Abstract
A number of extensive reviews are available discussing the roles of MMPs in various aspects of cancer progression from benign tumor formation to overt cancer present with deadly metastases. This review will focus specifically on the evidence functionally linking the MMPs and tumor-induced angiogenesis in various in vivo models. Emphasis has been placed on the cellular origin of the MMPs in tumor tissue, the requirement of proMMP activation and the resulting proteolytic activity for the induction and progression of tumor angiogenesis, and the pleiotropic roles for some of the MMPs. The functional mechanisms of the angiogenic MMPs are discussed as well as their catalytic detection in complex biological systems. In addition, the contribution of active MMPs to metastatic spread and establishment of secondary metastasis will be discussed in view of the findings indicating that MMPs are involved in the preparation of pre-metastatic niches. Finally, the most recent evidence, indicating the pro-metastatic consequences of anti-angiogenic therapies employing MMP inhibitors will be presented as examples highlighting possible outcomes of interfering with the pleiotropic nature of the MMP functionality.
Keywords: Cancer, Metastasis, Matrix metalloproteinases, Tumor angiogenesis, Angiogenic switch, VEGF, FGF-2, Neutrophil, MMP-9
Introduction
The growth and metastasis of solid tumors have been long associated with the formation of new vasculature, i.e. tumor neovascularization [1]. Neovascularization in malignant tissue occurs mostly through the process of angiogenesis, i.e. formation of new blood vessels from the pre-existing vascular network. Recently, evidence has emerged that tumor neovascularization can also involve vasculogenesis, a process of de novo formation of blood vessels from endothelial cell progenitors. Both vasculogenesis and angiogenesis are fundamental processes initiated at earliest stages of vertebrate animal development [2], however angiogenesis is considered as the prevalent process of new blood vessel formation in adult tissues [3].
Physiologic angiogenesis occurs during wound healing, during estrogen cycles in female reproductive tract or in placenta during pregnancy. Under these conditions, angiogenesis is strictly controlled by complex regulators defining the initiation and development of new blood vessels and their subsequent involution. Pathological angiogenesis is associated with numerous disease states, including inflammation, vascular retinopathy, rheumatoid arthritis and cancer [1,4–7]. The angiogenesis triggered in the benign solid tumor is a critical process in the initiation of malignant stage of cancer and its progression towards metastatic disease [1,8]. The establishment of new tumor blood vessels through vasculogenic process is associated with the development of endothelial cell networks from the recruited circulating endothelial cell progenitors [9,10]. In addition, vasculogenic mimicry is suggested to explain the mechanisms by which tumor cells initiate gene expression and phenotypic characteristics of endothelial cells [3,11,12].
Although pathological vascularization is frequently considered as a dysregulated process of new blood vessel formation, it is tightly regulated by a combination of interrelating factors similar to those governing physiologic angiogenesis. In this regard, matrix metalloproteinases (MMPs) are among the major players implicated in both malignant angiogenesis and vasculogenesis. From an initial recognition of a pro-angiogenic role for MMPs in angiogenesis, a full appreciation of a deep complexity of MMP roles in new vessel formation has accumulated in recent years. This complexity is underlined by a discovery of several MMPs with clearly protective, anti-malignant properties as well as a demonstration that individual MMPs can exhibit pro- or anti-angiogenic properties depending on the type of cancer or the stage of particular cancer development [13].
The family of mammalian MMPs comprises 24 endopeptidases characterized by a multi-domain structure, a Zn-containing active site and an overall requirement for activation of their zymogen forms prior to exerting their enzymatic functions [14–16]. The MMPs are represented by both secreted and membrane-tethered molecules, enzymatic activity of which is finely balanced by the complex interplay with their natural inhibitors, i.e. TIMPs (tissue inhibitors of metalloproteinases) and availability of specific substrates. During both embryonal and postnatal development, MMPs have been implicated in most physiological processes requiring tissue remodeling, including formation of new blood vessels and reorganization of the pre-existing vasculature.
1. The angiogenic switch: Induction of MMPs in the premalignant tumor
Vascular networks in most healthy tissues are relatively stable because of the quiescent state of adult endothelium. During tumor growth, however, endothelial cells are induced to proliferate and migrate and eventually, assemble in lumina-containing capillaries, i.e. form new angiogenic blood vessels. Tumor angiogenesis invariably results in the generation of distorted, irregular networks of tortuous, dilated blood vessels [3,17]. Physiologically, tumor angiogenesis appears to erupt at certain stage of tumor progression and therefore initiation of tumor angiogenesis has been described as an angiogenic switch [1]. The main mechanism underlying the angiogenic switch includes changing the balance between the levels of activators and inhibitors of angiogenesis in favor of activators [17]. Induction of tumor angiogenesis is followed by stabilization of newly-formed blood vessels and their maturation, resulting ultimately with switching-off the process of neovascularization. Among numerous strong regulators of tumor angiogenesis, MMPs have a special role as these proteolytic enzymes have been ascribed with both pro-angiogenic and anti-angiogenic functions.
Under steady state physiologic conditions, the expression of MMPs in most tissues is relatively low, with the possible exception of soluble MMP-2, which appears to be expressed constitutively and present in plasma at relatively high levels [18]. However, upon induction caused by such conditions as hypoxia and inflammation, the levels of certain MMPs are increased dramatically. Since the above-mentioned conditions are associated with physiologic angiogenesis, positive correlations have been suggested between MMPs and angiogenic switching in cancer. The experimental proof that MMPs are required and necessary for angiogenic induction came from transgenic mouse models and rescue experiments in MMP-deficient mice that directly linked MMP-9 and the appearance of new blood vessels in the developing tumor [17].
One of the first experimental reports describing the angiogenic switch was a study employing a multistage squamous epithelial carcinogenesis model [19]. In this model, the initiation of angiogenesis in the premalignant lesions was associated with the activation of MMP-9 delivered by inflammatory mast cells. Although initiation of angiogenesis was ablated in mast cell-deficient mice, it did not directly implicate MMP-9 in the angiogenic switch since in addition to proMMP-9 mast cells release a variety of other potent proteases. Such demonstration would require a specific ablation of MMP-9 in the leukocytes infiltrating tumors at the avascular stage.
The confirmation that MMP-9 triggers the angiogenic switch came from a model of multistage pancreatic carcinogenesis in transgenic RIP1-Tag2 mice [20]. In RIP1-Tag2 mice, MMP-2 and MMP-9 were found upregulated during angiogenic switching occurring in the hyperplastic islets. However, the genetic ablation of MMP-9, and not MMP-2 deficiency, achieved in corresponding knockout mice was associated with a coordinated lack of angiogenic induction. Of critical importance was the demonstration that the transplantation of wild type bone marrow rescued the induction of angiogenesis in pancreatic islets of MMP-9 null hosts, not only confirming that MMP-9 triggers the angiogenic switch, but that MMP-9 is delivered by bone marrow-derived hematopoietic cells [20]. Since its first introduction, the MMP-9-mediated angiogenic switching mechanism during tumor progression has been demonstrated in a number of mouse models systems, including models of ovarian cancer [21], cervical cancer [22,23], colorectal cancer [24], neuroblastoma progression [25], pancreatic cancer [26], glioblastoma development [27], mammary carcinoma vascularization [28] and breast carcinoma metastasis [29]. Interestingly, the effects of MMP-9 ablation on tumor growth and angiogenesis could depend on the genetic background of mice [29] or be not manifested at all and require genetic ablation of an additional MMP, e.g. MMP-2 [30].
The findings from the two above-mentioned independent models employing single ablation of MMP-2, i.e. the pancreatic islet carcinogenesis model [20] and malignant keratinocyte xenotransplantation [30], have indicated that MMP-2 apparently does not significantly contribute to the angiogenic switch. The functional role of MMP-2 and its proteolytic activity in angiogenic switching, however, has been proposed in the in vivo model of chondrosarcoma development, where suppression of tumor MMP-2 by specific antisense oligonucleotides led to a substantial inhibition of chondrosarcoma nodule neovascularization [31]. Although illustrating that MMP-2 could have contributed to tumor angiogenesis, this model did not provide experimental confirmation that MMP-2 was critically required to initiate the switch from the avascular stage to angiogenic phase. It is possible that the apparent discrepancy regarding the putative role of MMP-2 in a switching to an angiogenic phenotype could rely on the nature of the cancer model or the mode of MMP delivery to the site of tumor angiogenesis, i.e. either by the host, inflammatory leukocytes or tumor cells.
2. Cellular origin of MMPs in the angiogenic tumor
Tumor cells represent a powerful source of MMPs in the developing tumor. Although initially tumor-derived MMPs were regarded as those governing tumor metastasis and angiogenesis, accumulated evidence has indicated that the roles of tumor-derived MMPs is much more limited than first thought and appear to be restricted mainly to tumor cell migration and invasion [32,33]. Immunohistochemical and in situ hybridization analyses in breast carcinoma patients did not indicate MMP-9 expression in tumor cells, but conclusively associated MMP-9 with three different stromal cell types, i.e. neutrophils, macrophages and vascular pericytes [34]. In the pancreatic carcinogenesis model, immunohistochemistry revealed that MMP-9 was not expressed in normal non-transgenic islets, nor in the carcinoma epithelial cells, but in a small number of cells in close opposition to the developing vasculature [20]. Importantly, MMP-9 was also identified in the basement membrane and ECM, consistent with the localization of matrix-bound VEGF released by this proteinase [20].
Increase in local concentration of MMPs in tumors undergoing an angiogenic switch is closely associated with the inflammation driven by pro-inflammatory cytokines and factors produced by tumor and/or stromal cells. Among tumor-infiltrating leukocytes, the mononuclear cells (monocytes and macrophages) and granulocytes (neutrophils, eosinophils and basophils/mast cells) are considered to be the major sources of MMPs [4,35] (Table 1). Reconstitution experiments with MMP-9 null mice have demonstrated that MMP-9 produced by inflammatory leukocytes derived from the donor bone marrow significantly contributes to tumor development and angiogenesis [20–22,24,25].
Table 1.
Cellular origin of stromal MMPs implicated in tumor angiogenesis
| Cell Type | Functional expression of angiogenic MMP |
References |
|---|---|---|
| Monocyte/ macrophages |
MMP-9 | [21,22,38,43,228] |
| MMP-13 | [188] | |
| Neutrophils | MMP-9 | [23,26,29,30,48,50,51,143,189] |
| Mast cells | MMP-2 | [229] |
| MMP-9 | [19,52,230] | |
| B lymphocytes | MMP-9 | [60] |
| T lymphocytes | MMP-9 | [54,57,59] |
| Tumor-associated fibroblasts (mesenchymal cells) |
MMP-1 | [67] |
| MMP-7 | [67] | |
| MMP-9 | [64] | |
| MMP-14 | [65,66] | |
| MMP-19 | [213] | |
| Endothelial cells | MMP-1 | [68] |
| MMP-2 | [231] | |
| MMP-9 | [232] | |
| MMP-10 | [68] | |
| MMP-14 | [16,28,68,69,71,72,231] | |
| MMP-15 | [68] | |
| Perivascular cells (pericytes/smooth muscle cells) |
MMP-9 | [34] |
| MMP-14 | [77,233] |
Monocytes/macrophages are regarded as an obligatory cell type at the site of tumor formation and angiogenesis [36–39]. Direct involvement of macrophages in tumor progression and metastasis was demonstrated by introducing a null mutation in the CSF1 gene, encoding the macrophage colony-stimulating factor. In a mouse model of breast cancer, the lack of mature macrophages in the CSF-1 null mutants was associated with decreased mammary tumor growth and metastasis, but substantial defects in tumor-induced angiogenesis were not reported [40]. Later studies employing ablation of specific subsets of infiltrating monocytes demonstrated that Tie2-positive proangiogenic monocytes are required for tumor vessel formation [41]. Macrophages can produce a broad cohort of MMPs with potent matrix-degrading capabilities, including MMP-1, -2, -3, -7, -9, -10, -12, -13, -14, and -19 [42], among which, MMP-9 has been proven to be directly involved in the induction of tumor angiogenesis [22]. Recent evidence suggests that depending on the stage of differentiation or tissue localization, monocytes/macrophages may express characteristically different spectra of MMPs and their inhibitors [43]. In contrast to granulocytes poised to release their MMPs on demand, macrophages, differentiating from extravasated monocytes, require specific stimulation to up-regulate production of their MMPs. Interaction with activated endothelial cells and adhesion to ECM components appear to be major stimulators, which coordinately upregulate MMPs in the recruited monocytes/macrophages.
The initial studies have implicated tumor-associated macrophages as a major cell type delivering MMP-9 to the site of tumor progression and angiogenic switch [22,36,37], however the precise lineage of inflammatory MMP-9-delivering cells in the tumors sometimes was not determined [20,25,44]. On the other hand, evidence has accumulated indicating the persistent presence of infiltrating granulocytes, especially neutrophils in angiogenic tumors [4]. Histologically, infiltrating neutrophils and monocytes localize to the different areas in the angiogenic tumors: neutrophils are predominantly found in the tumor interior, whereas tumor-associated macrophages concentrate along the periphery, demarcating the tumor/stroma border [26,45,46]. The MMPs released by neutrophils include a neutrophil collagenase, MMP-8 and a neutrophil gelatinase, MMP-9, shown to be a uniquely potent pro-angiogenic enzyme [47,48]. Neutrophils, as opposed to other cell types, are capable of immediate release of their MMP cargo upon stimulation at the inflammatory site [49,50], which appears to contribute to unique functional potency of neutrophil MMP-9 in tumor growth and angiogenesis. In several model systems, neutrophils were shown to be the predominant source of MMP-9 in developing tumors [26,29,30,51]. Functional inhibition of macrophage influx with an antibody against monocyte-specific receptor CCR2, demonstrated that a compensatory neutrophil response could be elicited and infiltrating neutrophils could become an exclusive cellular source of angiogenic MMP-9 [23].
