Abstract
Arachidonic acid, a polyunsaturated fatty acid, can be metabolized to cardioprotective epoxyeicosatrienoic acids (EETs) by cytochrome P450 epoxygenases, which are subsequently hydrolyzed to less bioactive dihydroxyeicosatrienoic acids by soluble epoxide hydrolase (sEH). To study the effects of pharmacological inhibitor of sEH (sEHi), C57BL6 mice hearts were perfused in Langendorff mode for 40min of baseline and subjected to 30min of global no-flow ischemia followed by 40 min of reperfusion. Hearts were perfused with the sEHi, trans-4-[4-(3-adamantan-1-y1-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB; 0.05 µM, 0.1 µM, 0.5 µM and 1 µM). To study the mechanism(s), hearts were perfused with 0.1 µM t-AUCB in the presence or absence of putative EET receptor antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE, 10 µM) or phosphatidylinositol 3-kinase (PI3K) inhibitors wortmannin (200 nM) or LY294002 (5 µM). Infarct size was determined at the end of 2 h reperfusion by TTC staining. Inhibition of sEH by t-AUCB significantly improved postischemic left ventricular developed pressure (LVDP) recovery and reduced the infarct size following ischemia-reperfusion, as compared to control hearts. Perfusion with 14,15-EEZE, wortmannin or LY294002 prior to ischemia abolished the cardioprotective phenotype; however, co-perfusion of both t-AUCB and 11,12-EET did not result in an additive effect on improved LVDP recovery. Together, our data suggest that pharmacological inhibition of sEH by t-AUCB is cardioprotective.
Keywords: sEH inhibitors, Ischemia-reperfusion, Cardioprotection, Epoxyeicosatrienoic acid
INTRODUCTION
Arachidonic acid, is a polyunsaturated fatty acid that is present in the phospholipids of cell membranes which can be released into cytosol in response to stressors such as ischemia.1,2 Released free AA can then be metabolized by cyclooxygenases, lipoxygenases, and cytochrome P450 (CYP) monooxygenases.1,3 Biologically active regioisomers, epoxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EET) are products by CYP monooxygenases.2,3 EETs can be reincorporated into phospholipid membranes or metabolized to smaller reactive epoxides by β-oxidation.4,5 However, the predominant pathway of EET metabolism is conversion to the less active vicinal diols, dihydroxyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-DHET), by soluble epoxide hydrolases (sEH).4,5
EETs act as important cellular lipid mediators in the cardiovascular, renal and nervous systems.3,6–8 EETs have been shown to have protective effects against ischemia reperfusion injury.1,9–14 Our current understanding of the cardioprotective mechanism(s) of EETs suggest involvement of signaling pathways including phosphoinositide 3-kinase (PI3K) – Akt, increased secretion of cardiac hormones, and activation of cardiac ion channels such as ATP-sensitive K+ channels.1,9,10,12,13 Recent evidence indicates that PI3K/Akt or natriuretic peptide pathways activated by EETs converge onto the mitochondria thereby limiting mitochondrial damage from ischemia reperfusion injury.10,12
Previously we demonstrated that targeted deletion of sEH gene is protective against ischemia reperfusion injury.1,10 Recently, Motoki et al. has reported pharmacological inhibition of sEH with 12-(3-adamantan-1-yl-ureido)-dodecanoic acid n-butyl ester (AUDA-nBE) was cardioprotective.15 In the present study we report cardioprotective effects of more potent, water soluble and metabolically stable sEH inhibitor (sEHi), trans-4-[4-(3-adamantan-1-y1-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB) than AUDA-nBE. Moreover, our data demonstrate marked reduction in infarction and improved contractile function at nanomolar concentrations which involved the PI3K pathway.
