Abstract
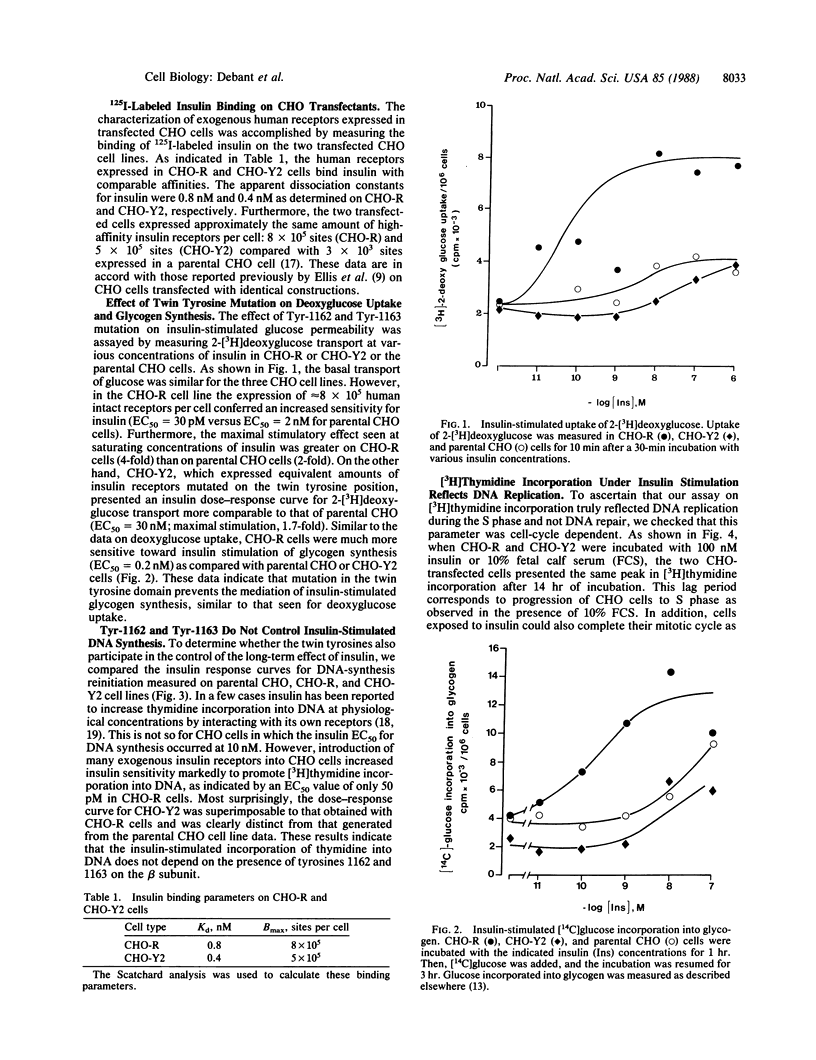
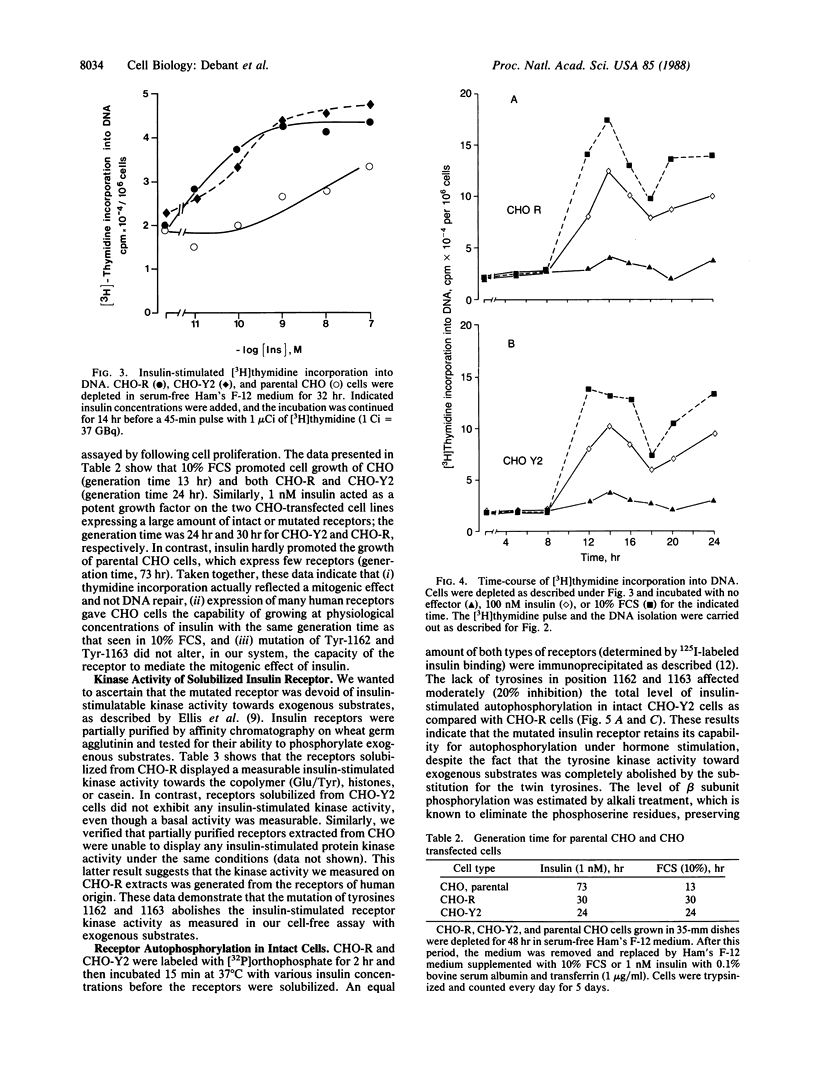
Chinese hamster ovary transfectants that express insulin receptors in which tyrosine residues 1162 and 1163 were replaced by phenylalanine exhibit a total inhibition of the insulin-mediated tyrosine kinase activity toward exogenous substrates [histone, casein, and poly(Glu/Tyr)]; this latter activity is associated with total inhibition of the hypersensitivity reported for insulin in promoting 2-deoxyglucose uptake. We now present evidence that the twin tyrosines also control the insulin-mediated stimulation of glycogen synthesis. Surprisingly, this type of Chinese hamster ovary transfectant is as hypersensitive to insulin for its mitogenic effect as are Chinese hamster ovary cells expressing many intact insulin receptors. Such data suggest that (i) the insulin mitogenic effect routes through a different pathway than insulin uses to activate the transport and metabolism of glucose and (ii) the mitogenic effect of insulin is not controlled by the twin tyrosines. At the molecular level, the solubilized mutated receptor has no insulin-dependent tyrosine kinase activity, whereas this receptor displays measurable insulin-stimulated phosphorylation of its beta subunit in 32P-labeled cells. We therefore propose that the autocatalytic phosphorylating activity of the receptor reports a cryptic tyrosine kinase activity that cannot be visualized by the use of classical exogenous substrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D. Oncogenes in development. Development. 1987 Apr;99(4):449–471. doi: 10.1242/dev.99.4.449. [DOI] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Regulation of a ribosomal protein S6 kinase activity by the Rous sarcoma virus transforming protein, serum, or phorbol ester. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7621–7625. doi: 10.1073/pnas.82.22.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz J. W., Iwahashi M. Insulin as a potent, specific growth factor in a rat hepatoma cell line. Science. 1981 Feb 27;211(4485):947–949. doi: 10.1126/science.7008195. [DOI] [PubMed] [Google Scholar]