Mast cells, closely related to basophils, constitute another leukocyte type present in angiogenic tumors and were shown to be a major supplier of MMP-9 in squamous epithelial carcinomas [19]. Interestingly, the accumulation of mast cells in the angiogenic tissue, e.g. in a limb ischemia model, was itself MMP-9-dependent and impaired in MMP-9 null mice [52].
Among hematopoietic cells of non-myeloid lineage, T and B lymphocytes are known to express an extensive number of MMPs, including MMP-1, -2, -9, and -14 [53–58]. Up-regulation of MMP-9 in splenic T lymphocytes via VEGF-mediated mechanisms was observed in mice bearing mammary tumors [59]. It has been also supposed that the combination of MMP-9 and VEGF secreted by leukemic B cells can augment an angiogenic switch during disease progression [60]. However, functional roles in tumor growth and angiogenesis for those MMPs that are derived from tumor-associated lymphocytes remain mainly unexplored.
The induction of MMPs in the angiogenic tissue has been also associated with increased production of MMPs by tumor- or cancer-associated fibroblasts. There is an increasing recognition of the importance of tumor-associated fibroblasts in cancer progression and the mechanisms involving fibroblast MMPs in the regulation of tumor angiogenesis are actively investigated. Stromal fibroblasts exert their cancer-promoting properties by stimulating tumor growth and angiogenesis, in part through elevated secretion of stromal cell-derived factor 1, CXCL12 [61]. Recently, tumor-associated fibroblasts were shown to produce PDGF-2 serving as an angiogenic factor conferring tumors with the ability to overcome anti-VEGF treatment [62]. There is a complex tumor-stroma cross-talk [63], and tumor cells can induce stromal fibroblasts to express MMPs, including MMP-9 [64]. MMP-14 activity in fibroblasts, regulated by one of the EMT factors, i.e. Snail1, positively contributes to the induction of angiogenesis [65,66]. Fibroblast-derived MMP-1 and MMP-7, overexpressed in transgenic mice, have been linked with the increased susceptibility to mammary cancer [67].
In the angiogenic tumor, the developing capillary network presents a rich source of proangiogenic MMPs. With the exception of MMP-2, endothelial cells are relatively deficient in MMP expression in quiescent state [18]. However, during sprouting and formation of lumina-containing tubules, activated endothelial cells over-express MMP-1, MMP-9 and -14 [15,16,68,69]. In the in vitro angiogenesis models this de novo expression of MMPs is associated with matrix proteolytic activity sensitive to natural and synthetic MMP inhibitors [16,68,70]. A critical role for MMP-14 expression and its proteolytic activity has been shown during neovessel formation in ex vivo and in vivo models [71]. MMP-14 vascular expression in vivo is largely confined to the sprouting tip of neocappillary structures, where endothelial cell proliferation and MMP-14-mediated collagen degradation are coordinately localized [72]. In the complex environment of primary tumors, in vivo expression of MMPs in the endothelial cells appears to be under tight temporal regulation between the onset of angiogenesis and completion of vascular maturation.
While newly-forming capillary network matures, the vasculature is stabilized by perivascular cells, enveloping the outer surface of endothelial lumina-containing tubules and stabilizing primitive endothelial cell network [73–75]. Pericytes and smooth muscle cells are the most common perivascular cell types implicated in tumor angiogenesis [6,76], and frequently smooth muscle cells expressing smooth muscle actin (SMA) are referred to as pericytes. Expression of MMP-9 in the vascular pericytes, but not in smooth muscle cells, was described in primary tumors from breast cancer patients, suggesting a putative role of MMP-9 produced by perivascular cells in ECM remodeling and degradation during tumor angiogenesis [34]. The experimental confirmation that perivascular cells play a pivotal role in tumor angiogenesis was obtained in the orthotopic metastasis model of human neuroblastoma, where MMP-9-deficiency was associated with significantly reduced numbers of SMA-positive pericytes along tumor microvessels, indicating the involvement of MMP-9 both in the recruitment of pericytes to angiogenic vasculature and the functional role of recruited pericytes in stabilization of vessel architecture [76]. An exclusive requirement for MMP-14 expression in vascular smooth muscle cells was shown for their PDGF-dependent recruitment to the sites of neovascularization in vivo [77]. There is also evidence that vascular maturation is coordinated by endothelial cell/mural cell interactions redirecting MT1-MMP expression to the neovessel tip [72]. The interactions between pericytes and endothelial cells are believed to result in the silencing of MMP activities upon maturation of newly-formed blood vessels [16], however the in vivo biochemical mechanisms for inhibition of the enzymatic activity associated with pericyte MMPs remain unclear.
The complex interplay at the level of multiple types of cells delivering and producing multiple MMPs also includes another level where different MMPs, their natural activators, inhibitors and substrates, all interact with each other. This complexity, in turn, would imply that the outcome of MMP functionality might vary from setting to setting, depending on a particular tissue/cellular milieu in either the normal or disease state.
3. In vivo activation of proMMPs during tumor angiogenesis
An increase in MMP expression is usually considered as a contributing factor in the induction of malignant angiogenesis [20]. However, it is the enzymatic activity of activated MMPs which actually contributes to the outcome of their angiogenic functions. Zymogen activation and enzymatic activity are regarded as the rate-limiting factors in MMP functioning, including MMP-induced or MMP-mediated angiogenesis [78]. However, most in vivo angiogenesis models are too complex to answer the question of how MMPs are activated and at best indicate how MMP zymogens could be activated [15].
To be activated, an MMP proenzyme should undergo the so called cysteine switch, upon which a latent MMP gains its catalytic activity. The mechanisms, attributed to MMP zymogen activation, involve direct activation by another proteinase, reduction by oxidants or nonphysiologic reagents, and allosteric perturbation. All six membrane-type MMPs (including MMP-14), a type II transmembrane MMP (MMP-23) and two soluble MMPs (MMP-11 and -28) contain a furin-cleavage motif and are activated intracellularly by one of the furin proprotein convertases. Therefore, being detected on the cell surface or in the ECM of angiogenic tissue, these MMPs could be considered as “intrinsically” activated. However, the in vivo activation mechanisms for most other, non-furin cleaved MMPs are largely unknown [15], with the exception of MMP-2: The activation mechanism of the secretory proMMP-2 by membrane-anchored MMP-14 in a complex with TIMP-2 was demonstrated in a number of in vitro settings [79,80]. That such mechanism exists in vivo is correlatively supported by independent observations showing decreased MMP-2 activation in MMP-14 null mice [81] and the lack of efficient proMMP-2 activation in TIMP-2 knockout mice [82], yet the functional importance of the MMP-14/TIMP-2/MMP-2 axis in growth and development throughout life is seriously debated [83].
There is evidence that plasmin and other serine proteinases such as uPA are in vivo activators of MMPs, however the data are often circumstantial and controversial [84]. Similarly, although proMMP activation by oxidants has been demonstrated in vitro, the role of this mechanism has not been established in the in vivo settings [15]. In view of the difficulties to identify and confirm in vivo activators of proMMPs, demonstrating the activation status and/or activity of individual MMPs at the site of angiogenesis appears to be an important, but severely underscored and often ignored aspect of MMP angiogenic functionality.
4. Detection of MMP proteolytic activity in angiogenic tumors
Initial detection of MMPs in solid tumors in patients and mouse models was focused on tumor cells. Elevated expression of many MMPs, usually at tumor/stroma border, was demonstrated by different methods, including immunohistochemistry and in situ hybridization. With the recognition of importance of tumor angiogenesis, more detailed analyses have localized MMPs in close association with blood vessels, and it becomes apparent that stromal cells, inflammatory cells and perivascular cells express MMPs more frequently than tumor cells. Double staining for cell lineage-specific markers and select MMPs allowed to demonstrate that recruited MMP-9-positive cells belonged to the monocyte/macrophage lineage in ovarian carcinoma [21], while MMP-9-positive cells were represented by neutrophils in the mammary gland, lung, and pancreatic islet tumors in transgenic mice [26,29].
Although quite informative, spatial localization of angiogenic MMPs, detected by immunohistochemistry or in situ hybridization, does not provide any information with regard to their activation status or actual activity. In order to determine activation status of MMPs in angiogenic tumors, tissue extracts have been subjected to SDS-PAGE western blot and gelatin zymography analyses to determine relative levels of the proenzyme and the activated species of lower molecular weight. In the mouse cancer progression models, gelatin zymography was employed to assess and confirm upregulation and activation of proMMP-2 and proMMP-9 during angiogenic switching in squamous epithelial carcinoma [19], pancreatic islet carcinoma [20], chondrosarcoma nodules [31], and cervical carcinoma [22,23]. Substrate gel proteolysis also can provide insight as to the origin of MMPs in tumors. Thus, gelatin zymography performed on neuroblastoma tumors indicated that MMP-9 originated form the host cells since the transplanted tumor cells were MMP-9 deficient [76]. Formally, substrate zymography of complex tissues, especially xenografts, should be accompanied by western blotting to confirm the MMP and species specificities of corresponding gelatinolytic bands, but such confirmatory analyses are performed rather rarely [31,85].
The measurement of MMP activity in complex tissues, however, is challenging [86]. Examples of direct and indirect methods employed for revealing MMP activity in live cells and unfixed tissues are listed in Table 2. An in vitro angiogenesis starburst model, involving the addition of enzymatically active MMPs with and without MMP inhibitors, allowed further discrimination between the activities of two gelatinases and confirmed that the activity of MMP-9 was responsible for endothelial cell sprouting and VEGF release in the pancreatic cancer model [20]. The functional link between activation and activity of MMP-9 and the levels of released VEGF was also demonstrated in ovarian carcinoma model where activation of gelatinases was shown by gelatin zymography and the requirement for the gelatinases’ activity was confirmed with synthetic MMP inhibitors [87].
Table 2.
Direct and indirect methods to measure MMP activity in live cells and unfixed tissues
| Direct Methods | Description |
|---|---|
| In situ gelatin (substrate) zymography |
Cell preparations or sections of unfixed tissues are brought into contact with a photographic emulsion containing gelatin or a layer of fluorescence-labeled gelatin or other substrates. After incubation, areas of enzymatic activity are revealed as white spots in a dark background or as black spots in a fluorescent background employing light or confocal fluorescent microscopy [88–90]. |
| In situ zymography using DQ-gelatin or other fluorescence- quenched substrates |
The method is based on use of dye-quenched (DQ) gelatin. DQ- gelatin is heavily labeled with FITC molecules so that its fluorescence is quenched. Cleavage of DQ-gelatin by active gelatinases produces fluorescent peptides that are visible against a weakly fluorescent background. The in situ zymography can be combined with simultaneous immunohistochemical detection of a specific MMP in the same section [90,91]. |
| Near-infrared optical imaging employing MMP- sensitive probes |
An optical imaging method designed to determine the activity of MMPs with specific MMP-sensitive probes by measuring near- infrared fluorescent signal intensity in live animals [234]. |
| Time-resolved, multimodal microscopy using confocal reflection and SHG |
MMP activity is visualized by the use of specific probes for detecting cleavage of interstitial collagens by live cells, for example the antibodies detecting the 3/4-1/4 cleavage epitope in type I collagen [235], while imaging native collagen fibers using confocal reflection or second harmonic generation (SHG) microscopy [100,236]. |
| Detection of whole- cell protease activity in live cells |
Engineered fusion proteins with altered protease cleavage specificity are used to detect specific cell-surface proteolytic activity. In conjunction with fluorescence microscopy, the assay is used to specifically image the activity of cell-surface proteases and metalloproteinases in live cells [98]. |
| Detection of MMP activity in live cells by FRET |
Visualization of MMP activity is based on the detection of fluorescence resonance energy transfer (FRET) induced by a cleavage of MMP-specific substrate in the biosensor [237,238]. |
| Imaging of MMP- mediated cleavage with fluorogenic biosensor probes |
Fluorescence is generated by MMP-mediated cleavage of specific sites introduced into interstitial collagen. In combination with high- resolution imaging, the fluorescent biosensor serves as a reporter of protease activity on the surface of cells migrating on 2-D or though 3-D collagen matrices [101]. |
| Indirect Methods | Description |
| Starburst assay | Endothelial cells are incorporated into collagen gels with or without individual MMPs and/or MMP inhibitors. In the presence of a localized source of pro-angiogenic factors, such as an angiogenic tumor, endothelial cells converge in a MMP-dependent manner to the source, forming tubes in a starburst pattern [239]. |
| Detection of cryptic epitopes in ECM substrates |
Proteolytic cleavage of substrates such as collagen type IV can expose cryptic sites which are normally hidden within its triple helical structure. These novel protease-exposed cryptic epitopes can be detected by immunohistochemistry with specific monoclonal antibodies [92,112]. |
| Immunodetection of activated MMPs |
Several monoclonal antibodies have been described as raised against activated forms of MMPs. However, the ability of any of these mAbs to differentially recognize only activated enzymes has not been demonstrated definitively. Nevertheless, detection of an activated MMP would not directly prove its functional proteolytic activity in the tissue. |
In situ gelatin zymography using two different approaches described in Table 2, provides the advantage of detecting within the tissue sections only proteolytically active MMPs [88–91]. However, resolution of these methods is relatively low and does not allow for unambiguous identification of the specific cell types exhibiting gelatinolytic activity.
An elegant way to show functional activity of MMP in the tissues is the use of mAbs or polypeptides specifically recognizing cryptic sites exposed by MMP-mediated proteolysis in collagen I and IV and laminin-containing ECM substrates [92–95]. A functional confirmation of the activation requirement for individual MMPs is offered by MMP-specific function-blocking mAbs specifically inhibiting activation of proMMP-9 in both in vitro and in vivo settings [48,96,97].
