Abstract
The phospholipase A2 (PLA2) superfamily consists of many different groups of enzymes that catalyze the hydrolysis of the sn-2 ester bond in a variety of different phospholipids. The products of this reaction, a free fatty acid, and lysophospholipid have many different important physiological roles. There are five main types of PLA2: the secreted sPLA2’s, the cytosolic cPLA2’s, the Ca2+ independent iPLA2’s, the PAF acetylhydrolases, and the lysosomal PLA2’s. This review focuses on the superfamily of PLA2 enzymes, and then uses three specific examples of these enzymes to examine the differing biochemistry of the three main types of these enzymes. These three examples are the GIA cobra venom PLA2, the GIVA cytosolic cPLA2, and the GVIA Ca2+-independent iPLA2.
1. Introduction
PLA2s form a superfamily that currently contains fifteen separate, identifiable groups and numerous subgroups of PLA2 [1–3]. These enzymes are characterized by their ability to specifically hydrolyze the sn-2 ester bond of phospholipid substrate as shown in Fig. 1. Enzymes are assigned to groups based on sequence, molecular weight, disulfide bonding patterns, the requirement for Ca2+, etc. There are five main categories of PLA2: the secreted small molecular weight sPLA2s, the larger cytosolic Ca2+-dependent cPLA2s, the Ca2+-independent iPLA2s, the PAF acetylhydrolases, and the lysosomal PLA2’s.
Figure 1.
Reaction catalyzed by the PLA2 superfamily of enzymes. Phospholipid on the left is hydrolyzed at the sn-2 position to yield lysophospholipid and free fatty acid on the right.
The products of the hydrolysis of the sn-2 ester bond of phospholipid are a free fatty acid and lysophospholipid. Both of these products represent the first step in generating important second messengers that play important physiological roles. Arachidonic acid (AA) when released from the sn-2 position of phospholipids can be converted into eicosanoids through the action of a variety of different downstream enzymes [4]. These eicosanoid molecules can exert a wide range of physiological and pathological effects. The lysophospholipid can be converted into lyso phosphatidic acid or be acetylated into platelet activating factor which also plays a variety of physiological roles [5, 6].
This review aims to introduce the superfamily of PLA2 enzymes, their mechanism of action, as well as focusing on three well defined enzymes of the sPLA2, cPLA2, and iPLA2 types.
2. The PLA2 Superfamily of Enzymes
2.1.Secreted PLA2 Enzymes
PLA2 activity was first studied in phenomenological detail as early as the 1890’s using “poison” or venom from cobras [7, 8]. The group numbering system was originally used to distinguish between different snake venoms, with the first use of the group numbering system seen in 1977 with Group I/II to distinguish between venoms from rattlesnakes and vipers from cobra and kraits [9], based on differences in disulfide bonding patterns. The secreted PLA2s are characterized by their requirement for histidine in the active site, low molecular weight, Ca2+ requirement for catalysis, and the presence of six conserved disulfide bonds, with one or two variable additional disulfide bonds [1, 2]. The mechanism of action/structure of the GIA PLA2 will follow in the following section, as well as mechanistic differences between the GIA sPLA2 and other group members. The first non-venom PLA2 named GIB was isolated from the pancreatic juices of cows, and was also found in many other animals [10]. There is strong evidence that this enzyme plays a major role in the digestion of phospholipids in the stomach [11, 12]. This was followed by the isolation of many other mammalian and other forms of secreted PLA2’ s shown in Table 1. The name sPLA2 was coined from the high content of GIIA PLA2 in the synovial fluid of patients with rheumatoid arthritis [13], but has come to stand for secreted.
Table 1.
Secreted phospholipases A2 (sPLA2)
| Group | Source | Molecular mass (kDa) |
Disulfide bonds |
|---|---|---|---|
| IA | Cobras and Kraits | 13–15 | 7 |
| IB | Human/porcine pancreas | 13–15 | 7 |
| IIA | Rattlesnakes: human synovial | 13–15 | 7 |
| IIB | Gaboon viper | 13–15 | 6 |
| IIC | Rat/murine testis | 15 | 8 |
| IID | Human/murine pancreas/spleen | 14–15 | 7 |
| IIE | Human/murine brain/heart/uterus | 14–15 | 7 |
| IIF | Human/murine testis/embryo | 16–17 | 6 |
| III | Human/murine/lizard/bee | 15–18 55 (human/murine) |
8 |
| V | Human/murine heart/lung/macrophage | 14 | 6 |
| IX | Snail venom (conodipine-M) | 14 | 6 |
| X | Human spleen/thymus/leukocyte | 14 | 8 |
| XIA | Green rice shoots (PLA2-I) | 12.4 | 6 |
| XIB | Green rice shoots (PLA2-II) | 12.9 | 6 |
| XII | Human/murine | 19 | 7 |
| XIII | Parvovirus | <10 | 0 |
| XIV | Symbiotic fungus/ bacteria | 13–19 | 2 |
All of the sPLA2 enzymes (except group III) [14] display a characteristic increase in activity when substrate is switched from monomeric to higher ordered lipid aggregates, and this is known as interfacial activation [15]. The mechanism of interfacial activation will be considered in the next section on the GIA PLA2.
The sPLA2 group of enzymes has a vast variety of physiological functions. One of the most defined biological functions is for Group IIA PLA2, which has been shown to be important as an antimicrobial agent by hydrolyzing the negatively charged membranes of gram negative bacteria [16, 17]. The exact physiological role of sPLA2 enzymes in production of eicosanoids has remained undefined. For more in depth analysis of the biological actions of the mammalian sPLA2 enzymes please see [18, 19],.
2.2 Cytosolic PLA2’s
The cytosolic PLA2’s are larger then the sPLA2 enzymes (61–114 kDa) and do not have the same disulfide bonding network as the sPLA2 enzymes. The first cytosolic PLA2 was isolated from neutrophils and platetlets in 1986 .The complete list of these enzymes is shown in Table 2. The detailed mechanism and biology of the GIVA PLA2 will be explained in detail in Section 4. These enzymes all function through the action of a serine/aspartic acid dyad. All of the cytosolic PLA2’s (except GIVC [20]) require Ca2+ for activity, due to the presence of C2 domains [21–24]. The different GIV enzymes have different specificity for fatty acids in the sn-2 position. GIVA is specific for AA containing phospholipids [25], GIVB and GIVC have very little specificity [22, 23], GIVD appears to be specific for linoleic acid (LA) containing fatty acids [24], while GIVE and GIVF hydrolyze both AA and LA [21]. For a more in-depth analysis of the biology of the other cytosolic enzymes see reviews[1, 26].
Table 2.
Cytosolic Group IV phospholipases A2 (cPLA2)
| Groups | Source | Molecular mass (kDa) | Features | Alternate names |
|---|---|---|---|---|
| IVA | Human/murine | 85 | C2 domain | cPLA2α |
| IVB | Human | 114 | C2 domain | cPLA2β |
| IVC | Human | 61 | acylated | cPLA2γ |
| IVD | Human/murine | 92–93 | C2 domain | cPLA2δ |
| IVE | Murine | 100 | C2 domain | cPLA2ε |
| IVF | Murine | 96 | C2 domain | cPLA2ξ |
2.3 Ca2+ Independent PLA2’s
The Ca2+ independent PLA2 (iPLA2s) includes six different types GVIA, GVIB, GVIC, GVID, GVIE, and GVIF PLA2 as shown in Table 3. The term Ca2+ independent PLA2 is misleading for the Group VIA enzymes. All of the enzymes in this group do not require Ca2+ for activity, but the GIVC enzyme also does not require Ca2+ for activity, but was placed in the Group IV of PLA2 enzymes due to homology to other GIV enzymes [20]. All of these enzymes function through a catalytic serine at the active site. There is no fatty acid chain specificity seen in any of the GVI enzymes. The inhibitor BEL inhibits specifically GVIA, and GVIB, however the inhibition is mediated through different enantiomers [27]. This property has allowed for studies focusing on specific GVI enzymes [28]. For reviews of the other GVI enzymes see [1].