- Livneh E., Reiss N., Berent E., Ullrich A., Schlessinger J. An insertional mutant of epidermal growth factor receptor allows dissection of diverse receptor functions. EMBO J. 1987 Sep;6(9):2669–2676. doi: 10.1002/j.1460-2075.1987.tb02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B. D., Pessin J. E. Insulin stimulation of the insulin receptor kinase can occur in the complete absence of beta subunit autophosphorylation. J Biol Chem. 1987 Feb 25;262(6):2861–2868. [PubMed] [Google Scholar]
- Nilsen-Hamilton M., Allen W. R., Hamilton R. T. Rapid and efficient method for analyzing phosphorylation of the S6 ribosomal protein in 32Pi-labeled, tissue culture cells. Anal Biochem. 1981 Aug;115(2):438–449. doi: 10.1016/0003-2697(81)90030-0. [DOI] [PubMed] [Google Scholar]
- Nilsen-Hamilton M., Hamilton R. T., Allen W. R., Potter-Perigo S. Synergistic stimulation of S6 ribosomal protein phosphorylation and DNA synthesis by epidermal growth factor and insulin in quiescent 3T3 cells. Cell. 1982 Nov;31(1):237–242. doi: 10.1016/0092-8674(82)90423-8. [DOI] [PubMed] [Google Scholar]
- Pierre M., Toru-Delbauffe D., Gavaret J. M., Pomerance M., Jacquemin C. Activation of S6 kinase activity in astrocytes by insulin, somatomedin C and TPA. FEBS Lett. 1986 Sep 29;206(1):162–166. doi: 10.1016/0014-5793(86)81361-8. [DOI] [PubMed] [Google Scholar]
- Podskalny J., McElduff A., Gorden P. Insulin receptors on Chinese hamster ovary (CHO) cells: altered insulin binding to glycosylation mutants. Biochem Biophys Res Commun. 1984 Nov 30;125(1):70–75. doi: 10.1016/s0006-291x(84)80335-6. [DOI] [PubMed] [Google Scholar]
- Ponzio G., Dolais-Kitabgi J., Louvard D., Gautier N., Rossi B. Insulin and rabbit anti-insulin receptor antibodies stimulate additively the intrinsic receptor kinase activity. EMBO J. 1987 Feb;6(2):333–340. doi: 10.1002/j.1460-2075.1987.tb04759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Phosphorylation of synthetic insulin receptor peptides by the insulin receptor kinase and evidence that the preferred sequence containing Tyr-1150 is phosphorylated in vivo. J Biol Chem. 1986 Jul 25;261(21):10000–10005. [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]





