Abstract
Brain-derived neurotrophic factor (BDNF), a cognate ligand for the tyrosine kinase receptor B (TrkB) receptor, mediates neuronal survival, differentiation, synaptic plasticity, and neurogenesis. However, BDNF has a poor pharmacokinetic profile that limits its therapeutic potential. Here we report the identification of 7,8-dihydroxyflavone as a bioactive high-affinity TrkB agonist that provokes receptor dimerization and autophosphorylation and activation of downstream signaling. 7,8-Dihydroxyflavone protected wild-type, but not TrkB-deficient, neurons from apoptosis. Administration of 7,8-dihydroxyflavone to mice activated TrkB in the brain, inhibited kainic acid-induced toxicity, decreased infarct volumes in stroke in a TrkB-dependent manner, and was neuroprotective in an animal model of Parkinson disease. Thus, 7,8-dihydroxyflavone imitates BDNF and acts as a robust TrkB agonist, providing a powerful therapeutic tool for the treatment of various neurological diseases.
Keywords: BDNF, neuroprotection, small molecule, binding
BDNF, a member of the neurotrophin family, exerts its biological functions through two transmembrane receptors: the p75 neurotrophin receptor and the tyrosine kinase receptor B (TrkB) (NGF binds to TrkA, BDNF and NT-4/5 bind to TrkB, and NT-3 preferentially binds to TrkC) (1). BDNF binding to TrkB triggers its dimerization and autophosphorylation, resulting in activation of the three major signaling pathways involving MAPK, PI3K, and phospholipase C-γ1. BDNF protects hippocampal neurons from glutamate toxicity (2) and reduces ischemic injury (3). BDNF is of particular therapeutic interest because of its neurotrophic actions on neuronal populations involved in several disorders, including amyotrophic lateral sclerosis (4), Parkinson disease, and Alzheimer’s disease (5). However, clinical trials using recombinant BDNF have been disappointingly negative (6), presumably because of poor delivery, short half-life, and other limitations. Although efforts have been made to circumvent these problems (7, 8), no exogenous agents have been identified that act as potent and selective in vivo agonists of TrkB.
Flavonoids, present in fruits and vegetables, are a diverse class of plant secondary metabolites and exert diverse biological effects, including acting as antioxidants and cancer-preventing agents (9). Flavonoids may improve cognitive performance by protecting vulnerable neurons, enhancing existing neuronal function, and stimulating neuronal regeneration (10). Flavonoids exert effects on long-term potentiation underlying learning and memory, on and consequently memory and cognitive performance, through their interactions with the signaling pathways including PI3K/Akt (11) and MAPK (12).
Results
A Cell-Based Screen for Protecting TrkB-Expressing Cells from Apoptosis.
To identify small molecules that activate TrkB, we prepared stably transfected TrkB murine cell lines T48 and T62, which originally were derived from basal forebrain SN56 cells that expressed negligible TrkB. BDNF provoked strong Trk-Y490 phosphorylation and Akt activation in both T48 and T62 cell lines, whereas only faint Trk activation and no Akt phosphorylation were demonstrated in T17 clones that stably express TrkA. As expected, BDNF substantially decreased apoptosis in T48 cells compared with the parental SN56 cells. Even in the absence of BDNF, T48 cells were slightly resistant to apoptosis, indicating that overexpression of TrkB weakly suppresses caspase activation. BDNF substantially enhanced the antiapoptotic effect (Fig. S1A). To screen a large number of chemicals, we developed a cell-based apoptotic assay using a cell-permeable fluorescent dye MR(DEVD)2, which turns red upon caspase cleavage in apoptotic cells (13). The positive compounds were validated further for TrkB activation in primary hippocampal neurons. The screening strategy scheme is depicted in Fig. S1B (Upper). The apoptotic cells are red, whereas the live cells have no signal. Using the caspase-activated fluorescent dye as a visual assay, we screened 2,000 biologically active compounds from the Spectrum Collection Library. Sixty-six compounds selectively protected T48 but not SN56 cells from staurosporine (STS)-initiated apoptosis, indicating that these compounds might act either directly through the TrkB receptor or through its downstream signaling effectors. The representative results from the screening are shown in Fig. S1B (Lower). The positive compounds were validated by an independent cell-viability assay.
Identification of Flavone Derivatives as Survival Enhancers.
