Abstract
Context: The human FSHB promoter polymorphism (rs10835638; −211 G/T) has been associated with serum FSH in a cohort of young Estonian men. The minor allele carriers had reduced serum FSH (15.7% in GT heterozygotes; 40% in TT homozygotes) compared with GG homozygotes.
Objective: Because FSH is essential for normal spermatogenesis and fertility, we speculated that abnormalities in FSH action could contribute to male infertility. We sought to study whether genetically inherited constitutively reduced FSH levels may affect male reproduction and replicate the association between rs10835638 and serum FSH among infertile male patients.
Design: Genotyping of rs10835638 in a cohort of infertile men (n = 1029; Andrology Center of the Tartu University Clinics, Estonia), including idiopathic infertility cases (IIFC; n = 750).
Patients: Patients included male partners (sperm concentration <20 × 106/ml) of infertile couples failing to conceive a child for 12 months or longer.
Results: A significant excess of TT homozygotes (1.1 vs. 2.4%) as well as GT heterozygotes (22.4 vs. 25.1%) was detected among infertile men compared with the young male cohort (χ2 test, P < 0.05). The T allele of rs10835638 was associated with reduced serum FSH (analysis of covariance; full cohort: P = 1.20 × 10−6, F = 13.8; IIFC: P = 7.70 × 10−7, F = 14.3) as well as with low FSH to LH ratio (full cohort: P = 1.52 × 10−11, F = 25.6; IIFC: P = 3.25 × 10−9, F = 20.4). The median serum FSH levels differed between the GG and TT carriers by 48.5%. All IIFC with TT genotype exhibited low (<1.8) FSH to LH ratio.
Conclusions: In perspective, this genetic marker may have clinical significance in molecular diagnostics of male reproductive success and a potential to identify positive responders to FSH treatment.
The −211 T allele of the FSHB promoter polymorphism is associated with significantly reduced serum FSH and is enriched among infertile male patients.
FSH is a pituitary-expressed glycoprotein hormone that contributes to the regulation of the development, pubertal maturation, and reproductive processes in both sexes in mammals (1). In females, FSH has a crucial role in stimulating the maturation of germ cells and initiating follicular growth, and it is widely used for the treatment of female infertility (2,3). In males, FSH affects during the fetal and neonatal development by activating the proliferation of the Sertoli cells as well as in the pubertal phase by inducing mitotic activity of the spermatogonia (4). The contribution of FSH in testicular function, spermatogenesis, and fertility in adult men has long been debated (5,6,7,8,9).
FSH is composed of an α-subunit shared with other glycoprotein hormones and a specific β-subunit coded by the FSHB gene. The transcription of FSHB is rate-limiting for FSH production and controls most of FSH secretion (10). Human FSHB gene (4.2 kb) encoding a 129-amino acid preprotein (11) is characterized by low genetic variation (12,13). The described FSHB inactivating mutations were associated with primary amenorrhea, disturbed pubertal development, and infertility in female patients (five subjects) and azoospermia, small testes, and affected pubertal development in male patients (three subjects) (14,15).
We recently reported the first human FSHB polymorphism (rs10835638; G/T; −211 from mRNA transcription start) showing significant association with serum FSH levels in a cohort of young men from Estonia (16). This variant is located within the evolutionary conserved progesterone response element, a DNA sequence motif 5′ upstream of the FSHB gene transcription site contributing to the regulation of FSHB expression in mammals (17). Compared with the wild-type homozygotes (GG), the heterozygotes (GT), and the homozygotes (TT) for the alternative allele had on average 15.7 and 40% lower levels of FSH in their bloodstream, respectively. Consistently, an independent functional study has demonstrated the differential effect of the two alleles (G, T) of rs10835638 on FSHB gene expression by the luciferase assay (18). The relative activity of the FSHB proximal promoter carrying of the rs10835638 T allele was only half (46–58%; P < 0.0005) compared with the activity of the wild-type promoter variant with the G allele.
Because FSH is essential for normal spermatogenesis and fertility, we speculated that abnormalities in FSH action due to genetically inherited constitutively (since fetal development) reduced hormone levels could affect male reproductive success. In the current study, we hypothesized that there might be an enrichment of the FSHB rs10835638 (G/T) T allele carriers among the male patients of infertile couples. Previously Mantovani et al. (19) reported a 19-yr-old infertile male, who was homozygous for the T allele of the FSHB −211 polymorphism and was described with normal virilization, azoospermia, and isolated FSH deficiency. The patient had no other mutations in the FSHB gene.
Consistent with the study hypothesis, we report significantly increased fraction of rs10835638 T allele carriers among the study group of Estonian infertile male patients (n = 1029) compared with the respective population cohort. Strong association was detected between the carrier status of the T allele and the reduction in serum FSH and in FSH to LH ratios.
Subjects and Methods
Study groups
The study has been approved by the Ethics Committee of Human Research of the University Clinic of Tartu, Estonia (permissions 112/3, 27.01.2003; 117/9, 16.06.03). The study group of infertile men (n = 1056) was recruited at the Andrology Center, Tartu University Clinics between June 2003 and August 2008 and consisted of male partners of couples failing to conceive a child for a period of 12 months or longer. The inclusion criterion for male partners of infertile couples entering the study was sperm count (in two consecutive semen analysis) less than 20 × 106/ml. All study participants were of white European ancestry, born and living in Estonia. In total, 27 subjects of the cohort with missing data (failed DNA extraction, missing or incomplete sperm analysis or hormonal data) were excluded from the analyses. The final number of eligible study subjects entering the genotyping and statistical analysis was 1029 (aged 31.7 ± 0.2 yr).
