Abstract
Four isoforms of actinoporins were isolated in 2002-2004 from the tropical sea anemone Heteractis crispa (=Radianthus macrodactylus). Their potent hemolytic activities and effects on Ehrlich ascites carcinoma bearing mice were also studied. In this study, the individual actinoporin (RTX-A) demonstrated potential cancer-preventive activity at extremely low and non-cytotoxic concentrations. The substance suppressed the malignant transformation of mouse JB6 P+ Cl41 cells stimulated by epidermal growth factor (EGF) in soft agar with the inhibition of number of the colonies C50 (INCC50) = 0.034 nM. Actinoporin RTX-A also was shown to inhibit the phenotype expression of HeLa human cancer cells with an INCC50 = 0.03 nM. The cytotoxic effect of RTX-A against JB6 P+ Cl41 cells and HeLa, THP-1, MDA-MB-231, and SNU-C4 human tumor cell lines was high (IC50 = 0.57, 2.26, 1.11, 30.0 and 4.66 nM), but significantly less than their capacity to suppress tumor cell colony formation or phenotype expression. RTX-A also induced apoptosis and inhibited basal AP-1, NF-κB, and p53-dependent transcriptional activity in JB6 Cl41 cells. These results confirmed that actinoporin RTX-A from H. crispa, at least partially, might exhibit cancer-preventive and anticancer cytotoxic properties through the induction of p53-independent apoptosis and inhibition of the oncogenic AP-1 and NF-κB nuclear factors activity.
Keywords: Actinoporin; Anticancer activity; Inhibition of malignant transformation; Apoptosis; AP-1, NF-κB, p53 nuclear factors
1. Introduction
Sea anemone actinoporins belong to a unique family of α-pore-forming toxins (PFT) that affect a majority of eukaryotic cells (Anderluh and Maček, 2002). Some bacterial α- and β-PFTs, including colicins of Escherichia coli, diphtheria toxin of Corynebacterium diphtheriae, α-hemolysin of Staphylococcus aureus, and some other bacterial toxins were formed as killing agents for target cells in a process of evolution (Parker and Feil, 2005). Actinoporins play an important role in defense against potential predators of the sea anemones (Maček, 1992). Unlike bacterial PFTs which have generally pore-forming, receptor and enzyme domains, actinoporins are single-domain sphingomyelin-dependent cytolysins with molecular weight of ~20 kDa and possess highly toxic and membranolytic activities. Many pharmacological effects of actinoporins such as cardiotoxicity, coronary vasospasm, and respiratory arrest are connected with their pore-forming action, causing the non-specific permeabilization of cell membranes (Anderluh and Macek, 2002).
Three isoforms of actinoporins, RTX-A, RTX-S, and RTX-G were isolated in 2002 from the tropical sea anemone Heteractis crispa (=Radianthus macrodactylus), and their potent hemolytic activities were established as well as the inhibition of these activities by sphingomyelin (Monastyrnaya et al., 2002). The effect of partially purified actinoporins from H. crispa on Ehrlich ascites carcinoma bearing mice was also studied. One fraction of actinoporins in vivo at a dose of 4.5 μg/kg increased the life span of these mice significantly (Monastyrnaya et al., 2002). Later a new isoform, actinoporin RTX-SII, was isolated from the same species and its properties and partial amino acid sequence were studied (Klyshko et al, 2004). Recently, the amino acid sequence of RTX-A was reported by amplification of its cDNA (Il’ina et al., 2006).
However, the cancer-preventive effects of actinoporins isolated from H. crispa and their cytotoxic effects against human tumor cells have not yet been well studied. In this manuscript, we report the cancer-preventive activity of actinoporin RTX-A using the mouse epidermal JB6 Cl 41 P+ cell transformation assay. We also demonstrate the anticancer effects of RTX-A against several human cancer cell lines as well as provide some details of probable molecular mechanisms of its action.