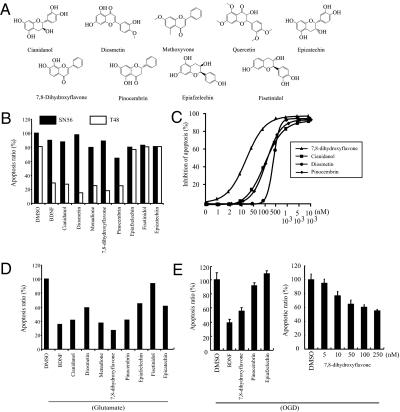
In our initial screening, 5 of 66 positive compounds were derivatives of flavone or its relative compounds. The library also contained numerous flavone derivatives that were inactive. The chemical structures of the nine representative compounds are depicted in Fig. 1A. To compare their activity in inhibiting apoptosis, we preincubated these flavone derivatives (0.5 μM) with T48 and SN56 cells, followed by 0.75 μM STS for 8 h. Quantitative analysis of the apoptosis inhibitory activities revealed that all these compounds barely protected SN56 cells from apoptosis. By contrast, 7,8-dihydroxyflavone, cianidanol, diosmetin, menadione, and pinocembrin strongly suppressed apoptosis in T48 cells. However, epiafzelechin, fisetinidol, and epicatechin failed to suppress apoptosis in T48 cells (Fig. 1B). The EC50 for 7,8-dihydroxyflavone, cianidanol, pinocembrin, and diosmetin was 35, 100, 100, and 500 nM, respectively (Fig. 1C). 7,8-Dihydroxyflavone displayed even stronger protective effect than BDNF, and cianidanol, menadione, and pinocembrin exhibited antiapoptotic activity comparable to that of BDNF (Fig. 1D). To explore whether 7,8-dihydroxyflavone exerts any neuroprotective effect, we pretreated the primary preparations with BDNF or various compounds 30 min before oxygen-glucose deprivation (OGD). 7,8-Dihydroxyflavone exhibited the most potent protective effects among the compounds (Fig. 1E, Left). The titration assay revealed that it protected neurons in a dose-dependent manner (Fig. 1E, Right). Human neurons are much less vulnerable than rodent neurons to apoptosis, and caspase-6 but not -3 is essential for human neuronal apoptosis (14). 7,8-Dihydroxyflavone protected primary human neurons from ES cells from H2O2-induced apoptosis (Fig. S2). Thus, 7,8-dihydroxyflavone selectively protects primary rodent and human neurons from apoptosis.
Fig. 1.
7,8-Dihydroxyflavone protects hippocampal neurons from apoptosis. (A) Chemical structures of flavones. (B) Flavone derivatives prevent apoptosis in T48 but not SN56 cells. (C) Titration of the EC50 for suppressing apoptosis in T48 cells. (D) 7,8-Dihydroxyflavone protects hippocampal neurons from glutamate-triggered apoptosis. Hippocampal neurons were pretreated with 500 nM flavone derivatives for 30 min, followed by 50 μM glutamate for 16 h. The cell lysates were analyzed by immunoblotting. (E) 7,8-Dihydroxyflavone protects rodent neurons from apoptosis triggered by OGD. Hippocampal neurons were seeded on coverslips in a 24-well plate. The neurons were pretreated with various flavones for 30 min followed by OGD at 37 °C for 3 h. Neuronal apoptosis was quantitatively analyzed (Left). 7,8-Dihydroxyflavone displayed a dose-dependent protection on neurons (Right). Data are expressed as mean ± SEM.
7,8-Dihydroxyflavone Provokes TrkB Activation.
