochemistry analyses (12–15). Furthermore, MMP-14 (membrane type 1 MMP), an important activator of MMP-2, also appears to be expressed at high levels in LAM lesions (15–17), whereas tissue inhibitor of metalloproteinase (TIMP) 3, an inhibitor of MMP-2, appears expressed at reduced levels in LAM lesions (15). The observation that TIMP3-null mice develop emphysema with pulmonary parenchymal destruction provides evidence of the importance of this enzymatic system in lung disease (18). In addition, blockade of MMP activity with doxycycline was associated with improvement in respiratory function in a woman with LAM in a case report (19).
The above circumstantial evidence suggests that MMPs may contribute to ECM degradation and development of cystic lesions, an important aspect of LAM pathology that likely contributes significantly to respiratory dysfunction. However, the full range of MMP expression abnormalities in LAM has not been studied in detail. Here, we use a recently developed AML cell line from a patient with LAM to explore the relationship between TSC1/TSC2 expression and MMP expression.
MATERIALS AND METHODS
Cell Culture
Cell culture media and supplements were from GIBCO (Frederick, MD). An immortalized TSC2-deficient human cell line derived from the AML of a patient with LAM (20), and its corresponding TSC2-rescued control cell line, has been described previously (21). These cells were cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 10% FBS, 0.2 μM hydrocortisone, 0.1 nM triiodothyronine, 0.01 μU/ml vasopressin, 1.6 μM FeSO4, cholesterol, insulin-transferrin-selenium, 100 ng/ml epidermal growth factor, 100 μg/ml zeomycin, and 1% penicillin-streptomycin-amphotericin B (PSA). Immortalized Tsc1−/− or Tsc2−/− murine embryonic fibroblast (MEF) cell lines, with their respective controls, have been described previously (22, 23). MEFs are maintained in Dulbecco's modified Eagle's medium, supplemented with 10% FBS and 1% PSA.
All experiments were performed in triplicate. For biochemical analyses and conditioned media analysis, cells were seeded at 2.5 × 105 cells/ml in six-well plates in their normal growth media for 24 hours. Media were replaced by serum-free media (Alpha-Eagle's minimum essential medium [MEM] with 1% PSA) with rapamycin (20 nM) or vehicle. After 24 hours, cell-free conditioned media were collected, and cell lysate was prepared with RIPA (Boston Bioproducts, Boston, MA) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN) and a phosphatase inhibitor (Thermo Scientific, Waltham, MA).
Long-term rapamycin treatment was performed in 10-mm culture dishes. Vehicle or rapamycin-containing media were replaced daily for 5 days. Cells were then detached and seeded at 2.5 × 105 cells/ml into six-well plates, as described previously here.
RNA Interference Studies
Small interfering RNA (siRNA) constructs were purchased from Ambion (Austin, TX), and used as instructed by the manufacturer. Briefly, 30–100 nM silencer siRNA constructs against Rheb (s12021), mTOR (s603) or nonsense negative control were incubated in Opti-MEM (Invitrogen, Carlsbad, CA) with NeoFX transfection agent (Ambion). The mixture was then plated into six-well plates and overlaid with 3 × 105 cells/well in Opti-MEM for 24 hours. Media was replaced with 2 ml/well of serum-free media and incubated for another 24 hours before collection of cell-free media and cell lysate, as described previously here.
Western Blotting
Protein samples were analyzed by SDS-PAGE using 4–12% NuPAGE Gel (Invitrogen), and transferred to a nitrocellulose membrane. Immunoblotting was performed by standard methods using horseradish peroxidase–conjugated secondary antibodies, and chemiluminescence using Supersignal West Pico Chemilumincesent substrate (Thermo Scientific) and exposure to film. Antibodies against pS6 (S240/244), mTOR, Rheb were purchased from Cell Signaling (Danvers, MA).
MMP-2 Assay Using Zymography
Gelatinase activity in conditioned media was assessed by zymography, as previously described (24). Serum-free, conditioned media, normalized to cell number, was mixed with nonreducing sample buffer (Boston Bioproducts), and loaded onto a 10% polyacrylamide gel containing 0.1% gelatin (Bio-Rad, Hercules, CA). After electrophoresis, the gels were soaked in 2.5% Triton X-100 with gentle shaking for 30 minutes at ambient temperature with one change of solution. The gels were then rinsed and incubated overnight at 37°C in developing buffer (Boston Bioproducts). Gels were then stained for 15–30 minutes in 0.5% Coomassie blue R-250 in acetic acid, isopropyl alcohol, and water (1:3:6), destained in acetic acid, ethanol, and water (1:3:6), and photographed. MMPs were identified by localized clearing of the gel. MMP-2 and -9 were identified by molecular weight, and confirmed as previously reported (24).
ELISA
A Quantikine Human MMP-2 ELISA kit (R&D Systems, Minneapolis, MN) was used as directed.