2. Materials and methods
2.1 Reagents
Minimum essential medium (MEM), DMEM and RPMI medium were purchased from Gibco Invitrogen Corporation (Carlsbad, CA, USA). Fetal bovine serum (FBS) was from Gemini Bio-Products (Calabasas, CA, USA); penicillin/streptomycin and gentamycin were from Bio-Whittaker (Walkersville, MD, USA); and L-glutamine was from Mediatech, Inc. (Herndon, VA, USA). Epidermal growth factor (EGF) was obtained from Collaborative Research (Bedford, MA, USA). The luciferase assay substrate and Cell Titer 96 Aqueous One Solution Reagent [5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl) -3-(4-sulfophenyl) tetrazolium, inner salt (MTS)] kit for the cell viability assay were from Promega (Madison, WI, USA). The Annexin V-FITC Apoptosis Detection kit was from Medical & Biological Laboratories (Watertown, MA, USA)
2.2 Isolation and purification of actinoporin RTX-A
The specimens of the sea anemone H. crispa (=R. macrodactylus) were collected in the coral reefs of the Seychelles during a marine expedition aboard the research vessel ‘Academik Oparin’. The species identification was carried out by Dr. Grybelniy C.D. (Zoological Institute of the Russian Academy of Sciences, Saint-Petersburg, Russia) and Dr. Kostina E.E. (Institute of Marine biology of the Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia). A highly purified mixture of actinoporins from H. crispa (RTXs) was isolated by hydrophobic chromatography on Polychrom-1 (Olaina, Latvia), gel-filtration on Akrilex P-4 (Reanal, Hungary), cation-exchange chromatography on Cellulose CM-32 (Whatman, England) followed by cation-exchange HPLC on Ultropac TSK CM-3CW column as previously described (Monastirnaya et al., 2002). The individual actinoporin RTX-A was obtained from this mixture by reversed-phase HPLC using Agilent 1100 Series HPLC system (Germany) and a Silasorb C18 column (10×250 mm, Elsico, NPO Diagnostikum, Russia). Elution of actinoporin RTX-A was performed using a linear gradient of concentration of acetonitrile (0.5–60%) in 0.1% trifluoroacetic acid for 60 min. The flow rate was 1 ml/min. RTX-A yield was 0.003% of dry weight of the animals. The homogeneity of RTX-A was confirmed by gradient SDS-PAGE, N-terminal amino acid sequencing, and mass spectrometry. Automatic amino acid sequence analysis of RTX-A (30 nmol) was performed using a Model 477A sequencer (Applied Biosystems, USA). Polybrene was used as a carrier. Amino acid phenilthiohydantoins were analysed by a Hewlett–Packard model 1090 liquid chromatograph equipped with a filter detector (254 nm) and a Microsil C18 column (10×250 mm, Machery Nagel, Germany). In accordance with data obtained from PDB UniProtKB/Swiss-Prot Acc. No. P58691, the molecular mass of RTX-A is 19275 Da (Klyshko et al, 2004). In result of MALDI-TOF-MS study (MALDI-TOF MS Vision 2000, Termo, England), the molecular mass of RTX-A was found to be 19207 Da. The hemolytic activity of RTX-A is 3.5×104 HU/mg.
2.3. Cell Culture
The JB6 P+ Cl 41 mouse epidermal cell line and its stable transfectants JB6-Luc AP-1, JB6-Luc NF-κB, or JB6-Luc p53 (PG-13) cells were cultured in monolayers at 37 °C and 5% CO2 in MEM containing 5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Huang et al., 1998). The human cancer cell lines, HL-60 (promyelocytic leukemia), THP-1 (monocytic leukemia), HeLa (cervix carcinoma), MDA MB 231 (breast cancer) and SNU-C4 (colon cancer) were obtained from the American Type Culture Collection (Rockville, MD, USA). The HL-60, THP-1, HeLa, and SNU-C4 cancer cell lines were cultured at 37°C and 5% CO2 in RPMI medium containing 10% FBS, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. The MDA-MB-231 cancer cell line was cultured at 37°C and 5% CO2 in DMEM medium containing 10% FBS, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin. Information regarding the genetic background of these cell lines is available online at the ATCC website.