A significant amount of effort was placed on a direct visualization of MMP activity in 3-dimensional in vitro and in vivo settings. The fluorescent probes for whole-cell protease activity and self-quenched probes for protease cleavage have been used to determine the location of proteolysis in migrating and invading tumor cells [91,98]. Three-dimensional in vitro model systems employing multimodal microscopic technologies gained a detailed knowledge about surface localization of proteolytic activity of MMP-14 during tumor cell migration and invasion [99,100]. Using analyses based on a DQ-collagen probe and antibody detection of cleaved collagen, MMP-14, overexpressed in tumor cells, was localized at two zones, i.e. the leading tip of the cell, allowing for directional migration, and also further back on the cell, allowing for the generation of a pulling force to protrude forward [100]. A newly-designed method allowing for high spatial and temporal resolution of MMP-mediated proteolysis is based on the use of a novel fluorogenic collagen substrate serving as a specific reporter of cell-associated protease activities [101]. By using this probe which yields fluorescence upon cleavage of a specific peptide site by MMP-2, -9, and -14, it has been demonstrated that the protease activity of MMP-14 in migrating tumor cells is localized only at the polarized leading edge [101]. The level of resolution offered by this novel probe appears to allow for further detection of MMP activity in the in vivo settings, including tumor angiogenesis.
An interesting experimental setting to study the role of MMPs in tumor cell invasion involves an addition of select reagents that results in a complete inhibition of protease activity. In the presence of a broad proteolytic inhibitor cocktail, tumor cells were shown both in vitro and, importantly, in vivo, to change their migration from a mesenchymal, protease-dependent mode to an amoeboid, non-proteolytic pattern. This compensatory type of migration was associated with the formation of a so called constriction ring allowing the cells to squeeze through the collagenous fibers [99,102]. An alternative point of view challenges the amoeboid mechanism of tumor cell migration in in vitro assays and also in some in vivo settings. According to this viewpoint, the activity of MMP-14, and only MMP-14, is an absolute requirement for transgressing physiologically relevant matrix barriers by tumor and endothelial cells [103]. This notion is further supported by in vitro data demonstrating that a proteolysis-independent pattern of cell invasion is observed only in artificially reconstituted collagen, which forms relatively porous and non-cross-linked fibrillar matrices [101,103].
The controversy in the above described experimental findings illustrates the urgent need for novel sophisticated approaches to dissect the in vivo matrix remodeling associated with the specific proteolytic activity of individual MMPs. Recent advances in proteomic technologies, including ABPP [104], PROTOMAP [105] and iTRAQ [106], will lead to identification on a global scale of distinct proenzyme activation events and specific substrate cleavage reactions that occur in complex biological systems. Application of these technologies, employing MMP-specific probes and inhibitors in the analysis of matching tissues that are undergoing or not undergoing angiogenic switching, will allow to determine which proMMPs become activated and which identifiable substrates are cleaved coordinately with the induction of angiogenesis.
5. MMP-mediated proteolysis in tumor angiogenesis
The notion that MMPs play a critical role in tumor angiogenesis comes from the initial appreciation of the potent matrix remodeling abilities of this class of proteases linked to the tissue remodeling necessary for angiogenesis [13,107–109].
5.a. MMP-mediated modifications of the angiogenic ECM
New vessel formation involves disassembling of the endothelial cell layer and its anchoring basement membrane, migration and proliferation of individual endothelial cells, followed by their subsequent re-assembling into lumina-containing blood vessels. Thus, being a spatial process, angiogenesis obviously requires disruption of physical barriers imposed by the dense extracellular matrix (ECM). Therefore, the capacity of MMPs to proteolytically target and modify many complex fibrillar proteins has long implicated MMPs in ECM modifications facilitating physiologic and tumor angiogenesis.
Generation of cryptic sites in the ECM
In addition to peptide bond cleavages of ECM molecules, MMP-mediated proteolytic remodeling also includes an exposing of cryptic epitopes in ECM proteins [110]. Proteolytic cleavage of collagen type IV results in the exposure of several cryptic sites hidden within the triple helical structure. These unmasked epitopes are recognized by specific mAbs raised by a subtractive immunization technique [111,112]. The HUIV26 cryptic epitope is exposed within the ECM of angiogenic but not quiescent blood vessels. In some in vivo animal model systems, interactions with this epitope was shown to regulate the development of angiogenic blood vessels through the ligation of αvβ3 integrin expressed de novo in the endothelial cells [92]. Detection of the HUIV26 cryptic site during retinal neovascularization was prevented in MMP-9-deficient, but not MMP-2-deficient mice, thereby implicating the proteolytic activity of MMP-9 in unmasking this epitope in vivo [93]. Another cryptic epitope, recognized by mAb HU177, could be exposed within three-dimensional collagen gels following proteolysis by tumor and vascular smooth muscle cells. The HU177 cryptic epitope is present in both interstitial collagen I and basement membrane collagen IV and is selectively expressed in the ECM of developing tumors and angiogenic vasculature in the chick embryo CAM model [94].
As was predicted in [92], a systematic search for cryptic sites resulted in identification of novel cryptic epitopes unmasked by MMPs in collagen IV and other ECM molecules, including collagen VIII, XV, and XVIII [110]. It has been also shown that MMP-2 and MMP-14 were capable of unmasking cryptic epitopes in laminin-5 in vitro, however the physiological relevance of these epitopes in vivo remained controversial [113]. In contrast, a potent peptide antagonist of a lamimin-1 sequence has been shown to block angiogenesis and tumor growth of breast carcinomas in the chick embryo assay [114]. Evidence has also been provided for another cryptic epitope within denatured and proteolyzed laminin-1, that is specifically recognized by a synthetic peptide STQ selected by phage display [95]. Blocking of this epitope with the STQ peptide inhibited in vivo angiogenesis in the CAM model, growth of s.c. melanoma xenografts in immunodeficient mice, and experimental metastasis in the chick embryo [95]. These inhibitory effects of laminin peptide antagonists indicate that during tumor angiogenesis laminin is proteolytically modified, possibly by select MMPs, presenting “pro-angiogenic” cryptic epitopes, which in turn regulate behavior of endothelial and tumor cells.
Directional proteolysis of ECM by endothelial and tumor MMP-14
Among all MMPs, MMP-14 is one of the most potent modifiers of the pericellular microenvironment [115]. MMP-14 has been ascribed with exceptional capability in the execution of directional matrix proteolysis, thereby governing migration and invasion of tumor and endothelial cells within proteolytically modified ECM [65,101,116]. A direct role for MMP-14 expressed on endothelial cells has been shown for endothelial tubular formation in 2- and 3-dimentional matrices in vitro [16,117,118], suggesting a functional role of MMP-14 in endothelial cell assembly in vivo. The findings generated in a number of in vitro, ex vivo and in vivo model systems have clearly implicated MMP-14 in matrix modifications that allow for coordinated migration and invasion of tumor and endothelial cells [71,100,101,103,119]. Moreover, there is strong evidence supporting the notion that MMP-14 orchestrates such coordinated interplay during in vivo angiogenesis induced in growing tumors [16,83,115,117,120].
Basement membrane remodeling by MMPs during angiogenesis
Basement membrane matrix, enriched in laminins and collagen IV, provides support for both endothelial cells and perivascular cells. During angiogenesis this basement membrane is proteolytically reorganized: first degraded at the onset of angiogenesis and then re-assembled during maturation and stabilization [121,122]. MMP-mediated degradation of basement membrane remodeling in turn leads to either release of pro-angiogenic factors, unmasking cryptic sites or generation of angiogenesis inhibitors [121]. Preferential basement membrane substrates for most MMPs were established in vitro. In the in vivo setting, the activity of MMP-2, -9 and -14, was implicated in the remodeling of the basement membrane during angiogenic vessel maturation and stabilization via their negative regulation by a distinct MMP inhibitor, the membrane-anchored glycoprotein RECK [123]. In MMP-14 null mice, impaired postnatal angiogenesis was demonstrated in models involving extensive proteolytic tissue remodeling [81,117]; however, little or no evidence was provided as to whether the observed angiogenic defects were specifically caused by the lack MMP-14-mediated remodeling of the basement membrane.
5.b. MMP-mediated modifications of cell surface molecules
The MMPs are able to proteolytically process a number of cell surface receptors and process and shed soluble forms of several membrane-bound growth factors and cytokines, which then directly affect endothelial cell functions. This mechanism underlies the important, although somewhat indirect functions of MMPs in tumor-induced angiogenesis. MMP-modified or shed molecules include both positive and negative regulators of angiogenic processes and therefore MMP modifying/shedding functions can have opposing effects on angiogenesis.
The processing/shedding functions of MMPs have been ascribed for both soluble and membrane-tethered MMPs, such as MMP-1, MMP-9 and MMP-14. Tumor-derived MMP-1 has been implicated in the proteolytic activation of protease-activated receptor 1 (PAR-1), a thrombin receptor, which is expressed at high levels on endothelial cells. MMP-1 activation of PAR-1 results in the activation of MAP kinase signaling cascades, causing transformation of the vascular endothelium from a quiescent state into prothrombotic or proinflammatory activated states [124,125]. MMP-9 has been shown to release sKitL, which plays an extremely important role in tumor growth and angiogenesis [126]. Direct MMP-14 proteolytic maturation of the αv integrin subunit of αvβ3 integrin [127] can influence tumor-induced angiogenesis through modulation of αvβ3 angiogenic and signaling functions [128], e.g. via activation of focal adhesion kinase [129]. Recently, MMP-14 expressed in head and neck squamous carcinoma cells was implicated in proteolytic processing and release of the cell membrane-tethered Semaphorin 4D, responsible for the induction of blood vessel growth into s.c. implanted angioreactors containing carcinoma cells [130].
5.c. MMP-mediated release of ECM-bound pro-angiogenic factors
MMP-mediated release and activation of matrix-bound growth factors and cytokines, subsequently acting as pro-angiogenic factors, is regarded as one of the major mechanisms in tumor angiogenesis [110].
MMP-mediated release of VEGF
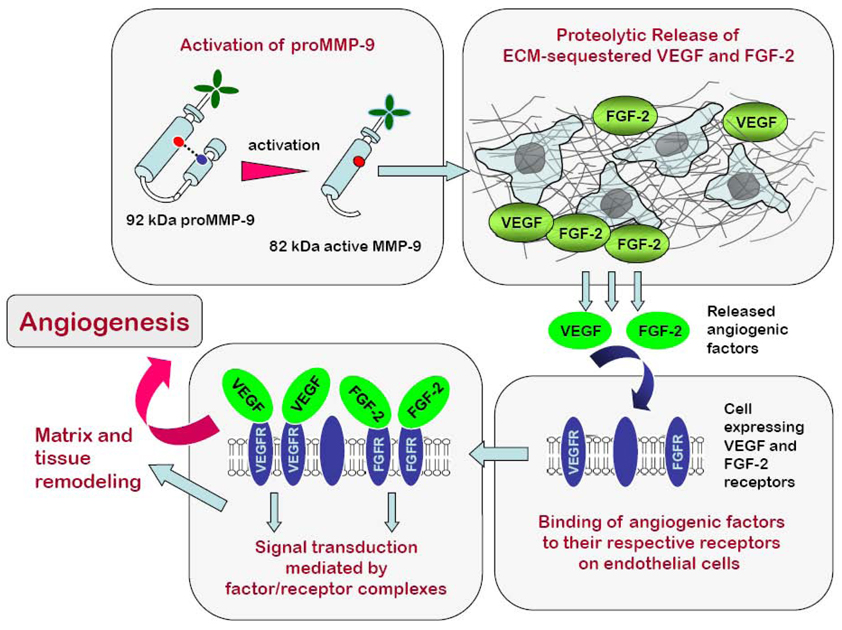
VEGF represents one of the most impressive examples of pro-angiogenic factors stored within the ECM and released by MMP proteolysis [32,110]. A number of MMPs have been implicated in VEGF release, although MMP-9 appears to be the most potent MMP functionally linked to tumor angiogenesis via VEGF-releasing mechanisms (Figure 1).
Figure 1. MMP-9 is a powerful proangiogenic factor acting via release of ECM-sequestered VEGF and FGF-2.
MMP-9 is produced as a zymogen and requires activation before exerting its angiogenic activity. Upon activation, MMP-9 enzyme is capable of releasing pro-angiogenic factors such as VEGF and FGF-2, which are sequestered in the ECM. Bioavailable VEGF and FGF-2 bind to the endothelial cells expressing corresponding receptors for the angiogenic factors. Complex formation between angiogenic factors and their tyrosine kinases receptors triggers signal transduction, which in turn leads to the angiogenic switch, involving endothelial cell reorganization and matrix and tissue remodeling.
Functional link between MMP-9 delivered to the pre-angiogenic site by inflammatory cells and the release of VEGF have been established in several genetic mouse models of carcinoma progression. Pancreatic carcinogenesis in RIP1-Tag2 transgenic mice was the first model, where MMP-9, produced by inflammatory leukocytes, was demonstrated to directly release VEGF and increase its bioavailability [20]. Increase in bioavailability of VEGF by MMP-9, likely produced by infiltrating macrophages, was also shown to contribute to the malignant progression of ovarian carcinoma by promoting neovessel sprouting and tumor growth [21]. Complicating the multipart scenario of MMP-9-mediated VEGF mobilization sustained by macrophages, additional mechanisms have emerged involving neutrophil MMP-9-mediated release of angiogenic growth factors. Thus, impaired macrophage recruitment and therefore, production of macrophage-derived MMP-9 to the sites of angiogenic switching, elicited a compensatory response of MMP-delivering neutrophils, providing an alternative paracrine support for tumor angiogenesis [23]. Illustrating the functional role of MMP-9 delivered by neutrophils in the release of VEGF, ablation of neutrophil infiltration and therefore, delivery of neutrophil MMP-9, resulted in the decrease of bioavailable VEGF in angiogenic islets and suppressed the angiogenic switch in the RIP1-Tag2 transgenic mouse model of pancreatic progression [26].