Within the study group, causal factors for male factor infertility (obstruction, cryptorchidism, chromosomal abnormalities, Y chromosome deletions, hypogonadotrophic hypogonadism, testicular diseases, sexual dysfunctions, androgen abuse, severe traumas and operation in genital area, chemo- and radiotherapy) were identified for 279 men following the diagnostic criteria and conditions as described elsewhere (20). The rest of the patients (n = 750) were classified as idiopathic infertility cases. Risk factors for male infertility with controversial evidence (such as leucocytospermia, n = 57; varicocele, n = 201; leucocytospermia and varicocele, n = 16) were not considered as conclusive causal factors for male fertility.
The population-based control cohort of young men was recruited at the Centre of Andrology, Tartu University Hospital between May 2003 and June 2004 and included 578 young men who participated in a prospective study Environment and Reproductive Health (EU 6th FP project QLRT-2001-02911). Study participation was voluntary and informed consent was obtained from all study subjects, all born and living in Estonia. Principles of the study group formation have been described previously (21). In total 24 subjects of the cohort with either severe pathologies in genital region (cryptorchidism, n = 9) or missing data (failed DNA extraction, n = 3; missing or incomplete sperm analysis, n = 9; or hormonal data, n = 3) were excluded from the analyses. The final number of eligible study subjects was 554 (aged 19.2 ± 1.7 yr; sperm concentration 86.4 ± 3.3 million/ml, sperm count 282.0 ± 12.3 × 106). The genotyping and detailed analyses of the FSHB promoter polymorphism (−211 G/T) in the population-based cohort are reported previously (16).
Hormone assays
Venous blood was obtained from the cubital vein between 0800 and 1100 h after overnight fasting or light morning meal. One sample was centrifuged and serum isolated, and the EDTA-blood sample was stored at −80 C until DNA extraction. The hormone assays of the population-based cohort of Estonian young men were conducted at the Department of Growth and Reproduction in Copenhagen, Rigshospitalet, Denmark and are described previously (16). Briefly, serum levels of FSH, LH, and testosterone were determined using a time-resolved immunofluorometric (FSH, LH) or flouroimmunoassay (testosterone) assay (Delfia; Wallac, Turku, Finland). Estradiol levels were measured by RIA (Pantex, Santa Monica, CA) and inhibin B by a specific two-sided enzyme immunometric assay (Serotec, Oxford, UK). In case of infertile male cohort described in this study, the Immulite tests for reproductive hormones were processed within 2 h at the United Laboratories, University of Tartu Clinics. Serum for Inhibin B test was stored at −80 C until assayed in four batches to decrease interassay influence. FSH, LH, testosterone, and estradiol levels of blood plasma were measured using the Immulite automated chemiluminescence immunoassay analyzer (Immulite; Diagnostic Products Corp., Los Angeles, CA) according to the manufacturer’s instructions. Inhibin B was determined in duplicate using a specific enzyme immunometric assay (Diagnostic Systems Laboratories, Inc., Webster, TX). The intra- and interassay coefficients of variation, respectively, are 4.2 and 8% for FSH; 4.0 and 7.1% for LH; 6.3 and 9.4% for testosterone; 7.5 and 13% for estradiol; 15 and 18% for inhibin B.
Semen analysis
Semen samples were obtained by masturbation and ejaculated into a sterile collection tube in a private room near the laboratory. After ejaculation, the semen was incubated at 37 C for 30–40 min for liquefaction. Recommended abstinence period was 3–4 d. The actual period of ejaculation abstinence was calculated as time in full days between current and previous ejaculation as reported by the men. Semen analysis was performed according to World Health Organization guidelines (22). Semen volume was estimated by weighing the collection tube with the semen sample and subsequently subtracting the predetermined weight of the empty tube, assuming 1 g = 1 ml. The motility assessment was performed in duplicate and the average value was calculated for both samples. For assessment of the sperm concentration, the samples were diluted in a solution of 0.6 mol/liter NaHCO3 and 0.4% (vol/vol) formaldehyde in distilled water. The sperm concentration was assessed using the improved Neubauer hemocytometers. Finally, smears for sperm morphology assessment were made. Morphology smears were Papanicolaou stained and spermatozoa were evaluated according to strict criteria (23) and The Nordic Association for Andrology suggestions (24), with the lowest limit of normal morphology set at 5% of all spermatozoa.
Physical examination
Physical examination for assessment of genital pathology and testicular size was performed with the man in standing position. If necessary, pathologies were clarified further with the men in supine position. The orchidometer (made of birch wood; Pharmacia & Upjohn, Copenhagen, Denmark) for assessment of testicular size was used. Participants of the study were examined by five investigators, who passed special training for standardization of the clinical examination immediately before the study. The combined testicular volume is the sum of the volume of the right and left testicles.
PCR and restriction fragment length polymorphism genotyping
The polymorphism located at the position −211 from FSHB mRNA start site (rs10835638) represents a restriction fragment length polymorphism. The genotyping of the alternative alleles (major G/minor T) was conducted by PCR (forward/reverse primers: 5′-GGAGCCAGATCATGAAATGTT-3′/5′-GACCAATGCTAGCCTGAAGC-3′) and restriction enzyme cutting (TatI; Fermentas, Vilnius, Lithuania) approach. The uncut PCR product (364 bp) representing the T allele was separated from the restricted fragments (233 and 131 bp) resulting from the G allele using electrophoreses in 2% agarose gel and 0.5 × Tris-borate EDTA buffer.