Gene Expression Analysis
Gene expression levels were measured using the CodeLink Human Whole Genome microarray with 53,423 30-mer oligonucleotide probes. In the microarray quality control studies, the measurements of gene expression changes on these microarrays exhibited a high level of concordance with other microarray platforms and with quantitative RT-PCR (25). Total RNA was extracted from cells using the RNeasy kit (Qiagen Inc., Valencia, CA). The quality of the total RNA was assessed by analysis on the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The ratio of the 28S/18S bands was required to be at least 2.0. Total RNA (0.75 μg) was reverse transcribed into cDNA, and the product used in an in vitro transcription reaction to generate cRNA labeled with biotin for hybridization to arrays following the CodeLink labeling protocol (Applied Microarrays, Inc., Tempe, AZ). Briefly, RNA was first reverse transcribed using a T7-oligo (dT) promoter primer, followed by second-strand cDNA synthesis using RNase H. Double-stranded cDNA was purified and used as a template in the in vitro transcription using T7 RNA polymerase and a biotinylated nucleotide analog/ribonucleotide mix to generate biotin-labeled cRNA.
For hybridization to the CodeLink arrays 10 μg of labeled cRNA was fragmented, denatured at 90°C for 5 minutes, and then hybridized at 37°C for 20 hours. After washing, the arrays were incubated with streptavidin–phycoerythrin and biotinylated anti-streptavidin antibodies, and the arrays scanned on a GenePix 4000B scanner (Axon Instruments, Sunnyvale, CA).
Global Gene Analysis
Gene expression data were obtained in triplicate on three groups of cells: TSC2− cells treated with rapamycin (group A), vehicle-treated TSC2− cells (group B), and TSC2+ cells (group C). The gene expression levels on each array were normalized to a median expression level of 1.0 using the CodeLink software. To focus on genes expressed well above background, we excluded genes from consideration if no sample had an expression level of at least 2. Coefficients of variation, intergroup P values (Student's t test), and gene ratios of two-group comparisons were calculated using Microsoft Excel (Microsoft Corp., Redmond, WA), and then imported to Microsoft Access for further analysis. To identify differentially expressed genes, we first required a within-group coefficient of variation of less than 0.2 to exclude highly variable genes, and then a P value of less than 0.05 for the Student's t test for the average expression between any pair of groups.
To identify statistically significant gene expression changes for the 23 MMP and 4 TIMP gene probes on the array, we imposed the Bonferroni multiple testing correction on the t test for statistically significant changes on the expression of these families of genes, corresponding to P values of 0.002 and 0.0125, respectively.
To identify additional genes, the expression of which is increased due to loss of TSC2, but independent of mTORC1 activation, we selected genes that met the following criteria: (1) expression ratio of group A:group C greater than 2, with a P value less than 0.05; (2) expression ratio of group B:group C greater than 2 with a P value less than 0.05; and (3) group A and group B expression not significantly different (P > 0.05). We also identified a subset of these genes in which expression levels were increased more than fivefold in the TSC2-deficient cells with or without rapamycin treatment (groups A and B) in comparison to TSC2+ cells (group C).
Statistical Analysis
The two-sided Student's t test was used to compare expression between pairs of groups. The simple Bonferroni correction was used to correct for multiple comparisons in gene expression data for the MMPs and TIMPs.
The gene expression levels in all three groups were compared in a pair-wise fashion. The TSC2-deficient cells with or without rapamycin treatment (groups A and B) were compared with each other to identify rapamycin-dependent genes, and both groups A and B were compared with the TSC2+ cells (group C) to identify TSC2-related genes.
RESULTS
TSC-Deficient Cell Lines Secrete More MMP-2 than Control Cell Lines
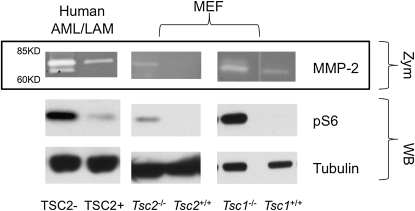
We used an immortalized cell line derived from an AML of a patient with LAM (20) to examine production of MMPs in a relevant cell type. Conditioned media from this line gave clear bands on zymogram gels in the range of 60–85 kD, corresponding to pro–MMP-2 (higher molecular weight) and active MMP-2 (lower molecular weight), which were clearly increased in comparison to media from the addback TSC2 cell line derived from this AML line (Figure 1A). MMP-9, the other highly gelatinolytic MMP, has a higher molecular weight (∼92 kD), and that region of the gel showed only a faint, nonreproducible signal. Similar findings were made in the analysis of MEF cell lines engineered to be null for either Tsc1 or Tsc2 (19, 20) (Figure 1). All three of the TSC2-, Tsc1-, or Tsc2-deficient cell lines showed increased levels of pS6 (S240/244) compared with controls, as expected.
Figure 1.
Secretion of matrix metalloproteinase (MMP)-2 by tuberous sclerosis complex (TSC) 1– or TSC2-deficient cells. Representative zymography (Zym: MMP-2) of conditioned media and Western blots (WB: pS6, tubulin) of cell lysates are shown. The first two lanes represent a TSC2-deficient human angiomyolipoma (AML) cell and addback control line; the last four lanes represent Tsc2- or Tsc1-null murine embryonic fibroblast (MEF) cells and respective controls. In TSC1/TSC2-deficient cells, pS6 (S240/244) levels are increased, reflecting mammalian target of rapamycin complex (mTORC) 1 activation.