MATERIAL AND METHOD
ANIMALS
All experiments used male and female mice aged 3–5 months, weighing 22–33 g and were treated in accordance with the guidelines of Health Science Laboratory Animal Services (HSLAS), University of Alberta. C57BL6 mice were purchased from Charles River Laboratories (Pointe Claire, PQ). A colony of mice with targeted disruption of the Ephx2 gene (sEH null) and backcrossed onto a C57BL6 genetic background for more than 7 additional generations, is maintained at the University of Alberta.
CHEMICALS
EETs were a kind gift from Dr. J. R. Falck (University of Texas Southwestern Medical Center, Dallas, TX). Stock solutions (5 mM) were dissolved in 100% ethanol and working solutions were diluted in perfusion buffer (1 µM). The sEHi, trans-4-[4-(3-adamantan-1-y1-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB), was synthesized in the laboratory of Dr. Bruce Hammock (UC Davis) and dissolved in DMSO to make 10 mM stock solution. The putative EET receptor antagonist, 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) was a kind gift from Dr. J. R. Falck and dissolved in 100% ethanol to make 5 mM stock solution. PI3K inhibitors, Wortmannin (Sigma-Aldrich, Oakville, ON) and LY294002 (Cell Signaling Technology, Inc., Danvers, MA), were dissolved in DMSO to make 5 mM and 50 mM stock solutions, respectively.
ISOLATED HEART PERFUSIONS
Hearts were perfused in the Langendorff mode as previously published.1,10 Briefly, hearts from age/sex-matched mice were perfused in a retrograde fashion at constant pressure (90 cmH2O) with continuously aerated (95%O2/5%CO2) Krebs-Henseleit buffer at 37°C. Hearts were perfused with buffer for 40 min of stabilization period and then subjected to 30 min global no-flow ischemia, followed by 40 min reperfusion. To determine t-AUCB dose-response, hearts from C57Bl6 mice were perfused with increasing concentrations of t-AUCB (0, 0.05, 0.1, 0.5 or 1µM). For some experiments, hearts were perfused for 40 min baseline, subjected to 30 min ischemia and then perfused with 0.1 µM t-AUCB in the presence or absence of putative EET receptor antagonist 14,15-EEZE (10 µM), PI3K inhibitors wortmannin (200 nM) or LY294002 (5 µM). The percentage of left ventricular developed pressure (%LVDP) at 40 min of reperfusion (R40), as compared to baseline LVDP, was taken as a marker for recovery of contractile function. After 40 min of reperfusion, hearts were immediately frozen and stored below −20°C.
INFARCT SIZE ANALYSIS
To determine the amount of infarction, following 40 min of stabilization period and 30 min global no-flow ischemia, hearts were reperfused for 2 h. After 2 h reperfusion, hearts were perfused with 1% solution of 2,3,5-triphenyltetrazolium chloride (TTC) dissolved in Krebs-Henseleit buffer at 37 °C for 10 min, then fixed in formalin and cut into thin cross-sectional slices. The area of infarction was quantified by measuring stained (red, live tissue) and unstained (white, necrotic) regions using Image J (NIH, USA).
STATISTICAL ANALYSIS
Values expressed as mean ± standard error of mean (SEM). Statistical significance was determined by the unpaired Student’s t-test and one-way ANOVA followed by Newman-Keuls and Duncan’s tests to assess differences between groups. Values were considered significant if p < 0.05.