2.4. Statistics
The statistical computer program Statistica 6.0 for Windows (StatSoft, Inc., Tulsa, OK, USA, 2001) was used for analysis of the obtained data. Six samples of two independent experiments were used for analysis. Nonparametric Mann - Whitney U test was used to compare two independent groups of data. The method of regressions was used to compute the IC50 or INCC50 in corresponding experiments.
2.5. Cell viability test
The effect of RTX-A on the viability of JB6 Cl41, HeLa, SNU-C4, THP-1, HL-60 and MDA-MB-231 cell lines was evaluated using MTS reduction into its formazan product as described previously (Fedorov et al., 2008; Baltrop et al., 1991). Briefly, corresponding cells were cultured for 12 h in 96-well plates (6,000 per well). The media were then replaced with fresh media containing RTX-A at various concentrations in a total volume of 0.1 ml and the cells were incubated for 22 h. Then, 20 μl of the MTS reagent were added into each well and MTS reduction was measured 2 h later spectrophotometrically at 492 and 690 nm as background using the μQuant microplate reader (Bio-Tek nstruments, Inc, USA). Results are shown in Table 1 and represent the IC50 of RTX-A against corresponding cells.
Table 1.
Cytotoxic activity of RTX-A against several human caner cell lines and mouse JB6 P+ Cl41 cells.
| Cell line | IC50, nM |
|---|---|
| HeLa | 2.26 |
| THP-1 | 1.11 |
| SNU-C4 | 4.66 |
| MDA MB 231 | 4.64 |
| HL-60 | 1.06 |
| JB6 P+ Cl41 | 0.57 |
2.6. Apoptosis assessed by flow cytometry
Early and late apoptosis induced by RTX-A in JB6 P+ Cl41 cells was analyzed by flow cytometry using the Becton Dickinson FACSCalibur (BD Biosciences, San Jose, CA, USA) as described previously (Fedorov et al., 2008). Briefly, the onset of early and late apoptosis was analyzed using Annexin V–FITC and propidium iodide (PI) double staining. JB6 P+ Cl41 cells, 1×106/10 cm dish, in 5% FBS-MEM were treated with various concentrations of RTX-A for 24 hours. After incubation, cells were washed with PBS by centrifugation at 1000 rpm (170 rcf) for 5 min, and processed for detection of apoptosis using Annexin V-FITC and PI staining. 1×105-5×105 cells were resuspended in 500 μl of 1x binding buffer. Then, 5 μl of Annexin V-FITC and 5 μl of PI were added, and the cells were incubated at room temperature for 15 min in the dark and were analyzed by flow cytometry.
2.7. Effect of RTX-A on the basal AP-1-, NF-κB-, or p53- nuclear factor-dependent transcriptional activity
The effect of RTX-A on the basal AP-1-, NF-κB-, or p53- nuclear factor-dependent transcriptional activities was evaluated using JB6 Cl41 cell lines stably expressing a luciferase reporter gene controlled by an AP-1, NF-κB, or p53 DNA binding sequence, as described previously (Fedorov et al., 2008). Briefly, viable cells (8×103) suspended in 100 μl of 5% FBS-MEM were added into each well of a 96-well plate. Plates were incubated overnight and then treated with various concentrations of RTX-A. After incubation with RTX-A for 24 h, the cells were disrupted for 1 h at room temperature with lysis buffer (0.1 M potassium phosphate buffer at pH 7.8, 1% Triton X-100, 1 mM DTT, 2 mM EDTA). Then, 30 μl of lysate from each well were transferred into a plate for luminescent analysis and luciferase activity was measured using 100 μl/well of the luciferase assay buffer (0.47 mM D-luciferin, 20 mM Tricin, 1.07 mM magnesium carbonate hydroxide pentahydrate (MgCO3)4 × Mg(OH)2 × 5H2O, 2.67 mM MgSO4 × 7H2O, 33.3 mM DTT, 0.53 mM ATP, 0.27 mM CoA, and 0.1 mM EDTA (pH 7.8)) and the Luminoscan Ascent Type 392 microplate reader (Labsystems, Helsinki, Finland). Results are expressed in Fig. 4 as a percentage of AP-1, NF-κB, or p53 -dependent transcriptional activity relative to untreated control cells (100%).