Table 3.
Ca2+ independent Group VI phospholipases A2 (iPLA2)
| Group | Source | Molecular mass (kDa) |
Features | Alternate names |
|---|---|---|---|---|
| VIA-1 | Human/murine | 84–85 | 8 ankyrin repeats | iPLA2 |
| VIA-2 | Human/murine | 88–90 | 7 ankyrin repeats | iPLA2β |
| VIB | Human/murine | 88–91 | Membrane-bound | iPLA2γ |
| VIC | Human/murine | 146 | Integral membrane protein |
iPLA2δ, neuropathy target esterase (NTE) |
| VID | Human | 53 | Acylglycerol transacylase, tricylglycerol lipase |
iPLA2ε, adiponutrin |
| VIE | Human | 57 | Acylglycerol transacylase, triacylglycerol lipase |
iPLA2ζ, TTS-2.2 |
| VIF | Human | 28 | Acylglycerol transacylase, triacyglycerol lipase |
iPLA2η, GS2 |
2.4 PAF acetylhydrolases
The PAF acetyldrolases are composed of two PLA2 groups that both hydrolyze the acetyl group from the sn-2 position of platelet activating factor (PAF) as shown in Table 4. The function of this class of enzymes is of high interest due to the important roles played by PAF in the body. All of these enzymes function through the action of a catalytic serine. The PAF acetylhydrolases all have a Ser/His/Asp catalytic triad mediating hydrolysis [29, 30]. These enzymes do not require Ca2+ for activity. The GVIIA PLA2 is a secreted enzyme that can also hydrolyze short chain fatty acids from the sn-2 position [31]. This enzyme is not interfacially activated [31]. This enzyme is also known as the lipoprotein associated PLA2. Recent work has identified regions in the catalytic domain important for binding to both HDL and LDL cholesterol molecules [32]. The mechanisms that impart preferences for HDL and LDL are currently poorly understood. The GVIII PAF acetylhydrolases are also regulated through aggregation of the catalytic and regulatory subunits [33]. For excellent reviews on the biology of lipoprotein associated PLA2 and relation to cardiovascular disease see[34, 35].
Table 4.
Group VII and Group VIII phospholipases A2 displaying PAF acetylhydrolase (PAF-AH) activity
| Group | Source | Molecular mass(kDa) |
Features | Alternate names |
|---|---|---|---|---|
| VIIA | Human, murine, porcine, bovine |
45 | Secreted, α/β hydrolase |
Lipoprotein-associated PLA2 (Lp-PLA2), Plasma PAF-AH |
| VIIB | Human, bovine |
40 | Intracellular, myristoylated, α/β hydrolase |
PAF-AH II |
| VIIIA | Human | 26 | Intracellular, Ser/His/Asp triad, homodimer or heterodimer with GVIIIB associates with regulatory β-subunit |
PAF-AH Ib (α1 subunit) |
| VIIIB | Human | 26 | Intracellular Ser/His/Asp triad, homodimer or heterodimer with GVIIIA associates with regulatory β-subunit |
PAF-AH Ib (α2 subunit) |
2.5 Lysosomal PLA2
The lysosomal PLA2 is the newest type; it was purified from bovine brain and acylates ceramide using the acyl group from the sn-2 position of phospholipid as substrate [36, 37]. This enzyme contains a conserved Ser-His-Asp triad and has four cysteine residues that are required for catalytic activity [38].
3. Group IA PLA2
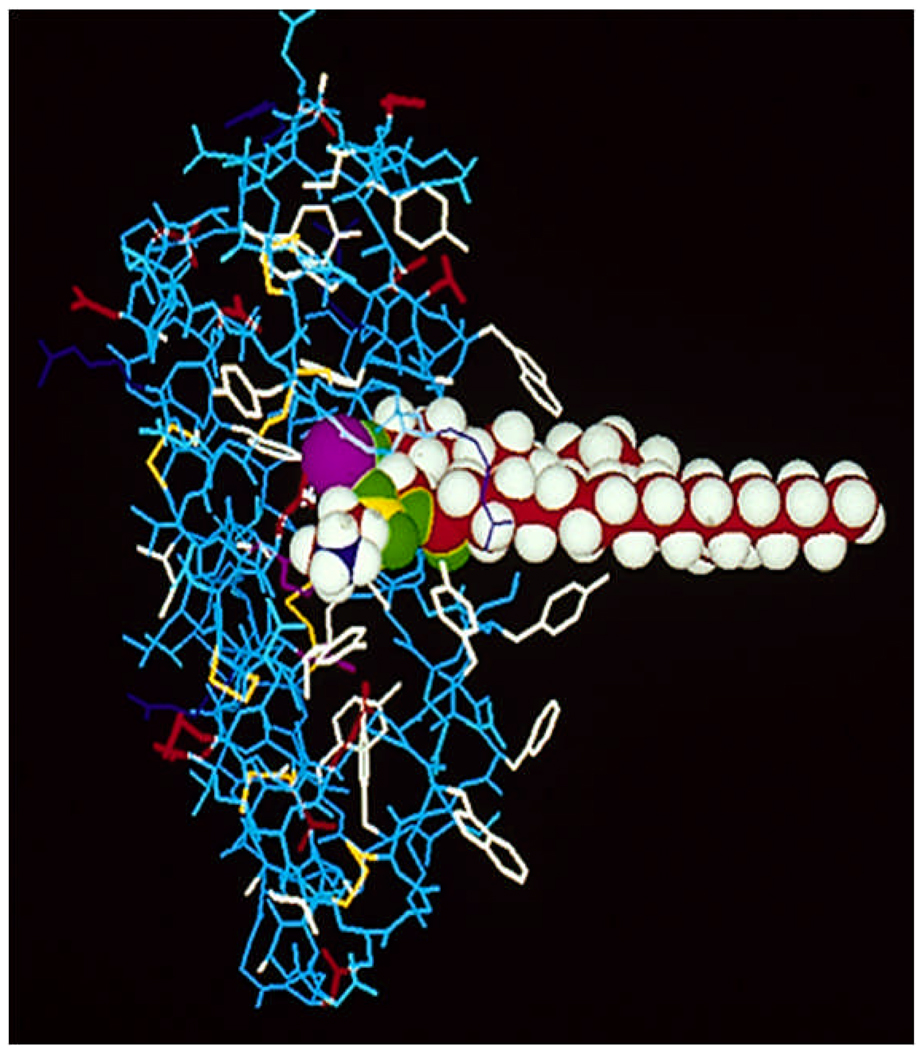
One of the best studied PLA2 enzymes is the cobra venom Group IA (GIA) PLA2. This enzyme has acted as not only an important model of phospholipid metabolizing enzymes, but of all lipid enzymology. Many different crystal structures of this enzyme exist from different venom sources [39–42]. These crystal structures all show some important traits as shown in Fig. 2. The enzyme contains the six conserved disulfide bonds from 28–44, 26–118, 43–99, 50–92, 60–85, and 78–80, as well as the additional disulfide bridge from 11–71 [39]. The active site dyad is composed of the conserved His-48, and Asp-99. The active site histidine is found to be conserved in all sPLA2 enzymes [15, 43–45]. The enzyme catalyzes hydrolysis through the activation of a water molecule by extraction of a proton, and attack at the sn-2 ester bond [15, 46, 47]. This mechanism explains the pH dependence of these enzymes at around 7–9. Recent work using unnatural phospholipid substrate with PC headgroups in the sn-2 position have shown that phospholipid hydrolysis is proportional to the ease of water accessibility to the active site [48, 49]. The enzyme binds Ca2+ through the conserved Asp-49 [50, 51], as well as the carbonyl oxygens of Tyr-28, Gly-30, and Gly-32 [39]. The Ca2+ ion is required for hydrolysis through orientation of the lipid substrate by coordination of the negative charge from the phosphate oxygen [40]. Some structures have shown the presence of a secondary Ca2+ ion that may act as a supplementary electrophile [40].