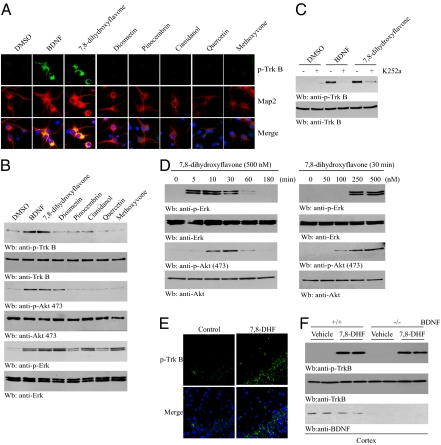
Immunofluorescent staining in hippocampal neurons revealed that 7,8-dihydroxyflavone, but not other flavone derivatives, specifically provoked TrkB tyrosine phosphorylation as well as BDNF (Fig. 2A). Immunoblotting validated this finding. Both Akt and Erk1/2 were robustly activated by 7,8-dihydroxyflavone. Although diosmetin barely activated TrkB, it triggered demonstrable Akt and Erk phosphorylation (Fig. 2B), perhaps explaining why it partially protected T48 and primary hippocampal neurons from apoptosis. K252a, a Trk receptor inhibitor, potently blocked TrkB phosphorylation by 7,8-dihydroxyflavone, indicating that the stimulatory effect is mediated mainly through TrkB autophosphorylation (Fig. 2C). 7,8-Dihydroxyflavone provoked Erk1/2 and Akt activation with a time course comparable to that of BDNF (Fig. 2D) and stimulated both Erk1/2 and Akt to activation in a dose-dependent manner. Pretreatment of primary cultures with either MEK1 inhibitor or PI3K inhibitors substantially blocked 7,8-dihydroxyflavone’s prosurvival activity (Fig. S3A). Moreover, infection of neurons with adenovirus expressing dominant-negative Akt (T308AK179AS473A) significantly impaired 7,8-dihydroxyflavone’s neuroprotective effect (Fig. S3B). Thus, 7,8-dihydroxyflavone–provoked PI3K/Akt and MAPK activation is necessary for its neuronal prosurvival actions. I.p. administration of 7,8-dihydroxyflavone provoked TrkB but not TrkA activation in mouse brain (Fig. S4), indicating that 7,8-dihydroxyflavone can penetrate the brain–blood barrier and provoke TrkB activation. Administration of 7,8-dihydroxyflavone into BDNF conditional knockout mice also triggered TrkB activation in the BDNF-depleted cortex (Fig. 2E). Hence, 7,8-dihydroxyflavone induces TrkB activation in mouse brain in a BDNF-independent manner.
Fig. 2.
7,8-Dihydroxyflavone elicits TrkB activation in hippocampal neurons. (A) 7,8-Dihydroxyflavone induces TrkB tyrosine phosphorylation in hippocampal neurons. Immunofluorescent staining was conducted with anti-phospho-TrkB Y816. (B) Immunoblotting shows 7,8-dihydroxyflavone triggers TrkB phosphorylation in hippocampal neurons. (C) K252a blocks 7,8-dihydroxyflavone’s agonistic effect on TrkB. Hippocampal neurons were pretreated with K252a (30 nM) for 30 min, followed by BDNF (100 ng/mL) or 7,8-dihydroxyflavone (500 nM) for 30 min. Cell lysates were analyzed by immunoblotting. (D) 7,8-Dihydroxyflavone stimulates Akt and ERK phosphorylation. (E) 7,8-Dihydroxyflavone induces TrkB phosphorylation in cortex of BDNF conditional knockout mice. Mice were injected i.p. with 5 mg/kg 7,8-dihydroxyflavone or vehicle. After 2 h, the mice were killed, and cortex lysates were analyzed by immunoblotting. (F) 7,8-dihydroxyflavone induces TrkB phosphorylation in cortex of BDNF conditional knockout mice. Mice were intraperitoneally injected with 5 mg/kg 7,8-dihydroxyflavone or vehicle. After 2 h, mouse cortex lysates were analyzed by immunoblotting.
7,8-Dihydroxyflavone Is a TrkB Receptor Agonist.
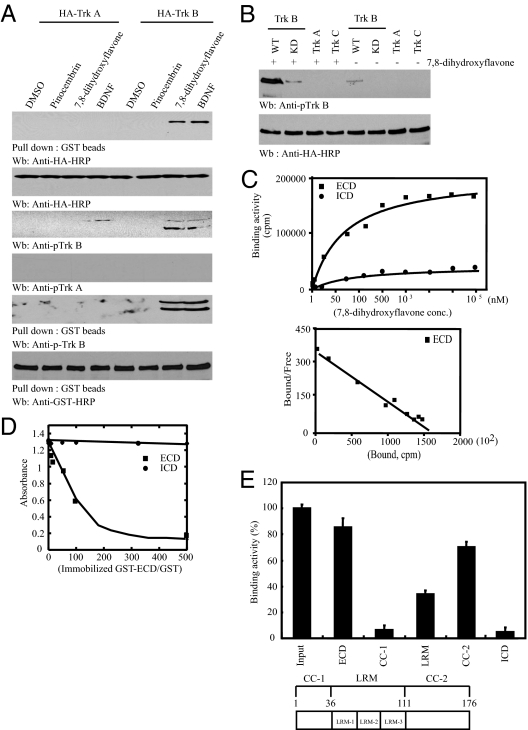
Receptor dimerization assay demonstrated that 7,8-dihydroxyflavone provoked TrkB dimerization as potently as BDNF, whereas the inactive flavone pinocembrin and DMSO did not. By contrast, the cotransfected TrkB receptor failed to dimerize with TrkA (Fig. 3A), supporting the notion that 7,8-dihydroxyflavone, like BDNF, selectively induces TrkB homodimerization. The compound also elicited phosphorylation in TrkB but not in TrkA or TrkC. Kinase-dead TrkB-KD displayed negligible phosphorylation compared with wild-type TrkB (Fig. 3B), indicating that TrkB phosphorylation by 7,8-dihydroxyflavone is through the receptor autophosphorylation but not by any other tyrosine kinases. A binding assay demonstrated that increasing concentrations of [3H]7,8-dihydroxyflavone progressively bound TrkB extracellular domain (ECD) but not intracellular domain (ICD). Scatchard analysis revealed that the ratio of ligand to the receptor is 1:1 with binding constant K d = 320 nM (Fig. 3C). By contrast, neither the ECD nor the ICD from TrkA receptor bound to 7,8-dihydroxyflavone or to p75NTR, supporting 7,8-dihydroxyflavone binding specificity. Glutathione bead-based column chromatography revealed that increasing concentration of GST-ECD, but not GST-ICD, retarded the elution of 7.8-dihydroxyflavone (Fig. 3D). A truncation assay showed that 7,8-dihydroxyflavone strongly associated with cysteine cluster (CC)-2 domain and partially interacted with leucine-rich motif domain but did not bind to the CC-1 or ICD domain (Fig. 3E). Hence, 7,8-dihydroxyflavone mimics BDNF in binding the ECD of TrkB and provoking its dimerization and autophosphorylation.
Fig. 3.
7,8-Dihydroxyflavone binds ECD of TrkB and provokes its dimerization and phosphorylation. (A) 7,8-Dihydroxyflavone provokes TrkB dimerization. mGST-TrkB and HA-TrkA or HA-TrkB were cotransfected into HEK293 cells and treated with 0.5 μM pinocembrin or 7,8-dihydroxyflavone for 30 min. GST-TrkB was pulled down and analyzed with anti-HA-HRP. (B) 7,8-Dihydroxyflavone induces TrkB autophosphorylation. (C) [3H]7,8-dihydroxyflavone binds the ECD but not ICD of TrkB. In vitro binding assay with purified TrkB ECD or ICD and [3H]7,8-dihydroxyflavone (Upper). Scatchard plot analysis of 7,8-dihydroxyflavone binding to TrkB (Lower). (D) In vitro binding assay with immobilized GST-TrkB ECD or ICD and 7,8-dihydroxyflavone. Gradual increments of GST-TrkB ECD but not ICD decreased 7,8-dihydroxyflavone in the eluted fractions. (E) Mapping assay with various ECD truncates and [3H]7,8-dihydroxyflavone. Data are expressed as mean ± SEM.
7,8-Dihydroxyflavone Protects Neurons from Apoptosis in a TrkB-Dependent Manner.
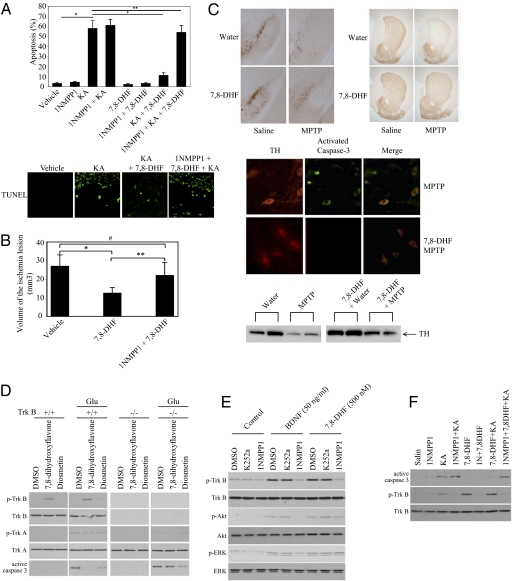
TrkB F616A knockin mice, when treated with 1NMPP1, exhibit an effective TrkB-null phenotype (15). We injected 5 mg/kg 7,8-dihydroxyflavone into TrkB F616A mice that were pretreated with saline or 1NMPP1, followed by kainic acid (KA). KA induced widespread apoptosis in the hippocampus; this apoptosis was substantially diminished by 7,8-dihydroxyflavone. Blocking TrkB F616A by 1NMPP1 significantly abolished the protective effect of 7,8-dihydroxyflavone (Fig. 4A), suggesting that TrkB activation by 7,8-dihydroxyflavone is essential for its neuroprotective action. The transient middle cerebral artery occlusion (MCAO) model of stroke showed that 7,8-dihydroxyflavone largely reduced infarct volumes, whereas inhibition of TrkB by 1NMPP1 abrogated the neuroprotective actions (Fig. 4B). Because BDNF has been shown to be protective in animal models of Parkinson disease, we administered the dopaminergic toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice that had been treated with 7,8-dihydroxyflavone. 7,8-Dihydroxyflavone attenuated the neurotoxic effects of MPTP as measured by preservation of tyrosine hydroxylase expression and reduction of activated caspase-3 (Fig. 4C). 7,8-Dihydroxyflavone specifically activated TrkB but not TrkA in wild-type but not in TrkB−/− cortical neurons. Glutamate-provoked caspase-3 activation was blocked substantially by 7,8-dihydroxyflavone in wild-type but not in TrkB−/− neurons. However, the control diosmetin weakly and nonselectively suppressed caspase-3 activation in both neurons (Fig. 4D). Moreover, 7,8-dihydroxyflavone provoked TrkB but not TrkA activation in both wild-type and TrkC-knockout neurons. Glutamate-triggered caspase-3 activation was significantly diminished by 7,8-dihydroxyflavone (Fig. S5A), demonstrating that 7,8-dihydroxyflavone represses neuronal apoptosis in a TrkB-specific manner. TrkC was robustly tyrosine phosphorylated by NT-3 but not by 7,8-dihydroxyflavone (Fig. S5B), supporting the specificity of 7,8-dihydroxyflavone for TrkB.