Fig. 4.
RTX-A inhibits basal AP-1 (a), NF-κB (c) or p53 (e) – dependent transcriptional activity in JB6 Cl41 mouse epidermal cells. The effects of RTX-A on the viability of JB6 Cl41 AP-1 (b), NF-κB (d), or p53 (f) cells. Data are represented as means ± S.D. of six samples from two independent experiments. The asterisk (*) indicates a significant decrease (p < 0.05) in AP-1 (b), NF-κB (d), or p53 (f) activation, or in JB6 Cl 41 cells viability.
2.8. Evaluation of RTX-A cancer-preventive effects
The cancer-preventive effects of RTX-A were evaluated using an anchorage-independent neoplastic transformation or phenotype expression assay in soft agar as described previously (Fedorov et al., 2007; Dong and Cmarik, 2002). Briefly, epidermal growth factor (EGF; 10 ng/ml) was used for stimulating neoplastic transformation of JB6 P+ Cl41 cells. The assay was carried out in six-well tissue culture plates. Mouse JB6 P+ Cl41 cells (8×103/ml) were treated with various concentrations of RTX-A in 1 ml of 0.33% basal medium Eagle (BME) agar containing 10% FBS over 3 ml of 0.5% BME agar containing 10% FBS and various concentration of RTX-A. The cultures were maintained in a 37°C, 5% CO2 incubator for 1 week and cell colonies were then scored using the LEICA DM IRB inverted research microscope (Leica Mikroskopie und Systeme GmbH, Germany) and Image-Pro Plus software, version 3.0 for Windows (Media Cybernetics, Silver Spring, MD, USA). The ability of RTX-A to inhibit the growth of human cancer HeLa cell colonies in soft agar was evaluated without EGF-stimulation. Results are shown in Fig. 2 as a number of colonies relative to EGF-control cells.
Fig. 2.
Inhibition of EGF-induced neoplastic transformation of JB6 P+ Cl41 cells (a) or phenotype expression of HeLa cells (b) by RTX-A. The asterisk (*) indicates a significant (p < 0.05) decrease in the number of colonies formed by treated and untreated cells. Data are shown as means ± S.D. of six samples from two independent experiments.
3. Results and discussion
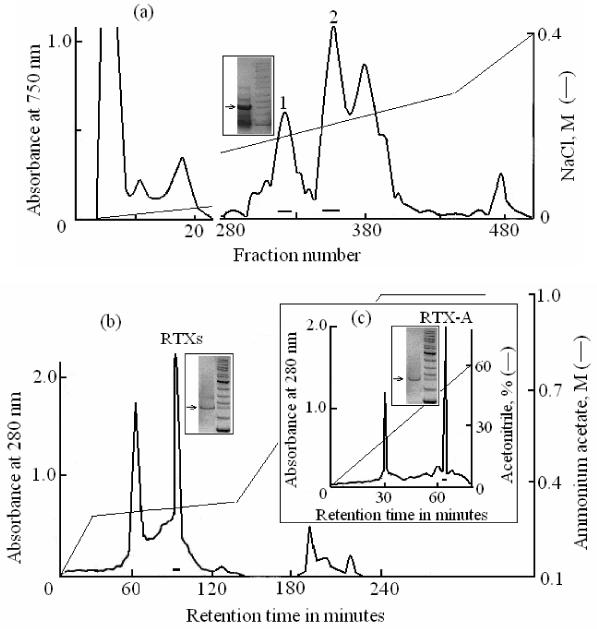
We studied the cancer-preventive and anticancer cytotoxic activities of the individual actinoporin RTX-A isolated from H. crispa. The isolation and purification of RTX-A included several steps (Monastirnaya et al., 2002). The results of the last three steps are shown in Fig. 1. The cation-exchange HPLC of H. crispa actinoporins mixture using cellulose CM-32 yielded several fractions (Fig. 1a). Further partition of the fraction 1 by cation-exchange HPLC using an Ultropac TSK CM-3SW column yielded four main fractions (Fig. 1b), one of which (RTXs) was then purified by reversed phase HPLC yielded pure RTX-A (Fig. 1c). The homogeneity of the obtained RTX-A was confirmed by gradient SDS-PAGE (Fig. 1c), N-terminal amino acid sequencing (Il’ina et al., 2006), and mass spectrometry.