RESULTS
CARDIOPROTECTIVE EFFECTS OF t-AUCB
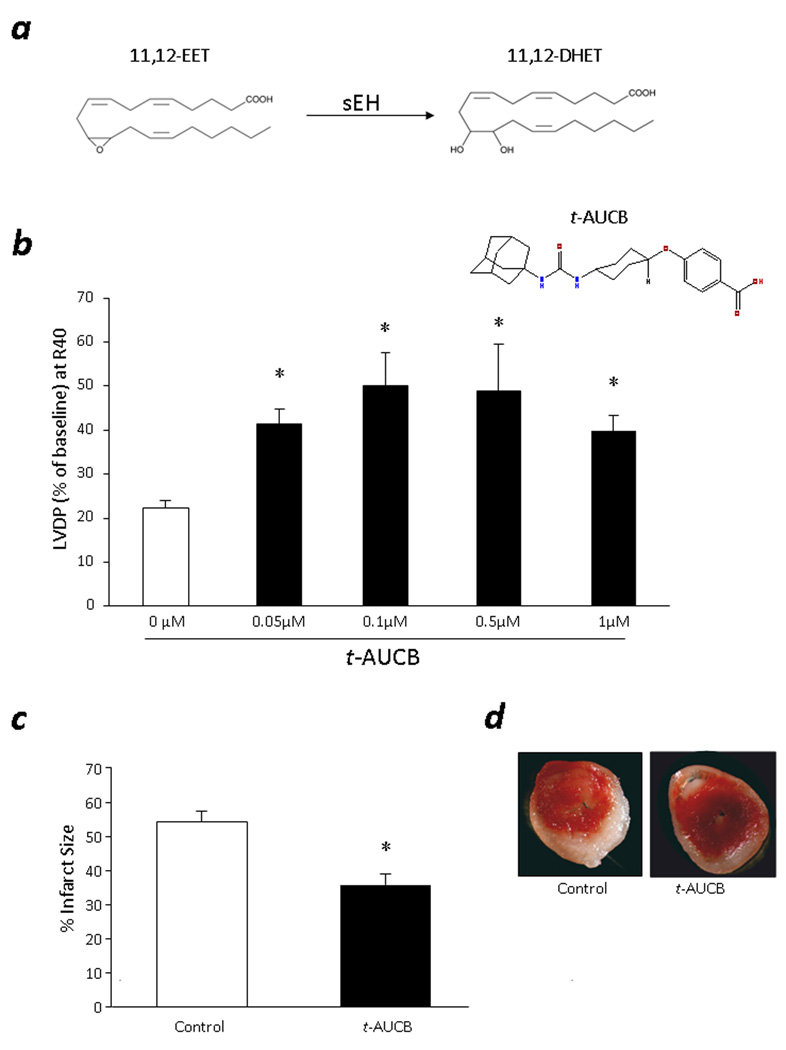
Recent evidence suggests that sEH is a good target in the prevention of ischemia reperfusion injury.1,10,15 To examine the effects of pharmacological inhibition of sEH on ischemia reperfusion injury, we first performed a dose-response study. We perfused the WT mouse hearts with 0, 0.05, 0.1, 0.5 and 1 µM of t-AUCB and monitored LVDP for postischemic functional recovery. No significant differences in baseline contractile function were observed between the groups, except LVDP in 1 µM t-AUCB treated hearts, during aerobic baseline perfusion (Table 1). Hearts perfused with t-AUCB had significantly improved postischemic recovery of LVDP compared to control mice (Fig 1b). The improved postischemic recovery followed a bell-shaped response, with the most improved functional recovery occurring at the mid-dose (0.1 µM). Therefore, 0.1 µM t-AUCB was used for further study the cardioprotective effects.
Table 1.
