Abstract
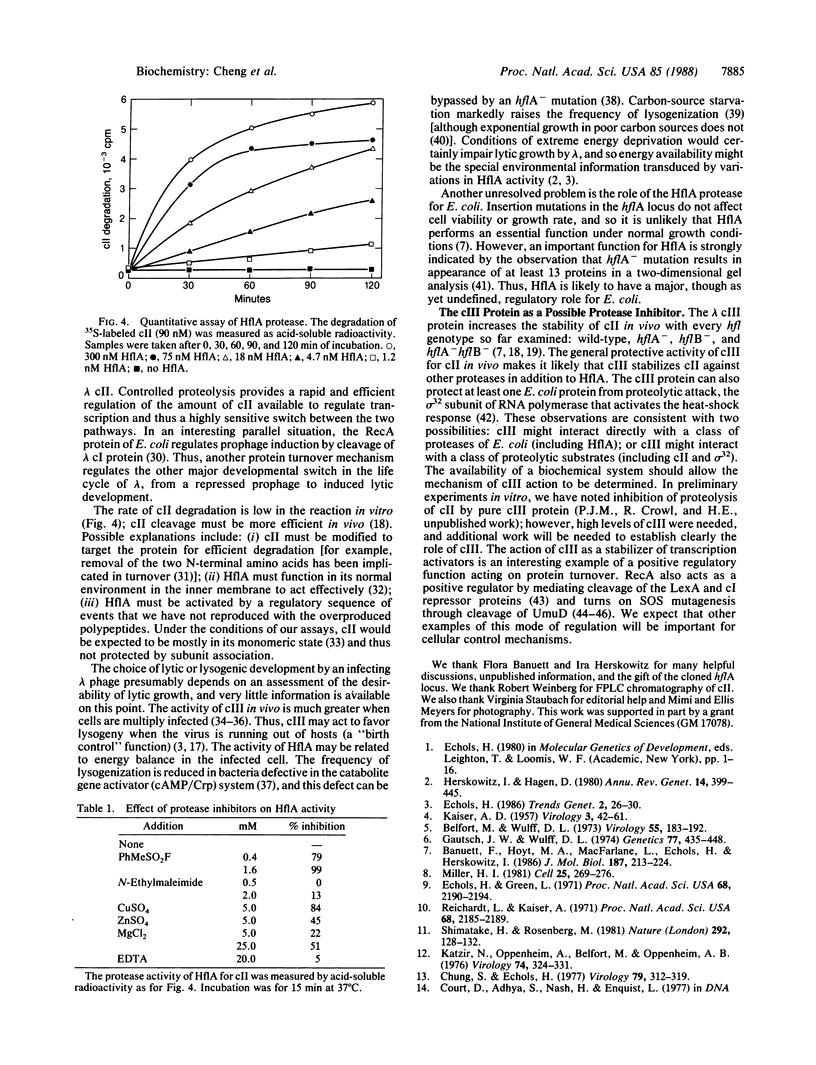
The activity of the cII protein of phage lambda is probably the critical controlling factor in the choice of the lytic or lysogenic pathway by an infecting virus. Previous work has established that cII activity is regulated through the turnover of cII protein; the products of the hflA and hflB loci of Escherichia coli are needed for a degradative reaction, and lambda cIII functions in stabilizing cII. By using the cloned hflA locus, we have purified a cII-cleaving enzyme that we term HflA. Purified HflA contains three polypeptides; at least two of the subunits are products of the hflA region, and the third is probably a cleavage product of the larger of these two hflA-encoded polypeptides. The HflA protease activity cleaves cII to small fragments. We conclude that the switch between lambda developmental pathways involves regulated cleavage of cII by the specific protease HflA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahl H., Echols H., Straus D. B., Court D., Crowl R., Georgopoulos C. P. Induction of the heat shock response of E. coli through stabilization of sigma 32 by the phage lambda cIII protein. Genes Dev. 1987 Mar;1(1):57–64. doi: 10.1101/gad.1.1.57. [DOI] [PubMed] [Google Scholar]
- Banuett F., Herskowitz I. Identification of polypeptides encoded by an Escherichia coli locus (hflA) that governs the lysis-lysogeny decision of bacteriophage lambda. J Bacteriol. 1987 Sep;169(9):4076–4085. doi: 10.1128/jb.169.9.4076-4085.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Hoyt M. A., McFarlane L., Echols H., Herskowitz I. hflB, a new Escherichia coli locus regulating lysogeny and the level of bacteriophage lambda cII protein. J Mol Biol. 1986 Jan 20;187(2):213–224. doi: 10.1016/0022-2836(86)90229-9. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. L. An analysis of the processes of infection and induction of E. coli mutant hfl-1 by bacteriophage lambda. Virology. 1973 Sep;55(1):183–192. doi: 10.1016/s0042-6822(73)81020-7. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. The roles of the lambda c3 gene and the Escherichia coli catabolite gene activation system in the establishment of lysogeny by bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Mar;71(3):779–782. doi: 10.1073/pnas.71.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. H., Echols H. A class of Escherichia coli proteins controlled by the hflA locus. J Mol Biol. 1987 Aug 5;196(3):737–740. doi: 10.1016/0022-2836(87)90046-5. [DOI] [PubMed] [Google Scholar]
- Chung S., Echols H. Positive regulation of integrative recombination by the cII and cIII genes of bacteriophase lambda. Virology. 1977 Jun 15;79(2):312–319. doi: 10.1016/0042-6822(77)90358-0. [DOI] [PubMed] [Google Scholar]