Fig.1.
The HPLC profile obtained by cation-exchange chromatography of the crude fraction of H. crispa actinoporins on cellulose CM-32 and the SDS-PAGE gel of the fraction 1(a). The HPLC profile obtained by cation-exchange chromatography of the fraction 1 on Ultropac TSK CM-3SW column and the SDS-PAGE gel of the RTXs fraction (b). Isolation of RTX-A from the RTXs fraction by reversed-phase HPLC and the SDS-PAGE gel of the pure RTX-A (c).
The investigation of the cancer preventive properties of RTX-A was carried out using mouse JB6 P+ Cl41 epidermal cells or the human cancer HeLa cell line and the anchorage independent transformation or phenotype expression assay in soft agar, respectively. The JB6 cell system of clonal genetic variants, including promotion-sensitive (P+), promotion-resistant (P−), or malignantly transformed cells, facilitates the search for chemopreventive compounds and helps to determine their cancer preventive properties at the molecular level. The JB6 P+, P−, and transformed variants are a series of cell lines representing earlier-to-late stages of preneoplastic-to-neoplastic progression (Bernstein and Colburn, 1989; Dong et al., 1994; Dong et al., 1995; Dong and Cmarik, 2002). JB6 P+ Cl41 cells undergo neoplastic transformation when stimulated with tumor promoters such as epidermal growth factor (EGF) or 12-O-tetradecanoylphorbol-13-acetate (TPA) resulting in the formation of colonies in soft agar. The transformation involves the activation of the activator protein-1 (AP-1) nuclear factor that regulates the transcription of various genes related to inflammation, proliferation and metastasis (Dong et al., 1994; Huang et al., 1997; Huang et al., 1998). Cancer cells, in contrast to JB6 P+ Cl41 cells, do not require tumor promoters like EGF to form colonies in soft agar.
Our experimental results showed that the actinoporin RTX-A can prevent malignant transformation of JB6 P+ Cl41 cells with an INCC50 = 0.034 nM (Fig. 2a). This concentration is 17 times less than the dose that induces cytotoxicity (IC50 = 0.57 nM) (Table 1).
The ability of RTX-A to suppress the growth of HeLa cell colony formation in soft agar was evaluated without EGF-stimulation. The active concentration, INCC50 = 0.03 nM (Fig. 2b), was determined to be 75 times less than the cytotoxic dose, IC50 = 2.26 nM (Table 1).
The cytotoxicities of RTX-A against JB6 P+ Cl41 cells and several human cancer cell lines were evaluated by the MTS cell viability assay (Baltrop et al., 1991). The results shown in Table 1 indicated that RTX-A demonstrated potent cytotoxic activity (IC50 = 1–5 nM) against human caner cell lines, including HL-60, MDA-MB-231, HeLa, THP-1, and SNU-C4. The active concentrations of RTX-A, 10−9 M, agree with the values of the cytotoxicity obtained earlier for the α-PFT of the actinoporin family, ranging from 10−10 to 10−7 M, according to reviewed data (Alvarez et al., 2009; Avila et al., 1988; Avila et al., 1989; Tejuca et al., 2004).