Figure 2.
Group IA PLA2 with phospholipid substrate modeled in the active site based on crystal structure by Fremont et al. [39], along with NOE measurements [52] using amide pseudo-substrate inhibitor [53]. The active site residues His-48 and Asp-93 and the bound Ca2+ is shown in purple. Ca2+ is bound by Asp-49 as well as the carbonyl oxygens of Tyr-28, Gly-30, and Gly-32. Aromatic residues are shown in white; of special interest are the aromatic residues on the interfacial binding surface Tyr-3, Trp-19, Trp 61, and Phe 64. Adapted from Dennis [3]
NMR studies of the GIA PLA2 with inhibitor bound in the active site have allowed for the creation of a model of substrate bound in the active site as shown in Fig. 2. [52, 53]. The fatty acid tails of the substrate are surrounded by the hydrophobic residues Leu-2, Phe-5, Trp-19, Tyr-52, and Tyr-69. Crystal structures of the GIA PLA2 with bound inhibitors closely matches this result [42]. The enzyme has very little selectivity for the fatty acid in the sn-2 position [54].
This enzyme is able to hydrolyze monomeric phospholipid substrates, but there is a substantial increase in activity when the enzyme acts on large lipid aggregates [55]. This enzyme has also been shown to be activated by phospholipids containing phosphatidylcholine (PC) head groups [56], and two possible sites for this interaction have been suggested [41, 57]. A combination of site-directed mutagenesis and equilibrium dialysis has identified and confirmed there is an activator site distinct from the catalytic site [58, 59].
The different sPLA2’s all share different preferences for the charge state of the lipid membrane. For an excellent analysis of the different mouse and human sPLA2 membrane preferences see [60]. The majority of sPLA2 enzymes preferentially hydrolyze anionic substrates [18]. The GIA enzyme however is able to hydrolyze zwitterionic substrate equally as well as negatively charged lipid surfaces [56, 61]. This is most likely due to the aromatic residues present on the interfacial binding surface of the GIA PLA2 as shown in Fig. 2. Mutation of these residues significantly decreases the membrane binding of this enzyme [59, 61].
In comparision, the crystal structures of the GIB and GIIA enzymes demonstrate these enzymes have a cationic interfacial binding surface, and this may play a large role in their preference for anionic lipids [44, 62]. It has been shown that a mutant form of the GIB enzyme with the pancreatic loop from 62–66 removed has increases in activity against zwitterionic substrate, and a decrease in activity against negatively charged substrate [63]. Recent studies using a GIIA enzyme with a Trp residue mutated into the interfacial binding region dramatically increased zwitterionic phospholipid hydrolysis [64], as well as penetration [65, 66]. The only other sPLA2 enzymes to have high affinity for zwitterionic vesicles are the GV, and GX enzymes [67–69] which also share the characteristic of having Trp residues in the interfacial binding region.
4. Group IVA PLA2
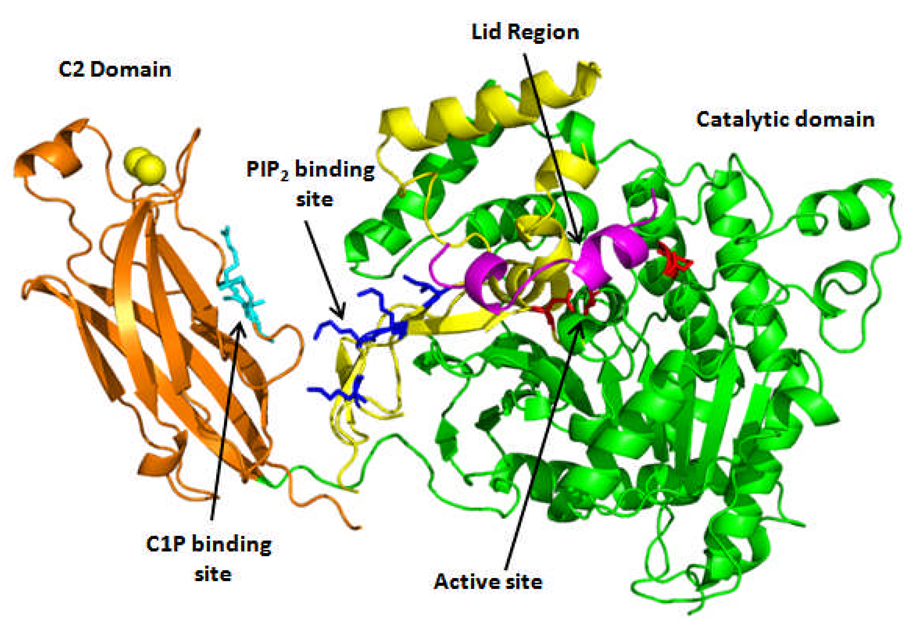
GIVA PLA2 is an 85 kDa enzyme that utilizes a catalytic serine for hydrolysis rather then histidine, as in the sPLA2 enzymes. This enzyme was initially isolated from human neutrophils [70], and platelets [71]. This enzyme was sequenced in 1991, and was shown to be specific for phospholipids containing arachidonic acid in the sn-2 position [72]. The crystal structure of the C2 domain was solved in 1998 [73] followed by the whole enzyme in 1999 and showed a Ca2+ binding C2 domain important for Ca2+ mediated membrane translocation, and a α/β hydrolase domain that contains the catalytic site [74]. The crystal structure is shown in Fig. 3. Of special note in this structure is the presence of a lid region that spans regions 415–432 that prevents the modeling of a phospholipid substrate in the active site. This structure confirmed previous work using mutant constructs showing the two independent functions of the C2, and catalytic domain [75]. This structure showed the presence of a novel Ser/Asp dyad that mediated the hydrolysis of phospholipid substrate. The enzyme hydrolyzes substrate through the formation of a serine-acyl intermediate [76, 77]. Previous work had suggested that the active site residues would consist of Ser-228, Asp-549, and Arg-200 due to inactivity of mutants containing mutations at any of these locations [78]. This crystal structure shows that Ser-228, and Asp-549 are in the correct orientation to act as an active site dyad, but Arg-200 is too far away to form any contacts with either Ser-228, or Asp-549. This led to the proposal that Arg-200 may be important in binding the charged headgroups of phospholipid substrate [74].
Figure 3.
Group IVA PLA2 crystal structure as determined by Dessen et. Al. [74]. The C2 domain is shown in orange, with two bound Ca2+ ions shown in light yellow. The catalytic domain is shown on the right with the cap region colored yellow, and the lid region 415–432 colored magenta. The active site residues Ser-228, Asp-549 and Arg-200 are shown in stick form colored red. The PIP2 binding site is shown in dark blue, and the C1P binding site is shown in cyan.
This enzyme also has both lysophospholipase, and transacylase activity [79, 80], however the lysophospholipase activity of this enzyme is insensitive to Ca2+ concentration. The PLA2 activity of the enzyme is active against monomeric substrate, but there is a substantial activation upon binding a membrane surface [75]. For the enzyme to be active, it must be sequestered to a phospholipid interface. The binding of the GIVA PLA2 to the membrane is mediated through three mechanisms: Ca2+ mediated translocation, binding of secondary lipid messengers, and phosphorylation.