Cardiac parameters for t-AUCB dose response
| Vehicle control (n=11) |
0.05 µM t-AUCB (n=4) |
0.1 µM t-AUCB (n=6) |
0.5 µM t-AUCB (n=5) |
1 µM t-AUCB (n=4) |
|
|---|---|---|---|---|---|
|
Isolated Perfused Heart – Preischemic | |||||
| LVDP (cmH2O) (Baseline) | 112.7±7.9 | 126.9±13.2 | 103.9±5.7 | 132.4±16.1 | 168.4±12.5* |
| Rate of contraction, dP/dtmax (cmH2O/msec) (Baseline) |
3249±294 | 5021±318 | 3223±213 | 3757±517 | 4089±451 |
| Rate of relaxation, −dP/dtmin (cmH2O/msec) (Baseline) |
−2683±188 | −3926±225 | −2670±168 | −3054±398 | −3688±333 |
| HR, perfused (beats/min) (Baseline) | 291±19 | 334±13 | 355±19 | 277±46 | 268±21 |
|
Isolated Perfused Heart – Postischemic | |||||
| LVDP (cmH2O) (R40) | 25.1±2.9 | 52.0±5.6* | 47.0±8.8* | 57.2±14.7* | 51.8±9.4* |
| Rate of contraction, dP/dtmax (cmH2O/msec) (R40) |
807±92 | 1582±115* | 1404±289* | 1697±415* | 1477±275* |
| Rate of relaxation, −dP/dtmin (cmH2O/msec) (R40) |
−688±82 | −1370±143* | −1326±169* | −1367±320* | −1285±203* |
| HR, perfused (beats/min) (R40) | 323±16 | 337±25 | 340±16 | 347±21 | 298±27 |
Hemodynamic parameters were measured in isolated-perfused hearts. Values represent mean±SEM,
p<0.05 vs vehicle control. LVDP, left ventricular pressure, HR, heart rate.
Figure 1. Cardioprotective effects of t-AUCB.
a, Metabolism of 11,12-EET to 11,12-DHET by sEH. b, LVDP recovery at 40min of reperfusion in WT hearts treated with t-AUCB (0, 0.05, 0.1, 0.5 or 1µM). Values represent mean±SEM; n=4–11 per group; *, p<0.05 vs. control. c, Quantification of infarct size. Values represent mean±SEM; n=5; *, p<0.05 vs. Control. d, Representative images of TTC staining in t-AUCB and vehicle control hearts. Surviving tissue stained red with TTC and infracted white tissue.
To further assess the cardioprotective effects of t-AUCB, we analyzed the infarct size following 2 h reperfusion in t-AUCB treated hearts and vehicle control hearts. Significant decrease in infarct size was observed in the hearts treated with 0.1 µM t-AUCB compared to vehicle controls (Fig 1c). Consistent with 40 min reperfusion, postischemic functional recovery remained significantly higher at 2 h reperfusion in t-AUCB treated hearts compared to vehicle controls (LVDP=43.3±13.3% vs. 21.2±1.8%).
PHARMACOLOGICAL OR GENETIC INHIBITION OF SEH AND CARDIOPROTECTION
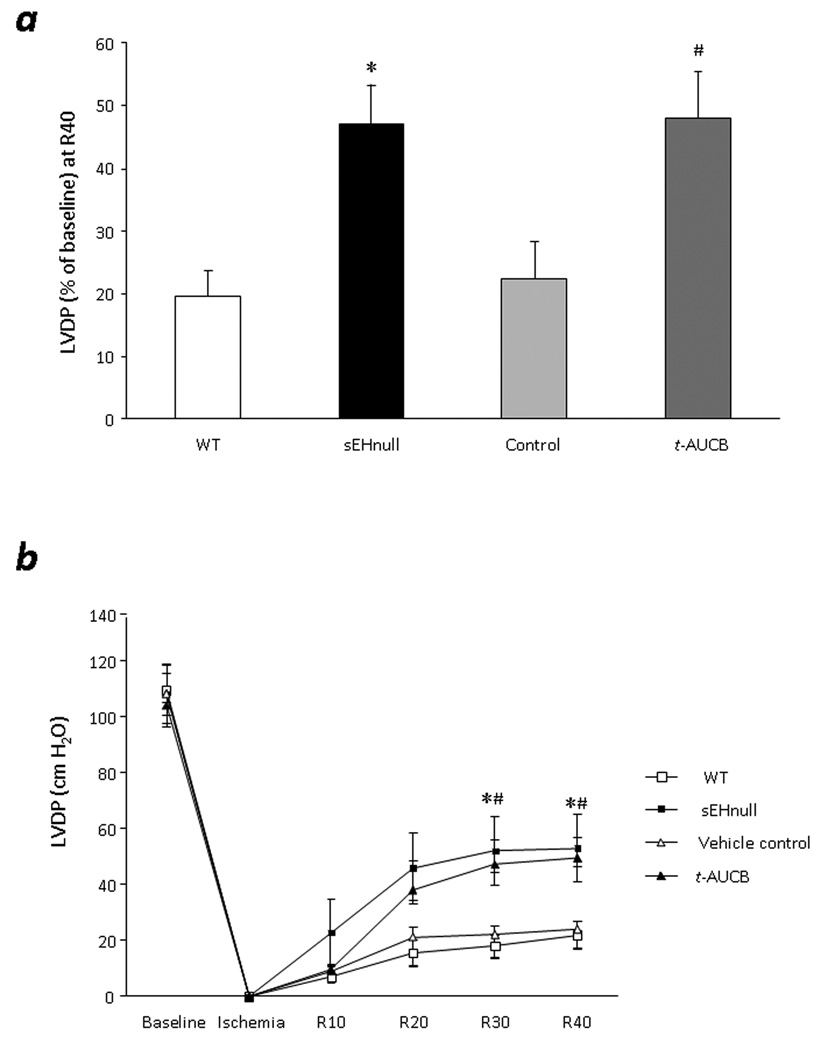