MMP-2 Secretion by TSC1/TSC2-Deficient Cells Is mTORC1-Independent
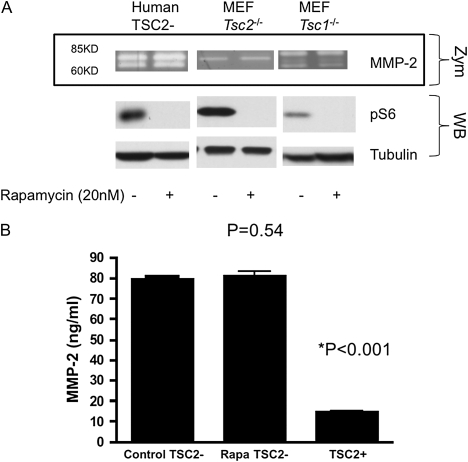
Because activation of mTORC1 is one of the signaling hallmarks of cells lacking TSC1 or -2, we examined whether MMP-2 secretion was dependent upon mTORC1 activity. Cells were treated with rapamycin during serum-free media incubation and collection of conditioned media for zymography. Rapamycin treatment at 20 nM for 24 hours had no effect on MMP-2 activity in the media, whereas it clearly blocked mTORC1 activity, as assessed by reduction in pS6 levels (Figure 2A). Long-term rapamycin treatment for 7 days similarly had no effect on MMP-2 activity (see Figure E1 in the online supplement).
Figure 2.
Lack of effect of rapamycin treatment on MMP-2 secretion by TSC1/TSC2–deficient cells. (A) Representative zymography (Zym: MMP-2) of conditioned media and Western blots (WB: pS6, tubulin) of lysates from TSC2-deficient human AML/lymphangioleiomyomatosis (LAM) cells and Tsc1- or Tsc2-null MEF cells with and without rapamycin treatment for 24 hours are shown. Rapamycin effectively suppressed pS6 (S240/244), but had no effect on MMP-2 secretion by the TSC1/TSC2-deficient cells. (B) ELISA quantification of MMP-2 in the conditioned media of human AML/LAM cells. Means (±SD) of triplicate experiments are presented. Rapamycin-treated TSC2-null cells secreted the same amount of MMP-2 as untreated TSC2-null cells (P = 0.54), whereas TSC2+ addbacks secreted much lower MMP-2 (*P < 0.001).
To confirm that MMP-2 was present in the conditioned media at higher levels in cells lacking TSC1/2, and to quantify this increase, an ELISA assay for MMP-2 was performed. MMP-2 levels were approximately 4.5-fold higher in the TSC2-null AML cell line in comparison to the addback TSC2-expressing line, and were not affected by rapamycin treatment for 24 hours (Figure 2B).
Increased MMP-2 Secretion Is mTOR- and Rheb-Independent
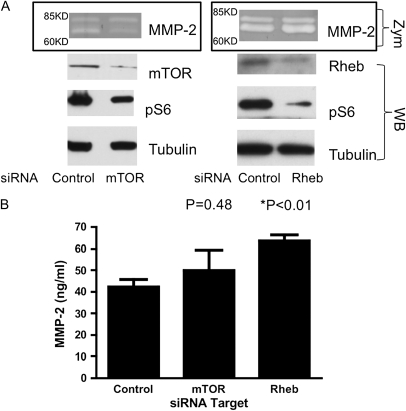
To confirm that MMP-2 secretion was independent of mTOR in these cells, we examined the effects of knockdown of mTOR or Rheb by siRNA. By densitometry estimates on the immunoblots, we achieved roughly 90% knockdown of mTOR protein with 40% lower pS6, and roughly 70% knockdown of Rheb protein with 70% lower pS6 in the siRNA experiments. siRNA knockdown of either mTOR or Rheb appeared to have no effect on MMP-2 secretion by the TSC2-null AML cell line, whereas each treatment clearly reduced pS6 levels (Figure 3A). Indeed, MMP-2 ELISA analysis showed that mTOR inhibition had no significant effect on secreted MMP-2 levels, whereas Rheb inhibition actually increased MMP-2 levels to a small but significant extent (Figure 3B). These data suggest that increased MMP-2 in TSC2-deficient cells is mTOR independent.
Figure 3.
Lack of effect of mTOR or Rheb knockdown on MMP-2 secretion by the TSC2-deficient AML cell line. (A) Representative zymography (Zym: MMP-2) of conditioned media and Western blots (WB: mTOR, Rheb, pS6, tubulin) of lysates from TSC2-deficient human AML cells. RNA interference with small interfering RNA (siRNA) targeting mTOR (left panel) or Rheb (right panel) successfully reduced expression of the target proteins and reduced pS6 (S240/244) levels, but had no effect on MMP-2 levels in the media. (B) ELISA quantification of MMP-2 levels in conditioned media of TSC2− AML cells treated with siRNA targeting mTOR, or in Rheb. Means (±SD) of triplicate experiments are shown. Compared with control cells, knockdown of mTOR did not affect MMP-2 secretion (P = 0.48), whereas knockdown of Rheb caused a minor increase in MMP-2 production (P < 0.01).
