Abstract
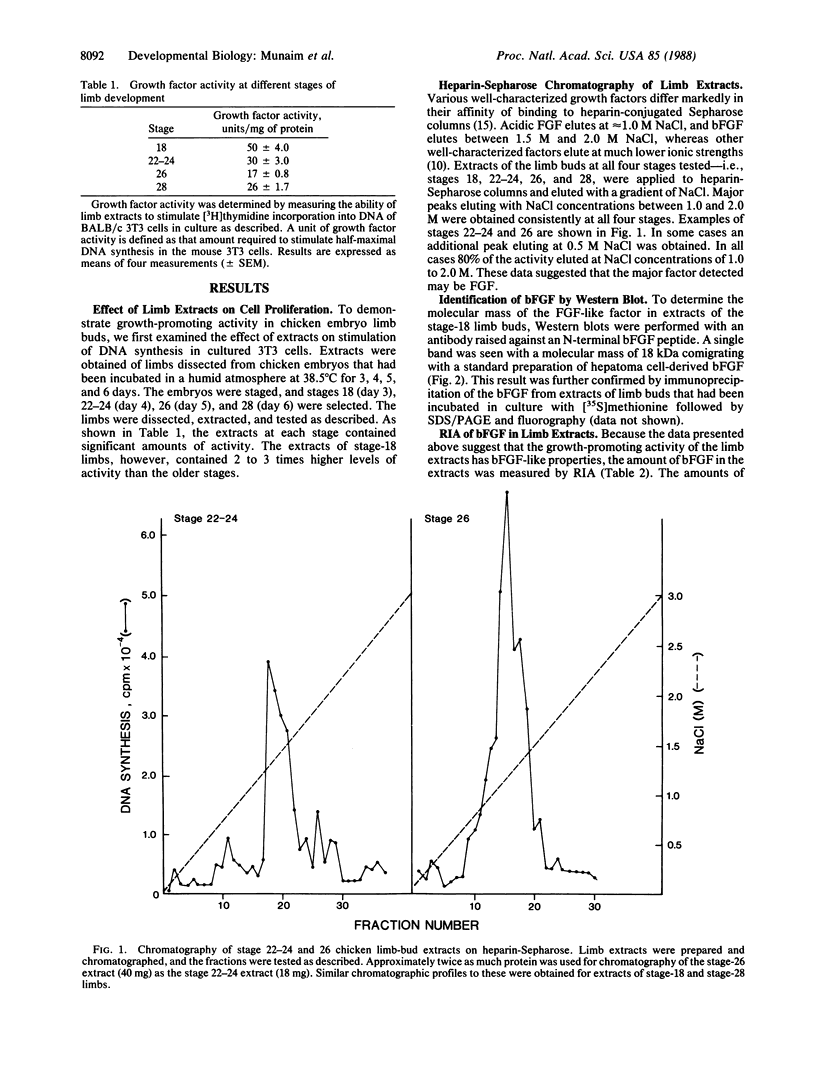
Cell proliferation is a major event during early limb development. Significant levels of growth factor activity, as measured by stimulation of DNA synthesis in mouse BALB/c 3T3 cells, were found in extracts of chicken embryo limb buds at early stages of development. Extracts from stage-18 limbs (3 days of incubation) were 2 to 3 times more potent than were extracts from older stages, namely 22-24 (4 days), 26 (5 days), and 28 (6 days). Basic fibroblast growth factor (bFGF) was measured specifically using an RIA, and the amounts of factor obtained corresponded to the activities measured by the 3T3 cell-growth assay. In addition, most growth factor in the extracts bound with high affinity to heparin-Sepharose columns. Western (immunologic) blotting and immunoprecipitation with an antibody specific for bFGF revealed a protein of identical size to bFGF--i.e., 18 kDa, in the extracts. Thus, a growth factor with the properties of bFGF is present in the early limb, and the level of this factor is highest when proliferation is a predominant cellular event in the developing limb. These and other data suggest that fibroblast growth factor is a key regulatory factor in embryonic growth and morphogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Böhlen P., Ling N., Guillemin R. Radioimmunoassay for fibroblast growth factor (FGF): release by the bovine anterior pituitary in vitro. Regul Pept. 1985 Apr;10(4):309–317. doi: 10.1016/0167-0115(85)90043-6. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. The vasculature and limb development. Cell Differ. 1985 Feb;16(1):1–11. doi: 10.1016/0045-6039(85)90602-5. [DOI] [PubMed] [Google Scholar]
- Chevallier A., Kieny M., Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol. 1977 Oct;41:245–258. [PubMed] [Google Scholar]
- Cooke J., Summerbell D. Cell cycle and experimental pattern duplication in the chick wing during embryonic development. Nature. 1980 Oct 23;287(5784):697–701. doi: 10.1038/287697a0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Sasse J., Klagsbrun M. A cartilage-derived growth factor enhances hyaluronate synthesis and diminishes sulfated glycosaminoglycan synthesis in chondrocytes. J Cell Physiol. 1986 May;127(2):317–322. doi: 10.1002/jcp.1041270220. [DOI] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Capillary endothelial cell migration: loss of stress fibres in response to retina-derived growth factor. J Muscle Res Cell Motil. 1984 Dec;5(6):697–709. doi: 10.1007/BF00713928. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Langer R., Levenson R., Smith S., Lillehei C. The stimulation of DNA synthesis and cell division in chondrocytes and 3T3 cells by a growth factor isolated from cartilage. Exp Cell Res. 1977 Mar 1;105(1):99–108. doi: 10.1016/0014-4827(77)90155-0. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C. B., Toole B. P. Changes in the pericellular matrix during differentiation of limb bud mesoderm. Dev Biol. 1985 Dec;112(2):308–318. doi: 10.1016/0012-1606(85)90401-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D., Dodge L., Fujii D. K. Heparin modulation of the neurotropic effects of acidic and basic fibroblast growth factors and nerve growth factor on PC12 cells. J Cell Physiol. 1987 Apr;131(1):131–140. doi: 10.1002/jcp.1041310119. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Hauschka S. D. Identification of the fibroblast growth factor receptor of Swiss 3T3 cells and mouse skeletal muscle myoblasts. Biochemistry. 1986 Jun 17;25(12):3487–3492. doi: 10.1021/bi00360a001. [DOI] [PubMed] [Google Scholar]
- Reiter R. S., Solursh M. Mitogenic property of the apical ectodermal ridge. Dev Biol. 1982 Sep;93(1):28–35. doi: 10.1016/0012-1606(82)90235-4. [DOI] [PubMed] [Google Scholar]
- Risau W., Ekblom P. Production of a heparin-binding angiogenesis factor by the embryonic kidney. J Cell Biol. 1986 Sep;103(3):1101–1107. doi: 10.1083/jcb.103.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Slack J. M., Darlington B. G., Heath J. K., Godsave S. F. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987 Mar 12;326(6109):197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Wadzinski M. G., Folkman J., Sasse J., Devey K., Ingber D., Klagsbrun M. Heparin-binding angiogenesis factors: detection by immunological methods. Clin Physiol Biochem. 1987;5(3-4):200–209. [PubMed] [Google Scholar]