Data analysis
Estimation of allele frequencies, conformance with Hardy-Weinberg equilibrium, and tests for population differentiation were computed by Fisher’s exact tests (α = 0.05) using Genepop software (version 4.0.10, Laboratorie de Genetique, Montpellier, France) (25). The testing conditions were as follows: dememorization = 10,000; batches = 1000; iterations = 10,000. The distribution of rs10835638 genotypes did not deviate from the Hardy-Weinberg equilibrium in the full study group (P = 0.626) as well as among the patients with idiopathic male factor infertility (P = 0.475).
Differences in hormone levels (FSH, LH, testosterone, inhibin B, and estradiol) and testicular parameters (semen and combined testes volume, sperm motility, concentration, and morphology) were tested between groups of GG, GT, or TT genotype carriers using one-way analysis of covariance (ANCOVA). Analysis was adjusted by age (all parameters) and abstinence period (only semen parameters). The quantitative phenotypes [except body mass index (BMI) and age] were nonnormally distributed and were analyzed using naturally logged transformed data to normalize their distribution. Data are presented as median and mean ± sem. A P ≤ 0.05 was considered as significant and a P < 0.1 was considered suggestive. The F ratio represents between-group variance divided by within-group variance. The association tests were implemented in Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL).
Results
The −211 T allele of FSHB promoter polymorphism (rs10835638) is overrepresented among infertile male patients
The Estonian patients with severe male factor infertility (n = 1029; aged 31.7 ± 0.2 yr) were recruited to the study during their visit to the Andrology Center of the Tartu University Clinics, Estonia over a 5-yr period. The entire study group of Estonian infertile male patients was genotyped for the human FSHB promoter polymorphism rs10835638 (G/T; −211 from mRNA transcription start). The genotyped cohort consisted of 746 major allele homozygotes (GG; 72.5%), 258 heterozygotes (GT; 25.1%), and 25 minor allele homozygotes (TT; 2.4%). The frequencies for G and T alleles were 85 and 15%, respectively. Allele and genotype distributions among the men with idiopathic infertility (T/T, 2.5%; G/T, 24.8%) compared with the patients with identified causal factors for infertility (T/T, 2.2%; G/T, 25.8%) did not exhibit significant differences.
When the genotype data of the studied FSHB promoter variant was compared with the cohort of young Estonian men (n = 554; aged 19.2 ± 0.1 yr) (16), there was a significant difference in both allele and genotype frequencies between the two studies. Compared with the male cohort, the patients with male factor infertility exhibited a significant excess of TT homozygotes (1.1 vs. 2.4%) as well as GT heterozygotes (22.4 vs. 25.1%) (P < 0.05; Table 1).
Table 1.
FSHB rs10835638 allele and genotype frequencies among male patients of infertile couples compared with the cohort of young men
| n | Allele frequencies
|
P valuea | Genotype frequencies
|
P valueb | ||||
|---|---|---|---|---|---|---|---|---|
| G (%) | T (%) | G/G (%) | G/T (%) | T/T (%) | ||||
| Population-based cohort of menc | 554 | 87.7 | 12.3 | 76.5 (n = 423) | 22.4 (n = 125) | 1.1 (n = 6) | ||
| Full cohort of infertile male patients | 1029 | 85.0 | 15.0 | 0.042 | 72.5 (n = 746) | 25.1 (n = 258) | 2.4 (n = 25) | 0.043 |
| Patients with idiopathic infertility | 750 | 85.1 | 14.9 | 0.066 | 72.7 (n = 545) | 24.8 (n = 186) | 2.5 (n = 19) | 0.066 |
| Patients with causal factors for infertilityd | 279 | 84.9 | 15.1 | 0.146 | 72.0 (n = 201) | 25.8 (n = 72) | 2.2 (n = 6) | 0.141 |
P value of χ2 test for allele frequencies in population-based cohort of men vs. infertile male patients;
P value of χ2 test for genotype frequencies in population-based cohort of men vs. infertile male patients;
from Grigorova et al. (16);
detailed description in Materials and Methods.
Strong association of rs10835638 with serum FSH and FSH to LH ratio
Statistical analysis using ANCOVA revealed an extremely strong association between the carrier status of rs10835638 variants and serum FSH levels among the full group of infertile male patients (P = 1.20 × 10−6, F = 13.8; Table 2) and more remarkably among the patients with idiopathic infertility (P = 7.70 × 10−7, F= 14.3; Table 3). Despite the high variation in FSH measurements among the patients with male factor infertility (Fig. 1A and supplemental Fig. S1A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), the association of T allele with lowered serum FSH was consistent with the seminal study conducted on a cohort of young men (16). There was a gradient of declining FSH levels among the three subgroups of wild-type homozygotes (GG), heterozygotes (GT), and homozygotes for the minor allele (TT) of rs10835638 (Tables 2 and 3). Consistently, when the study subjects were subgrouped based on normal/low (<7 IU/liter) and elevated (≥7 IU/liter) serum FSH (26), there was about 10% higher fraction of T allele carriers among the patients in the first group (P < 0.0005; Table 4).
Table 2.
