Abstract
Various membrane ATPases have been tested for their sensitivity to bafilomycin A1, a macrolide antibiotic. F1F0 ATPases from bacteria and mitochondria are not affected by this antibiotic. In contrast, E1E2 ATPases--e.g., the K+-dependent (Kdp) ATPase from Escherichia coli, the Na+,K+-ATPase from ox brain, and the Ca2+-ATPase from sarcoplasmic reticulum--are moderately sensitive to this inhibitor. Finally, membrane ATPases from Neurospora vacuoles, chromaffin granules, and plant vacuoles are extremely sensitive. From this we conclude that bafilomycin A1 is a valuable tool for distinguishing among the three different types of ATPases and represents the first relatively specific potent inhibitor of vacuolar ATPases.
Full text
PDF
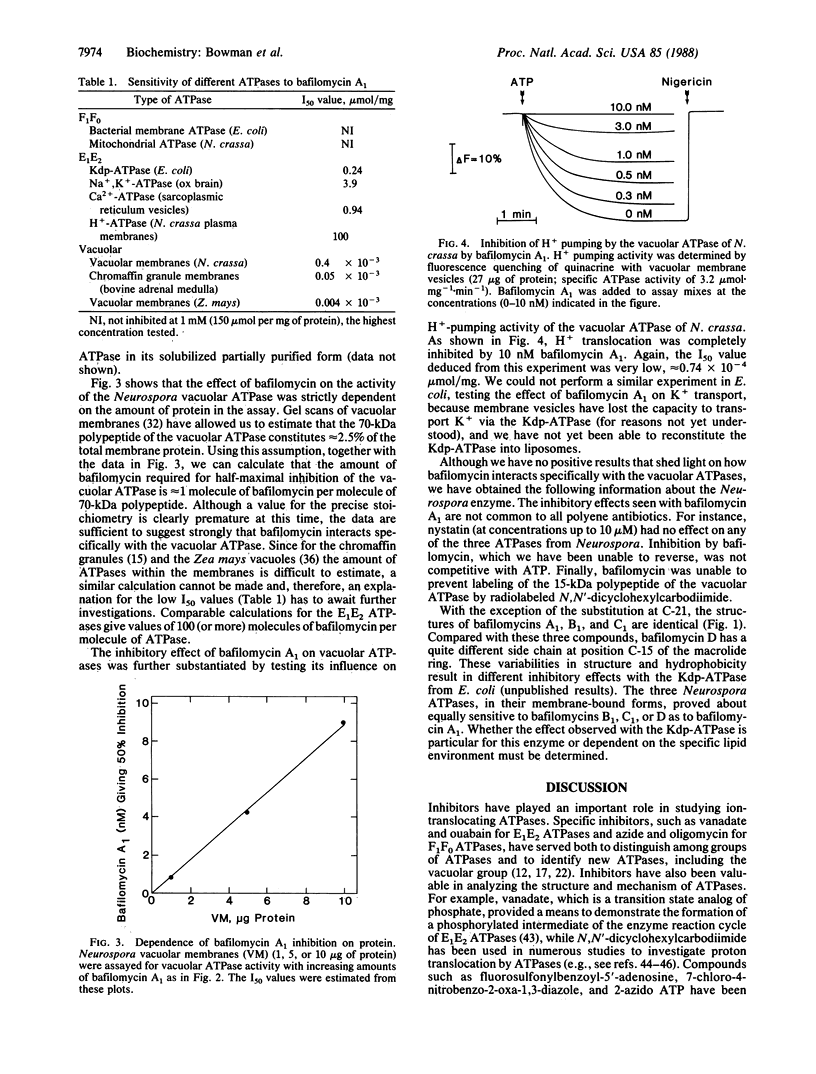


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Berne M., Terres G., Terres H., Puopolo K., Forgac M. Subunit composition and ATP site labeling of the coated vesicle proton-translocating adenosinetriphosphatase. Biochemistry. 1987 Oct 20;26(21):6632–6638. doi: 10.1021/bi00395a011. [DOI] [PubMed] [Google Scholar]
- Arnold A., Wolf H. U., Ackermann B. P., Bader H. An automated continuous assay of membrane-bound and solube ATPases and related enzymes. Anal Biochem. 1976 Mar;71(1):209–213. doi: 10.1016/0003-2697(76)90029-4. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Borchard A., Michels M., Altendorf K., Siebers A. High-affinity potassium uptake system in Bacillus acidocaldarius showing immunological cross-reactivity with the Kdp system from Escherichia coli. J Bacteriol. 1987 Sep;169(9):4342–4348. doi: 10.1128/jb.169.9.4342-4348.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Berenski C. J., Jung C. Y. Size of the plasma membrane H+-ATPase from Neurospora crassa determined by radiation inactivation and comparison with the sarcoplasmic reticulum Ca2+-ATPase from skeletal muscle. J Biol Chem. 1985 Jul 25;260(15):8726–8730. [PubMed] [Google Scholar]
- Bowman B. J., Blasco F., Slayman C. W. Purification and characterization of the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1981 Dec 10;256(23):12343–12349. [PubMed] [Google Scholar]
- Bowman B. J., Bowman E. J. H+-ATPases from mitochondria, plasma membranes, and vacuoles of fungal cells. J Membr Biol. 1986;94(2):83–97. doi: 10.1007/BF01871190. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Bowman B. J. Purification of vacuolar membranes, mitochondria, and plasma membranes from Neurospora crassa and modes of discriminating among the different H+-ATPases. Methods Enzymol. 1988;157:562–573. doi: 10.1016/0076-6879(88)57104-5. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Mandala S., Taiz L., Bowman B. J. Structural studies of the vacuolar membrane ATPase from Neurospora crassa and comparison with the tonoplast membrane ATPase from Zea mays. Proc Natl Acad Sci U S A. 1986 Jan;83(1):48–52. doi: 10.1073/pnas.83.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R. J., Slayman C. W. Inhibition of the plasma membrane [H+]-ATPase of Neurospora crassa by N-ethylmaleimide. Protection by nucleotides. J Biol Chem. 1982 Oct 25;257(20):12051–12055. [PubMed] [Google Scholar]