- Court D., Green L., Echols H. Positive and negative regulation by the cII and cIII gene products of bacteriophage lambda. Virology. 1975 Feb;63(2):484–491. doi: 10.1016/0042-6822(75)90321-9. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Green L., Kudrna R., Edlin G. Regulation of phage lambda development with the growth rate of host cells: a homeostatic mechanism. Virology. 1975 Jul;66(1):344–346. doi: 10.1016/0042-6822(75)90207-x. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wulff D. L. Fine structure mapping, complementation, and physiology of Escherichia coli hfl mutants. Genetics. 1974 Jul;77(3):435–448. doi: 10.1093/genetics/77.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Ho Y., Lewis M., Rosenberg M. Purification and properties of a transcriptional activator. The cII protein of phage lambda. J Biol Chem. 1982 Aug 10;257(15):9128–9134. [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Jones M. O., Herskowitz I. Mutants of bacteriophage lambda which do not requre the cIII gene for efficient lysogenization. Virology. 1978 Jul 15;88(2):199–212. doi: 10.1016/0042-6822(78)90277-5. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Katzir N., Oppenheim A., Belfort M., Oppenheim A. B. Activation of the lambda int gene by the cii and ciii gene products. Virology. 1976 Oct 15;74(2):324–331. doi: 10.1016/0042-6822(76)90339-1. [DOI] [PubMed] [Google Scholar]
- Knoll B. J. Isolation and characterization of mutations in the cIII gene of bacteriophage lambda which increase the efficiency of lysogenization of Escherichia coli K-12. Virology. 1979 Jan 30;92(2):518–531. doi: 10.1016/0042-6822(79)90154-5. [DOI] [PubMed] [Google Scholar]
- Kourilsky P. Lysogenization by bacteriophage lambda. I. Multiple infection and the lysogenic response. Mol Gen Genet. 1973 Apr 12;122(2):183–195. doi: 10.1007/BF00435190. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the c2 and c3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J Mol Biol. 1970 May 14;49(3):639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Miller H. I. Multilevel regulation of bacteriophage lambda lysogeny by the E. coli himA gene. Cell. 1981 Jul;25(1):269–276. doi: 10.1016/0092-8674(81)90252-x. [DOI] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray A., Altuvia S., Mahajna G., Oppenheim A. B., Gottesman M. Control of bacteriophage lambda CII activity by bacteriophage and host functions. J Bacteriol. 1984 Jul;159(1):238–242. doi: 10.1128/jb.159.1.238-242.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F. Control of bacteriophage lambda repressor synthesis after phage infection: the role of the N, cII, cIII and cro products. J Mol Biol. 1975 Apr 5;93(2):267–288. doi: 10.1016/0022-2836(75)90132-1. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuermann R. H., Echols H. A separate editing exonuclease for DNA replication: the epsilon subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]