To compare the cardioprotective effects of genetic and pharmacological inhibition of sEH, we perfused WT mice with 0.1 µM t-AUCB and sEH null mice with vehicle. Significant increase in postischemic functional recovery of sEH null (LVDP= 47.3±6.2%) and t-AUCB perfused WT (LVDP= 48.0±7.2%) was observed following ischemia reperfusion protocol, compared to vehicle controls (LVDP= 22.4±1.7%) and WT (LVDP= 19.7±3.8) (Fig 2a, Table 2). No significant differences were observed in cardiac parameters during baseline (Table 2). Consistent with LVDP, rate of contraction and relaxation were significantly higher in sEH null hearts and t-AUCB treated hearts following ischemia reperfusion (Table 2). No significant differences were observed in heart rate during baseline or following ischemia reperfusion (Table 2). The improved function was evident within 30 min of reperfusion following treatment with 0.1 µM t-AUCB and sEH null mice, which persisted throughout the recovery period (Fig 2b).
Figure 2. Effect of genetic or pharmacological inhibition of sEH on postischemic contractile function.
a, LVDP recovery at 40min of reperfusion in WT hearts, sEH null, vehicle control and WT treated with t-AUCB (0.1µM). Values represent mean±SEM; n=5–11 per group; *, p<0.05 vs. WT; #, p<0.05 vs. Vehicle control. b, LVDP in perfused hearts from WT, sEH null, vehicle control and WT treated with t-AUCB (0.1µM). Values represent mean ± SEM; n=5–11 per group; *, p<0.05 vs. WT; #, p<0.05 vs. vehicle control.
Table 2.
Cardiac parameters for pharmacological and genetic inhibition of sEH
| WT (n=5) |
sEH null (n=9) |
Vehicle control (n=11) |
WT + t-AUCB (1µM) (n=5) |
|
|---|---|---|---|---|
|
Isolated Perfused Heart - Preischemic | ||||
| LVDP (cmH2O) (Baseline) | 114.1±9.0 | 112.5±10.8 | 112.7±7.9 | 108.4±3.6 |
| Rate of contraction, dP/dtmax (cmH2O/msec) (Baseline) |
3384±332 | 3416±507 | 3249±294 | 3361±198 |
| Rate of relaxation, −dP/dtmin (cmH2O/msec) (Baseline) |
−2920±257 | −2809±448 | −2683±188 | −2786±148 |
| HR, perfused (beats/min) (Baseline) | 325±28 | 297±29 | 291±19 | 368±17# |
|
Isolated Perfused Heart - Postischemic | ||||
| LVDP (cmH2O) (R40) | 22.8±4.7 | 55.2±12.4* | 25.1±2.9 | 51.5±7.8# |
| Rate of contraction, dP/dtmax (cmH2O/msec) (R40) | 587±142 | 1377±162* | 807±92 | 1549±306# |
| Rate of relaxation, −dP/dtmin (cmH2O/msec) (R40) | −578±119 | −1291±181* | −688±82 | −1387±194# |
| HR, perfused (beats/min) (R40) | 244±42 | 251±23 | 323±16 | 333±18 |
Hemodynamic parameters were measured in isolated-perfused hearts. Values represent mean±SEM,
p<0.05 vs WT;
p<0.05 vs. Vehicle control. LVDP, left ventricular pressure, HR, heart rate.