MMP-2 mRNA Levels Are Increased in TSC2-Deficient Cells
To examine the mechanism of increased MMP-2 secretion by the TSC2-null AML cell line, we performed gene expression analysis using CodeLink microarrays. To assess the potential effects of treatment with rapamycin on gene expression, three sets of RNA samples were studied: (sample A) the TSC2-null AML cell line treated with rapamycin for 24 hours; (sample B) the TSC2-null AML cell line treated with vehicle; and (sample C) the vehicle-treated TSC2 addback AML cell line. All cells were maintained in serum-free conditions for 24 hours before RNA isolation. Probes for 23 MMPs were present on the microarray. Even after Bonferroni multiple testing correction, the difference in expression levels of MMP-2 between samples B and C (neither treated with rapamycin) was statistically significant, with an average increase of 2.34 between the TSC2-null AML cell line and the TSC2-expressing addback cell line (Table 1; P = 0.0003). The mRNA levels of MMP-15, also known as membrane type 2 MMP, and a reported activator of MMP-2 (26), were also increased in the TSC2-null AML cell line, with a ratio of 3.44 (P = 0.0004). Rapamycin treatment had no significant effect on MMP-2 levels in the TSC2-null line (Table 1), consistent with our zymography and ELISA results. Multiple other expression differences were seen among the MMPs, but these were not significant after correction for multiple testing. In particular, comparing TSC2− cells to TSC2+ cells, MMP-1 and -3 mRNA levels were reduced (ratios of 0.4883 and 0.6667, with P values of 0.0029 and 0.0063, respectively), whereas MMP-13, -17, and -25 were increased (ratios of 4.53, 5.05, and 1.42, with P values of 0.0030, 0.0035, and 0.0031, respectively). Among these, only MMP-13 mRNA levels were reduced by rapamycin (Table 1).
TABLE 1.
GENE EXPRESSION ANALYSIS OF MMP GENES IN THREE DIFFERENT GROUPS OF ANGIOMYOLIPOMA/LYMPHANGIOLEIOMYOMATOSIS CELL LINES
| Ratio |
P Value |
|||||
|---|---|---|---|---|---|---|
| MMP Name | A:B | A:C | B:C | A and B | A and C | B and C |
| MMP1 | 1.15 | 0.56 | 0.49 | 0.3125 | 0.0045 | 0.0029 |
| MMP2 | 1.14 | 2.66 | 2.34 | 0.3971 | 0.0094 | 0.0003 |
| MMP3 | 1.27 | 0.85 | 0.67 | 0.0262 | 0.0266 | 0.0063 |
| MMP7 | 0.82 | 0.80 | 0.98 | 0.1011 | 0.1359 | 0.7752 |
| MMP8 | 1.93 | 1.34 | 0.69 | 0.3522 | 0.6231 | 0.4585 |
| MMP9 | 1.12 | 1.38 | 1.23 | 0.2796 | 0.0414 | 0.0161 |
| MMP10 | 1.05 | 1.09 | 1.04 | 0.3922 | 0.1230 | 0.1999 |
| MMP11 | 1.57 | 1.69 | 1.08 | 0.0254 | 0.0336 | 0.7037 |
| MMP12 | 2.89 | 5.13 | 1.77 | 0.0374 | 0.0275 | 0.4306 |
| MMP13 | 0.20 | 0.91 | 4.53 | 0.0183 | 0.9253 | 0.0030 |
| MMP14 | 1.12 | 1.34 | 1.19 | 0.1749 | 0.0143 | 0.0846 |
| MMP15 | 0.85 | 2.91 | 3.44 | 0.4740 | 0.0510 | 0.0004 |
| MMP16 | 0.97 | 1.10 | 1.13 | 0.9109 | 0.8767 | 0.8245 |
| MMP17 | 1.14 | 5.75 | 5.05 | 0.4888 | 0.0032 | 0.0035 |
| MMP19 | 1.44 | 1.67 | 1.16 | 0.0030 | 0.0010 | 0.1317 |
| MMP20 | 1.07 | 1.06 | 0.99 | 0.3777 | 0.4013 | 0.9130 |
| MMP21 | 1.17 | 1.05 | 0.89 | 0.7837 | 0.9037 | 0.8257 |
| MMP23 | 0.64 | 1.08 | 1.67 | 0.3521 | 0.9012 | 0.3332 |
| MMP24 | 1.28 | 1.86 | 1.45 | 0.6926 | 0.4654 | 0.4756 |
| MMP24 | 1.02 | 1.01 | 0.99 | 0.7416 | 0.8682 | 0.8282 |
| MMP25 | 0.95 | 1.35 | 1.42 | 0.0982 | 0.0091 | 0.0031 |
| MMP26 | 0.85 | 0.95 | 1.11 | 0.0638 | 0.3624 | 0.0802 |
| MMP27 | 3.04 | 1.48 | 0.49 | 0.1176 | 0.3704 | 0.0759 |
| MMP28 | 1.82 | 0.84 | 0.46 | 0.1045 | 0.4865 | 0.0879 |
Definition of abbreviation: MMP, matrix metalloproteinase.
Group A, tuberous sclerosis complex (TSC) 2− cells treated with rapamycin; group B, TSC2− cells treated with vehicle; and group C, TSC2+ treated with vehicle. Gene expression ratios and P values for comparisons of pairs of the three groups are presented. Comparing TSC2− (group B) to TSC2+ (group C), we found that MMP2 and MMP15 expression was significantly increased. Rapamycin treatment had no significant effect on any MMP expression (comparing groups A and B). The Bonferroni correction was used to conservatively correct for multiple comparisons, so that a P value of less than 0.002 was considered significant. Boldface type denotes meeting statistical significance.
TIMP Expression Analysis
Four TIMPs were analyzed in the gene expression analysis (Table 2). Comparing TSC2− to TSC2+ cells, TIMP1, -2, and -3 showed no statistically significant changes. Interestingly, only the TIMP4 mRNA expression was significantly different. Although it has not been previously implicated in LAM, TIMP4 was increased 3.71-fold in the TSC2− AML line (P < 0.0001). Rapamycin treatment had no significant effect.