Fig. 4.
7,8-Dihydroxyflavone is neuroprotective in models of neuronal injury. (A ) 7,8-Dihydroxyflavone decreases KA-induced apoptosis in mouse brain in a TrkB-dependent manner. TrkB F616A mice were injected with various indicated solutions, and the brain slides were analyzed with TUNEL assay. Results are expressed as mean ± SEM (ANOVA; n = four to five mice/group; #, P < 0.005; *, P < 0.01; **, P < 0.1;). (B) 7,8-Dihydroxyflavone diminishes stroke damage in a TrkB-dependent manner. Infarct volumes after 24 h MCAO were substantially decreased by 7,8-dihydroxyflavone. Pretreatment with 1NMPP1 impaired the protective effect of 7,8-dihydroxyflavone. Results are expressed as mean ± SEM (One way ANOVA, n = 8–12 mice/group; #, not significant; *, P < 0.01; **, P < 0.02). (C) 7,8-Dihydroflavone is neuroprotective in a model of Parkinson disease. Mice were administered 7,8-dihydroxyflavone in drinking water for 14 days. On day 7, the mice were given two doses of MPTP (20 mg/kg, i.p.) 2 h apart. On day 14, mice were killed. Immunostaining (substantia nigra, striatum) (Upper panels) and immunoblotting (striatal homogenates, lanes from the same gel at the same exposure) of tyrosine hydroxylase (TH) (Lower panels), and fluorescence microscopy of activated caspase-3 in TH+ nigral neurons revealed reduced toxicity in 7,8-dihydroxyflavone-treated mice (Lower panels). (D) 7,8-Dihydroxyflavone prevents glutamate-triggered neuronal apoptosis in wild-type but not TrkB-null neurons. Cortical neurons were prepared from the P0 pups. The neurons were pretreated with indicated compounds, followed by glutamate (50 μM). The cell lysates were analyzed by immunoblotting. (E) 7,8-Dihydroxyflavone selectively activates TrkB F616A, which can be blocked by 1NMPP1. The primary cortical cultures were pretreated for 30 min with either K252a (100 nM) or 1NMPP1 inhibitor (100 nM), followed by 7,8-dihydroxyflavone. Immunoblotting with cell lysates was performed. (F) 7,8-Dihydroxyflavone suppresses KA-induced neuronal cell death in TrkB F616A mutant mice, which can be blocked by 1NMPP1.
To determine if 7,8-dihydroxyflavone can mimic BDNF in vivo, we prepared cortical neurons from TrkB F616A knockin mice. 7,8-Dihydroxyflavone- and BDNF-provoked TrkB phosphorylation was selectively blocked by 1NMPP1 but not by K252a (Fig. 4E). Because 1NMPP1 selectively inhibits TrkB F616A activation by 7,8-dihydroxyflavone, we reasoned that blockade of TrkB F616A signaling by 1NMPP1 in mice would make the neurons vulnerable to KA-provoked neuronal cell death. As expected, treatment with 1NMPP1 alone, with 7,8-dihydroxyflavone alone, or with 1NMPP1 plus 7,8-dihydroxyflavone had no effect on apoptosis in TrkB F616A mice. KA caused significant caspase-3 activation, and pretreatment with 1NMPP1 elevated KA-provoked apoptosis in TrkB F616A, underscoring that TrkB signaling is essential for neuronal survival. 7,8-Dihydroxyflavone markedly suppressed KA-provoked apoptosis, whereas 1NMPP1 pretreatment abolished 7,8-dihydroxyflavone’s protective effect in F616A mice (Fig. 4F). Hence, 7,8-dihydroxyflavone selectively activates TrkB and enhances neuronal survival in mice.