Characteristics of the study group (n = 1029) stratified by FSHB rs10835638 genotype
| Parameters | G/G homozygotes (n = 746; 72.5%) median (mean ± sem) | G/T heterozygotes (n = 258; 25.1%) median (mean ± sem) | T/T homozygotes (n = 25; 2.4%) median (mean ± sem) |
P values of statistical analysis testing genotype effect by AN(C)OVAa
|
||
|---|---|---|---|---|---|---|
| All subjects | T/T vs. G/G carriers | T/T vs. G/G + G/T carriers | ||||
| Age (yr) | 31.1 (31.7 ± 0.2) | 30.7 (31.4 ± 0.3) | 31.7 (32.5 ± 1.3) | 5.76 × 10−1 | 5.80 × 10−1 | 5.33 × 10−1 |
| BMI (kg/m2) | 26.1 (27.3 ± 0.5) | 25.9 (26.3 ± 0.4) | 26.3 (26.2 ± 0.9) | 4.02 × 10−1 | 5.53 × 10−1 | 6.04 × 10−1 |
| Abstinence time (d) | 3.0 (3.8 ± 0.1) | 4.0 (4.0 ± 0.2) | 4.5 (6.2 ± 1.1) | 6.28 × 10−6 | 7.64 × 10−7 | 1.34 × 10−6 |
| FSH (IU/liter) | 6.8 (11.1 ± 0.4) | 5.4 (8.1 ± 0.5) | 3.5 (6.5 ± 1.4) | 1.20 × 10−6 | 3.95 × 10−2 | 6.74 × 10−2 |
| Inhibin B (pg/ml) | 77.8 (88.4 ± 3.8) | 75.2 (85.6 ± 5.6) | 58.2 (73.7 ± 16.4) | 8.50 × 10−1 | 6.61 × 10−1 | 6.47 × 10−1 |
| LH (IU/liter) | 4.3 (5.2 ± 0.1) | 4.2 (4.9 ± 0.2) | 4.3 (5.1 ± 1.6) | 9.32 × 10−1 | 8.61 × 10−1 | 9.18 × 10−1 |
| FSH/LH | 1.8 (2.1 ± 0.0) | 1.4 (1.7 ± 0.1) | 1.1 (1.2 ± 0.1) | 1.52 × 10−11 | 1.37 × 10−4 | 5.47 × 10−4 |
| Testosterone (nmol/liter) | 17.7 (18.1 ± 0.3) | 18.8 (19.1 ± 0.5) | 17.3 (17.4 ± 1.1) | 1.46 × 10−1 | 6.32 × 10−1 | 5.20 × 10−1 |
| Estradiol (pmol/liter) | 88.7 (102.1 ± 1.6) | 87.0 (98.6 ± 2.3) | 91.3 (106.9 ± 8.1) | 5.15 × 10−1 | 5.92 × 10−1 | 5.12 × 10−1 |
| Combined testicular volume (ml) | 38.0 (37.6 ± 0.5) | 38.0 (37.5 ± 0.7) | 34.0 (33.2 ± 1.9) | 9.97 × 10−1 | 9.05 × 10−2 | 8.11 × 10−2 |
| Semen volume (ml) | 3.7 (3.9 ± 0.1) | 3.8 (4.2 ± 0.1) | 4.8 (4.7 ± 0.3) | 4.02 × 10−1 | 1.76 × 10−1 | 2.37 × 10−1 |
| Sperm concentration (106/ml) | 3.7 (5.7 ± 0.2) | 4.2 (6.2 ± 0.4) | 5.6 (6.6 ± 1.2) | 1.85 × 10−1 | 5.54 × 10−1 | 5.94 × 10−1 |
| Total sperm count (×106) | 13.9 (24.9 ± 1.1) | 15.3 (27.8 ± 2.0) | 27.5 (30.0 ± 5.9) | 2.58 × 10−1 | 5.77 × 10−1 | 6.33 × 10−1 |
| Sperm A+B motility (%) | 22.0 (23.8 ± 0.7) | 22.0 (24.6 ± 1.3) | 13.0 (16.2 ± 3.1) | 1.02 × 10−1 | 4.90 × 10−2 | 4.65 × 10−2 |
| Sperm morphology | 1.0 (2.2 ± 0.2) | 1.0 (2.3 ± 0.2) | 2.00 (2.7 ± 0.7) | 4.91 × 10−1 | 5.37 × 10−1 | 5.29 × 10−1 |
Significant difference has been indicated in bold: P < 0.05, P < 0.001. Evidence for suggestive difference is underlined (P < 0.1).
One-way ANCOVA for genotype-trait associations were performed with the age as a covariate. Analysis of seminal parameters (semen volume, sperm concentration, total sperm count, sperm A+B motility) were additionally adjusted by abstinence time.
Table 3.