Furthermore, MMP-9 derived from Gr+/CD11b+ cells of myeloid lineage co-injected with colorectal MC26 tumor cells, was demonstrated to directly promote tumor angiogenesis via an increase in bioavailability of VEGF [24]. In addition, MMP-9 produced at the site of primary tumor formation by recruited CD45+ myeloid cells was shown to release ECM-sequestered VEGF during angiogenic switching in glioblastoma [27]. Importantly, the total or bound amounts of VEGF detected by immunohistochemistry or immunoblotting were comparable in tumors regardless of the presence of MMP-9, indicating that angiogenic switching is triggered by the soluble, bioavailable VEGF released by MMP-9 [24,27].
In agreement with a requirement of MMP-9 proteolytic activity for release of VEGF, the inhibition of MMP-9 results in the reduction of VEGF bioavailability and a corresponding inhibition of tumor-induced angiogenesis. Thus, the reduction in the levels of active MMP-9 due to overexpression of thrombospondin in mice prone to generate mammary gland tumors was concomitant with the decrease in bioavailable VEGF and inhibition of tumor angiogenesis, and vice versa, thrombospondin-deficiency resulted in higher levels of active MMP-9 and increased VEGF/VEFGR2 complex formation [131]. In a cervical carcinogenesis model, chemical suppression of MMP-9 in infiltrating MMP-9-positive macrophages inhibited the expression and activation MMP-9 and diminished VEGF mobilization to its receptor [22]. Similarly, chemical repression of MMP-9 production in a human prostate cancer model resulted in the reduced levels of bioavailable VEGF and inhibited tumor angiogenesis in a Matrigel plug assay [132]. Interestingly, only a few studies demonstrated that the tumor-derived MMP-9 could also increase release of VEGF: Thus, VEGF release in ovarian carcinoma model was attributed to the activity of MMP-9 produced by tumor cells [87].
In contrast to MMP-9, another gelatinase, i.e. MMP-2, sharing some substrate specificity and biochemical modes of activities with MMP-9, does not appear to strongly contribute to release of ECM-sequestered VEGF. Thus, knock-down of MMP-2 in mice did not change the expression of VEGF in retinal neovascularization model [133]. However, the ovarian carcinoma model in mice presented evidence implicating tumor-derived MMP-2 in the release of VEGF [87]. Moreover, recent analysis of proteome signatures of MMP-2-null versus MMP-2-rescued mouse embryonic fibroblasts indicated that MMP-2 is involved in the processing of several VEGF-binding proteins, proteolytic degradation of which ultimately results in the mobilization of VEGF [134,135].
MMP-14 was also linked to a possible regulation of VEGF during angiogenesis. Thus, overexpression of MMP-14 in human glioma and breast carcinoma cells resulted in accelerated tumor growth and angiogenesis attributed to up-regulation of VEGF in xenografts [136,137]. In addition, MMP-3, -7, and -19 have been shown to cleave in vitro matrix-bound isoforms of VEGF, releasing it from the matrix as soluble fragments [138]. In this study, soluble and sequestered VEGF appeared to exert different patterns of tumor neovascularization, i.e. vascular hyperplasia (soluble VEGF) or vascular sprouting and branching (matrix-bound VEGF). It was suggested that two forms of VEGF are involved in different signaling pathways, although both matrix-bound and soluble VEGF bind to the same cell surface receptor [138].
MMP-mediated release of FGF-2
The role of FGF-2 in tumor angiogenesis is often overshadowed by studies implicating VEGF in the angiogenic switch and complicated by a wide redundancy in the FGF family [139,140]. A cross-talk between VEGF and FGF-2, their corresponding growth factor/receptor systems along with inflammatory cells delivering MMPs that release ECM-bound angiogenic factors, may play important roles in the modulation of blood vessel growth. Functional roles for FGF-2 in angiogenesis were demonstrated in the chick embryo and mouse tumor angiogenesis models [141–143]. With regard of tumor angiogenesis, modulation of FGF-2/FGFR2 system showed overall correlative effects on tumor neovascularization. Thus, inhibition of FGF-2 and interference with the FGFR2 pathway concomitantly decreased tumor angiogenesis, while FGF-2 overexpression or exogenous delivery of FGF-2 resulted in the accelerated development of new blood vessels in tumors [142].
FGF-2 is found in association with endothelial basement membranes and appears to be sequestered in the ECM as a stable but inactive form, requiring release for biological activity. The mechanisms of FGF-2 release from ECM include proteolytic cleavage of heparan sulphate proteoglycans, a major anchor of FGF-2, by plasmin, thrombin or MMPs [144,145] (Figure 1). MMP involvement in the FGF-2-mediated angiogenesis was suggested in the corneal neovascularization model in MMP-14 knockout mice, where the lack of MMP-14, either directly or through impaired MMP-2 activation, resulted in a complete inhibition of angiogenesis response to FGF-2 [81]. In agreement with this suggestion, MMP-2 proteolytic activity was implicated in the release of FGF-2 from the cell-ECM interface in the lens capsule model [146]. Recently, MMP-9 has been shown to directly induce release of FGF-2 and mediate FGF-2-dependent physiologic angiogenesis in the chick embryo model [143].
Despite a general acceptance that FGF-2 release from the ECM is a prerequisite for exhibiting its biological function, the experimental evidence implementing such release mechanism in angiogenesis in vivo and especially in tumor-induced angiogenesis is limited. A possible explanation may be in that an initial blocking of the VEGF/VEGFR2 pathway must first occur before switching to the alternative FGF/FGFR pathway of angiogenesis. This switching mechanism was demonstrated in the pancreatic carcinogenesis model, where development of late-stage tumors, emerging after VEGFR2 blockade and initial tumor suppression, was associated with reactivation of VEGF-independent angiogenesis induced by the members of FGF family [147]. In this regard, the natural dependence of CAM vascularization on FGF-2 rather than on VEGF [148], might have allowed for the demonstration of MMP-9-mediated FGF-2 release and involvement of FGF-2/FGFR2 pathway elicited by MMP-9 proteolytic activity in the chick embryo angiogenesis model [143].
5.d. MMP-mediated generation of anti-angiogenic factors
The existence of a fine balance in the regulation of pleiotropic functions of MMPs is highlighted by the fact that the same individual MMPs, clearly implicated in pro-angiogenic activity can be implicated as well in an anti-angiogenic “conspiracy”. In addition to the proteolytic release of matrix-bound pro-angiogenic factors, MMPs have been also demonstrated to generate a number of anti-angiogenic molecules [121]. Therefore, different cell types responsible for delivery and production of certain MMPs in the pre-angiogenic site could present quite different scenarios for tumor-associated angiogenesis.
Several MMPs, including MMP-2, -7, -9 and -12, are capable of cleaving plasminogen and producing angiostatin, a circulating polypeptide fragment with anti-angiogenesis capabilities [149–151]. Macrophage metalloelastase, MMP-12 proved to be one of the most efficient angiostatin-producing MMPs [152]. In human hepatocellular carcinomas, high expression of MMP-12 was associated with high levels of angiostatin and diminished vascularization [153]. Mice lacking α1 integrin and expressing high plasma levels of MMP-9 and MMP-9-generated angiostatin, demonstrated auto-inhibition of tumor angiogenesis [154,155], suggesting that diminishment of MMP activity by MMP inhibitors may contribute to the acceleration of tumor progression.
Endostatin, another reported inhibitor of blood vessel growth, was shown to be produced in vitro from basement membrane type XVIII collagen by several MMPs such as MMP-3, -7, -9, -12, and -20 [150,151,156,157]. However, MMP-1, -2, -8 and -12 failed to show any significant activity against collagen XVIII [158]. Anti-angiogenic mechanisms of endostatin involves direct inhibition of endothelial cell proliferation, migration and capillary formation, in part via binding to α5β1 integrin and blocking of downstream phosphorylation of FAK [159]. Interestingly, in a study where endostatin inhibited human tongue carcinoma invasion and dissemination in the chick embryo CAM model, it was also shown to block activation of several MMPs, including MMP-9 and -13 [151], i.e. those MMPs which are initially involved in the production of endostatin.
The anti-angiogenic role of select MMPs, e.g. MMP-9, also has been attributed to their ability to generate tumstatin from the α3 chain of collagen IV [150,151,160]. Decreased levels of circulating tumstatin in MMP-9 null mice were linked to accelerated growth and pathological angiogenesis of subcutaneously developing Lewis lung carcinomas [160]. Although anti-angiogenic effects of tumstatin in the in vitro settings are direct and involve direct binding to αvβ3 integrin expressed on endothelial cells and inhibition of their proliferation [160], it is difficult to explain why MMP-9 deficiency in many in vivo mouse models has been repeatedly associated with a substantial inhibition of tumor-induced angiogenesis (due to the lack of pro-angiogenic MMP-9), rather than with accelerated tumor neovascularization (due to the absence of MMP-9-generated tumstatin or other MMP-9-cleaved angiogenesis inhibitors). There is also not much experimental evidence to mechanistically link the enzymatic activity of MMPs required for generation of the specific anti-angiogenic factors in vivo with a decrease in a MMP activity observed during the switching-off of angiogenesis.
6. Cell surface localization of soluble MMPs in the angiogenic tissue
Specific cellular and pericellular localization is viewed as a means to compartmentalize MMPs and regulate their activity in vivo. Understanding MMP functions during tumor-induced angiogenesis in vivo depends on identification of the mechanisms of how the released, secretory MMPs are activated and targeted to the right location and how MMP activity is regulated at the pericellular space.
Initially, localized proteolytic activity of soluble MMPs was associated with the surface expression of membrane-tethered MMP-14 mediating activation of secretory proMMP-2 [161]. Focalized distribution of cell surface MMP-14 [72,100,101], facilitates compartmentalization of proMMP-2 and it subsequent activation at specific areas such as the leading edge of tumor and endothelial cells [115]. MMP-2 zymogen also binds to the αvβ3 integrin on activated endothelial cells, which was reported to facilitate proMMP-2 activation [162]. Although initially described as αvβ3-mediated [162], the activation of proMMP-2 by αvβ3-expressing cells is actually mediated by MMP-14 since cells expressing αvβ3 and no MMP-14 do not generate activated MMP-2, but gain this ability after transfection with MMP-14 cDNA [163,164]. The functional role of cell surface bound MMP-2 in tumor-induced angiogenesis was highlighted in a study where lentiviral delivery of a noncatalytic fragment of MMP-2 (PEX) inhibited tumor-induced angiogenesis and tumor growth in a nude mouse model [85].
Another example of docking of soluble MMPs to cell surface molecules that lack any proteolytic activity, but promote zymogen activation through autolytic cleavage, is provided by the binding of MMP-9 to the hyaluronan receptor CD44 [165]. By gelatin zymography, MMP-9 bound to CD44 was identified as an activated enzyme (i.e. 82 kDa processed form), but the mechanism of zymogen activation and whether it occurs prior to CD44 binding remained unresolved. CD44-facilitated activation of MMP-9 resulted in modification of tumor invasion and angiogenesis, likely via activation of latent TGFβ [166]. A membrane-bound form of MMP-9 detected in a variety of normal and tumor cells, including neutrophils, endothelial, and carcinoma cells, constitutes only a small fraction of the total enzyme, while the major soluble form has long been regarded as the biologically relevant form of the proteinase [167]. It would be plausible to suggest that the cell surface-localized portion of MMP-9 might represent the enzyme bound to a putative membrane-tethered protease, which directly activates the MMP-9 zymogen. This membrane-associated portion of MMP-9 enzyme can then serve as a potent catalyst of a MMP-9 activation cascade. However, a study in which human MCF-7 breast carcinoma cells were generated to express MMP-9 either as a constitutively active, membrane-anchored enzyme or as a secreted proenzyme provided experimental evidence in favor of a view that it is the secreted form of MMP-9 which promotes tumor growth and the angiogenic switch in s.c. xenografts [168]. Importantly, this study also demonstrated that enhanced MMP-9-dependent angiogenesis required activation of proMMP-9 (by yet unidentified in vivo activators) and the activity of MMP-9 enzyme. Furthermore, the inhibition of MMP-9 activation and activity resulted in the decrease of tumor angiogenesis concomitant with reduced microvessel density. All these results provide additional evidence for the critical role that MMP-9, more than any other MMP, plays in tumor angiogenesis.
7. MMP-9 and the recruitment of bone marrow-derived progenitor cells to the sites of tumor angiogenesis and vasculogenesis
A rapidly increasing volume of studies indicate that in addition to local cellular resources to build new blood vessels, tumor neovascularization also depends on circulating cell progenitors, mainly of bone marrow origin, mobilized into the circulation by cytokines and growth factors such as VEGF [169,170]. These circulating progenitor cells are recruited by primary tumors in a MMP-9-dependent manner through a complex system of chemokines [9,10,171,172].
7.a. MMP-9-dependent mobilization of angiogenic and vasculogenic progenitor cells
Initial experiments have demonstrated that MMP-9, induced in the bone marrow by circulating cytokines such as SDF-1, VEGF or G-CSF, releases soluble Kit ligand (sKitL), which in turn, permits the transfer of quiescent, c-Kit-positive endothelial (VEGFR1+) and hematopoietic (VEGFR2+) stem and progenitor cells from the osteoblastic zone to the vascular zone. Translocation to the vascular-enriched zone induces proliferation and differentiation of hematopoietic progenitor cells (HPCs) and endothelial progenitor cells (EPCs) and their mobilization to the peripheral circulation [126,173]. Such mobilization of bone marrow derived HPCs and EPCs was inefficient in MMP-9 null mice due to the lack of sKitL release, confirming the critical dependence of the process on MMP-9 activity, although the precise cellular source of MMP-9 responsible for release of sKitL was not identified in this study. The requirement of MMP-9 activity for the mobilization of HPCs was also confirmed by administrating a synthetic MMP inhibitor in vivo, which blocked chemokine-induced mobilization in MMP-9-competent mice [126].