Discussion
BDNF is a homodimer and binds amino acid residues 103–181 that contain the third leucine-rich motif and the CC-2 domain and the Ig2 domain in TrkB receptor and stimulates the receptor dimerization (16). Here, we show that 7,8-dihydroxyflavone strongly associates with the same region in the TrkB ECD and induces TrkB dimerization. It remains unknown exactly how 7,8-dihydroxyflavone provokes TrkB dimerization and autophosphorylation. Presumably, it provokes TrkB conformational change and reduces the autoinhibitory effect by the Ig2 domain, which can block TrkB dimerization in the absence of BDNF (17). It is noteworthy that 7,8-dihydroxyflavone can provoke only wild-type TrkB but not TrkB-KD tyrosine phosphorylation, suggesting that TrkB tyrosine phosphorylation is exerted by the receptor itself and not by any other tyrosine kinases.
Because of their neurotrophic activities in promoting neuronal survival and preventing neurodegeneration, neurotrophins and their receptors are targets for therapeutic intervention in neurodegeneration (18, 19). Nevertheless, neurotrophin clinical trials have been disappointing. Through screening chemical library and follow-up validation, we obtained a few potent and selective TrkB agonists that virtually mimic BDNF’s biochemical and physiological actions, and 7,8-dihydroxyflavone is one of them. The hits obtained from screening the 2,000 drugs and the natural product library are the most noteworthy, because such a pilot screen can be expanded to the larger and more complex chemical libraries. Furthermore, the relatively low cellular EC50 for this drug in neuronal survival and its robust neuroprotective effect with a 5-mg/kg dosage make it an ideal candidate to follow up in animal or clinical studies. The structure–activity relationship study reveals that chrysin (5,7-dihydroxyflavone) fails to activate TrkB receptor, indicating that the 8-position hydroxy group is essential for flavone derivatives to bind TrkB receptor (Fig. S6). Chrysin and 7,8-dihydroxyflavone have been proposed as aromatase inhibitors (20). However, in vivo studies do not show proof of aromatase inhibitor activity by chrysin in rats or humans (21, 22). Presumably, 7,8-dihydroxyflavone might not inhibit aromatase, either. In addition to the activity described above, 7,8-dihydroxyflavone also has been shown to inhibit aldehyde dehydrogenase and estrogen sulfotransferase in vitro with K i values of 35 μM and 1–3 μM, respectively (23, 24). However, these actions have not been confirmed in animals.
Selectivity, rather than binding affinity, is the more important feature that affects drug efficacy. Despite its high TrkB binding affinity (K d = 9.9 × 10−10 M) (25), BDNF has a poor therapeutic effect partly because of poor pharmacokinetic properties. By contrast, the dissociation constant for 7,8-dihydroxyflavone to TrkB is ≈320 nM, and 7,8-dihydroxyflavone has better pharmacokinetic properties. i.p. injected 7,8-dihydroxyflavone can strongly activate TrkB in hippocampus, indicating that it can penetrate the brain–blood barrier. 7,8-Dihydroxyflavone showed great therapeutic potential in animal models of excitotoxicity, stroke, and Parkinson disease. Because BDNF possesses a broad spectrum of physiological activities, and its dysregulation is involved in numerous neurological disorders, flavonoid-based TrkB agonists have the potential to be developed into a powerful class of therapeutic drugs.
Materials and Methods
Cells, Reagents, and Mice.
Mouse septal neuron × neuroblastoma hybrid SN56 cells were created by fusing N18TG2 neuroblastoma cells with murine (strain C57BL/6) neurons from postnatal day 21- septa. SN56 cells were maintained at 37 °C with 5% CO2 atmosphere in DMEM medium containing 1 mM pyruvate and 10% FBS. T48 and T62 cells stably transfected with rat TrkB were cultured in the same medium containing 300 μg/mL G418. NGF and BDNF were from Roche. Phospho-Akt-473 or 308, Akt, and lamin A/C antibodies were from Cell Signaling. Anti-phospho-Erk1/2, anti-phospho-TrkA Y490, and anti-phospho-Akt 473 antibodies were from Upstate Biotechnology, Inc. Anti-TrkA antibody was from Santa Cruz. Anti-TrkB antibody was from Biovision. The chemical library containing 2,000 biologically active compounds was from the Spectrum Collection (MicroSource Discovery System). TrkBF616A mice and TrkB+/−, TrkA+/−, and BDNF+/− C57BL/6 mice were bred in a pathogen-free environment in accordance with Emory Medical School guidelines. All other chemicals were purchased from Sigma.