EET MEDIATED EFFECTS OF t-AUCB
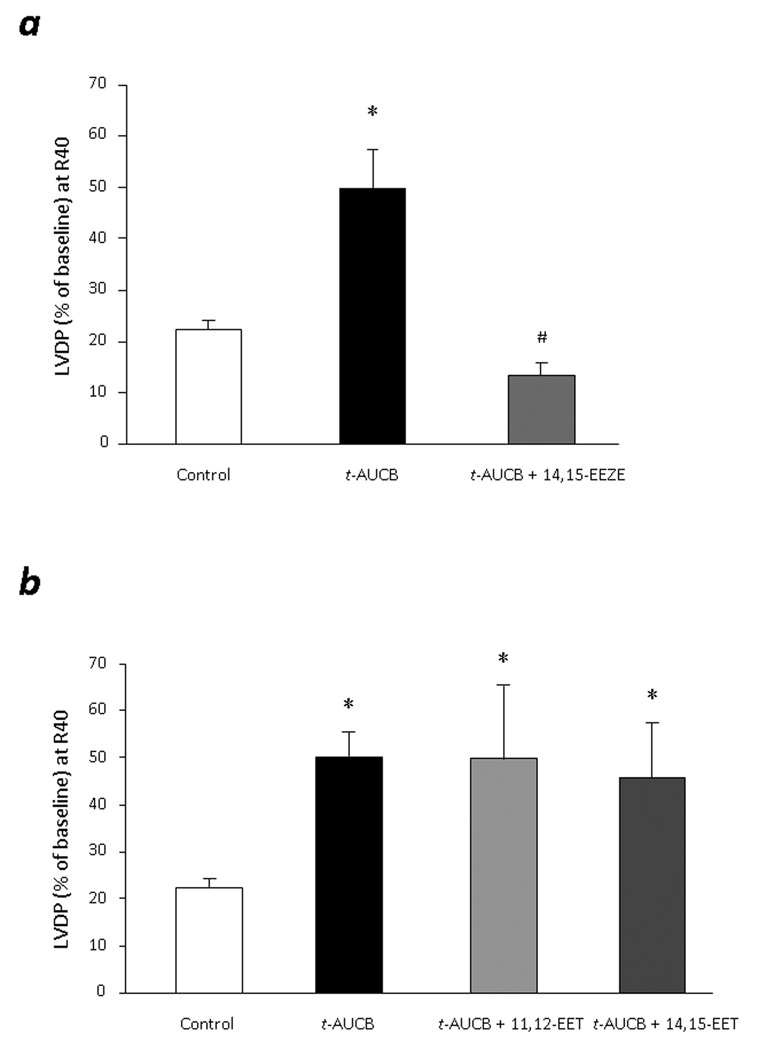
As previously reported, targeted deletion or pharmacological inhibition of sEH leads to increase in intracellular EET levels which can further produce cardioprotective effects following ischemia reperfusion injury.1 To confirm the EET mediated effects of t-AUCB, we perfused the WT hearts with 0.1 µM t-AUCB in presence or absence of putative pan-EET receptor antagonist 14, 15-EEZE (10 µM). 14,15-EEZE did not affect the baseline function of the WT mice hearts (Data not shown). However, postischemic functional recovery of t-AUCB treated hearts (LVDP= 49.9 ± 7.5%) was completely abolished in the hearts co-perfused with 14,15-EEZE (LVDP= 13.3±2.5%) (Fig 3a). To assess the effects of exogenous EET cardioprotective effects of t-AUCB, we perfused the WT hearts with t-AUCB in presence or absence of 11,12-EET (1 µM) and 14,15-EET (1 µM). Interestingly, no additive effects of exogenous EET was observed on t-AUCB treated hearts (Fig 3b). These data suggests that maximum recovery was achieved by treating the hearts with 0.1 µM t-AUCB and therefore addition of exogenous EET does not show any additional effect on functional recovery.