In the study where mobilization of HPCs was induced by a combination of G-CSF and GROβ, the cellular source of MMP-9 required for HPC egress from bone marrow into circulation surprisingly, but importantly, was attributed to neutrophils [97]. HPC mobilization correlated with elevated levels of neutrophil-derived MMP-9 in marrow and was blocked by a function-blocking anti-MMP-9 mAb, preventing activation of proMMP-9. The lack of HPC egress in response to chemokines in MMP-9 knockout mice also confirms MMP-9-dependency of HPCs mobilization [97].
7.b. MMP-9-mediated recruitment of bone marrow-derived progenitor cells
Both HPCs and EPCs, mobilized into the circulation from bone marrow, can participate in neovascularization at the primary tumor site. The relative contribution of each cell type could be dictated by the cytokine repertoire and matrix composition of a particular tumor type [10,174,175]. Circulating HPCs recruited to the site of adult neovascularization are usually distributed around blood vessels with clear affinity to retain a close proximity to vessels, and do not incorporate into the endothelial lining of the blood vessel wall [170]. However, tumor-retained HPCs can functionally mimic EPCs by enhancing tumor angiogenesis [28]. In contrast, circulating EPCs are intimately integrated within endothelium and often are found colocalizing with endothelial cells [10,176].
Recent studies also indicated that more mature cell subpopulations belonging to myeloid (CD45+) or myelomonocytic (CD11b+) lineages could be mobilized from bone marrow and contribute to tumor angiogenesis. Thus, mobilized CD45+ myeloid cells were shown to include MMP-9-positive monocytic cells which can be traced to the site of glioblastoma development, where they were found randomly distributed within the tumor [27]. In this orthotopic glioblastoma model, the recruitment of bone marrow-derived CD45+ myeloid cells is driven by HIF1α, the direct effector of hypoxia, whereas MMP-9 activity of recruited MMP-9-positive cells has been demonstrated to be essential and sufficient to initiate the angiogenic switch. The mechanism of angiogenic switching included the earlier-discovered MMP-9-mediated release of soluble, bioavailable VEGF [27]. Besides releasing VEGF, the MMP-9 produced by HPCs recruited to neoangiogenic sites in the primary tumors, positively contributes to tumor angiogenesis by increasing the bioavailability of stem cell active chemocytokines, including SDF-1 and sKitL [177].
7.c. The role of MMP-9 in tumor vasculogenesis
In addition to tumor-induced angiogenesis involving endothelial cell sprouting and capillary formation from pre-existing blood vessels, recent years brought about data, which have led to a new concept of tumor vasculogenesis as an alternative mechanism of tumor vessel generation. This concept is supported by the findings demonstrating that at a certain stage of tumorigenesis, circulating bone marrow-derived cells, including HPCs, EPCs and pericyte progenitor cells (PPCs), may contribute to the assembly of new tumor blood vessels. Immunohistological studies demonstrated that the recruited cells were often found being incorporated into vasculature wall and observed in the perivascular regions of the endothelium or colocalized with endothelial cells. Although the ontogeny and differentiation of mobilized progenitor populations and the expression of lineage-specific markers are not well established and remain controversial [10,170,178,179], it appears that tumor vasculogenesis requires the expression of MMP-9 for efficient integration of recruited cells to the tumor vasculature. Thus, in the HIF1α model of glioblastoma development, the expression of MMP-9 enabled EPCs (and apparently also PPCs) to incorporate into tumor vessels and sustain their pericyte coverage [27].
An alternative scenario of tumor vasculogenesis was demonstrated in a model system where mammary tumors were transplanted into wild type or MMP-9 knockout mice pre-irradiated to prevent angiogenesis at the site of implantation [28]. In this model, genetic depletion of MMP-9 abrogated tumor growth and development of new, vasculogenic blood vessels. However in wild-type recipients, MMP-9-positive, bone marrow-derived CD11b+ myelomonocytic cells, which essentially are HPCs, but not EPCs, infiltrated the tumors and were responsible for tumor growth and development of immature blood vessels [28].
8. MMPs at the metastatic site: MMP-9 and the establishment of the pre-metastatic niche
In addition to the initial concept of mobilization and recruitment of bone marrow-derived cells to the primary tumor, a novel concept has recently emerged whereby the primary tumor induces the recruitment of HPCs to distant sites, where permissive pre-metastatic niches are created, providing a favorable soil to seed the tumor cells which will eventually escape the primary tumor [180]. The contribution of bone marrow-derived HPCs in the generation of pre-metastatic niches was analyzed in a spontaneous metastasis model where Lewis lung carcinoma or B16 melanoma cells were implanted subcutaneously and allowed to form primary tumors, eventually metastasizing to the distant sites [181]. It has been demonstrated that primary tumors produced soluble factors which by unknown mechanisms induced recruitment of bone marrow-derived HPCs to preferential metastatic organs. Of critical importance was the identification that HPC arrived to the putative metastatic site and generated a permissive niche well before tumor cell dissemination. Interestingly, that in this model EPCs did not contribute to the generation of pre-metastatic niches, but rather accompanied the arriving tumor cells. In addition to other critical factors such as the expression of integrin α4β1, apparently required for adhesion of HPCs to the niche fibronectin, the whole process of niche generation appeared to depend on MMP-9 produced by HPC clusters. The functional role of MMP-9 in establishing the pre-metastatic niche was confirmed by using MMP-9 null mice demonstrating impaired HPC cluster formation and reduced metastatic spread. However, the precise catalytic activities of MMP-9 during generation of and within the pre-metastatic niche remain unknown, although the breakdown of basement membranes and the altering of local microenvironment by releasing sKitL and VEGF have been proposed [180,181]. The TIMP-1 status of MMP-9 delivered by these critical HPCs has yet to be determined.
9. Biochemical mechanisms underlying the distinct angiogenic potency of MMP-9
An extensive volume of experimental evidence, much of which has already been discussed in previous sections of this review, indicates that MMP-9 is clearly one of the most potent pro-angiogenic MMPs. The direct contribution of MMP-9 to angiogenesis and vascular performance is thought to involve the catalytic activity of the enzyme resulting either in the cleavage of ECM components such as native and denatured collagens [93,182,183], processing of various cytokines and chemokines [47,50], or release of angiogenic growth factors such as VEGF [20,24,26,27,52,132,138] and also FGF-2 [143]. Although MMP-9 has been strongly associated with angiogenesis, its direct catalytic reactions in vivo and regulation of its activity during the angiogenic process remain largely undefined. Moreover, the actual in vivo substrates for MMP-9’s pro-angiogenic activity have not been demonstrated (Figure 1).
It has been generally accepted that at the site of angiogenic switch, MMP-9 is produced mainly by inflammatory monocytes [17,38,43]. However, inflammatory neutrophils could serve as an immediate and major source of MMP-9 in pre-angiogenic tissue [184]. Recent findings from Hanahan’s laboratory demonstrated a close link between the presence of MMP-9-expressing neutrophils and the onset of the angiogenic switch in developing tumors [23,26,185]. The close association of neutrophil-derived MMP-9 with angiogenesis was also found in tumors where monocyte influx had been greatly diminished by specific blocking reagents and a compensatory neutrophil response was evoked [23].
The functional role of distinct inflammatory cells and their associated MMPs during physiologic and tumor-induced angiogenesis was demonstrated by using a quantitative in vivo angiogenesis model [186], modified from the original assay described by Folkman and colleagues [187]. In this model angiogenesis involves the influx of inflammatory cells, including monocyte/macrophages and avian neutrophils, and is facilitated by MMPs released or produced by these cells, i.e. monocyte MMP-13 and neutrophil MMP-9 [188,189]. A question remained, however, as to whether inflammatory cell-derived MMP-9 itself is uniquely proangiogenic in vivo.
We have recently probed the regulatory mechanisms determining the pro-angiogenic status of neutrophil MMP-9 and demonstrated a direct functional role of neutrophils and neutrophil MMP-9 in physiologic angiogenesis. By employing a modified chick embryo chorioallantoic membrane (CAM) angiogenesis assay, it was demonstrated that human neutrophil proMMP-9 is potently angiogenic at low to sub nanomolar levels [48]. This angiogenic proMMP-9 is released from human neutrophils in a unique, TIMP-free form, which is in contrast to the TIMP-1-complexed proMMP-9 produced by most other cell types, including macrophages, B lymphocytes and tumor cells [49,56]. The potent angiogenic characteristics of neutrophil MMP-9 can be attributed directly to its TIMP-free nature and also to its immediate availability at relatively high concentrations upon granulae release from the stimulated neutrophils (Figure 2). In angiogenesis-reconstitution experiments, the substitution of neutrophil TIMP-free proMMP-9 with equimolar and even 20-fold molar excess levels of the neutrophil proMMP-9/TIMP-1 complex has confirmed that it is the TIMP-free nature that determines the potent proangiogenic potential of neutrophil proMMP-9 [48,143].
Figure 2. Efficient and rapid activation of neutrophil proMMP-9 due to its unique TIMP-free status.
Neutrophils synthesize and store MMP-9 in their secretory granules, readily available for release on demand. Since TIMP-1 production in neutrophils is very low or even undetectable, MMP-9 zymogen is released in the TIMP-free form, immediately accessible to proteolytic activation. Therefore, activation of TIMP-free neutrophil MMP-9 is relatively rapid and activated MMP-9 enzyme becomes readily available to trigger the angiogenic switch. In contrast to neutrophils, stimulated monocytes/macrophages and tumors cells synthesize MMP-9 de novo. Usually, induction of MMP-9 is concomitant with an increased expression of TIMP-1 and therefore, MMP-9 zymogen at least partially is complexed with TIMP-1. Complexing with TIMP-1 slows activation of MMP-9 zymogen and makes it relatively inefficient as compared with activation rates of TIMP-free MMP-9. This delayed activation, might make monocyte and tumor-derived MMP-9 less efficient in the induction of angiogenesis.
These findings also demonstrate that neutrophil MMP-9 induces a differential influx of additional protein components into angiogenic tissue and proteolytically causes a release of bioactive basic FGF (FGF-2). The released bioavailable FGF-2, acting through its cognate receptor FGFR2, is the actual inducer of angiogenesis that functions downstream of the released proMMP-9, which is very effectively activated in vivo because of its TIMP-free status. The TIMP-free MMP-9/FGF-2 pathway of angiogenic induction was confirmed to operate not only in the chick CAM assay, but also in vivo in a mammalian setting, i.e. in a mouse angiotube assay recently established in our laboratory [143,190].
Overall our results indicate that neutrophil TIMP-free proMMP-9 is potently and uniquely angiogenic and is distinct from the low- or non-angiogenic proMMP-9 secreted by other cell types, including activated macrophages which appear to produce proMMP-9 molecules stoichiometrically bound to TIMP-1. In addition, even upon stimulation required for the induction of expression and secretion of MMP-9 by monocytes/macrophages, the levels of MMP-9 production by these cells are much lower than those provided by degranulating neutrophils. It has been determined that in 30 min 1 × 106 neutrophils release 5 to 20 times more proMMP-9 than 1 × 106 PMA-stimulated monocytes secrete over 24–48 hours (our unpublished observations). Therefore, it appears that inflammatory neutrophils uniquely provide immediate and high levels of the angiogenic TIMP-free proMMP-9 [48,143]. However, it is unclear mechanistically how neutrophil proMMP-9 becomes activated and when and how active neutrophil MMP-9 enzyme does induce angiogenesis. Identification of the relevant in vivo substrate(s) of angiogenic MMP-9 will help clarify these unanswered issues.
10. Pleiotropic functions of individual MMPs in malignant angiogenesis
The multiple and sometimes contrasting aspects of diversified functionality within the MMP family in angiogenesis-related processes imply that MMPs, acting as a cohort of enzymes or as individual enzymes, exhibit pleiotropic roles in tumor neovascularization. Below, we will highlight contrasting and synergistic functions of individual MMPs as they relate directly to malignant angiogenesis (Table 3).
Table 3.
Evidence for the roles of individual MMPs in tumor angiogenesis
| MMP | Evidence |
|---|---|
| MMP-1 |
|
| MMP-2 |
|
| MMP-3 | |
| MMP-7 | |
| MMP-8 | |
| MMP-9 |
|
| MMP-12 | |
| MMP-14 | |
| MMP-19 |
|
MMP-1
MMP-1, an interstitial collagenase, can be produced by inflammatory monocytes/macrophages infiltrating the primary tumors as well as by tumor cells themselves. In breast cancer, MMP-1 belongs to a family of metastasis signature genes governing lung metastasis [191]. In combination with MMP-2, the cyclooxygenase COX-2 and the EGFR ligand epiregulin, MMP-1 can regulate the structure of the vascular network in the primary tumor and promote the formation of the dilated, tortuous and leaky blood vessels, typical of aggressive primary tumors [192]. While single down-regulation of the above-mentioned genes in MDA-MB-231 breast carcinoma cells did not considerably alter the overall vascular network in the primary tumor, the combined knock-down of all 4 genes attenuated vascular permeability and also diminished the ability of escaped tumor cells to extravasate in the lung capillaries, resulting in substantially inhibited lung metastasis [191,192], suggesting a cooperative, possibly synergistic role for MMP-1.
MMP-2
In the above-described model, single down-regulation of MMP-2 in tumor cells did not produce significant effects on tumor angiogenesis and cancer progression, demonstrating a relative lack of tumor-derived MMP-2 involvement in angiogenesis [192]. Moreover, MMP-2 deficiency did not affect the course of angiogenic switching in the pancreatic carcinoma islets [20] nor did it affect angiogenesis in malignant keratinocyte transplants [30], thus questioning the putative angiogenic capabilities of stromal MMP-2 in vivo. However, reduced angiogenesis in subcutaneous tumors developing from the B16-BL6 melanoma or Lewis lung carcinoma cells was reported in MMP-2 knockout mice [193], highlighting a possible functional role of stromal MMP-2 in tumor neovascularization. In addition, targeting of MMP-2 with soluble PEX also reduced tumor angiogenesis in the pre-established s.c. melanoma transplants [85]. Overall, these opposing results may indicate that MMP-2 contribution to tumor angiogenesis apparently depends on the model and the mode of MMP delivery to the site of primary tumor formation.