Ca2+ binding in the GIVA PLA2 is not required for catalysis as in the sPLA2 enzymes, but is required for translocation to the membrane surface [81–86]. Ca2+ binding is mediated through the C2 domain of the enzyme. C2 domains are conserved domains present on many different lipid binding proteins (For an excellent review on membrane binding domains see [87]). The mechanism of Ca2+ binding to the C2 domain, and how this mediates phospholipid binding, has been studied through a variety of techniques, including x-ray reflectivity, site directed mutagenesis, NMR, EPR, and computational methods[88–94]. These studies have shown that Ca2+ binding to this domain sequesters the protein to the lipid surface through penetration of Ca2+ binding loops one and three, composed of amino acids 35–39, and 96–98, into the interface. The C2 domain of the GIVA PLA2 is specific for membranes with PC headgroups [92, 95].
The GIVA PLA2 is also activated by binding many different lipid second messengers. It has been shown that phosphatidylinositol (4,5) bis phosphate (PIP2) significantly activates the enzyme in a Ca2+ independent manner [96, 97]. The location of the PIP2 binding site was identified through the use of site directed mutagenesis and is located at four lysines at position 485, 541, 543, and 544 [97, 98] as shown in Fig. 3. We have also shown that this PIP2 activation requires the presence of the C2 domain, even though the PIP2 binding site is completely contained on the catalytic domain [97]. Recently the lipid ceramide 1 kinase was discovered to also be an activator of GIVA PLA2 [99–101]. Ceramide 1-phosphate (C1P) binds to the enzyme at a specific site in the C2 domain consisting of Arg-57, Lys-58, and Arg-59 shown in Fig. 3. [102]. Studies have also shown that the mechanism of C1P activation is Ca2+ dependent, and decreases the kinetic dissociation constant from the membrane surface [103] while PIP2 activation is caused by an increase in the catalytic efficiency, potentially through a conformational change [103].
The phosphorylation state of the GIVA PLA2 also plays an important role in mediating lipid-enzyme interactions. Many different residues on the GIVA enzyme can be phosphorylated by a myriad of different kinases. The main residues found phosphorylated are Ser-505, Ser-515, and Ser-727. These residues are phosphorylated by mitogen activated protein kinases (MAPKs), mitogen activated protein kinase interacting kinase (MNK1), calmodulin kinase II (CamKII), and mitogen activated protein kinase interacting kinase (MNK1) respectively [104–107]. Other phosphorylation sites have been reported at Ser-437, and Ser-454 in Sf9 cells [108], but there is currently no information on the effects of phosphorylation at these residues. Interestingly all of the residues that have been found to be phosphorylated are located in areas of the crystal structure with no traceable electron density [74]. It has been shown that Ser-505, and Ser-727 are common phosphorylation sites in agonist-stimulated human platelets and HeLa cells [104], while Ser-505, and Ser-515 phosphorylation are found in vascular smooth muscle cells [109]. Ser-505 phosphorylation has been shown to cause a very small increase in activity [110, 111], however recent work studying membrane binding found a 60 fold increase in membrane affinity at 2.5 μM Ca2+, and it is suggested that it induces a conformational change that causes tighter binding to the lipid surface [112]. It has recently been shown that Ser-727 phosphorylation mediates GIVA PLA2 activity through disruption of the complex formed between annexin A2, p11, and GIVA PLA2 [113]. Ser-515 phosphorylation has been shown to increase in-vitro activity of the enzyme 3 fold [106], and may also activate the enzyme through a conformational change.
The importance of GIVA PLA2 in many different inflammatory processes has been proven through the use of knockout mice deficient in GIVA PLA2. These mice showed significant decreases in allergic response, damage from acute lung injury, and postischaemic brain injury [114–116]. For review see [117].
5. GVIA PLA2
The human Group VIA PLA2 gene yields multiple splice variants, including GVIA-1, GVIA-2, GVIA-3 PLA2, GVIA Ankyrin-1 and GVIA Ankyrin-2 [118, 119]. At least two of these isoforms, GVIA-1 and GVIA-2 iPLA2 are active. The human GVIA iPLA2 contains 7–8 ankyrin repeats, a linker region, and a catalytic domain. The 85-kDa GVIA-2 iPLA2 was first purified and isolated from the p388D1 cell line [120, 121], which possesses PLA2 activity as well as lysophospholipase and transacylase activity [122]. The active site serine of the GVIA iPLA2 lies within a lipase consensus sequence (Gly-X-Ser519-X-Gly) [123]. The enzyme is not specific in what fatty acid is being released [120, 123]. The activity of GVIA iPLA2 has been reported to be regulated through many mechanisms. The enzyme possesses a caspase-3 cleavage site that is clipped in vitro [124–126]. The caspase truncated enzyme was hyperactive and reduced cell viability when overexpressed in HEK293 cells [124]. Caspase mediated activation has also been recently shown to be important in mediating cell migration in ovarian cancer cells [127]. The enzyme is also regulated through ATP binding. ATP binding seems to protect the GVIA PLA2 from losing activity [122]. Fatty acyl-CoA was also shown to be a substrate for GVIA iPLA2, showing a potential role for nucleotide binding [128].
The GVIA PLA2 contains multiple ankyrin repeats which may be important in mediating protein-protein interactions. The enzyme when originally isolated was shown to be active as a tetramer [120]. The splice variant GVI Ankyrin-1 was also suspected to be a negative regulator of GVIA PLA2 through blocking potential formation of the active aggregate.[119]. The importance of the ankyrin repeats is shown by studies done on the catalytic domain alone of GVIA PLA2 showing no activity [119]. The enzyme has also been shown to be regulated by calmodulin which negatively regulates GVIA PLA2 through direct binding on the residues 694–705 of GVIA-1 PLA2 [129, 130].
GVIA-2 iPLA2 is membrane associated when overexpressed in COS-7 cells as well as rat vascular smooth muscle cells [118, 131]. The other active splice variant, GVIA-1, is cytosolic and not specific in targeting membrane surfaces [118, 131]. This enzyme has been shown to be important in membrane homeostasis and remodeling [132], and it appears that this enzyme is the primary PLA2for day to day metabolic functions within the cell. More recently, others have found that GVIA iPLA2 is involved in cell proliferation [133–136], mediating cell growth [28], apoptosis [137] and glucose-induced insulin secretion [138].
Conclusion
The PLA2 superfamily of enzymes mediates a variety of important cellular functions. Research in this field has continued to expand with the discovery of important new functions for many of these enzymes. With the continued work of dedicated researchers this field of study will most certainly continue to generate important discoveries of novel PLA2 activities, as well as understanding the mechanism and function of these enzymes. Further knowledge of this superfamily of enzymes should raise the potential for many possible new and exciting drug targets in the years to come.
Table 5.