Apoptosis is a general mechanism for removal of unwanted cells from organisms and plays a protective role against carcinogenesis (Bursch et al., 1992). Evidence from both in vivo and in vitro experiments shows that apoptosis is involved in successful cancer treatment using many drugs and other chemical substances (Hickman, 1992). Our experiments using flow cytometry showed that RTX-A induced apoptosis in JB6 P+ Cl41 cells. The JB6 P+ Cl41 cells, treated with 0.125 – 1 nM of RTX-A, were harvested after 24 h. Results indicated that apoptosis was clearly induced by RTX-A in a dose-dependent manner (Fig. 3). Results are shown as the percentage of early (bottom right) or late (top right) apoptosis.
Fig. 3.
The induction of apoptosis by RTX-A in JB6 P+ Cl41 cells. A representative experiment is shown.
To elucidate a possible mechanism of the anticancer activity of actinoporins, the effect of RTX-A on the basal AP-1, NF-κB, and p53 transcriptional activation was investigated in JB6 Cl41 cells stably expressing a luciferase reporter gene controlled by an AP-1, NF-κB, or p53 DNA binding sequence. The role of the AP-1 or NF-κB protein transcriptional activation in the expression of many genes involved in proliferation, differentiation, tumor promotion, suppression of apoptosis, inflammation, and malignant transformation of normal cells is well known (Dong et al., 1994; Dong et al., 1995; Bernstein and Colburn, 1989; Young et al., 1999; Amit and Ben-Neriah, 2003; Beg and Baltimore, 1996). The search for natural compounds that suppress AP-1- or NF-κB- dependent transcriptional activation in normal cells and induce apoptosis of tumor cells creates opportunities for selection of new anticancer agents. Our results show that RTX-A at concentrations 0.1-1.6 nM time- and dose-dependently inhibits basal AP-1- and NF-κB- dependent transcriptional activity (Fig. 4a, c). Beside AP-1- or NF-κB- dependent transcriptional activity, the time- and dose-dependent effects of RTX-A on the viability of JB6 Cl41 AP-1 or JB6 Cl41 NF-κB cells (Fig. 4b, d) was also evaluated. As shown, about 100-70% of cells were still alive after 6 h of the treatment with the active concentrations 0.1-1.6 nM of RTX-A. At these conditions, the AP-1- or NF-κB -dependent transcriptional activity was inhibited to the level of 60-10%, when compared with untreated control cells. After 24 h of the incubation, cells treated with the concentrations 0.1-0.8 nM of RTX-A also showed 2 – 8-fold decrease in the AP-1- or NF-κB -dependent transcriptional activity compared to untreated cells (Fig. 4a-d).
The tumor suppressor protein p53 is part of the cell’s emergency response team that functions to negatively regulate cell growth following damage by inducing cell cycle arrest and apoptosis (Levine, 1997; Prives and Hall, 1999; Vousden, 2000). Nevertheless, in our study, RTX-A at concentrations 0.1-1.6 nM in time and dose-dependent manner also suppressed basal p53-dependent transcriptional activity in JB6 Cl41 cells (Fig. 4e, f) indicating that the apoptosis induced by RTX-A in JB6 Cl41 cells might be independent of p53. Results (Fig. 4) are expressed as a percentage of AP-1-, NF-κB-, or p53-dependent transcriptional activity relative to untreated control cells. Some of the known anticarcinogenic compounds, including gingerol, curcumin, 3-demethylubiquinone Q2, and genistein were also reported to decrease p53 expression and/or induce apoptosis in cancer cells through a p53-independent pathway (Park et al., 2006; Tsvetkov et al., 2005; Fedorov et al., 2006, Lian et al., 1999). Taking into account that many neoplasms have a deficiency or mutations of the p53 protein, the observation that apoptosis induced by RTX-A might be p53-independent is important and interesting. For instance, gingerol was reported to decrease p53 expression in two human pancreatic cancer cell lines, HPAC cells that express wild-type p53 and BxPC-3 cells that express a mutant p53 (Park et al., 2006). However the most interesting finding in that study was that gingerol induced p53-independent apoptotic death in the mutant p53-expressing BxPC-3 cancer cells, whereas no signs of apoptosis were detected in wild-type p53-expressing HPAC cells. These results suggest that gingerol can overcome the resistance of mutant p53-expressing cancer cells towards chemotherapy by inducing p53-independent apoptosis (Park et al., 2006). This might explain why the low concentration 1 nM of RTX-A (Table 1) induces cytotoxic effects in THP-1 or HL-60 cells, which are p53-deficient.