Hormonal and testicular parameters of the male patients with idiopathic infertility (n = 750) grouped by FSHB rs10835638 genotype
| Parameters | G/G homozygotes (n = 545; 72.6%) median (mean ± sem) | G/T heterozygotes (n = 186; 24.8%) median (mean ± sem) | T/T homozygotes (n = 19; 2.5%) median (mean ± sem) |
P values of statistical analysis testing genotype effect by AN(C)OVAa
|
||
|---|---|---|---|---|---|---|
| All subjects | T/T vs. G/G carriers | T/T vs. G/G + G/T carriers | ||||
| Age (yr) | 31.2 (31.8 ± 0.3) | 30.3 (31.0 ± 0.4) | 29.9 (31.2 ± 1.3) | 3.72 × 10−1 | 6.43 × 10−1 | 7.25 × 10−1 |
| BMI (kg/m−2) | 26.0 (26.7 ± 0.3) | 26.0 (26.5 ± 0.4) | 26.3 (26.2 ± 0.9) | 2.42 × 10−1 | 5.80 × 10−1 | 6.07 × 10−1 |
| Abstinence time (d) | 3.0 (3.8 ± 0.1) | 3.0 (3.8 ± 0.1) | 4.0 (6.4 ± 1.4) | 3.76 × 10−7 | 4.27 × 10−7 | 5.47 × 10−8 |
| FSH (IU/liter) | 6.4 (9.5 ± 0.4) | 5.3 (7.1 ± 0.5) | 3.3 (4.8 ± 1.1) | 7.70 × 10−7 | 2.99 × 10−2 | 4.11 × 10−2 |
| Inhibin B (pg/ml) | 80.6 (91.0 ± 4.2) | 75.2 (85.5 ± 6.3) | 58.2 (76.2 ± 21.5) | 9.75 × 10−1 | 5.96 × 10−1 | 6.30 × 10−1 |
| LH (IU/liter) | 4.1 (4.7 ± 0.1) | 4.1 (4.5 ± 0.2) | 4.1 (4.8 ± 0.7) | 8.29 × 10−1 | 9.35 × 10−1 | 8.40 × 10−1 |
| FSH/LH | 1.7 (2.0 ± 0.1) | 1.3 (1.6 ± 0.1) | 0.9 (1.0 ± 0.1) | 3.25 × 10−9 | 4.52 × 10−4 | 1.06 × 10−3 |
| Testosterone (nmol/liter) | 17.8 (18.4 ± 0.3) | 18.3 (19.4 ± 0.5) | 17.3 (17.6 ± 1.2) | 1.92 × 10−1 | 5.89 × 10−1 | 4.93 × 10−1 |
| Estradiol (pmol/liter) | 87.7 (101.3 ± 1.8) | 87.2 (99.3 ± 2.7) | 97.6 (17.6 ± 9.4) | 5.12 × 10−1 | 3.46 × 10−1 | 3.07 × 10−1 |
| Combined testicular volume (ml) | 40.0 (39.7 ± 0.5) | 39.0 (39.1 ± 0.7) | 34.0 (34.4 ± 1.9) | 2.25 × 10−1 | 3.99 × 10−2 | 3.98 × 10−2 |
| Semen volume (ml) | 3.9 (4.1 ± 0.1) | 3.9 (4.2 ± 0.1) | 5.0 (4.9 ± 0.4) | 6.48 × 10−1 | 4.99 × 10−1 | 5.66 × 10−1 |
| Sperm concentration (106/ml) | 5.0 (6.7 ± 0.3) | 6.3 (7.4 ± 0.4) | 5.6 (6.9 ± 1.5) | 4.77 × 10−1 | 9.96 × 10−1 | 9.07 × 10−1 |
| Total sperm count (×106) | 19.1 (29.3 ± 1.3) | 20.2 (32.7 ± 2.4) | 28.6 (32.9 ± 7.6) | 3.99 × 10−1 | 8.73 × 10−1 | 7.72 × 10−1 |
| Sperm A+B motility (%) | 23.0 (24.5 ± 0.8) | 23.0 (25.1 ± 1.4) | 11.0 (15.3 ± 3.6) | 6.75 × 10−2 | 2.07 × 10−2 | 2.39 × 10−2 |
| Sperm morphology | 1.0 (2.2 ± 0.2) | 1.0 (2.3 ± 0.2) | 1.0 (1.9 ± 0.6) | 6.25 × 10−1 | 7.24 × 10−1 | 6.91 × 10−1 |
Significant difference has been indicated in bold: P < 0.05, P < 0.001. Evidence for suggestive difference is underlined (P < 0.1).
One-way ANCOVA for genotype-trait associations were performed with the age as a covariate. Analysis of seminal parameters (semen volume, sperm concentration, total sperm count, sperm A+B motility) were additionally adjusted by abstinence time.
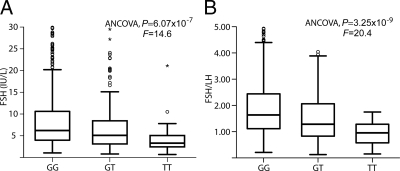
Figure 1.
Serum FSH (A) and serum FSH to LH ratio values (B) in patients with idiopathic infertility (n = 750) grouped according to their FSHB promoter single-nucleotide polymorphism rs10835638 genotype (GG, n = 545; GT, n = 186; TT, n = 19). The extreme outlier values (above +2.5 sd from the total mean) were excluded from the figure. The boxes represent the 25th and 75th percentiles; whiskers are lines extending from each end of the box covering the extent of the data on 1.5 × interquartile range. The median value is denoted as the line that bisects the boxes. Circles represent the outlier values. For each box plot P values of one-way ANCOVA are shown. The F ratio represents between-group variance divided by within-group variance.
Table 4.