TABLE 2.
GENE EXPRESSION ANALYSIS OF TISSUE INHIBITOR OF METALLOPROTEINASE GENES IN THREE DIFFERENT GROUPS OF ANGIOMYOLIPOMA/LYMPHANGIOLEIOMYOMATOSIS CELL LINES
| Ratio |
P Value |
|||||
|---|---|---|---|---|---|---|
| TIMP Name | A:B | A:C | B:C | A and B | A and C | B and C |
| TIMP1 | 1.29 | 1.59 | 1.24 | 0.1696 | 0.0299 | 0.1257 |
| TIMP2 | 1.22 | 1.03 | 0.85 | 0.0259 | 0.7392 | 0.1575 |
| TIMP3 | 1.05 | 1.25 | 1.19 | 0.4789 | 0.5966 | 0.6807 |
| TIMP4 | 0.89 | 3.30 | 3.71 | 0.2524 | 0.0016 | <0.0001 |
Definition of abbreviation: TIMP, tissue inhibitor of metalloproteinase.
Group A, tuberous sclerosis complex (TSC) 2− cells treated with rapamycin; group B, TSC2− cells treated with vehicle; and group C, TSC2+ treated with vehicle. Gene expression ratios and P values for comparisons of pairs of the three groups are presented. Comparing TSC2− (group B) to TSC2+ (group C), we found that TIMP4 was expressed at a significantly higher level, and rapamycin treatment had little effect on this difference. The Bonferroni correction was used to conservatively correct for multiple comparisons, so that a P value of 0.0125 was considered significant. Boldface type denotes meeting statistical significance.
Global Gene Analysis to Identify Other Genes the Expression of which Was also Up-Regulated in a TSC2-Dependent, but Rapamycin-Insensitive, Manner
The full gene expression data set was also analyzed to identify genes that appear to increase along with MMP2 and -15. A total of 271 genes were identified for which expression levels were increased twofold or greater in the TSC2-null cell line compared with the addback cell line, independent of rapamycin treatment (Table 3). A total of 22 genes were identified for which expression levels were increased fivefold or greater in the TSC2-null cell line compared with the addback cell line, independent of rapamycin treatment (Table 4). In these cases, the Bonferroni correction for the testing of the 50,000 gene probes on the array would require a P value of less than 0.000001 for statistical significance at the 0.05 level. Because of the small sample size (n = 3) for each group, only two genes (CCL7 and SLC16A6) met this strict requirement. Nevertheless, many genes exhibited large increases, from 10- to 65-fold.
TABLE 3.
SELECTION CRITERIA FOR CANDIDATE GENES THAT ARE UP-REGULATED AND INSENSITIVE TO RAPAMYCIN TREATMENT IN THE ABSENCE OF TUBEROUS SCLEROSIS COMPLEX 2
|
P Value |
Ratio |
No. of Unique Genes | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Group A vs. B | Group A vs. C | Group B vs. C | A:B | A:C | B:C | |
| First selection | >0.05 | <0.05 | <0.05 | >2 | >2 | 271 | |
| Second selection | >0.05 | <0.05 | <0.05 | >5 | >5 | 22 | |
Group A, tuberous sclerosis complex (TSC) 2− cells treated with rapamycin; group B, TSC2− cells treated with vehicle; and group C, TSC2+ treated with vehicle. P values and expression ratio of intergroup comparisons used as selection criteria, and the number of candidate genes identified, are presented.
TABLE 4.
HIGHLY OVEREXPRESSED GENES THAT ARE TUBEROUS SCLEROSIS COMPLEX 2–DEPENDENT RAPAMYCIN-INSENSITIVE IN THE ANGIOMYOLIPOMA/LYMPHANGIOLEIOMYOMATOSIS CELL LINES
| Ratio |
P Value |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Description | A:B | A:C | B:C | A and B | A and C | B and C |
| ARL7 | ADP-ribosylation factor-like 7 | 1.12 | 6.03 | 5.35 | 0.32 | 0.000141 | 0.000936 |
| CCL7 | Chemokine (C-C motif) ligand 7 | 0.98 | 63.93 | 65.06 | 0.67 | 0.000015 | <0.000001 |
| CKLFSF8 | Chemokine-like factor super family 8 | 1.3 | 12.43 | 9.49 | 0.07 | 0.000079 | 0.001112 |
| CNIH3 | Cornichon homolog 3 | 0.92 | 26.29 | 28.56 | 0.22 | 0.000004 | 0.000042 |
| COL1A2 | Collagen, type I, alpha 2 | 1.1 | 8.57 | 7.76 | 0.34 | 0.000285 | 0.000065 |
| COLEC12 | Collectin subfamily member 12 | 1.17 | 9.82 | 8.37 | 0.14 | 0.00008 | 0.000224 |
| CYB5R2 | Cytochrome b5 reductase b5R2 | 1.08 | 20.29 | 18.72 | 0.2 | 0.000001 | 0.000052 |
| CYP1B1 | Cytochrome P450, family 1, subfamily B, polypeptide 1 | 1.05 | 57.32 | 54.28 | 0.55 | 0.000011 | 0.000224 |
| EPHB2 | EPH receptor B2 | 0.89 | 5.41 | 6.05 | 0.1 | 0.000109 | 0.000004 |
| G1P3 | IFN-α–inducible protein | 1.15 | 6.06 | 5.25 | 0.1 | 0.000068 | 0.000135 |
| GJA1 | Gap junction protein, alpha 1 | 0.96 | 13.07 | 13.48 | 0.62 | 0.000053 | 0.000008 |
| HGF | Hepatocyte growth factor | 1.09 | 6.47 | 5.93 | 0.44 | 0.000231 | 0.000479 |
| INPP5F | Inositol polyphosphate-5-phosphatase F | 1.07 | 9.32 | 8.68 | 0.38 | 0.00001 | 0.000206 |