MMP-3
MMP-3 (stromelysin-1) represents one of the MMPs with clearly pleiotropic functionality in tumor progression. MMP-3 is found almost exclusively in the tumor stroma and is expressed by fibroblasts, endothelial cells and infiltrating leukocytes. In transgenic mice, MMP-3 has been demonstrated to act as a natural mammary tumor promoter [194] and overexpression of MMP-3 positively contributed to mammary carcinogenesis [195]. Surprisingly, the lack of MMP-3 alone or in combination with MMP-9 deficiency did not affect tumor vascularization in malignant keratinocyte transplantation model [30]. However, a protective role for MMP-3 was demonstrated in a squamous cell carcinoma model, where the lack of MMP-3 in the host stroma in null mice led to elevated tumor growth [196]. Tumor progression in this model was inversely associated with the leukocyte infiltration, specifically with an overall reduction in tumor-associated macrophages and neutrophils, suggesting that expression of MMP-3, probably in stromal fibroblasts, might regulate leukocyte infiltration and thus indirectly influences inflammatory leukocyte-mediated angiogenesis.
MMP-7
MMP-7 (matrilysin) is produced by a variety of cells and its association with cancer metastasis and progression was demonstrated in a number of studies [197]. In immunodeficient mice, exogenously-delivered recombinant MMP-7 was demonstrated to induce human colon tumor angiogenesis through direct stimulation of endothelial cell proliferation [198]. In contrast, in the multistage MMTV-PyVT mammary carcinogenesis model, the lack of MMP-7 in knockout mice did not have effect on the development of primary mammary tumors or lung metastasis [29].
MMP-8
MMP-8 (collagenase-2), produced mainly by neutrophils, is a potent protease, which exhibits strong protective functions during chemically-induced skin carcinogenesis [199] and also in breast cancer metastasis models [200]. However, it remained unclear whether the anti-tumor effects of MMP-8 were associated with the modulation of tumor angiogenesis. The data obtained in MMP-8 null mice indicated that MMP-8 appears as contributing to the resolution of inflammation [201]. More recently it has been demonstrated that by generating chemotactic factors during inflammation, MMP-8 can also promote, apparently in a paracrine fashion, neutrophil migration through the collagenous stroma of cornea [202]. Therefore, any putative role of MMP-8 in angiogenesis will likely be linked to MMP-8-mediated regulation of the inflammatory response.
MMP-9
The initial observation that knockout of MMP-9 in mice leads to developmentally delayed angiogenesis in bone-growth plates [203], provided a strong link of this MMP to vascular angiogenesis. This finding was followed by the demonstration of impaired tumor neovascularization in several metastasis models. Thus, microvessel density in intraosseous tumors developing from the human prostate cancer cell line PC-3 in RAG-1/MMP-9 double knockout mice is lower compared with control MMP-9-positive, RAG-1-negative counterpart [204]. The genetic ablation of MMP-9 abrogated angiogenic switching during carcinogenesis of pancreatic islets in transgenic RIP1-Tag2 model [20]. Similarly, the ablation of MMP-9-delivery to primary tumors by genetic manipulations involving other than MMP-9 genes, e.g. by ablation of CSF-1 [40], by chemical treatments, e.g. with bisphosphonate [22], or using antibodies against granulocytes, e.g. anti-Gr-1 mAb [26], provided additional strong links supporting the concept that MMP-9 is an MMP with unique pro-angiogenic properties.
The proof-of-principle providing a direct functional link between MMP-9 and the formation, structure and remodeling of new blood vessels, however, came from the phenotypical rescue of vasculogenic and angiogenic defects manifested in MMP-9 null mice by the transplantation of wild-type bone marrow [21,25,28,52,76]. All these studies have unambiguously demonstrated that MMP-9, delivered to the primary tumors by the progenies of donor hematopoietic cells, directly contribute to tumor neovascularization. The rescue of impaired angiogenesis in colorectal tumors developing in the MMP-9-deficient mice can also be achieved by a co-injection of myeloid Gr/CD11b-positive cells isolated from spleens of wild-type donor mice used as a source of MMP-9-delivering cells [24]. The precise lineage of these myeloid cells, i.e. their terminal differentiation into monocytes or neutrophils has not been defined. Noteworthy, the induction of angiogenesis in vivo by purified preparations of TIMP-free MMP-9 provided evidence that neutrophil MMP-9 itself is a potent angiogenic factor [48,143].
In addition to genetic ablation of MMP-9 in knockout mice, down-regulation of MMP-9 expression in tumor cells provided valuable information regarding essential functions of tumor-derived MMP-9 in tumor neovascularization. Inhibition of MMP9 gene expression in conjunction with the downregulation of cathepsin B or uPAR in glioblastoma cells resulted in reduction of tumor growth and angiogenesis in the mouse dorsal window assay [205–207]. Anti-angiogenic effects of MMP-9 deficiency in tumor cells were manifested in the disruption of capillary network formation, suggesting that the MMP-9-mediated proteolytic modifications of ECM are required for proper blood vessel formation.
Of significance to the pleiotropic nature of MMPs, MMP-9 may also exhibit protective functions in tumor angiogenesis, which are associated mostly with its ability to proteolytically generate inhibitors of angiogenesis. Those protective properties of MMP-9 have been discussed in section 5.d.
MMP-12
MMP-12 (macrophage metalloelastase), expressed almost exclusively in macrophages, represents an MMP with cancer protective roles. The protective function of MMP-12 in tumor progression was suggested by the ability of this MMP to generate the anti-angiogenic fragment angiostatin from plasminogen [208] and the resulting anti-angiogenic effects of angiostatin (previously discussed in 5.d). This suggestion was further affirmed by suppression of primary tumor growth and halted angiogenesis in the mice grafted with melanoma cells overexpressing MMP-12 [153]. In agreement with the anti-angiogenic function of MMP-12, MMP-12 null mice demonstrated increased tumor growth and blood vessel formation in the orthotopic Lewis lung carcinoma model and decreased levels of circulating angiostatin [209]. Similar results were obtained in the Lewis lung carcinoma model employing MMP-12 null and wild type mice, where presence of MMP-12 was associated with decreased tumor-associated microvessel density, resulting in suppressed growth of lung metastasis [210]. Interestingly, some MMP-12-mediated effects on tumor growth and angiogenesis were independent of the MMP-12-mediated production of angiostatin. That the macrophages express a potent anti-angiogenic MMP, i.e. MMP-12, along with the production of pro-angiogenic MMPs, emphasizes the notion that depending on the relative composition of the induced MMP secretate, this inflammatory cell type might exhibit opposing angiogenic roles.
MMP-14
Although embryonal vasculogenesis appeared to be MMP-14-independent [83], apparent defects in neonatal angiogenesis of MMP-14 knockout mice have been observed, however mostly in ex vivo and in vitro models [117]. Angiogenic defects in early neonatal development of MMP-14 null mice were described only in a few in vivo models, e.g. a corneal angiogenesis assay [81]. Due to the early lethality of mice deficient in MMP-14, limited evidence has accumulated supporting the role of MMP-14 in tumor-induced angiogenesis models. A few studies providing some evidence for the pro-angiogenic role of tumor MMP-14 in angiogenesis in vivo, demonstrated the enhanced vascularization in tumors over-expressing MMP-14, which was clearly linked to up-regulation of VEGF [136,137]. In vitro analyses of mechanisms, which could underlie MMP-14-mediated enhancement of tumor angiogenesis, implicated activation of Src tyrosine kinases by MMP-14 [120] or release and reactivation of VEGF from the connective tissue growth factor [211]. Another mechanism for regulation of tumor-induced angiogenesis by tumor MMP-14 involves release of Semaphorin 4D, a potent promoter of neovascularization [130]. Recent findings demonstrated that selective inhibition of MMP-14 with a function-blocking mAb, generated by phage display technology, inhibited tumor-induced angiogenesis and slowed tumor progression and metastasis [212].
MMP-19
MMP-19 is reported to be expressed in normal human epidermis, but down-regulated during malignant transformation and dedifferentiation [213]. Following acute inflammation or injury, MMP-19 was found expressed in the endothelial cells of synovial capillaries [214] and was also reported to be expressed in peripheral blood monocytes [53]. In Matrigel plugs, tumor-induced angiogenesis is accompanied by MMP-19 expression in the host mesenchymal cells but not by endothelial capillary cells or CD11b-positive inflammatory cells [213]. The initial observations in MMP-19 null mice indicated that a lack of MMP-19 resulted in a decreased susceptibility to skin tumors chemically induced by carcinogens [215], i.e. pointing only to a positive role of MMP-19 in carcinogenesis. However, during FGF-2-induced angiogenesis in Matrigel plugs containing malignant keratinocytes and implanted into MMP-19 deficient mice, host MMP-19 deficiency was associated with an increased early angiogenic response and enhanced tumor invasion [213]. Therefore, MMP-19 can also function as a negative regulator of early steps of tumor angiogenesis and invasion.
11. MMPs as targets of anti-angiogenic therapy: interfering with pleiotropic functions
Inhibition of angiogenesis during malignant progression by targeting of VEGF/VEGFR and FGF-2/FGFR2 pathways with corresponding therapeutics was considered for a long time the most reasonable way for controlling metastatic disease at the level of primary tumor development [185,216,217]. However, from the initial reports that tumors might become refractory to anti-VEGF therapy, the concept has emerged that anti-angiogenic therapy may result in increased malignant progression. In this regard, the most recent studies have presented evidence indicating that targeting the VEGF pathway in pancreatic neuroendocrine carcinomas and glioblastomas in mice actually increased local invasion and distant metastasis [218]. Similarly, in metastasis models involving intravenous injection of cells or removal of orthotopic primary tumors, treatments with potent anti-angiogenic VEGFR/PDGFR inhibitors were shown to accelerate metastasis of breast cancer and melanoma cells [219]. In addition, VEGF has been recently described as a negative regulator of pericyte function and vessel maturation [220] and its deletion in myeloid cells was shown to increase pericyte coverage and normalize tumor vasculature [221]. In these studies, ablation of VEGF was associated with accelerated tumorigenesis and tumor progression, which might provide, at least in part, mechanistic explanation for undesirable consequences of anti-VEGF therapies. These disillusioning findings once again indicate that anti-angiogenic therapy either with inhibitors targeting directly endothelial cells or targeting the molecules triggering the angiogenic switch could have different outcomes in the course of malignant progression.
Since MMPs are potent triggers and contributors of the angiogenic switch, it was a long-standing notion that blocking MMPs at the site of tumor development would substantially inhibit angiogenesis and also disrupt already established angiogenic networks. The initial promising observations in mice, however, were not supported by clinical trials with general or partially selective inhibitors of MMPs [222–224]. Several reasons were implicated in the failure of MMP inhibitors in patients, including the sole targeting of only late stages of cancer disease and the relative lack of MMP specificity of the inhibitors. However, the overall pleiotropic nature of MMP functionality at the levels of their cell origin, e.g. stromal vs. tumor, their cellular or extracellular targets, e.g. endothelial cells vs. tumor cells vs. ECM, their biochemistry, e.g. compensatory overexpression, alternative modes of activation and processing of multiple substrates, may essentially pre-determine the non-unilateral consequences of inhibition of MMP functions. Thus, the abrogation of the influx of MMP-9-delivering macrophages in CCLR2-deficient, K14-HPV/E2 mice, genetically prone to develop cervical cancer, was associated with a compensatory infiltration of developing tumors with MMP-9-positive neutrophils [23]. These findings suggest that if tumor-promoting, pro-angiogenic macrophages are suppressed, then MMP-9 delivering neutrophils are recruited to deliver highly angiogenic, TIMP-free proMMP-9 and elicit an alternative mechanism, supporting tumor angiogenesis and cancer progression [23,48,143]. In addition, adaptive-evasive responses by tumors leading to a resistance to anti-angiogenic therapy involving growth factor/receptor inhibitors have been recently demonstrated to include up-regulation of alternative pro-angiogenic signaling pathways, recruitment of bone marrow-derived vascular and hematopoietic progenitors and stabilization of newly-developed tumor blood vessels via pericyte coverage [185]. In view of the above discussed properties and angiogenic functions of neutrophil MMP-9, inhibition of the VEGF/VEGFR pathway driven by MMP-9-producing macrophages may induce the compensatory FGF/FGFR pathway driven by MMP-9-delivering infiltrating neutrophils and thus specific targeting of inflammatory cell MMP-9 and not tumor-derived MMP-9 (as was anticipated in the initial clinical trials) might represent an alternative therapeutic approach.