The distribution of the FSHB rs10835638 genotypes among the study subjects subgrouped based on normal/low (<7 IU/liter) and elevated (≥7 IU/liter) serum FSHa
| Subgroup definition | All infertile men (n = 1029)
|
Men with idiopathic infertility (n = 750)
|
Men with causal factors for infertility (n = 279)
|
|||
|---|---|---|---|---|---|---|
| GG/GT/TT (%) | χ2 test P valueb | GG/GT/TT (%) | χ2 test P valueb | GG/GT/TT (%) | χ2 test P valueb | |
| FSH less than 7 (IU/liter) | 68.0/29.0/3.0 | Pallellic = 4.01 × 10−4 | 68.2/28.4/3.4 | Pallellic = 9.49 × 10−4 | 67.2/31.2/1.6 | Pallellic = 1.89 × 10−1 |
| FSH 7 or greater (IU/liter) | 77.8/20.4/1.7 | Pgenotypic = 4.93 × 10−4 | 78.5/20.2/1.3 | Pgenotypic = 1.12 × 10−4 | 76.3/21.2/2.6 | Pgenotypic = 1.89 × 10−1 |
Definition following Ahda et al. (26).
Comparison between subgroups defined based on serum FSH.
Compared with the carriers of the wild-type GG genotype (median = 6.8 IU/liter), the serum FSH levels of TT homozygotes were approximately 50% lower (median 3.5 IU/liter; P = 3.95 × 10−2, F = 4.3). As the carrier status of rs10835638 variants had no significant effect on serum LH levels, the declining gradient of serum FSH among the GG, GT, and TT carriers was correlated with the FSH to LH ratio, a prognostic value to reproductive health (P = 1.52 × 10−11, F = 25.6; Table 2; Fig. 1B; supplemental Fig. S1B). Compared with the wild-type GG genotype group (median 1.8), the FSH to LH ratio was reduced in GT heterozygotes (median 1.4) and more remarkably in TT homozygotes (median 1.1; P = 1.37 × 10−4, F = 14.7). The association between rs10835638 variants and serum FSH to LH ratio remained highly significant in case only patients with idiopathic fertility were analyzed (P = 3.25 × 10−9, F = 20.4; Table 3). Among the TT genotype carriers, 96% exhibited FSH to LH ratios less than 2 compared with 68% of GT heterozygotes and 56% of wild-type carriers. All the TT subjects with diagnosis of idiopathic infertility group had low (<1.8) FSH to LH ratio (Fig. 1B).
In addition to highly significant reduction in serum FSH and FSH to LH ratio, the rs10835638 minor allele homozygosity (TT) revealed a possible effect on other markers of male reproductive function. Among the patients diagnosed with idiopathic infertility, TT carriers had significantly smaller combined testes volume (GG: median 40.0 ml > TT: median 34.0 ml; P = 3.99 × 10−2, F = 4.2; Table 3) and sperm A+B motility (GG-GT: median 23.0% > TT: median 11.0%; P = 2.39 × 10−2, F = 5.4). Concordant to the population-based male cohort study, a nonsignificant trend was observed among the TT homozygotes for the increase in semen volume (GG-GT: median 3.9 ml < TT: median 5.0 ml) and total sperm count (GG: median 19.1 × 106 < GT: median 20.2 × 106 ≪ TT: median 28.6 × 106). The carrier status of rs10835638 variants had no statistically significant effect on serum testosterone, estradiol, and inhibin B levels; sperm concentration; and morphology.
Discussion
In the seminal study, we identified and described the first polymorphism in human FSHB gene (rs10835638; −211 G/T) showing significant association with serum FSH levels in a cohort of young men from Estonia (16). The carriers of the minor allele T had significantly reduced serum FSH (15.7% in the group of GT heterozygotes; 40% among TT homozygotes) compared with the wild-type homozygotes (GG). Because FSH is essential for normal spermatogenesis and fertility, we speculated that abnormalities in FSH action could contribute to male factor infertility. In the current study, we asked two questions: 1) does genetically inherited reduced FSH level in FSHB rs10835638 T allele carriers affect male reproductive success and 2) is the association between FSHB promoter variant and serum FSH reproducible in the cohort of infertile male patients characterized by wide variance in serum FSH levels in response to disturbed spermatogenesis?
Consistent with the study hypothesis, we detected significant enrichment in the minor allele (T) carriers (GT heterozygotes and TT homozygotes) of FSHB promoter variant rs10835638 among the large cohort of Estonian patients with male factor infertility (n = 1029). We are aware of the limitations of the current study comparing the group of infertile male patients with a population-based cohort of young men. First, information about the fertility of the control subjects could not be collected due to their youth (19.2 ± 1.7 yr). Second, it was a population-based cohort with no selection for predicted fertility, e.g. based on the quality of seminal parameters. Further case-control studies including age-matched group of normozoospermic men with proven fertility are expected to provide additional data about the association of the FSHB promoter polymorphism with male reproductive success.