| MAGEH1 | Melanoma antigen, family H, 1 | 1.16 | 5.96 | 5.1 | 0.19 | 0.000386 | 0.000246 |
| MT1L | Metallothionein 1L | 1.13 | 6.49 | 5.73 | 0.14 | 0.000092 | 0.000053 |
| PKIB | Protein kinase (cAMP-dependent, catalytic) inhibitor β | 0.94 | 5.47 | 5.77 | 0.48 | 0.000151 | 0.000033 |
| PYCARD | PYD and CARD domain containing | 1.06 | 5.76 | 5.4 | 0.42 | 0.000186 | 0.000034 |
| SLC16A6 | Solute carrier family 16 (monocarboxylic acid transporters), member 6 | 1.14 | 9.08 | 7.94 | 0.22 | 0.000549 | <0.000001 |
| SOX4 | SRY (sex determining region Y)-box 4 | 0.87 | 13.22 | 15.15 | 0.16 | 0.00018 | 0.000029 |
| WISP2 | WNT1-inducible signaling pathway protein 2 | 0.94 | 7.07 | 7.51 | 0.5 | 0.000006 | 0.000354 |
| WNT9A | Wingless-type MMTV integration site family, member 9A | 0.89 | 9.59 | 10.75 | 0.15 | 0.000024 | 0.000056 |
| ZNF537 | Zinc finger protein 537 | 1.23 | 6.73 | 5.44 | 0.08 | 0.000415 | 0.00006 |
Definition of abbreviations: CARD, caspase-recruitment domain; EPH, ephrin; MMTV, mouse mammary tumor virus; PYD, PYRIN-PAAD-DAPIN domain; WNT, wingless-type MMTV integration site.
Global gene expression analysis was performed as described in Materials and Methods and Table 3. Genes listed here are: (1) expressed at fivefold or greater increase in tuberous sclerosis complex (TSC)-2–null cells with (group A) or without (group B) rapamycin treatment as compared to TSC2+ cells (group C); and (2) not differentially expressed between groups A and B (P > 0.05). Gene expression ratios and P values of intergroup comparisons are presented.
Because all of the CodeLink microarray data are publically available in the Gene Expression Omnibus database at the National Center for Biotechnology Information, this data set can be examined in more detail for up- and down-regulated genes in future work.
DISCUSSION
The lack of reliable cell lines to serve as an in vitro model system to study aspects of the pathogenesis of LAM has been a major limitation. Here, we use a recently derived TSC2-null AML cell line to explore the regulation of expression of MMPs. This cell line was derived from an AML resected from a patient with sporadic (non–TSC-associated) LAM, and has been shown to have biallelic inactivation of TSC2 (R611Q on one allele, and loss of the second TSC2 allele) (20). The identical mutations were identified in the LAM cells of this patient, consistent with the “benign metastasis” model of LAM development, with migration of cells from some initial site to both the renal and lung lesions in this patient (3, 27).
We have shown that this TSC2-null AML cell line secretes higher levels of MMP-2 than its TSC2 addback derivative, and that rapamycin inhibition of mTORC1 does not affect the increased MMP-2 secretion. In addition, knockdown of either mTOR or Rheb has no major effect on MMP-2 secretion by this line. Similar observations were made on MMP-2 secretion by pairs of MEF lines that are null for either Tsc1 or Tsc2. In aggregate, these data strongly suggest that MMP-2 production is increased in TSC2-null LAM cells, at least partly through a transcriptional mechanism. These data lend support to previous immunohistochemistry observations on the expression of MMPs in LAM lesions, to the speculation that MMPs may play a role in the pathogenesis of pulmonary LAM, and to the finding that MMPs are elevated in the urine of patients with LAM (2, 12–15, 19). As with other chronic lung diseases and cancers, the ability of MMP-2 to degrade ECM may directly contribute to lung cyst formation in pulmonary LAM. However, it is important to note that how MMP-2 plays a role in the pathogenesis of LAM remains unclear, and thus the therapeutic potential for MMP-2 inhibition is also uncertain.
Another possible role for MMPs in LAM pathogenesis is through stimulation of angiogenesis and lymphangiogenesis. MMPs have been shown to enhance vascular endothelial growth factor (VEGF) expression and regulate angiogenesis, and TIMPs are known to suppress the angiogenesis phenotype at a number of stages of the angiogenesis program (28–32). The up-regulation of MMP in LAM may contribute to lymphangiogenesis through the expression of VEGF-C, VEGF-D, and the corresponding receptors (5, 33). The observations that VEGF-D is expressed by LAM lesions, and that increased circulating VEGF-D levels may be found in patients with LAM, suggest the importance of prolymphangiogenesis factors in development of LAM (34, 35).