Lysosomal Phospholipase A2
| Group | Source | Molecular mass (kDa) |
Features | Alternate names |
|---|---|---|---|---|
| XV | Human, murine, bovine |
45 (deglycosylated) | Ser/His/Asp triad, glycosylated, N-terminal signal sequence |
ACS, lysosomal PLA2(LPLA2), LLPL |
REFERENCES
- 1.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. BiochimBiophysActa. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 3.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 4.Funk CD. Prostaglandins and Leukotrienes: Advances in Eicosanoid Biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 5.Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- 6.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 7.Fairbairn D. The phospholipase of the venom of the cottonmouth moccasin (Agkistrodon Piscivorus L) J Biol Chem. 1945;157:633–644. [Google Scholar]
- 8.Stephens WW, Walker JL, Myers W. The action of cobra poison on the blood: a contribution to the study of passive immunity. J Pathol Bacteriol. 1898;5:279–301. [Google Scholar]
- 9.Heinrikson RL, Krueger ET, Keim PS. Amino acid sequence of phospholipase A2-alpha from the venom of Crotalus adamanteus. A new classification of phospholipases A2 based upon structural determinants. J Biol Chem. 1977;252:4913–4921. [PubMed] [Google Scholar]
- 10.Puijk WC, Verheij HM, De Haas GH. The primary structure of phospholipase A2 from porcine pancreas. A reinvestigation. Biochim Biophys Acta. 1977;492:254–259. doi: 10.1016/0005-2795(77)90076-9. [DOI] [PubMed] [Google Scholar]
- 11.Eerola LI, Surrel F, Nevalainen TJ, Gelb MH, Lambeau G, Laine VJ. Analysis of expression of secreted phospholipases A2 in mouse tissues at protein and mRNA levels. Biochim Biophys Acta. 2006;1761:745–756. doi: 10.1016/j.bbalip.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Richmond BL, Boileau AC, Zheng S, Huggins KW, Granholm NA, Tso P, et al. Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A(2)-deficient mice. Gastroenterology. 2001;120:1193–1202. doi: 10.1053/gast.2001.23254. [DOI] [PubMed] [Google Scholar]
- 13.Seilhamer JJ, Pruzanski W, Vadas P, Plant S, Miller JA, Kloss J, et al. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989;264:5335–5338. [PubMed] [Google Scholar]
- 14.Lin G, Noel J, Loffredo W, Stable HZ, Tsai MD. Use of short-chain cyclopentano-phosphatidylcholines to probe the mode of activation of phospholipase A2 from bovine pancreas and bee venom. J Biol Chem. 1988;263:13208–13214. [PubMed] [Google Scholar]
- 15.Verheij HM, Slotboom AJ, de Haas GH. Structure and function of phospholipase A2. Rev Physiol Biochem Pharmacol. 1981;91:91–203. doi: 10.1007/3-540-10961-7_3. [DOI] [PubMed] [Google Scholar]
- 16.Buckland AG, Heeley EL, Wilton DC. Bacterial cell membrane hydrolysis by secreted phospholipases A(2): a major physiological role of human group IIa sPLA(2) involving both bacterial cell wall penetration and interfacial catalysis. Biochim Biophys Acta. 2000;1484:195–206. doi: 10.1016/s1388-1981(00)00018-4. [DOI] [PubMed] [Google Scholar]
- 17.Buckland AG, Wilton DC. The antibacterial properties of secreted phospholipases A(2) Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 18.Lambeau G, Gelb MH. Biochemistry and Physiology of Mammalian Secreted Phospholipases A(2) Annu Rev Biochem. 2008 doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 19.Biology of Secretory Phospholipase A2. Cardiovascular Drugs and Therapy. 2008 doi: 10.1007/s10557-008-6134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underwood KW, Song C, Kriz RW, Chang XJ, Knopf JL, Lin LL. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J Biol Chem. 1998;273:21926–21932. doi: 10.1074/jbc.273.34.21926. [DOI] [PubMed] [Google Scholar]
- 21.Ohto T, Uozumi N, Hirabayashi T, Shimizu T. Identification of novel cytosolic phospholipase A(2)s, murine cPLA(2){delta}, {epsilon}, and {zeta}, which form a gene cluster with cPLA(2){beta} J Biol Chem. 2005;280:24576–24583. doi: 10.1074/jbc.M413711200. [DOI] [PubMed] [Google Scholar]
- 22.Pickard RT, Strifler BA, Kramer RM, Sharp JD. Molecular cloning of two new human paralogs of 85-kDa cytosolic phospholipase A2. J Biol Chem. 1999;274:8823–8831. doi: 10.1074/jbc.274.13.8823. [DOI] [PubMed] [Google Scholar]
- 23.Song C, Chang XJ, Bean KM, Proia MS, Knopf JL, Kriz RW. Molecular characterization of cytosolic phospholipase A2-beta. J Biol Chem. 1999;274:17063–17067. doi: 10.1074/jbc.274.24.17063. [DOI] [PubMed] [Google Scholar]
- 24.Chiba H, Michibata H, Wakimoto K, Seishima M, Kawasaki S, Okubo K, et al. Cloning of a gene for a novel epithelium-specific cytosolic phospholipase A2, cPLA2delta, induced in psoriatic skin. J Biol Chem. 2004;279:12890–12897. doi: 10.1074/jbc.M305801200. [DOI] [PubMed] [Google Scholar]
- 25.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A2 family. Prog Lipid Res. 2006;45:487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2gamma, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J Biol Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra G, Zhang W, Peterson B, Cummings BS. Differential roles for cytosolic and microsomal Ca2+-independent phospholipase A2 in cell growth and maintenance of phospholipids. J Pharmacol Exp Ther. 2006;318:1211–1219. doi: 10.1124/jpet.106.105650. [DOI] [PubMed] [Google Scholar]
- 29.Tjoelker LW, Eberhardt C, Unger J, Trong HL, Zimmerman GA, McIntyre TM, et al. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J Biol Chem. 1995;270:25481–25487. doi: 10.1074/jbc.270.43.25481. [DOI] [PubMed] [Google Scholar]
- 30.Tjoelker LW, Wilder C, Eberhardt C, Stafforini DM, Dietsch G, Schimpf B, et al. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature. 1995;374:549–553. doi: 10.1038/374549a0. [DOI] [PubMed] [Google Scholar]
- 31.Min JH, Jain MK, Wilder C, Paul L, Apitz-Castro R, Aspleaf DC, et al. Membrane-bound plasma platelet activating factor acetylhydrolase acts on substrate in the aqueous phase. Biochemistry. 1999;38:12935–12942. doi: 10.1021/bi991149u. [DOI] [PubMed] [Google Scholar]
- 32.Gardner AA, Reichert EC, Topham MK, Stafforini DM. Identification of a domain that mediates association of platelet-activating factor acetylhydrolase with high density lipoprotein. J Biol Chem. 2008;283:17099–17106. doi: 10.1074/jbc.M802394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manya H, Aoki J, Kato H, Ishii J, Hino S, Arai H, et al. Biochemical characterization of various catalytic complexes of the brain platelet-activating factor acetylhydrolase. J Biol Chem. 1999;274:31827–31832. doi: 10.1074/jbc.274.45.31827. [DOI] [PubMed] [Google Scholar]
- 34.Biology of Lipoprotein Associated Phospholipase A2. Cardiovascular Drugs and Therapy. 2008 [Google Scholar]
- 35.Epidemiology of Secretory and Lipoprotein Associated Phospholipase A2 Activity and Mass with Cardiovascular Disease. Cardiovascular Drugs and Therapy. 2008 [Google Scholar]
- 36.Abe A, Shayman JA. Purification and characterization of 1-O-acylceramide synthase, a novel phospholipase A2 with transacylase activity. J Biol Chem. 1998;273:8467–8474. doi: 10.1074/jbc.273.14.8467. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka M, Abe A, Shayman JA. Cloning and characterization of a lysosomalphospholipase A2, 1-O-acylceramide synthase. J Biol Chem. 2002;277:10090–10099. doi: 10.1074/jbc.M111977200. [DOI] [PubMed] [Google Scholar]