On the basis of the results described above (Figs. 1-4 and Table 1), we conclude that the cancer-preventive and anticancer effects of RTX-A, might be explained, at least in part, by the induction of p53-independent apoptosis and inhibition of the activation of the oncogenic AP-1 and NF-κB nuclear transcriptional factors.
Acknowledgements
This work was supported in part by The Hormel Foundation and National Institutes of Health grants CA81064, CA77646 and CA88961; by the Grant 2813.2008.4 for Support of the Leading Russian Science Schools, Program of Presidium of RAS “Molecular and Cell Biology” 09-I-Π22-05 and 09-I-Π22-06, Grant RFBR 08-04-01052-a and FEB RAS Grants 09-III-B-05-155, 09-III-A-05-146, 09-IIIA-05-141. The Korean co-authors are grateful for financial support from the National Research Foundation of Korea (NRF) (No. R13-2002-044-04001-0).
Abbreviations
- INCC50
inhibition of number of colonies formed in soft agar C50
- AP-1
activator protein-1
- PFT
pore-forming toxins
- EGF
epidermal growth factor
- FBS
fetal bovine serum
Footnotes
Conflicts of interest The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Álvarez C, Mancheño JM, Martínez D, Tejuca M, Pazos F, Lanio ME. Sticholysins, two pore-forming toxins produced by the Caribbean Sea anemone Stichodactyla helianthus: their interaction with membranes. Toxicon. 2009 doi: 10.1016/j.toxicon.2009.02.022. doi:10.1016/j.toxicon.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Amit S, Ben-Neriah Y. NF-κB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Seminars in Cancer Bioogyl. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- Anderluh G, Maček P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria) Toxicon. 2002;40:111–124. doi: 10.1016/s0041-0101(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Avila AD, de Acosta C. Mateo, Lage A. A new immunotoxin built by linking a hemolytic toxin to a monoclonal antibody specific for immature T lymphocytes. Int. J. Cancer. 1988;142:568–571. doi: 10.1002/ijc.2910420417. [DOI] [PubMed] [Google Scholar]
- Avila AD, de Acosta MC, Lage A. A carcinoembryonic antigendirected immunotoxin built by linking a monoclonal antibody to a hemolytic toxin. Int. J. Cancer. 1989;43:926–929. doi: 10.1002/ijc.2910430533. [DOI] [PubMed] [Google Scholar]
- Baltrop JA, Owen TC, Cory AH, Cory JG. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl) tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991;1:611–614. [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- Bursch W, Oberhammer F, Schulte-Hermann R. Cell death by apoptosis and its protective role against disease. Trends Pharmacol. Sci. 1992;13:245–251. doi: 10.1016/0165-6147(92)90077-j. [DOI] [PubMed] [Google Scholar]
- Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc. Natl. Acad. Sci. USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Watts SG, Sun Y, Colburn NH. Progressive elevation of AP-1 activity during preneoplastic-to-neoplastic progression as modeled in mouse JB6 cell variants. Int. J. Oncol. 1995;7:359–364. doi: 10.3892/ijo.7.2.359. [DOI] [PubMed] [Google Scholar]
- Dong Z, Cmarik JL. Harvesting cells under anchorage-independent cell transformation conditions for biochemical analyses. Sci. STKE. 2002:PL7. doi: 10.1126/stke.2002.130.pl7. (2002) [DOI] [PubMed] [Google Scholar]
- Fedorov SN, Radchenko OS, Shubina LK, Balaneva NN, Bode AM, Stonik VA, Dong Z. Evaluation of cancer-preventive activity and structure-activity relationships of 3-demethylubiquinone Q2, isolated from the ascidian Aplidium glabrum, and its synthetic analogs. Pharm. Res. 2006;23:70–81. doi: 10.1007/s11095-005-8813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov SN, Shubina LK, Bode AM, Stonik VA, Dong Z. Dactylone inhibits epidermal growth factor-induced transformation and phenotype expression of human cancer cells and induces G1-S arrest and apoptosis. Cancer Res. 2007;67:5914–5920. doi: 10.1158/0008-5472.CAN-06-3723. [DOI] [PubMed] [Google Scholar]