Cell-Based Screen.
T48 cells were seeded in a 96-well plate at 10,000 cells/well in 100 μL complete medium. Cells were incubated overnight, followed by 30 min pretreatment with 10 μM compounds in DMSO (10 mM stock concentration from the Spectrum Collection library). The cells then were treated with 0.75 μM STS for 9 h. One h before the termination of the experiment, 10 μM MR(DEVD)2, a cell-permeable caspase-3–activated fluorescent dye, was introduced. Cells were fixed with 4% paraformaldehyde for 15 min. Cells were washed with PBS and incubated with 1 μg/mL of Hoechst 33342 for 10 min. Cover slides were washed with PBS, mounted, and examined using a fluorescence microscope.
Binding Constant Determination.
Purified TrkB ECD or ICD proteins (10 μg/each) were incubated with different concentrations of [3H]7,8-dihydroxyflavone in 1 mL binding buffer [0.05 M Na/K phosphate buffer (pH 7.1), 200 mM NaCl] (1 nM [3H]7,8-dihydroxyflavone ∼61948 counts per minute [cpm]) at 4 °C for 10 min. After the incubation, the reaction mixture was loaded on filter paper. The mixture was washed with 3 × 5 mL Tris buffer (100 mM Tris, pH 7.1). The dried filter paper was put into a small vial and subjected to liquid scintillation counter analysis. The value of the dissociate constant and the number of sites were obtained from Scatchard plots by using the equation r/[L]free = n/K d − r/K d, where r is the ratio of the concentration of bound ligand to the total protein concentration and n is the number of binding sites.
7,8-Dihydroxyflavone Suppresses KA-Induced Neuronal Death in TrkB F616A Mice.
TrkB F616A knockin mice (2–3 months old) were fed with 1NMPP1 (25 μM) in drinking water 1 day before pharmacological reagent treatment. On the next day, the mice were injected i.p. with KA (20 mg/kg) or with 7,8-dihydroxyflavone (5 mg/kg) 4 h before KA treatment. The control mice without 1NMPP1 were injected with either KA or 7,8-dihydroxyflavone alone or were given 7,8-dihydroxyflavone 4 h before KA administration. After 4 days, the mice were killed, and the brains were extracted, homogenized, and ultracentrafuged. The supernatant was used for SDS/PAGE and immunoblotting analysis.
Stroke Experiment: The MCAO Model.
Eight TrkB F616A knockin male mice in each group were treated with saline or 5 mg/kg 7,8-dihydroxyflavone 2 h before the experiment. Twelve mice were treated with 1NMPP1 (20 μM) in drinking water 1 day before the experiment. The mice were injected with 5 mg/kg 7,8-dihydroxyflavone 2 h before the experiment. The mice were anesthetized with 4% chloral hydrate, and rectal and masseter muscle temperatures were controlled at 37 °C with a homeothermic blanket. Cerebral perfusion in the distribution of the middle cerebral artery was monitored throughout the surgical procedure with a laser Doppler (Perimed Inc.), and only animals with a >80% decrease in cerebral perfusion were included in this study. After 48 h, MCAO mice were killed, and brains were cut onto 5-μm sections and stained with TUNEL assay.
MPTP Administration.
7,8-Dihydroxyflavone or water was administered to 8-week-old C57BL/6 mice for 14 days. On day 7, mice (n = 5 per group) were treated with either saline or MPTP-HCl (2 × 20 mg/kg, 2 h apart; Sigma). On day 14, the mice were killed, and brains were prepared for immunochemical analysis. Briefly, one hemisphere was taken for immunoblotting of tyrosine hydroxylase on striatal homogenates, and one hemisphere was drop-fixed and sectioned (40 μm) for tyrosine hydroxylase and activated caspase-3 immunohistochemistry.
Supplementary Material
Acknowledgments
The authors thank Dr. D. Ginty, Johns Hopkins University, for providing TrkA F592A and TrkB F616A knockin mice. This work was supported by National Institute of Health Grants RO1 NS045627 to K.Y. and P01 ES016731 to G.W.M.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online atwww.pnas.org/cgi/content/full/0913572107/DCSupplemental.