We found that the increased expression of MMP-2 by the AML/LAM cell line appeared to be independent of mTORC1, as assessed by the lack of effect of rapamycin treatment, as well as siRNA knockdown of mTOR and Rheb. Although most biochemical and signaling effects in cells lacking TSC1 or TSC2 are thought to occur through activation of mTORC1, there is previous evidence that several abnormalities in TSC2-deficient cells are independent of mTORC1 activation. B-Raf kinase activity is reduced in TSC2-deficient cells due to Rheb-GTP, but independent of mTORC1 (36, 37). In addition, Tsc2-null MEFs exhibit reduced Akt kinase activation, partially due to impaired mTORC2 activity (38). Finally, Tsc1- or Tsc2-null MEFs have a higher percentage of cilium-containing cells compared to the respective controls, and rapamycin treatment has no effect on this result (39). However, none of these observations seems to be connected to increased expression of MMP-2 seen in this TSC2-null cell line, and, indeed, the lack of effect of Rheb knockdown on MMP-2 levels argues against this being due to Rheb.
Much enthusiasm has been generated by the prospect that inhibitors of mTOR may represent ideal therapeutic agents in the treatment of LAM as an mTOR pathway disease. Initial clinical trials have indicated that rapamycin may have some benefit in LAM/TSC-related diseases, but further investigation is required (8, 9). The existence of pathways aberrantly activated in TSC2-null cells that do not respond to rapamycin treatment suggest that rapamycin treatment alone may be inadequate for disease control. Treatments aimed at inhibiting MMP thus may have potential benefit in LAM. Clinically available agents, such as doxycycline and HMG-CoA (3-hydroxy-3-methyl-glutaryl-CoA) reductase (statins), reductase inhibitors, and statins, have been shown to reduce MMP production and pathologic proliferation in vascular smooth muscle cells (19, 40–43), and may have benefit for clinical LAM in combination with rapamycin. Statins may be particularly attractive, as they also have some ability to reduce Rheb-GTP and Rho-GTP levels in cells lacking TSC1/2 (44).
In addition to MMPs, gene expression analysis identified several genes the expression of which is increased in TSC2− cells compared with TSC2+ controls. The expression of these genes also did not appear to be affected significantly by rapamycin treatment. Although these differences in gene expression had extremely low P values (P = 10−3−10−6; Table 4), it is possible that some of these have occurred by chance, given the large number of comparisons being made. Nonetheless, they suggest that there are additional, mTORC1-independent effects of TSC1/2 loss in this AML/LAM cell line. WISP2, WNT9A, and SOX4 are increased in expression in the AML/LAM cell line, suggesting that there may be aberration of Wnt signaling in these cells, consistent with a previous observation of increased β-catenin levels within LAM lesion by immunohistochemistry (45). Wnt activation has been reported to induce MMP-2 expression (46), suggesting a possible link between Wnt and MMP in LAM. Further investigation into their potential role in the pathogenesis of LAM is required.
In conclusion, we report that MMP-2 expression is significantly increased in TSC2-deficient cells derived from a LAM-associated AML, and is not affected by rapamycin treatment. In addition, several other genes show expression changes that fit a similar pattern, suggesting the existence of mTORC1-independent pathway events in LAM-like cells lacking TSC2. Finally, our observations raise the possibility that rapamycin treatment of LAM may have limited clinical effectiveness.
Supplementary Material
This work was supported by LAM Foundation pilot award LAM065P07-06, American Heart Association Scientist Development grant 0735620N (P.-S.L.), and National Institutes of Health/National Cancer Institute grants 1P01CA120964 (D.J.K.) and 1RO1CA118764-01 (M.A.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0050OC on April 24, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008;133:507–516. [DOI] [PubMed] [Google Scholar]
- 2.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol 2007;36:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet 1998;62:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumasaka T, Seyama K, Mitani K, Sato T, Souma S, Kondo T, Hayashi S, Minami M, Uekusa T, Fukuchi Y, et al. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol 2004;28:1007–1016. [DOI] [PubMed] [Google Scholar]
- 6.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–1356. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008;412:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 2008;358:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, Doyle T, Elmslie F, Saggar A, de Vries PJ, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 2008;358:200–203. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res 2005;31:599–621. [DOI] [PubMed] [Google Scholar]
- 11.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 2004;48:411–424. [DOI] [PubMed] [Google Scholar]
- 12.Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell–mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol 2004;164:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Travis WD, Moss J. Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch 2000;67:311–329. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi T, Fleming MV, Stetler-Stevenson WG, Liotta LA, Moss J, Ferrans VJ, Travis WD. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM). Hum Pathol 1997;28:1071–1078. [DOI] [PubMed] [Google Scholar]