- 38.Hiraoka M, Abe A, Shayman JA. Structure and function of lysosomal phospholipase A2: identification of the catalytic triad and the role of cysteine residues. J Lipid Res. 2005;46:2441–2447. doi: 10.1194/jlr.M500248-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Fremont DH, Anderson D, Wilson IA, Dennis EA, Xuong NH. The crystal structure of phospholipase A2 from Indian cobra reveals a novel trimeric association. Proc Natl Acad Sci U S A. 1993;90:342–346. doi: 10.1073/pnas.90.1.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott DL, White SP, Otwinowski Z, Yuan W, Gelb MH, Sigler PB. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990;250:1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segelke BW, Nguyen D, Chee R, Xuong NH, Dennis EA. Structures of two novel crystal forms of Naja naja naja phospholipase A2 lacking Ca2+ reveal trimeric packing. J Mol Biol. 1998;279:223–232. doi: 10.1006/jmbi.1998.1759. [DOI] [PubMed] [Google Scholar]
- 42.White SP, Scott DL, Otwinowski Z, Gelb MH, Sigler PB. Crystal structure of cobra-venom phospholipase A2 in a complex with a transition-state analogue. Science. 1990;250:1560–1563. doi: 10.1126/science.2274787. [DOI] [PubMed] [Google Scholar]
- 43.Verheij HM, Volwerk JJ, Jansen EH, Puyk WC, Dijkstra BW, Drenth J, et al. Methylation of histidine-48 in pancreatic phospholipase A2. Role of histidine and calcium ion in the catalytic mechanism. Biochemistry. 1980;19:743–750. doi: 10.1021/bi00545a021. [DOI] [PubMed] [Google Scholar]
- 44.Dijkstra BW, Drenth J, Kalk KH. Active site and catalytic mechanism of phospholipase A2. Nature. 1981;289:604–606. doi: 10.1038/289604a0. [DOI] [PubMed] [Google Scholar]
- 45.Dijkstra BW, Kalk KH, Hol WG, Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981;147:97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- 46.Lombardo D, Fanni T, Pluckthun A, Dennis EA. Rate-determining step in phospholipase A2 mechanism. 18O isotope exchange determined by 13C NMR. J Biol Chem. 1986;261:11663–11666. [PubMed] [Google Scholar]
- 47.Yu L, Dennis EA. Critical role of a hydrogen bond in the interaction of phospholipase A2 with transition-state and substrate analogues. Proc Natl Acad Sci U S A. 1991;88:9325–9329. doi: 10.1073/pnas.88.20.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linderoth L, Andresen TL, Jorgensen K, Madsen R, Peters GH. Molecular basis of phospholipase A2 activity toward phospholipids with sn-1 substitutions. Biophys J. 2008;94:14–26. doi: 10.1529/biophysj.107.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters GH, Moller MS, Jorgensen K, Ronnholm P, Mikkelsen M, Andresen TL. Secretory phospholipase A2 hydrolysis of phospholipid analogues is dependent on water accessibility to the active site. J Am Chem Soc. 2007;129:5451–5461. doi: 10.1021/ja067755b. [DOI] [PubMed] [Google Scholar]
- 50.Fleer EA, Verheij HM, de Haas GH. Modification of carboxylate groups in bovine pancreatic phospholipase A2. Identification of aspartate-49 as Ca2+-binding ligand. Eur J Biochem. 1981;113:283–288. doi: 10.1111/j.1432-1033.1981.tb05064.x. [DOI] [PubMed] [Google Scholar]
- 51.van den Bergh CJ, Slotboom AJ, Verheij HM, de Haas GH. The role of Asp-49 and other conserved amino acids in phospholipases A2 and their importance for enzymatic activity. J Cell Biochem. 1989;39:379–390. doi: 10.1002/jcb.240390404. [DOI] [PubMed] [Google Scholar]
- 52.Plesniak LA, Yu L, Dennis EA. Conformation of micellar phospholipid bound to the active site of phospholipase A2. Biochemistry. 1995;34:4943–4951. doi: 10.1021/bi00015a005. [DOI] [PubMed] [Google Scholar]
- 53.Yu L, Deems RA, Hajdu J, Dennis EA. The interaction of phospholipase A2 with phospholipids analogues and inhibitors. J Biol Chem. 1990;265:2657–2664. [PubMed] [Google Scholar]
- 54.Roberts MF, Otnaess AB, Kensil CA, Dennis EA. The specificity of phospholipase A2 and phospholipase C in a mixed micellar system. J Biol Chem. 1978;253:1252–1257. [PubMed] [Google Scholar]
- 55.Pluckthun A, Rohlfs R, Davidson FF, Dennis EA. Short-chain phosphatidylethanolamines: physical properties and susceptibility of the monomers to phospholipase A2 action. Biochemistry. 1985;24:4201–4208. doi: 10.1021/bi00336a058. [DOI] [PubMed] [Google Scholar]
- 56.Adamich M, Roberts MF, Dennis EA. Phospholipid activation of cobra venom phospholipase A2. 2. Characterization of the phospholipid--enzyme interaction. Biochemistry. 1979;18:3308–3314. doi: 10.1021/bi00582a017. [DOI] [PubMed] [Google Scholar]
- 57.Ortiz AR, Pisabarro MT, Gallego J, Gago F. Implications of a consensus recognition site for phosphatidylcholine separate from the active site in cobra venom phospholipases A2. Biochemistry. 1992;31:2887–2896. doi: 10.1021/bi00126a007. [DOI] [PubMed] [Google Scholar]
- 58.Boegeman SC, Deems RA, Dennis EA. Phospholipid binding and the activation of group IA secreted phospholipase A2. Biochemistry. 2004;43:3907–3916. doi: 10.1021/bi035921b. [DOI] [PubMed] [Google Scholar]
- 59.Lefkowitz LJ, Deems RA, Dennis EA. Expression of group IA phospholipase A2 in Pichia pastoris: identification of a phosphatidylcholine activator site using site-directed mutagenesis. Biochemistry. 1999;38(43):14174–14184. doi: 10.1021/bi991432t. [DOI] [PubMed] [Google Scholar]
- 60.Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277:48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 61.Sumandea M, Das S, Sumandea C, Cho W. Roles of aromatic residues in high interfacial activity of Naja naja atra phospholipase A2. Biochemistry. 1999;38:16290–16297. doi: 10.1021/bi9921384. [DOI] [PubMed] [Google Scholar]
- 62.Scott DL, White SP, Browning JL, Rosa JJ, Gelb MH, Sigler PB. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science. 1991;254:1007–1010. doi: 10.1126/science.1948070. [DOI] [PubMed] [Google Scholar]
- 63.Kuipers OP, Thunnissen MM, de Geus P, Dijkstra BW, Drenth J, Verheij HM, et al. Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop. Science. 1989;244:82–85. doi: 10.1126/science.2704992. [DOI] [PubMed] [Google Scholar]
- 64.Beers SA, Buckland AG, Giles N, Gelb MH, Wilton DC. Effect of tryptophan insertions on the properties of the human group IIA phospholipase A2: mutagenesis produces an enzyme with characteristics similar to those of the human group V phospholipase A2. Biochemistry. 2003;42:7326–7338. doi: 10.1021/bi0343222. [DOI] [PubMed] [Google Scholar]
- 65.Nemec KN, Pande AH, Qin S, Bieber Urbauer RJ, Tan S, Moe D, et al. Structural and functional effects of tryptophans inserted into the membrane-binding and substrate-binding sites of human group IIA phospholipase A2. Biochemistry. 2006;45:12448–12460. doi: 10.1021/bi061440r. [DOI] [PubMed] [Google Scholar]
- 66.Pande AH, Qin S, Nemec KN, He X, Tatulian SA. Isoform-specific membrane insertion of secretory phospholipase A2 and functional implications. Biochemistry. 2006;45:12436–12447. doi: 10.1021/bi060898q. [DOI] [PubMed] [Google Scholar]
- 67.Han SK, Kim KP, Koduri R, Bittova L, Munoz NM, Leff AR, et al. Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2. J Biol Chem. 1999;274:11881–11888. doi: 10.1074/jbc.274.17.11881. [DOI] [PubMed] [Google Scholar]
- 68.Pan YH, Yu BZ, Singer AG, Ghomashchi F, Lambeau G, Gelb MH, et al. Crystal structure of human group X secreted phospholipase A2. Electrostatically neutral interfacial surface targets zwitterionic membranes. J Biol Chem. 2002;277:29086–29093. doi: 10.1074/jbc.M202531200. [DOI] [PubMed] [Google Scholar]
- 69.Bezzine S, Bollinger JG, Singer AG, Veatch SL, Keller SL, Gelb MH. On the binding preference of human groups IIA and X phospholipases A2 for membranes with anionic phospholipids. J Biol Chem. 2002;277:48523–48534. doi: 10.1074/jbc.M203137200. [DOI] [PubMed] [Google Scholar]