References
- 1.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 2.Lindholm D, Dechant G, Heisenberg CP, Thoenen H. Brain-derived neurotrophic factor is a survival factor for cultured rat cerebellar granule neurons and protects them against glutamate-induced neurotoxicity. Eur J Neurosci. 1993;5:1455–1464. doi: 10.1111/j.1460-9568.1993.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 3.Schäbitz WR, et al. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 2000;31:2212–2217. doi: 10.1161/01.str.31.9.2212. [DOI] [PubMed] [Google Scholar]
- 4.Askanas V. Neurotrophic factors and amyotrophic lateral sclerosis. Adv Neurol. 1995;68:241–244. [PubMed] [Google Scholar]
- 5.Siegel GJ, Chauhan NB. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev. 2000;33:199–227. doi: 10.1016/s0165-0173(00)00030-8. [DOI] [PubMed] [Google Scholar]
- 6.Ochs G, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary PD, Hughes RA. Design of potent peptide mimetics of brain-derived neurotrophic factor. J Biol Chem. 2003;278:25738–25744. doi: 10.1074/jbc.M303209200. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JM, Hughes RA. Novel monocyclic and bicyclic loop mimetics of brain-derived neurotrophic factor. J Pept Sci. 2006;12:515–524. doi: 10.1002/psc.760. [DOI] [PubMed] [Google Scholar]
- 9.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 10.Spencer JP. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc Nutr Soc. 2008;67:238–252. doi: 10.1017/S0029665108007088. [DOI] [PubMed] [Google Scholar]
- 11.Vauzour D, Vafeiadou K, Rice-Evans C, Williams RJ, Spencer JP. Activation of pro-survival Akt and ERK1/2 signalling pathways underlie the anti-apoptotic effects of flavanones in cortical neurons. J Neurochem. 2007;103:1355–1367. doi: 10.1111/j.1471-4159.2007.04841.x. [DOI] [PubMed] [Google Scholar]
- 12.Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SW, et al. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc Natl Acad Sci USA. 2007;104:16329–16334. doi: 10.1073/pnas.0706662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo H, et al. Caspase-1 activation of caspase-6 in human apoptotic neurons. Cell Death Differ. 2006;13:285–292. doi: 10.1038/sj.cdd.4401753. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, et al. A chemical-genetic approach to studying neurotrophin signaling. Neuron. 2005;46:13–21. doi: 10.1016/j.neuron.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Haniu M, et al. Interactions between brain-derived neurotrophic factor and the TRKB receptor. Identification of two ligand binding domains in soluble TRKB by affinity separation and chemical cross-linking. J Biol Chem. 1997;272:25296–25303. doi: 10.1074/jbc.272.40.25296. [DOI] [PubMed] [Google Scholar]
- 17.Arevalo JC, et al. TrkA immunoglobulin-like ligand binding domains inhibit spontaneous activation of the receptor. Mol Cell Biol. 2000;20:5908–5916. doi: 10.1128/mcb.20.16.5908-5916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hefti F. Neurotrophic factor therapy for nervous system degenerative diseases. J Neurobiol. 1994;25:1418–1435. doi: 10.1002/neu.480251109. [DOI] [PubMed] [Google Scholar]
- 19.Thoenen H, Sendtner M. Neurotrophins: From enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci. 2002;5(Suppl):1046–1050. doi: 10.1038/nn938. [DOI] [PubMed] [Google Scholar]
- 20.Kellis JT, Jr, Vickery LE. Inhibition of human estrogen synthetase (aromatase) by flavones. Science. 1984;225:1032–1034. doi: 10.1126/science.6474163. [DOI] [PubMed] [Google Scholar]
- 21.King DS, et al. Effect of oral androstenedione on serum testosterone and adaptations to resistance training in young men: A randomized controlled trial. JAMA. 1999;281:2020–2028. doi: 10.1001/jama.281.21.2020. [DOI] [PubMed] [Google Scholar]
- 22.Saarinen N, et al. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J Steroid Biochem Mol Biol. 2001;78:231–239. doi: 10.1016/s0960-0760(01)00098-x. [DOI] [PubMed] [Google Scholar]
- 23.Keung WM, Klyosov AA, Vallee BL. Daidzin inhibits mitochondrial aldehyde dehydrogenase and suppresses ethanol intake of Syrian golden hamsters. Proc Natl Acad Sci USA. 1997;94:1675–1679. doi: 10.1073/pnas.94.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris RM, et al. Phytoestrogens are potent inhibitors of estrogen sulfation: Implications for breast cancer risk and treatment. J Clin Endocrinol Metab. 2004;89:1779–1787. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- 25.Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the Trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.