- Cantley L. C., Jr, Cantley L. G., Josephson L. A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications. J Biol Chem. 1978 Oct 25;253(20):7361–7368. [PubMed] [Google Scholar]
- Cross R. L., Cunningham D., Miller C. G., Xue Z. X., Zhou J. M., Boyer P. D. Adenine nucleotide binding sites on beef heart F1 ATPase: photoaffinity labeling of beta-subunit Tyr-368 at a noncatalytic site and beta Tyr-345 at a catalytic site. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5715–5719. doi: 10.1073/pnas.84.16.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Edwards D. L., Unger B. W. Nuclear mutations conferring oligomycin resistance in Neurospora crassa. J Biol Chem. 1978 Jun 25;253(12):4254–4258. [PubMed] [Google Scholar]
- Fillingame R. H. Identification of the dicyclohexylcarbodiimide-reactive protein component of the adenosine 5'-triphosphate energy-transducing system of Escherichia coli. J Bacteriol. 1975 Nov;124(2):870–883. doi: 10.1128/jb.124.2.870-883.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Friedl P., Schairer H. U. Preparation and reconstitution of F1F0 and F0 from Escherichia coli. Methods Enzymol. 1986;126:579–588. doi: 10.1016/s0076-6879(86)26060-7. [DOI] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Solioz M. Formation of a beta-aspartyl phosphate intermediate by the vanadate-sensitive ATPase of Streptococcus faecalis. J Biol Chem. 1985 Jan 10;260(1):50–52. [PubMed] [Google Scholar]
- Fürst P., Solioz M. The vanadate-sensitive ATPase of Streptococcus faecalis pumps potassium in a reconstituted system. J Biol Chem. 1986 Mar 25;261(9):4302–4308. [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Albers-Schonberg G., Monaghan R. L., Jakubas K., Pong S. S., Hensens O. D., Burg R. W., Ostlind D. A., Conroy J., Stapley E. O. Discovery, production and purification of the Na+, K+ activated ATPase inhibitor, L-681,110 from the fermentation broth of Streptomyces sp. MA-5038. J Antibiot (Tokyo) 1984 Sep;37(9):970–975. doi: 10.7164/antibiotics.37.970. [DOI] [PubMed] [Google Scholar]
- Hugentobler G., Heid I., Solioz M. Purification of a putative K+-ATPase from Streptococcus faecalis. J Biol Chem. 1983 Jun 25;258(12):7611–7617. [PubMed] [Google Scholar]
- Ikemoto N. Structure and function of the calcium pump protein of sarcoplasmic reticulum. Annu Rev Physiol. 1982;44:297–317. doi: 10.1146/annurev.ph.44.030182.001501. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ plus K+ )-ATPase. 3. Purification from the outer medulla of mammalian kidney after selective removal of membrane components by sodium dodecylsulphate. Biochim Biophys Acta. 1974 Jul 12;356(1):36–52. doi: 10.1016/0005-2736(74)90292-2. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Mandala S., Taiz L. Partial purification of a tonoplast ATPase from corn coleoptiles. Plant Physiol. 1985 Jun;78(2):327–333. doi: 10.1104/pp.78.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker J. H., Perlin D. S., Haber J. E. Pleiotropic plasma membrane ATPase mutations of Saccharomyces cerevisiae. Mol Cell Biol. 1987 Nov;7(11):4082–4088. doi: 10.1128/mcb.7.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. The purified ATPase from chromaffin granule membranes is an anion-dependent proton pump. J Biol Chem. 1987 Jul 5;262(19):9175–9180. [PubMed] [Google Scholar]