12. Perspectives
The functional roles of MMPs in tumor neovascularization are intrinsically associated with a specific aspect of aggressive cancer, i.e. the nature of vessels utilized for metastatic tumor cell dissemination [225,226]. Addressing this aspect poises additional questions pertinent to the regulation of tumor vascularization by MMPs, including: 1) Do tumor cells intravasate into the newly-formed vasculature of the primary tumor, i.e. does the angiogenic vasculature serve as conduits for tumor cell dissemination? If so, the pro-metastatic role of MMPs in tumor cell dissemination would critically depend on the overall outcome of positive and negative MMP contributions to tumor angiogenesis and vasculogenesis. Although there is general agreement that tumor neovascularization is linked to and required for tumor metastasis, there is a relative paucity of experimental evidence directly demonstrating intravasation of tumor cells into angiogenic capillaries, rather than into pre-established blood vessels [45]. Moreover, recent evidence indicates that even if cancer dissemination occurs through tumor-induced angiogenic vessels, it might be efficient only at the early stages of angiogenic network development, i.e. before endothelial cell normalization [227]. It would be of particular interest to analyze whether MMPs are involved in this normalization of the vasculature. 2) If disseminating tumor cells, however, intravasate into the pre-established vasculature (either hematogenous or lymphatic), do MMPs facilitate cancer dissemination via those vessels co-opted by the tumor or surrounding the tumor? If implemented, this scenario apparently would critically depend on MMP-mediated proteolytic modifications of tumor ECM, especially at the tumor border, assisting the escape, invasion, directional migration towards and subsequent intravasation of tumor cells into pre-existing blood vessels. This scenario would also imply that MMP-assisted tumor dissemination might, paradoxically, be independent of those functions of MMPs, which are directly related to tumor neovascularization such as angiogenic switching, growth factor release and recruitment of progenitor cells. These aspects of MMP pleiotropic functionality remain experimentally unexplored, awaiting further research for their resolution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Eichmann A, Pardanaud L, Yuan L, Moyon D. Vasculogenesis and the search for the hemangioblast. J Hematother Stem Cell Res. 2002;11:207–214. doi: 10.1089/152581602753658411. [DOI] [PubMed] [Google Scholar]
- 3.Tang DG, Conti CJ. Endothelial cell development, vasculogenesis, angiogenesis, and tumor neovascularization: an update. Semin Thromb Hemost. 2004;30:109–117. doi: 10.1055/s-2004-822975. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 10.Papaspyridonos M, Lyden D. Chapter 11. The role of bone marrow-derived cells in tumor angiogenesis and metastatic progression. Methods Enzymol. 2008;444:255–269. doi: 10.1016/S0076-6879(08)02811-5. [DOI] [PubMed] [Google Scholar]
- 11.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald DM, Foss AJ. Endothelial cells of tumor vessels: abnormal but not absent. Cancer Metastasis Rev. 2000;19:109–120. doi: 10.1023/a:1026529222845. [DOI] [PubMed] [Google Scholar]
- 13.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 14.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 15.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 17.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 18.Zucker S, Lysik RM, Zarrabi MH, Stetler-Stevenson W, Liotta LA, Birkedal-Hansen H, Mann W, Furie M. Type IV collagenase/gelatinase (MMP-2) is not increased in plasma of patients with cancer. Cancer Epidemiol Biomarkers Prev. 1992;1:475–479. [PubMed] [Google Scholar]
- 19.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, Fidler IJ. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 22.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, Declerck YA. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005;65:3200–3208. doi: 10.1158/0008-5472.CAN-04-3770. [DOI] [PubMed] [Google Scholar]
- 26.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin MD, Carter KJ, Jean-Philippe SR, Chang M, Mobashery S, Thiolloy S, Lynch CC, Matrisian LM, Fingleton B. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008;68:6251–6259. doi: 10.1158/0008-5472.CAN-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masson V, de la Ballina LR, Munaut C, Wielockx B, Jost M, Maillard C, Blacher S, Bajou K, Itoh T, Itohara S, Werb Z, Libert C, Foidart JM, Noel A. Contribution of host MMP-2 and MMP-9 to promote tumor vascularization and invasion of malignant keratinocytes. FASEB J. 2005;19:234–236. doi: 10.1096/fj.04-2140fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 33.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen BS, Sehested M, Kjeldsen L, Borregaard N, Rygaard J, Dano K. Expression of matrix metalloprotease-9 in vascular pericytes in human breast cancer. Lab Invest. 1997;77:345–355. [PubMed] [Google Scholar]
- 35.van Kempen LC, de Visser KE, Coussens LM. Inflammation, proteases and cancer. Eur J Cancer. 2006;42:728–734. doi: 10.1016/j.ejca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 37.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 39.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009 doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 40.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2008;28:2108–2114. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 43.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 44.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 46.Deryugina EI, Quigley JP. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem Cell Biol. 2008;130:1119–1130. doi: 10.1007/s00418-008-0536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270:3739–3749. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 48.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- 50.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 51.Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res. 2006;66:259–266. doi: 10.1158/0008-5472.CAN-05-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–750. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bar-Or A, Nuttall RK, Duddy M, Alter A, Kim HJ, Ifergan I, Pennington CJ, Bourgoin P, Edwards DR, Yong VW. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 54.Owen JL, Iragavarapu-Charyulu V, Lopez DM. T cell-derived matrix metalloproteinase-9 in breast cancer: friend or foe? Breast Dis. 2004;20:145–153. doi: 10.3233/bd-2004-20115. [DOI] [PubMed] [Google Scholar]
- 55.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 56.Gaudin P, Trocme C, Berthier S, Kieffer S, Boutonnat J, Lamy C, Surla A, Garin J, Morel F. TIMP-1/MMP-9 imbalance in an EBV-immortalized B lymphocyte cellular model: evidence for TIMP-1 multifunctional properties. Biochim Biophys Acta. 2000;1499:19–33. doi: 10.1016/s0167-4889(00)00084-7. [DOI] [PubMed] [Google Scholar]
- 57.Yakubenko VP, Lobb RR, Plow EF, Ugarova TP. Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp Cell Res. 2000;260:73–84. doi: 10.1006/excr.2000.5002. [DOI] [PubMed] [Google Scholar]
- 58.Di Girolamo N, Tedla N, Lloyd A, Wakefield D. Expression of matrix metalloproteinases by human plasma cells and B lymphocytes. Eur J Immunol. 1998;28:1773–1784. doi: 10.1002/(SICI)1521-4141(199806)28:06<1773::AID-IMMU1773>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 59.Owen JL, Iragavarapu-Charyulu V, Gunja-Smith Z, Herbert LM, Grosso JF, Lopez DM. Up-regulation of matrix metalloproteinase-9 in T lymphocytes of mammary tumor bearers: role of vascular endothelial growth factor. J Immunol. 2003;171:4340–4351. doi: 10.4049/jimmunol.171.8.4340. [DOI] [PubMed] [Google Scholar]
- 60.Kay NE. The angiogenic status of B-CLL B cells: role of the VEGF receptors. Leuk Res. 2004;28:221–222. doi: 10.1016/j.leukres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 62.Crawford Y, Kasman I, Yu L, Zhong C, Wu X, Modrusan Z, Kaminker J, Ferrara N. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15:21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stuelten CH, DaCosta Byfield S, Arany PR, Karpova TS, Stetler-Stevenson WG, Roberts AB. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-alpha and TGF-beta. J Cell Sci. 2005;118:2143–2153. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 65.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rowe RG, Li XY, Hu Y, Saunders TL, Virtanen I, Garcia de Herreros A, Becker KF, Ingvarsen S, Engelholm LH, Bommer GT, Fearon ER, Weiss SJ. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J Cell Biol. 2009;184:399–408. doi: 10.1083/jcb.200810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 68.Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galvez BG, Genis L, Matias-Roman S, Oblander SA, Tryggvason K, Apte SS, Arroyo AG. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/ccl2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. J Biol Chem. 2005;280:1292–1298. doi: 10.1074/jbc.M408673200. [DOI] [PubMed] [Google Scholar]
- 70.Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yana I, Sagara H, Takaki S, Takatsu K, Nakamura K, Nakao K, Katsuki M, Taniguchi S, Aoki T, Sato H, Weiss SJ, Seiki M. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007;120:1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 73.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 74.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 75.Chantrain CF, Henriet P, Jodele S, Emonard H, Feron O, Courtoy PJ, DeClerck YA, Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: a new role for metalloproteinases. Eur J Cancer. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Chantrain CF, Shimada H, Jodele S, Groshen S, Ye W, Shalinsky DR, Werb Z, Coussens LM, DeClerck YA. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004;64:1675–1686. doi: 10.1158/0008-5472.can-03-0160. [DOI] [PubMed] [Google Scholar]
- 77.Lehti K, Allen E, Birkedal-Hansen H, Holmbeck K, Miyake Y, Chun TH, Weiss SJ. An MT1-MMP-PDGF receptor-beta axis regulates mural cell investment of the microvasculature. Genes Dev. 2005;19:979–991. doi: 10.1101/gad.1294605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. doi: 10.1111/j.1699-0463.1999.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 79.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 80.Cao J, Rehemtulla A, Bahou W, Zucker S. Membrane type matrix metalloproteinase 1 activates pro-gelatinase A without furin cleavage of the N-terminal domain. J Biol Chem. 1996;271:30174–30180. doi: 10.1074/jbc.271.47.30174. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200:11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 84.Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- 85.Pfeifer A, Kessler T, Silletti S, Cheresh DA, Verma IM. Suppression of angiogenesis by lentiviral delivery of PEX, a noncatalytic fragment of matrix metalloproteinase 2. Proc Natl Acad Sci U S A. 2000;97:12227–12232. doi: 10.1073/pnas.220399597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lombard C, Saulnier J, Wallach J. Assays of matrix metalloproteinases (MMPs) activities: a review. Biochimie. 2005;87:265–272. doi: 10.1016/j.biochi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 88.George SJ, Johnson JL. In Situ Zymography. In: C I, editor. Methods in Molecular Biology. Vol 151. Totowa, NJ: Humana Pres Inc; 2000. pp. 411–415. [DOI] [PubMed] [Google Scholar]
- 89.Yan SJ, Blomme EA. In situ zymography: a molecular pathology technique to localize endogenous protease activity in tissue sections. Vet Pathol. 2003;40:227–236. doi: 10.1354/vp.40-3-227. [DOI] [PubMed] [Google Scholar]
- 90.Frederiks WM, Mook OR. Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histochem Cytochem. 2004;52:711–722. doi: 10.1369/jhc.4R6251.2004. [DOI] [PubMed] [Google Scholar]
- 91.Sloane BF, Sameni M, Podgorski I, Cavallo-Medved D, Moin K. Functional imaging of tumor proteolysis. Annu Rev Pharmacol Toxicol. 2006;46:301–315. doi: 10.1146/annurev.pharmtox.45.120403.095853. [DOI] [PubMed] [Google Scholar]
- 92.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, Ryan SJ, Brooks PC. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–1437. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cretu A, Roth JM, Caunt M, Akalu A, Policarpio D, Formenti S, Gagne P, Liebes L, Brooks PC. Disruption of endothelial cell interactions with the novel HU177 cryptic collagen epitope inhibits angiogenesis. Clin Cancer Res. 2007;13:3068–3078. doi: 10.1158/1078-0432.CCR-06-2342. [DOI] [PubMed] [Google Scholar]
- 95.Akalu A, Roth JM, Caunt M, Policarpio D, Liebes L, Brooks PC. Inhibition of angiogenesis and tumor metastasis by targeting a matrix immobilized cryptic extracellular matrix epitope in laminin. Cancer Res. 2007;67:4353–4363. doi: 10.1158/0008-5472.CAN-06-0482. [DOI] [PubMed] [Google Scholar]
- 96.Ramos-DeSimone N, Moll UM, Quigley JP, French DL. Inhibition of matrix metalloproteinase 9 activation by a specific monoclonal antibody. Hybridoma. 1993;12:349–363. doi: 10.1089/hyb.1993.12.349. [DOI] [PubMed] [Google Scholar]
- 97.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 98.Hobson JP, Liu S, Rono B, Leppla SH, Bugge TH. Imaging specific cell-surface proteolytic activity in single living cells. Nat Methods. 2006;3:259–261. doi: 10.1038/nmeth862. [DOI] [PubMed] [Google Scholar]
- 99.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multistep pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 101.Packard BZ, Artym VV, Komoriya A, Yamada KM. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009;28:3–10. doi: 10.1016/j.matbio.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 103.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 105.Dix MM, Simon GM, Cravatt BF. Global mapping of the topography and magnitude of proteolytic events in apoptosis. Cell. 2008;134:679–691. doi: 10.1016/j.cell.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 108.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopez-Otin C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brooks PC, Lin JM, French DL, Quigley JP. Subtractive immunization yields monoclonal antibodies that specifically inhibit metastasis. J Cell Biol. 1993;122:1351–1359. doi: 10.1083/jcb.122.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu J, Rodriguez D, Kim JJ, Brooks PC. Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma. 2000;19:375–385. doi: 10.1089/02724570050198893. [DOI] [PubMed] [Google Scholar]
- 113.Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- 114.Ponce ML, Hibino S, Lebioda AM, Mochizuki M, Nomizu M, Kleinman HK. Identification of a potent peptide antagonist to an active laminin-1 sequence that blocks angiogenesis and tumor growth. Cancer Res. 2003;63:5060–5064. [PubMed] [Google Scholar]
- 115.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 116.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 117.Genis L, Galvez BG, Gonzalo P, Arroyo AG. MT1-MMP: universal or particular player in angiogenesis? Cancer Metastasis Rev. 2006;25:77–86. doi: 10.1007/s10555-006-7891-z. [DOI] [PubMed] [Google Scholar]
- 118.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 119.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 120.Sounni NE, Roghi C, Chabottaux V, Janssen M, Munaut C, Maquoi E, Galvez BG, Gilles C, Frankenne F, Murphy G, Foidart JM. A Noel, Up-regulation of vascular endothelial growth factor-A by active membrane-type 1 matrix metalloproteinase through activation of Src-tyrosine kinases. J Biol Chem. 2004;279:13564–13574. doi: 10.1074/jbc.M307688200. [DOI] [PubMed] [Google Scholar]
- 121.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 122.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 123.Oh J, Takahashi R, Kondo S, Mizoguchi A, Adachi E, Sasahara RM, Nishimura S, Imamura Y, Kitayama H, Alexander DB, Ide C, Horan TP, Arakawa T, Yoshida H, Nishikawa S, Itoh Y, Seiki M, Itohara S, Takahashi C, Noda M. The membrane-anchored MMP inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell. 2001;107:789–800. doi: 10.1016/s0092-8674(01)00597-9. [DOI] [PubMed] [Google Scholar]