Figure 3. EET mediated cardioprotection in t-AUCB treated hearts.
a. LVDP recovery at 40min of reperfusion in WT hearts treated with t-AUCB (0.1µM) in presence or absence of putative EET-receptor antagonist, 14,15-EEZE (10 µM). Values represent mean ± SEM; n=5 per group; *, p<0.05 vs. Vehicle control; #, p<0.05 vs. t-AUCB treated hearts. b. LVDP recovery at 40min of reperfusion as percentage of baseline. Hearts were perfused with t-AUCB (0.1µM) in presence or absence of exogenous 11,12-EET (1µM) and 14,15-EET (1µM). Values represent mean ± SEM; n=3–5 per group; *, p<0.05 vs. vehicle control.
PI3K MEDIATED EFFECTS OF t-AUCB
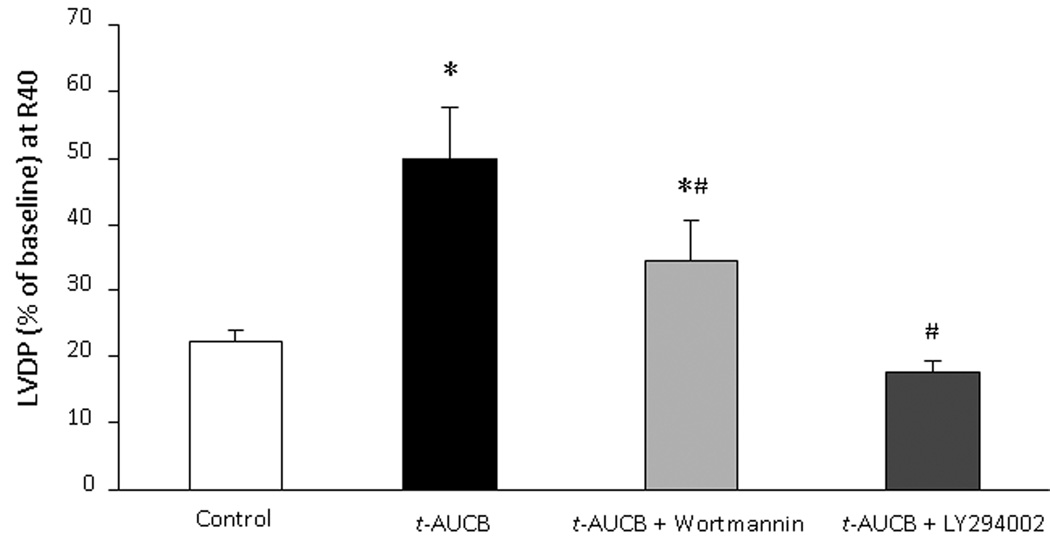
To determine the role of the PI3K signaling cascade in t-AUCB mediated cardioprotective response, we performed isolated perfused heart experiments in the presence or absence of the PI3K inhibitors wortmannin or LY294002. Neither inhibitor had a significant effect on baseline LVDP (data not shown). Perfusion with either wortmannin or LY294002 for 5 min prior to ischemia had no effect on postischemic LVDP recovery in vehicle controls; however, both inhibitors significantly reduced the improved postischemic functional recovery in t-AUCB treated hearts (Fig 4). Thus, percent LVDP recovery at 40 min reperfusion was comparable in both the groups after treatment with either wortmannin or LY294002 (Fig 4). These data suggest the involvement of the PI3K cascade in the cardioprotective effects of pharmacological inhibition of sEH.