- 70.Alonso F, Henson PM, Leslie CC. A cytosolic phospholipase in human neutrophils that hydrolyzes arachidonoyl-containing phosphatidylcholine. Biochim Biophys Acta. 1986;878:273–280. doi: 10.1016/0005-2760(86)90156-6. [DOI] [PubMed] [Google Scholar]
- 71.Kramer RM, Checani GC, Deykin A, Pritzker CR, Deykin D. Solubilization and properties of Ca2+-dependent human platelet phospholipase A2. Biochim Biophys Acta. 1986;878:394–403. doi: 10.1016/0005-2760(86)90248-1. [DOI] [PubMed] [Google Scholar]
- 72.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- 73.Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2. JBiolChem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- 74.Dessen A, Tang J, Schmidt H, Stahl M, Clark JD, Seehra J, et al. Crystal structure of human cytosolic phospholipase A2 reveals a novel topology and catalytic mechanism. Cell. 1999;97:349–360. doi: 10.1016/s0092-8674(00)80744-8. [DOI] [PubMed] [Google Scholar]
- 75.Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, et al. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca(2+)-dependent lipid-binding domain and a Ca(2+)-independent catalytic domain. JBiolChem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- 76.Hanel AM, Gelb MH. Multiple enzymatic activities of the human cytosolic 85-kDa phospholipase A2: hydrolytic reactions and acyl transfer to glycerol. Biochemistry. 1995;34:7807–7818. doi: 10.1021/bi00024a004. [DOI] [PubMed] [Google Scholar]
- 77.Trimble LA, Street IP, Perrier H, Tremblay NM, Weech PK, Bernstein MA. NMR structural studies of the tight complex between a trifluoromethyl ketone inhibitor and the 85-kDa human phospholipase A2. Biochemistry. 1993;32:12560–12565. doi: 10.1021/bi00210a002. [DOI] [PubMed] [Google Scholar]
- 78.Pickard RT, Chiou XG, Strifler BA, DeFelippis MR, Hyslop PA, Tebbe AL, et al. Identification of essential residues for the catalytic function of 85-kDa cytosolic phospholipase A2. Probing the role of histidine, aspartic acid, cysteine, and arginine. JBiolChem. 1996;271:19225–19231. doi: 10.1074/jbc.271.32.19225. [DOI] [PubMed] [Google Scholar]
- 79.Leslie CC. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. J Biol Chem. 1991;266:11366–11371. [PubMed] [Google Scholar]
- 80.Reynolds L, Hughes L, Louis AI, Kramer RA, Dennis EA. Metal ion and salt effects on the phospholipase A2, lysophospholipase, and transacylase activities of human cytosolic phospholipase A2. BiochimBiophysActa. 1993;1167:272–280. doi: 10.1016/0005-2760(93)90229-3. [DOI] [PubMed] [Google Scholar]
- 81.Evans JH, Leslie CC. The cytosolic phospholipase A2 catalytic domain modulates association and residence time at Golgi membranes. J Biol Chem. 2004;279:6005–6016. doi: 10.1074/jbc.M311246200. [DOI] [PubMed] [Google Scholar]
- 82.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 83.Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, et al. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- 84.Hirabayashi T, Kume K, Hirose K, Yokomizo T, Iino M, Itoh H, et al. Critical duration of intracellular Ca2+ response required for continuous translocation and activation of cytosolic phospholipase A2. J Biol Chem. 1999;274:5163–5169. doi: 10.1074/jbc.274.8.5163. [DOI] [PubMed] [Google Scholar]
- 85.Peters-Golden M, Song K, Marshall T, Brock T. Translocation of cytosolic phospholipase A2 to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem J. 1996;318(Pt 3):797–803. doi: 10.1042/bj3180797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shirai Y, Balsinde J, Dennis EA. Localization and functional interrelationships among cytosolic Group IV, secreted Group V, and Ca2+-independent Group VI phospholipase A2s in P388D1 macrophages using GFP/RFP constructs. Biochim Biophys Acta. 2005;1735:119–129. doi: 10.1016/j.bbalip.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 87.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 88.Ball A, Nielsen R, Gelb MH, Robinson BH. Interfacial membrane docking of cytosolic phospholipase A2 C2 domain using electrostatic potential-modulated spin relaxation magnetic resonance. ProcNatlAcadSciUSA. 1999;96:6637–6642. doi: 10.1073/pnas.96.12.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2. Identification of essential calcium ligands and hydrophobic membrane binding residues. JBiolChem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- 90.Malkova S, Long F, Stahelin RV, Pingali SV, Murray D, Cho W, et al. X-ray reflectivity studies of cPLA2{alpha}-C2 domains adsorbed onto Langmuir monolayers of SOPC. BiophysJ. 2005;89:1861–1873. doi: 10.1529/biophysj.105.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malmberg NJ, Van Buskirk DR, Falke JJ. Membrane-docking loops of the cPLA2 C2 domain: detailed structural analysis of the protein-membrane interface via site-directed spin-labeling. Biochemistry. 2003;42:13227–13240. doi: 10.1021/bi035119+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nalefski EA, McDonagh T, Somers W, Seehra J, Falke JJ, Clark JD. Independent folding and ligand specificity of the C2 calcium-dependent lipid binding domain of cytosolic phospholipase A2. JBiolChem. 1998;273:1365–1372. doi: 10.1074/jbc.273.3.1365. [DOI] [PubMed] [Google Scholar]
- 93.Stahelin RV, Rafter JD, Das S, Cho W. The molecular basis of differential subcellular localization of C2 domains of protein kinase C-alpha and group IVa cytosolic phospholipase A2. JBiolChem. 2003;278:12452–12460. doi: 10.1074/jbc.M212864200. [DOI] [PubMed] [Google Scholar]
- 94.Jaud S, Tobias DJ, Falke JJ, White SH. Self-induced docking site of a deeply embedded peripheral membrane protein. BiophysJ. 2007;92:517–524. doi: 10.1529/biophysj.106.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corbin JA, Evans JH, Landgraf KE, Falke JJ. Mechanism of specific membrane targeting by C2 domains: localized pools of target lipids enhance Ca2+ affinity. Biochemistry. 2007;46:4322–4336. doi: 10.1021/bi062140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. JBiolChem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- 97.Six DA, Dennis EA. Essential Ca(2+)-independent role of the group IVA cytosolic phospholipase A(2) C2 domain for interfacial activity. JBiolChem. 2003;278:23842–23850. doi: 10.1074/jbc.M301386200. [DOI] [PubMed] [Google Scholar]
- 98.Das S, Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. JBiolChem. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- 99.Lamour NF, Chalfant CE. Ceramide-1-phosphate: the "missing" link in eicosanoid biosynthesis and inflammation. Mol Interv. 2005;5:358–367. doi: 10.1124/mi.5.6.8. [DOI] [PubMed] [Google Scholar]
- 100.Pettus BJ, Bielawska A, Subramanian P, Wijesinghe DS, Maceyka M, Leslie CC, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–11326. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 101.Subramanian P, Stahelin RV, Szulc Z, Bielawska A, Cho W, Chalfant CE. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J Biol Chem. 2005;280:17601–17607. doi: 10.1074/jbc.M414173200. [DOI] [PubMed] [Google Scholar]
- 102.Stahelin RV, Subramanian P, Vora M, Cho W, Chalfant CE. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. JBiolChem. 2007;282:20467–20474. doi: 10.1074/jbc.M701396200. [DOI] [PubMed] [Google Scholar]