- Fedorov SN, Shubina LK, Kicha AA, Ivanchina NV, Kwak JY, Jin JO, Bode AM, Dong Z, Stonik VA. Proapoptotic and anticarcinogenic activities of leviusculoside G from the starfish Henricia leviuscula and probable mechanism. Nat. Prod. Commun. 2008;3:1575–1580. [Google Scholar]
- Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma W-Y, Dawson MI, Rincon M, Flavell RA, Dong Z. Blocking activator protein – 1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc. Natl. Acad. Sci.USA. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ma W-Y, Young MR, Colburn N, Dong Z. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouseJB6 cells. Proc. Natl. Acad. Sci. USA. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il’ina A, Lipkin A, Barsova E, Issaeva M, Leychenko E, Gusev K, Monastyrnaya M, Lukyanov S, Kozlovskaya E. Amino acid sequence of RTX-A’s isoform actinoporin from the sea anemone Radianthus macrodactylus. Toxicon. 2006;47:517–520. doi: 10.1016/j.toxicon.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Klyshko EV, Issaeva MP, Monastyrnaya MM, Il’ina AP, Guzev KV, Vakorina TI, Dmitrenok PS, Zykova TA, Kozlovskaya EP. Isolation, properties and partial amino acid sequence of a new actinoporin from the sea anemone Radianthus macrodactylus. Toxicon. 2004;44:315–324. doi: 10.1016/j.toxicon.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Lian F, Li Y, Bhuiyan M, Sarkar FH. p53-independent apoptosis induced by genistein in lung cancer cells. Nutrition and Cancer. 1999;33:125–131. doi: 10.1207/S15327914NC330202. [DOI] [PubMed] [Google Scholar]
- Maček P. Polypeptide cytolytic toxins from sea anemones (Actinaria) FEMS Microbiol. Immunol. 1992;105:121–130. doi: 10.1111/j.1574-6968.1992.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Monastyrnaya MM, Zykova TA, Apalikova OW, Shwets TW, Kozlovskaya EP. Biologically active polypeptides from the tropical sea anemone Radianthus macrodactylus. Toxicon. 2002;40:1197–1217. doi: 10.1016/s0041-0101(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Park YJ, Wen J, Bang S, Park SW, Song SY. [6]-Gingerol Induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med. J. 2006;47:688–697. doi: 10.3349/ymj.2006.47.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Prog. Biophys. Mol. Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Prives C, Hall PA. The p53 pathway. J. Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tejuca M, Díaz I, Figueredo R, Roque L, Pazos F, Martínez D, Iznaga-Escobar N, Pérez R, Alvarez C, Lanio ME. Construction of an immunotoxin with the pore forming protein StI and ior C5, a monoclonal antibody against a colon cancer cell line. Int. Immunopharmacol. 2004;6:731–744. doi: 10.1016/j.intimp.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Tsvetkov P, Asher G, Reiss V, Shaul Y, Sachs L, Lotem J. Inhibition of NAD(P)H: quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound cucrcumin. Proc. Natl. Acad. Sci. USA. 2005;102:5535–5540. doi: 10.1073/pnas.0501828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden KH. p53: death star. Cell. 2000;103:691–694. doi: 10.1016/s0092-8674(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Young MR, Li JJ, Rincon M, Flavell RA, Sathyanarayana BK, Hunziker R, Colburn N. Transgenic mice demonstrate AP-1 (activator protein–1) transactivation is required for tumor promotion. Proc. Natl. Acad. Sci. USA. 1999;96:9827–9832. doi: 10.1073/pnas.96.17.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]