Simultaneously an extremely strong association was detected between rs10835638 variants and serum FSH levels (P = 0.0000012) and serum FSH to LH ratio (P = 0.000000000152) in this study group. The median serum FSH values in the heterozygotes (GT) and the homozygotes (TT) for the minor allele were 1.4 IU/liter (20.6%) and 3.3 IU/liter (48.5%) lower compared with wild-type homozygotes (GG). The levels of serum LH were not affected by the carrier status of the −211 G/T SNP. The overrepresentation of T allele carriers among infertile men may reflect the effect of understimulation of testicular function and spermatogenesis by reduced FSH during fetal/neonatal development in pubertal age and/or in adulthood. The role of FSH in contributing to human testicular function, spermatogenesis, and fertility has been discussed for decades (6). Findings in genetically manipulated mice suggested that FSH action is not needed for spermatogenesis per se and male fertility (27,28). Transgenic FSH-deficient male mice lacking FSH are fertile, although with reduction in testicular size, sperm count, and motility (28). Consistently in this study we detected significant reduction in combined testes volume and sperm motility among the Estonian patients diagnosed for idiopathic infertility and carrying the rs10835638 TT genotype. Significantly reduced testes volume of T/T carriers (P < 0.05, ANOVA) was also reported in the previous study for the population-based cohort of young men (16). In the normal men, baseline serum inhibin correlates with testicular volume in a positive and with FSH in a negative fashion (29). In contrast, we observed a nonsignificant decline of serum inhibin B concentration in parallel with the reduction of serum FSH among T allele carriers of the FSHB promoter variant in population-based cohort (16) and even more remarkably among infertile men. We propose that the understimulation of Sertoli cell proliferation during fetal and neonatal development may also affect the capacity of reduced testes to provide a negative feedback loop for the normal hormonal regulation. This is in concordance with the suggestion that serum inhibin levels reflect to some extent the integrity of seminiferous tubule function (29).
The interpretation of available clinical data about human patients with altered FSH action is not straightforward. Already as early as 1983, Matsumoto et al. (30) showed that in gonadotropin-suppressed normal men, the administration of FSH alone was sufficient to reinitiate sperm production (30). In hypogonadotrophic patients FSH treatment alone failed to induce spermatogenesis. On the other hand, hypogonadal patients with isolated lack of LH/testosterone action showed active spermatogenesis (31) supported by FSH action only (32). After careful analysis of all published evidence and available data, it was suggested that fully normal spermatogenesis requires the combined effects of FSH and LH/testosterone (6). However, the initiation of spermatogenesis may have different proportional hormonal requirements than maintenance of this process.
Numerous attempts have been made to identify a role of FSH in the treatment of male fertility. Some studies have shown that FSH treatment is able to stimulate spermatogonial population and increase sperm concentration and pregnancy rate in oligozoospermic patients, whereas other reports do not support these data. Recent reviews of the progress in FSH treatment of male infertility came to the conclusion that it should be performed on selected patients using some predictive parameters able to identify a priori responder patients with high probability (8,9). The systematic analysis of conducted clinical studies concluded that significant increase in the concentration of ejaculated spermatozoa could be expected only in the cases in which oligozoospermia is sustained by hypospermatogenesis without maturation alterations in the germ line. At the same time, elevated plasma levels of FSH have been shown as a negative predictive factor for the usefulness of FSH treatment. Based on the data of the current study, we suggest that one potential group of responders to the FSH therapy may be men diagnosed with idiopathic infertility but exhibiting genetically inherited, constitutively lower serum FSH. Determination of the genotype of the FSHB promoter polymorphism (−211 G/T) would allow the identification of the patients, whose primary cause of the infertility problems may be too low FSH production. Significantly reduced FSH to LH ratio (all but one T/T genotype carriers had serum FSH/LH ≤1.78) and also often small testes provide as additional markers to identify infertile patients with inherited low FSH.
In summary, we have described the FSHB gene promoter variant, which contributed to the determination of serum FSH levels both in a cohort of young men and among infertile male patients. The minor allele of this polymorphism was associated with reduced hormone levels in additive manner and was enriched among male partners of infertile couples. The significance of the finding arises from the fact that in contrast to the conclusive association of FSHR isoforms with serum FSH level in women, no corresponding genetic variants had been identified in men (33). The clinical significance of the FSHB promoter polymorphism is to be uncovered in further case-control studies as well as longitudinal studies following the dynamics of male reproductive parameters and fertility. In addition, this genetic marker may offer a potential for molecular diagnostics of male factor infertility and identification of potential responders to FSH treatment.
Supplementary Material
Acknowledgments
We are thankful for the participants of the study. Helle-Mai Tabo and Kairi Pramann are thanked for technical assistance.
Footnotes
This work was supported by HHMI International Scholarship Grant 55005617 (to M.L.). Additional support was provided by Wellcome Trust International Senior Fellowship Grant 070191/Z/03/Z in Biomedical Science in Central Europe; the Estonian Science Foundation Grant 7471; and Estonian Ministry of Science and Education Core Grant 0182721s06 (to M.L. and M.G.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 6, 2009
Abbreviations: ANCOVA, Analysis of covariance; BMI, body mass index.