- 15.Zhe X, Yang Y, Jakkaraju S, Schuger L. Tissue inhibitor of metalloproteinase-3 downregulation in lymphangioleiomyomatosis: potential consequence of abnormal serum response factor expression. Am J Respir Cell Mol Biol 2003;28:504–511. [DOI] [PubMed] [Google Scholar]
- 16.Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med 2000;124:267–275. [DOI] [PubMed] [Google Scholar]
- 17.Matsui K, Takeda K, Yu ZX, Valencia J, Travis WD, Moss J, Ferrans VJ. Downregulation of estrogen and progesterone receptors in the abnormal smooth muscle cells in pulmonary lymphangioleiomyomatosis following therapy: an immunohistochemical study. Am J Respir Crit Care Med 2000;161:1002–1009. [DOI] [PubMed] [Google Scholar]
- 18.Leco KJ, Waterhouse P, Sanchez OH, Gowing KL, Poole AR, Wakeham A, Mak TW, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest 2001;108:817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med 2006;354:2621–2622. [DOI] [PubMed] [Google Scholar]
- 20.Yu J, Astrinidis A, Howard S, Henske EP. Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol 2004;286:L694–L700. [DOI] [PubMed] [Google Scholar]
- 21.Hong F, Larrea MD, Doughty C, Kwiatkowski DJ, Squillace R, Slingerland JM. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell 2008;30:701–711. [DOI] [PubMed] [Google Scholar]
- 22.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 2002;11:525–534. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Cicchetti G, Onda H, Koon HB, Asrican K, Bajraszewski N, Vazquez F, Carpenter CL, Kwiatkowski DJ. Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J Clin Invest 2003;112:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL): modulation of MMP-9 activity by NGAL. J Biol Chem 2001;276:37258–37265. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longveville F, Kawasaki ES, Lee KY, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 2006;24:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2–independent pathway. J Biol Chem 2001;276:47402–47410. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Astrinidis A, Henske EP. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med 2001;164:1537–1540. [DOI] [PubMed] [Google Scholar]
- 28.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA 2000;97:3884–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res 2003;63:5224–5229. [PubMed] [Google Scholar]
- 30.Sounni NE, Baramova EN, Munaut C, Maquoi E, Frankenne F, Foidart JM, Noel A. Expression of membrane type 1 matrix metalloproteinase (MT1-MMP) in A2058 melanoma cells is associated with MMP-2 activation and increased tumor growth and vascularization. Int J Cancer 2002;98:23–28. [DOI] [PubMed] [Google Scholar]
- 31.Harper J, Moses MA. Molecular regulation of tumor angiogenesis: mechanisms and therapeutic implications. EXS 2006;96:223. [DOI] [PubMed] [Google Scholar]
- 32.Moses M, Sudhalter J, Langer R. Identification of an inhibitor of neovascularization from cartilage. Science 1990;248:1408–1410. [DOI] [PubMed] [Google Scholar]
- 33.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005;438:946–953. [DOI] [PubMed] [Google Scholar]
- 34.Seyama K, Kumasaka T, Souma S, Sato T, Kurihara M, Mitani K, Tominaga S, Fukuchi Y. Vascular endothelial growth factor–D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat Res Biol 2006;4:143–152. [DOI] [PubMed] [Google Scholar]
- 35.Young LR, Inoue Y, McCormack FX. Diagnostic potential of serum VEGF-D for lymphangioleiomyomatosis. N Engl J Med 2008;358:199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karbowniczek M, Cash T, Cheung M, Robertson GP, Astrinidis A, Henske EP. Regulation of B-Raf kinase activity by tuberin and Rheb is mammalian target of rapamycin (mTOR)–independent. J Biol Chem 2004;279:29930–29937. [DOI] [PubMed] [Google Scholar]
- 37.Karbowniczek M, Robertson GP, Henske EP. Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J Biol Chem 2006;281:25447–25456. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1–TSC2 complex is required for proper activation of mTOR complex 2. Mol Cell Biol 2008;28:4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1–independent pathway. Hum Mol Genet 2009;18:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandrasekar B, Mummidi S, Mahimainathan L, Patel DN, Bailey SR, Imam SZ, Greene WC, Valante AJ. Interleukin-18–induced human coronary artery smooth muscle cell migration is dependent on NF-kappa B– and AP-1–mediated matrix metalloproteinase-9 expression, and is inhibited by atorvastatin. J Biol Chem 2006;281:15099–15109. [DOI] [PubMed] [Google Scholar]
- 41.Ercan E, Tengiz I, Altuglu I, Sekuri C, Aliyev E, Ercan HE, Akin M. Atorvastatin treatment decreases inflammatory and proteolytic activity in patients with hypercholesterolemia. Kardiol Pol 2004;60:454–458. [PubMed] [Google Scholar]
- 42.Gomez-Hernandez A, Sanchez-Galan E, Martin-Ventura JL, Vidal C, Blanco-Colio LM, Ortego M, Vega M, Serrano J, Ortega L, Hernandez G, et al. Atorvastatin reduces the expression of prostaglandin E2 receptors in human carotid atherosclerotic plaques and monocytic cells: potential implications for plaque stabilization. J Cardiovasc Pharmacol 2006;47:60–69. [DOI] [PubMed] [Google Scholar]
- 43.Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol 2002;160:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finlay GA, Malhowski AJ, Liu Y, Fanburg BL, Kwiatkowski DJ, Toksoz D. Selective inhibition of growth of tuberous sclerosis complex 2 null cells by atorvastatin is associated with impaired Rheb and Rho GTPase function and reduced mTOR/S6 kinase activity. Cancer Res 2007;67:9878–9886. [DOI] [PubMed] [Google Scholar]
- 45.Mak BC, Kenerson HL, Aicher LD, Barnes EA, Yeung RS. Aberrant beta-catenin signaling in tuberous sclerosis. Am J Pathol 2005;167:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 2007;26:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.