- 124.Goerge T, Barg A, Schnaeker EM, Poppelmann B, Shpacovitch V, Rattenholl A, Maaser C, Luger TA, Steinhoff M, Schneider SW. Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66:7766–7774. doi: 10.1158/0008-5472.CAN-05-3897. [DOI] [PubMed] [Google Scholar]
- 125.Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173:1736–1746. doi: 10.2353/ajpath.2008.080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, Chestukhina GG, Smith JW, Deryugina EI, Strongin AY. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J Biol Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- 128.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 129.Deryugina EI, Ratnikov BI, Postnova TI, Rozanov DV, Strongin AY. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J Biol Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- 130.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282:6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 133.Ohno-Matsui K, Uetama T, Yoshida T, Hayano M, Itoh T, Morita I, Mochizuki M. Reduced retinal angiogenesis in MMP-2-deficient mice. Invest Ophthalmol Vis Sci. 2003;44:5370–5375. doi: 10.1167/iovs.03-0249. [DOI] [PubMed] [Google Scholar]
- 134.Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, Overall CM. Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/Connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–8465. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dean RA, Overall CM. Proteomics discovery of metalloproteinase substrates in the cellular context by iTRAQ labeling reveals a diverse MMP-2 substrate degradome. Mol Cell Proteomics. 2007;6:611–623. doi: 10.1074/mcp.M600341-MCP200. [DOI] [PubMed] [Google Scholar]
- 136.Deryugina EI, Soroceanu L, Strongin AY. Up-regulation of vascular endothelial growth factor by membrane-type 1 matrix metalloproteinase stimulates human glioma xenograft growth and angiogenesis. Cancer Res. 2002;62:580–588. [PubMed] [Google Scholar]
- 137.Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- 138.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ozaki H, Okamoto N, Ortega S, Chang M, Ozaki K, Sadda S, Vinores MA, Derevjanik N, Zack DJ, Basilico C, Campochiaro PA. Basic fibroblast growth factor is neither necessary nor sufficient for the development of retinal neovascularization. Am J Pathol. 1998;153:757–765. doi: 10.1016/S0002-9440(10)65619-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bikfalvi A, Klein S, Pintucci G, Rifkin DB. Biological roles of fibroblast growth factor-2. Endocr Rev. 1997;18:26–45. doi: 10.1210/edrv.18.1.0292. [DOI] [PubMed] [Google Scholar]
- 142.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284:25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 146.Tholozan FM, Gribbon C, Li Z, Goldberg MW, Prescott AR, McKie N, Quinlan RA. FGF-2 release from the lens capsule by MMP-2 maintains lens epithelial cell viability. Mol Biol Cell. 2007;18:4222–4231. doi: 10.1091/mbc.E06-05-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 148.Ribatti D, Presta M. The role of fibroblast growth factor-2 in the vascularization of the chick embryo chorioallantoic membrane. J Cell Mol Med. 2002;6:439–446. doi: 10.1111/j.1582-4934.2002.tb00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 150.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–860. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 151.Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- 152.Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. Matrix metalloproteinases generate angiostatin: effects on neovascularization. J Immunol. 1998;161:6845–6852. [PubMed] [Google Scholar]
- 153.Gorrin-Rivas MJ, Arii S, Furutani M, Mizumoto M, Mori A, Hanaki K, Maeda M, Furuyama H, Kondo Y, Imamura M. Mouse macrophage metalloelastase gene transfer into a murine melanoma suppresses primary tumor growth by halting angiogenesis. Clin Cancer Res. 2000;6:1647–1654. [PubMed] [Google Scholar]
- 154.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–2207. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21:272–281. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- 156.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 157.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 158.Heljasvaara R, Nyberg P, Luostarinen J, Parikka M, Heikkila P, Rehn M, Sorsa T, Salo T, Pihlajaniemi T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res. 2005;307:292–304. doi: 10.1016/j.yexcr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 159.Wickstrom SA, Alitalo K, Keski-Oja J. Endostatin associates with integrin alpha5beta1 and caveolin-1, and activates Src via a tyrosyl phosphatase-dependent pathway in human endothelial cells. Cancer Res. 2002;62:5580–5589. [PubMed] [Google Scholar]
- 160.Hamano Y, Kalluri R. Tumstatin, the NC1 domain of alpha3 chain of type IV collagen, is an endogenous inhibitor of pathological angiogenesis and suppresses tumor growth. Biochem Biophys Res Commun. 2005;333:292–298. doi: 10.1016/j.bbrc.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 161.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 162.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 163.Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 164.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 165.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 167.Fridman R, Toth M, Chvyrkova I, Meroueh SO, Mobashery S. Cell surface association of matrix metalloproteinase-9 (gelatinase B) Cancer Metastasis Rev. 2003;22:153–166. doi: 10.1023/a:1023091214123. [DOI] [PubMed] [Google Scholar]
- 168.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jimenez-Baranda S, Gomez-Mouton C, Martinez AC, Manes S. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. J Cell Sci. 2004;117:1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 169.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 171.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 172.Rafii S, Lyden D. Cancer. A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rafii S, Avecilla S, Shmelkov S, Shido K, Tejada R, Moore MA, Heissig B, Hattori K. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann N Y Acad Sci. 2003;996:49–60. doi: 10.1111/j.1749-6632.2003.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 174.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 175.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–166. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 176.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 177.Seandel M, Butler J, Lyden D, Rafii S. A catalytic role for proangiogenic marrow-derived cells in tumor neovascularization. Cancer Cell. 2008;13:181–183. doi: 10.1016/j.ccr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 179.Larrivee B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, Olive PL, Karsan A. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J Immunol. 2005;175:2890–2899. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- 180.Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Van den Steen PE, Proost P, Grillet B, Brand DD, Kang AH, Van Damme J, Opdenakker G. Cleavage of denatured natural collagen type II by neutrophil gelatinase B reveals enzyme specificity, post-translational modifications in the substrate, and the formation of remnant epitopes in rheumatoid arthritis. FASEB J. 2002;16:379–389. doi: 10.1096/fj.01-0688com. [DOI] [PubMed] [Google Scholar]
- 183.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- 187.Nguyen M, Shing Y, Folkman J. Quantitation of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994;47:31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- 188.Zijlstra A, Aimes RT, Zhu D, Regazzoni K, Kupriyanova T, Seandel M, Deryugina EI, Quigley JP. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3) J Biol Chem. 2004;279:27633–27645. doi: 10.1074/jbc.M313617200. [DOI] [PubMed] [Google Scholar]
- 189.Zijlstra A, Seandel M, Kupriyanova TA, Partridge JJ, Madsen MA, Hahn-Dantona EA, Quigley JP, Deryugina EI. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107:317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Deryugina EI, Quigley JP. Chapter 2. Chick embryo chorioallantoic membrane models to quantify angiogenesis induced by inflammatory and tumor cells or purified effector molecules. Methods Enzymol. 2008;444:21–41. doi: 10.1016/S0076-6879(08)02802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 193.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 194.Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19:1102–1113. doi: 10.1038/sj.onc.1203347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McCawley LJ, Crawford HC, King LE, Jr, Mudgett J, Matrisian LM. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res. 2004;64:6965–6972. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- 197.Wilson CL, Matrisian LM. Matrilysin. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. Academic Press; 1998. pp. 149–184. [Google Scholar]
- 198.Nishizuka I, Ichikawa Y, Ishikawa T, Kamiyama M, Hasegawa S, Momiyama N, Miyazaki K, Shimada H. Matrilysin stimulates DNA synthesis of cultured vascular endothelial cells and induces angiogenesis in vivo. Cancer Lett. 2001;173:175–182. doi: 10.1016/s0304-3835(01)00634-6. [DOI] [PubMed] [Google Scholar]
- 199.Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 200.Montel V, Kleeman J, Agarwal D, Spinella D, Kawai K, Tarin D. Altered metastatic behavior of human breast cancer cells after experimental manipulation of matrix metalloproteinase 8 gene expression. Cancer Res. 2004;64:1687–1694. doi: 10.1158/0008-5472.can-03-2047. [DOI] [PubMed] [Google Scholar]
- 201.Gutierrez-Fernandez A, Inada M, Balbin M, Fueyo A, Pitiot AS, Astudillo A, Hirose K, Hirata M, Shapiro SD, Noel A, Werb Z, Krane SM, Lopez-Otin C, Puente XS. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) Faseb J. 2007;21:2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE, Pearlman E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. Am J Pathol. 2008;173:144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nabha SM, Bonfil RD, Yamamoto HA, Belizi A, Wiesner C, Dong Z, Cher ML. Host matrix metalloproteinase-9 contributes to tumor vascularization without affecting tumor growth in a model of prostate cancer bone metastasis. Clin Exp Metastasis. 2006;23:335–344. doi: 10.1007/s10585-006-9042-x. [DOI] [PubMed] [Google Scholar]
- 205.Lakka SS, Gondi CS, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, Rao JS. Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 2003;63:2454–2461. [PubMed] [Google Scholar]
- 206.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 207.Lakka SS, Gondi CS, Dinh DH, Olivero WC, Gujrati M, Rao VH, Sioka C, Rao JS. Specific interference of urokinase-type plasminogen activator receptor and matrix metalloproteinase-9 gene expression induced by double-stranded RNA results in decreased invasion, tumor growth, and angiogenesis in gliomas. J Biol Chem. 2005;280:21882–21892. doi: 10.1074/jbc.M408520200. [DOI] [PubMed] [Google Scholar]
- 208.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 209.Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, Sloane BF, Matrisian LM. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. doi: 10.1158/0008-5472.CAN-05-4279. [DOI] [PubMed] [Google Scholar]
- 210.Houghton AM, Grisolano JL, Baumann ML, Kobayashi DK, Hautamaki RD, Nehring LC, Cornelius LA, Shapiro SD. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 211.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 212.Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N, Chang E, Tao Q, Vanhove M, Lejeune A, van Gool R, Sexton DJ, Kuang G, Rank D, Hogan S, Pazmany C, Ma YL, Schoonbroodt S, Nixon AE, Ladner RC, Hoet R, Henderikx P, Tenhoor C, Rabbani SA, Valentino ML, Wood CR, Dransfield DT. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
- 213.Jost M, Folgueras AR, Frerart F, Pendas AM, Blacher S, Houard X, Berndt S, Munaut C, Cataldo D, Alvarez J, Melen-Lamalle L, Foidart JM, Lopez-Otin C, Noel A. Earlier onset of tumoral angiogenesis in matrix metalloproteinase-19-deficient mice. Cancer Res. 2006;66:5234–5241. doi: 10.1158/0008-5472.CAN-05-4315. [DOI] [PubMed] [Google Scholar]
- 214.Kolb C, Mauch S, Krawinkel U, Sedlacek R. Matrix metalloproteinase-19 in capillary endothelial cells: expression in acutely, but not in chronically, inflamed synovium. Exp Cell Res. 1999;250:122–130. doi: 10.1006/excr.1999.4493. [DOI] [PubMed] [Google Scholar]
- 215.Pendas AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodriguez F, Astudillo A, Noel A, Birkedal-Hansen H, Lopez-Otin C. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 2004;24:5304–5313. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Quesada AR, Medina MA, Alba E. Playing only one instrument may be not enough: limitations and future of the antiangiogenic treatment of cancer. Bioessays. 2007;29:1159–1168. doi: 10.1002/bies.20655. [DOI] [PubMed] [Google Scholar]
- 217.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 218.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–813. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–818. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 223.Molina JR, Reid JM, Erlichman C, Sloan JA, Furth A, Safgren SL, Lathia CD, Alberts SR. A phase I and pharmacokinetic study of the selective, non-peptidic inhibitor of matrix metalloproteinase BAY 12-9566 in combination with etoposide and carboplatin. Anticancer Drugs. 2005;16:997–1002. doi: 10.1097/01.cad.0000176504.86551.5c. [DOI] [PubMed] [Google Scholar]
- 224.Fingleton B. MMP Inhibitor Clinical Trials - The Past, Present, and Future. In: DEe al., editor. The Cancer Degradome. Vol Part V. New York: Springer; 2008. pp. 759–785. [Google Scholar]
- 225.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5:812–817. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis:do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, De Smet F, Vinckier S, Aragones J, Debackere K, Luttun A, Wyns S, Jordan B, Pisacane A, Gallez B, Lampugnani MG, Dejana E, Simons M, Ratcliffe P, Maxwell P, Carmeliet P. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 230.Vacca A, Ribatti D, Roccaro AM, Ria R, Palermo L, Dammacco F. Bone marrow angiogenesis and plasma cell angiogenic and invasive potential in patients with active multiple myeloma. Acta Haematol. 2001;106:162–169. doi: 10.1159/000046612. [DOI] [PubMed] [Google Scholar]
- 231.Lafleur MA, Forsyth PA, Atkinson SJ, Murphy G, Edwards DR. Perivascular cells regulate endothelial membrane type-1 matrix metalloproteinase activity. Biochem Biophys Res Commun. 2001;282:463–473. doi: 10.1006/bbrc.2001.4596. [DOI] [PubMed] [Google Scholar]
- 232.Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- 233.Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, Roberts JD, Fay WP, Birkedal-Hansen H, Holmbeck K, Sabeh F, Allen ED, Weiss SJ. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005;202:663–671. doi: 10.1084/jem.20050607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bremer C, Bredow S, Mahmood U, Weissleder R, Tung CH. Optical imaging of matrix metalloproteinase-2 activity in tumors: feasibility study in a mouse model. Radiology. 2001;221:523–529. doi: 10.1148/radiol.2212010368. [DOI] [PubMed] [Google Scholar]
- 235.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carragher NO. Profiling distinct mechanisms of tumour invasion for drug discovery: imaging adhesion, signalling and matrix turnover. Clin Exp Metastasis. 2009;26:381–397. doi: 10.1007/s10585-008-9222-y. [DOI] [PubMed] [Google Scholar]
- 237.Yang J, Zhang Z, Lin J, Lu J, Liu BF, Zeng S, Luo Q. Detection of MMP activity in living cells by a genetically encoded surface-displayed FRET sensor. Biochim Biophys Acta. 2007;1773:400–407. doi: 10.1016/j.bbamcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 238.Ouyang M, Lu S, Li XY, Xu J, Seong J, Giepmans BN, Shyy JY, Weiss SJ, Wang Y. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J Biol Chem. 2008;283:17740–17748. doi: 10.1074/jbc.M709872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 240.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4:1399–1408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]