- 103.Subramanian P, Vora M, Gentile LB, Stahelin RV, Chalfant CE. Anionic lipids activate group IVA cytosolic phospholipase A2 via distinct and separate mechanisms. J Lipid Res. 2007;48:2701–2708. doi: 10.1194/jlr.M700356-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Borsch-Haubold AG, Bartoli F, Asselin J, Dudler T, Kramer RM, Apitz-Castro R, et al. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J Biol Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- 105.Hefner Y, Borsch-Haubold AG, Murakami M, Wilde JI, Pasquet S, Schieltz D, et al. Serine 727 phosphorylation and activation of cytosolic phospholipase A2 by MNK1-related protein kinases. J Biol Chem. 2000;275:37542–37551. doi: 10.1074/jbc.M003395200. [DOI] [PubMed] [Google Scholar]
- 106.Muthalif MM, Hefner Y, Canaan S, Harper J, Zhou H, Parmentier JH, et al. Functional interaction of calcium-/calmodulin-dependent protein kinase II and cytosolic phospholipase A(2) J Biol Chem. 2001;276:39653–39660. doi: 10.1074/jbc.M103136200. [DOI] [PubMed] [Google Scholar]
- 107.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 108.de Carvalho MG, McCormack AL, Olson E, Ghomashchi F, Gelb MH, Yates JR, 3rd, et al. Identification of phosphorylation sites of human 85-kDa cytosolic phospholipase A2 expressed in insect cells and present in human monocytes. J Biol Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- 109.Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res. 2008;49:724–737. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- 110.Bayburt T, Gelb MH. Interfacial catalysis by human 85 kDa cytosolic phospholipase A2 on anionic vesicles in the scooting mode. Biochemistry. 1997;36:3216–3231. doi: 10.1021/bi961659d. [DOI] [PubMed] [Google Scholar]
- 111.de Carvalho MG, Garritano J, Leslie CC. Regulation of lysophospholipase activity of the 85-kDa phospholipase A2 and activation in mouse peritoneal macrophages. J Biol Chem. 1995;270:20439–20446. doi: 10.1074/jbc.270.35.20439. [DOI] [PubMed] [Google Scholar]
- 112.Das S, Rafter JD, Kim KP, Gygi SP, Cho W. Mechanism of group IVA cytosolic phospholipase A(2) activation by phosphorylation. JBiolChem. 2003;278:41431–41442. doi: 10.1074/jbc.M304897200. [DOI] [PubMed] [Google Scholar]
- 113.Tian W, Wijewickrama GT, Kim JH, Das S, Tun MP, Gokhale N, et al. Mechanism of regulation of group IVA phospholipase A2 activity by Ser727 phosphorylation. J Biol Chem. 2008;283:3960–3971. doi: 10.1074/jbc.M707345200. [DOI] [PubMed] [Google Scholar]
- 114.Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 115.Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, et al. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. NatImmunol. 2000;1:42–46. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- 116.Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 117.Uozumi N, Shimizu T. Roles for cytosolic phospholipase A2alpha as revealed by gene-targeted mice. Prostaglandins Other Lipid Mediat. 2002;68–69:59–69. doi: 10.1016/s0090-6980(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 118.Larsson Forsell PK, Kennedy BP, Claesson HE. The human calcium-independent phospholipase A2 gene multiple enzymes with distinct properties from a single gene. Eur J Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 119.Larsson PK, Claesson HE, Kennedy BP. Multiple splice variants of the human calciumindependent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 120.Ackermann EJ, Kempner ES, Dennis EA. Ca(2+)-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 121.Balboa MA, Balsinde J, Jones SS, Dennis EA. Identity between the Ca2+-independent phospholipase A2 enzymes from P388D1 macrophages and Chinese hamster ovary cells. J Biol Chem. 1997;272:8576–8580. doi: 10.1074/jbc.272.13.8576. [DOI] [PubMed] [Google Scholar]
- 122.Lio YC, Dennis EA. Interfacial activation, lysophospholipase and transacylase activity of group VI Ca2+-independent phospholipase A2. Biochim Biophys Acta. 1998;1392:320–332. doi: 10.1016/s0005-2760(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 123.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 124.Atsumi G, Murakami M, Kojima K, Hadano A, Tajima M, Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. Proteolytic fragment of type IVA cytosolic phospholipase A2alpha inhibits stimulus-induced arachidonate release, whereas that of type VI Ca2+-independent phospholipase A2 augments spontaneous fatty acid release. J Biol Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 125.Peterson B, Knotts T, Cummings BS. Involvement of Ca2+-independent phospholipase A2 isoforms in oxidant-induced neural cell death. Neurotoxicology. 2007;28:150–160. doi: 10.1016/j.neuro.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 126.Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA. Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J Biol Chem. 2006;281:22275–22288. doi: 10.1074/jbc.M604330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao X, Wang D, Zhao Z, Xiao Y, Sengupta S, Zhang R, et al. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J Biol Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 128.Jenkins CM, Yan W, Mancuso DJ, Gross RW. Highly selective hydrolysis of fatty acyl-CoAs by calcium-independent phospholipase A2beta. Enzyme autoacylation and acyl-CoA-mediated reversal of calmodulin inhibition of phospholipase A2 activity. J Biol Chem. 2006;281:15615–15624. doi: 10.1074/jbc.M511623200. [DOI] [PubMed] [Google Scholar]
- 129.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. Identification of the calmodulin-binding domain of recombinant calcium-independent phospholipase A2beta. implications for structure and function. J Biol Chem. 2001;276:1729–1735. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 130.Wang Z, Ramanadham S, Ma ZA, Bao S, Mancuso DJ, Gross RW, et al. Group VIA phospholipase A2 forms a signaling complex with the calcium/calmodulin-dependent protein kinase IIbeta expressed in pancreatic islet beta-cells. J Biol Chem. 2005;280:6840–6849. doi: 10.1074/jbc.M405287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–9616. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balsinde J, Bianco ID, Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc Natl Acad Sci U S A. 1995;92:8527–8531. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herbert SP, Walker JH. Group VIA calcium-independent phospholipase A2 mediates endothelial cell S phase progression. J Biol Chem. 2006;281:35709–35716. doi: 10.1074/jbc.M600699200. [DOI] [PubMed] [Google Scholar]
- 134.Roshak AK, Capper EA, Stevenson C, Eichman C, Marshall LA. Human calcium-independent phospholipase A2 mediates lymphocyte proliferation. J Biol Chem. 2000;275:35692–35698. doi: 10.1074/jbc.M002273200. [DOI] [PubMed] [Google Scholar]
- 135.Song Y, Wilkins P, Hu W, Murthy KS, Chen J, Lee Z, et al. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem J. 2007;406:427–436. doi: 10.1042/BJ20070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang XH, Zhao C, Seleznev K, Song K, Manfredi JJ, Ma ZA. Disruption of G1-phase phospholipid turnover by inhibition of Ca2+-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J Cell Sci. 2006;119:1005–1015. doi: 10.1242/jcs.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochim Biophys Acta. 2006;1761:1344–1350. doi: 10.1016/j.bbalip.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 138.Bao S, Li Y, Lei X, Wohltmann M, Jin W, Bohrer A, et al. Attenuated free cholesterol loading-induced apoptosis but preserved phospholipid composition of peritoneal macrophages from mice that do not express group VIA phospholipase A2. J Biol Chem. 2007;282:27100–27114. doi: 10.1074/jbc.M701316200. [DOI] [PMC free article] [PubMed] [Google Scholar]