- Muñoz E. Polymorphism and conformational dynamics of F1-ATPases from bacterial membranes. A model for the regulation of these enzymes on the basis of molecular plasticity. Biochim Biophys Acta. 1982 May 12;650(4):233–265. doi: 10.1016/0304-4157(82)90018-1. [DOI] [PubMed] [Google Scholar]
- Percy J. M., Pryde J. G., Apps D. K. Isolation of ATPase I, the proton pump of chromaffin-granule membranes. Biochem J. 1985 Nov 1;231(3):557–564. doi: 10.1042/bj2310557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. Proton-conducting portion (F0) from Escherichia coli ATP synthase: preparation, dissociation into subunits, and reconstitution of an active complex. Methods Enzymol. 1986;126:569–578. doi: 10.1016/s0076-6879(86)26059-0. [DOI] [PubMed] [Google Scholar]
- Schoner W., von Ilberg C., Kramer R., Seubert W. On the mechanism of Na+- and K+-stimulated hydrolysis of adenosine triphosphate. 1. Purification and properties of a Na+-and K+-activated ATPase from ox brain. Eur J Biochem. 1967 May;1(3):334–343. doi: 10.1007/978-3-662-25813-2_45. [DOI] [PubMed] [Google Scholar]
- Serrano R. Plasma membrane ATPase of fungi and plants as a novel type of proton pump. Curr Top Cell Regul. 1984;23:87–126. doi: 10.1016/b978-0-12-152823-2.50007-6. [DOI] [PubMed] [Google Scholar]
- Siebers A., Wieczorek L., Altendorf K. K+-ATPase from Escherichia coli: isolation and characterization. Methods Enzymol. 1988;157:668–680. doi: 10.1016/0076-6879(88)57114-8. [DOI] [PubMed] [Google Scholar]
- Sun S. Z., Xie X. S., Stone D. K. Isolation and reconstitution of the dicyclohexylcarbodiimide-sensitive proton pore of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1987 Oct 25;262(30):14790–14794. [PubMed] [Google Scholar]
- Sussman M. R., Slayman C. W. Modification of the Neurospora crassa plasma membrane [H+]-ATPase with N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1983 Feb 10;258(3):1839–1843. [PubMed] [Google Scholar]
- Vignais P. V., Lunardi J. Chemical probes of the mitochondrial ATP synthesis and translocation. Annu Rev Biochem. 1985;54:977–1014. doi: 10.1146/annurev.bi.54.070185.004553. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sze H. Similarities and differences between the tonoplast-type and the mitochondrial H+-ATPases of oat roots. J Biol Chem. 1985 Sep 5;260(19):10434–10443. [PubMed] [Google Scholar]
- Werner G., Hagenmaier H., Drautz H., Baumgartner A., Zähner H. Metabolic products of microorganisms. 224. Bafilomycins, a new group of macrolide antibiotics. Production, isolation, chemical structure and biological activity. J Antibiot (Tokyo) 1984 Feb;37(2):110–117. doi: 10.7164/antibiotics.37.110. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Stone D. K. Isolation and reconstitution of the clathrin-coated vesicle proton translocating complex. J Biol Chem. 1986 Feb 25;261(6):2492–2495. [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]