References
- Moyle WR, Campbell RK 1996 Gonadotropins. In: Adashi EY, Rock JA, Rosenwaks Z, eds. Reproductive endocrinology, surgery and technology. Philadelphia: Lippincott-Raven Publishers; 684–724 [Google Scholar]
- Howles CM 2000 Role of LH and FSH in ovarian function. Mol Cell Endocrinol 161:25–30 [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ 2000 Initial and cyclic recruitment of ovarian follicles. Endocr Rev 21:200–214 [DOI] [PubMed] [Google Scholar]
- van Alphen MM, van de Kant HJ, de Rooij DG 1988 Follicle-stimulating hormone stimulates spermatogenesis in the adult monkey. Endocrinology 123:1449–1455 [DOI] [PubMed] [Google Scholar]
- Moudgal NR, Sairam MR 1998 Is there a true requirement for follicle stimulating hormone in promoting spermatogenesis and fertility in primates? Hum Reprod 13:916–919 [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Simoni M, Gromoll J, Weinbauer GF 1999 Role of FSH in the regulation of spermatogenesis: clinical aspects. Clin Endocrinol (Oxf) 51:139–146 [DOI] [PubMed] [Google Scholar]
- Plant TM, Marshall GR 2001 The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev 22:764–786 [DOI] [PubMed] [Google Scholar]
- Foresta C, Selice R, Ferlin A, Arslan P, Garolla A 2007 Hormonal treatment of male infertility: FSH. Reprod Biomed Online 15:666–672 [DOI] [PubMed] [Google Scholar]
- Foresta C, Selice R, Garolla A, Ferlin A 2008 Follicle-stimulating hormone treatment of male infertility. Curr Opin Urol 18:602–607 [DOI] [PubMed] [Google Scholar]
- Miller WL, Shafiee-Kermani F, Strahl BD, Huang HJ 2002 The nature of FSH induction by GnRH. Trends Endocrinol Metab 13:257–263 [DOI] [PubMed] [Google Scholar]
- Jameson JL, Becker CB, Lindell CM, Habener JF 1988 Human follicle-stimulating hormone β-subunit gene encodes multiple messenger ribonucleic acids. Mol Endocrinol 2:806–815 [DOI] [PubMed] [Google Scholar]
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I 2005 Human FSHβ subunit gene is highly conserved. Mol Hum Reprod 11:601–605 [DOI] [PubMed] [Google Scholar]
- Grigorova M, Rull K, Laan M 2007 Haplotype structure of FSHB, the β-subunit gene for fertility-associated follicle-stimulating hormone: possible influence of balancing selection. Ann Hum Genet 71:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I 2003 Mutations affecting gonadotropin secretion and action. Horm Res 60(Suppl 3):21–30 [DOI] [PubMed] [Google Scholar]
- Berger K, Souza H, Brito VN, d'Alva CB, Mendonca BB, Latronico AC 2005 Clinical and hormonal features of selective follicle-stimulating hormone (FSH) deficiency due to FSH β-subunit gene mutations in both sexes. Fertil Steril 83:466–470 [DOI] [PubMed] [Google Scholar]
- Grigorova M, Punab M, Ausmees K, Laan M 2008 FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod 23:2160–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Pedersen NR, Edwards DP, Beck CA, Miller WL 1995 The 5′-flanking region of the ovine follicle-stimulating hormone-β gene contains six progesterone response elements: three proximal elements are sufficient to increase transcription in the presence of progesterone. Endocrinology 136:1049–1058 [DOI] [PubMed] [Google Scholar]
- Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, O'Donovan MC 2003 Functional analysis of human promoter polymorphisms. Hum Mol Genet 12:2249–2254 [DOI] [PubMed] [Google Scholar]
- Mantovani G, Borgato S, Beck-Peccoz P, Romoli R, Borretta G, Persani L 2003 Isolated follicle-stimulating hormone (FSH) deficiency in a young man with normal virilization who did not have mutations in the FSHβ gene. Fertil Steril 79:434–436 [DOI] [PubMed] [Google Scholar]
- Punab M, Korrovits P, Peetsalu A 2003 Diseases influencing male infertility (in Estonian). Eesti Arst 82:181–187 [Google Scholar]
- Punab M, Zilaitiene B, Jørgensen N, Horte A, Matulevicius V, Peetsalu A, Skakkebaek NE 2002 Regional differences in semen qualities in the Baltic region. Int J Androl 25:243–252 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1999 Laboratory manual for examination of human semen and sperm-cervical mucus interaction. 4th ed. New York: Cambridge University Press [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA 1990 The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod 5:586–592 [DOI] [PubMed] [Google Scholar]
- Kvist U, Björndahl L, eds. 2002 Manual on basic semen analysis. ESHRE Monographs. Oxford, United Kingdom: Oxford University Press [Google Scholar]
- Rousset F 2008 Genepop ’007: A complete reimplementation of the Genepop software for Windows and Linx. Mol Ecol Resources 8:103–106 [DOI] [PubMed] [Google Scholar]
- Ahda Y, Gromoll J, Wunsch A, Asatiani K, Zitzmann M, Nieschlag E, Simoni M 2005 Follicle-stimulating hormone receptor gene haplotype distribution in normozoospermic and azoospermic men. J Androl 26:494–499 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur, M, Sassone-Corsi P 1998 Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 95:13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Paulsen CA, McLachlan RI 1992 Relationship of serum inhibin levels to serum follicle stimulating hormone and sperm production in normal men and men with varicoceles. J Clin Endocrinol Metab 74:859–864 [DOI] [PubMed] [Google Scholar]
- Matsumoto AM, Karpas AE, Paulsen CA, Bremner WJ 1983 Reinitiation of sperm production in gonadotropin-suppressed normal men by administration of follicle-stimulating hormone. J Clin Invest 72:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaison G, Young J, Pholsena M, Nahoul K, Couzinet B 1993 Failure of combined follicle-stimulating hormone-testosterone administration to initiate and/or maintain spermatogenesis in men with hypogonadotropic hypogonadism. J Clin Endocrinol Metab 77:1545–1549 [DOI] [PubMed] [Google Scholar]
- Pasqualini RQ, Burr GE 1950 Sindrome hypoandrogenico con gametogenesis conservada. Classificación de la insuficiencia testicular. Rev Assoc Med Argent 64:6–19 [Google Scholar]
- Gromoll J, Simoni M 2005 Genetic complexity of FSH receptor function. Trends Endocrinol Metab 16:368–373 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.