Abstract
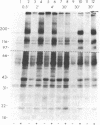
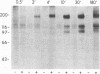
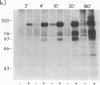
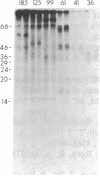
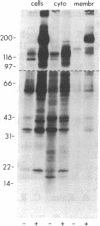
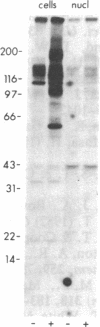
To investigate growth factor-mediated signal transduction, we have studied phosphorylation events that take place within seconds of the binding of colony-stimulating factor 1 (CSF-1) to its cell-surface receptor. CSF-1 stimulated rapid tyrosine phosphorylation of cellular proteins in murine BAC1.2F5 macrophages at 37 degrees C and 4 degrees C. The pattern of CSF-1-stimulated tyrosine phosphorylation of at least 15 different proteins at both temperatures was similar and unchanged by treatment of the lysate with reducing agent. With the exception of the 185-kDa CSF-1 receptor, a 260-kDa protein and a 133-kDa protein, the proteins were predominantly cytoplasmic. At 37 degrees C, all the proteins were phosphorylated within 30 sec of addition of growth factor. At 4 degrees C, CSF-1 receptor sites were saturated after 2 min of incubation in the presence of high concentrations of CSF-1 and differences in the order of appearance of phosphorylated proteins were observed: 185 kDa (CSF-1 receptor) (by 2 min); 99 kDa (by 4 min); 125 kDa (by 10 min); 61 kDa (by 30 min); and 260 kDa, 84 kDa, and 41 kDa (by 180 min). In addition to stimulating the phosphorylation of these proteins in tyrosine, CSF-1 caused dephosphorylation of phosphorylated serine residues on the receptor. As neither CSF-1 nor its receptor is internalized at 4 degrees C, analysis of these early reactions and the phosphotyrosine-containing proteins in intact cells under these conditions should lead to an understanding of the early events in growth factor receptor-mediated signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cooper J. A., Bowen-Pope D. F., Raines E., Ross R., Hunter T. Similar effects of platelet-derived growth factor and epidermal growth factor on the phosphorylation of tyrosine in cellular proteins. Cell. 1982 Nov;31(1):263–273. doi: 10.1016/0092-8674(82)90426-3. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Gabrilove J. L., Tam J. P., Moore M. A., Hanafusa H. Specific expression of the human cellular fps/fes-encoded protein NCP92 in normal and leukemic myeloid cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2379–2383. doi: 10.1073/pnas.82.8.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Tremble P. M., Williams L. T. Evidence for the platelet-derived growth factor-stimulated tyrosine phosphorylation of the platelet-derived growth factor receptor in vivo. Immunopurification using a monoclonal antibody to phosphotyrosine. J Biol Chem. 1984 Jun 25;259(12):7909–7915. [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Honegger A. M., Szapary D., Schmidt A., Lyall R., Van Obberghen E., Dull T. J., Ullrich A., Schlessinger J. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol. 1987 Dec;7(12):4568–4571. doi: 10.1128/mcb.7.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn R. D., Posner M. R., Rayter S. I., Foulkes J. G., Frackelton A. R., Jr Cell lines and peripheral blood leukocytes derived from individuals with chronic myelogenous leukemia display virtually identical proteins phosphorylated on tyrosine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4408–4412. doi: 10.1073/pnas.84.13.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Izumi T., White M. F., Kadowaki T., Takaku F., Akanuma Y., Kasuga M. Insulin-like growth factor I rapidly stimulates tyrosine phosphorylation of a Mr 185,000 protein in intact cells. J Biol Chem. 1987 Jan 25;262(3):1282–1287. [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan C., Pollard J. W., Stanley E. R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987 Mar;130(3):420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Browning P. J., White M. F., Roberts T. M. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. doi: 10.1128/mcb.8.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Dull T. J., Rettenmier C. W., Ralph P., Ullrich A., Sherr C. J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor). Nature. 1987 Feb 5;325(6104):549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- Sacca R., Stanley E. R., Sherr C. J., Rettenmier C. W. Specific binding of the mononuclear phagocyte colony-stimulating factor CSF-1 to the product of the v-fms oncogene. Proc Natl Acad Sci U S A. 1986 May;83(10):3331–3335. doi: 10.1073/pnas.83.10.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Epidermal growth factor stimulates the phosphorylation of the calcium-dependent 35,000-dalton substrate in intact A-431 cells. J Biol Chem. 1985 Jul 15;260(14):8233–8236. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Stanley E. R. Solubilization and assay of a colony-stimulating factor receptor from murine macrophages. J Cell Biochem. 1986;31(4):259–269. doi: 10.1002/jcb.240310403. [DOI] [PubMed] [Google Scholar]