Figure 4. Role of PI3K in t-AUCB mediated cardioprotection.
LVDP recovery at 40min of reperfusion as percentage of baseline. Hearts were perfused with t-AUCB (0.1µM) in presence or absence of PI3K inhibitor wortmannin (200 nM) or LY294002 (5µM). Values represent mean ± SEM; n=5 per group; *, p<0.05 vs. vehicle control; #, p<0.05 vs. t-AUCB treated hearts.
DISCUSSION
In this paper, we report improved postischemic contractile function and reduced infarct size in isolated mice hearts perfused with a novel water-soluble and potent pharmacological sEHi, t-AUCB. The effects of t-AUCB were attenuated by the putative EET-receptor antagonist 14,15-EEZE and inhibition of PI3K. Taken together, our data suggest that pharmacological inhibition of sEH by t-AUCB can be protective against ischemia reperfusion injury and the effects are mediated through EETs and PI3K pathway.
sEH is a bifunctional enzyme with C-terminus hydrolase activity and N-terminus phosphatase activity.5,16 While the functional effects of phosphatase are not well known, much evidence implicates the importance of its hydrolase activity in cardiovascular diseases.1,5,11,15,17,18 Two different approaches have been used to study the effects of sEH on cardiovascular system: genetic modification and pharmacological inhibition.1,11,15 Genetic modulation by targeted deletion of Ephx2 gene results in lack of sEH expression and reported to produce anti-arrhythmic, antihypertensive and cardioprotective effects.1,19 On the other hand, sEHi causes inhibition of hydrolase activity exerting similar protective effects in the cardiovascular system.11,15,20,21 Consistent with the previous observations, the data in this manuscript indicate that both genetic and chemical knockout of sEH lead to a dramatic improvement on the cardiac parameters measure. However, neither approach resulted in full return to normal function indicating that additional approaches could be helpful in restoring cardiac function. The fact that both the chemical and genetic knockout experiments resulted in similar improvements in function indicates that a near maximum effect for a sEHi was reached with t-AUCB in this system.
Small molecule inhibitors are valuable tools to study the role of sEH in the pathophysiology of cardiovascular diseases. The first generation of sEHi were epoxide-containing compounds that turned out to be substrates of EH metabolism.16 These compounds tended to only have a transient inhibitory effect in vitro and were not effective in vivo.16,22 EH inhibitory properties were shown with urea, amide and carbamate functionalities as the central pharmacophore. The ureas were in general the most active with similar R groups, but amide and carbamate derivatives with optimized substituents can be as active as ureas.16 The next generation of sEHi demonstrated that 1,3-Disubstituted urea derivatives were stable and more potent sEHi than previous compounds.16,23 t-AUCB has a lipophilic adamantane on the N of the urea and a bicyclic system with a polar ether and acid on the N' position which dramatically increase water solubility.24–26 Formation of hydrogen bonds and salt bridges between urea functionality and active cites of sEH results in inhibition of hydrolase activity.16 sEH has a high Vmax and low KM for endogenous epoxides of arachidonic and linoleic acid which results in diminished biological activity. Emerging evidence demonstrates that sEHi are potential therapeutic compounds for the treatment of several diseases. cis-isomer of t-AUCB (cic-4-[4-(3-adamantan-1-y1-ureido)-cyclohexyloxy]-benzoic acid; c-AUCB) has recently been demonstrated to attenuate monocrotalin induced pulmonary hypertension in rats.27 Xu et al. has reported importance of sEH inhibition prevented and reversed cardiac hypertrophy using 1-adamantan-3-(5-(2-(2-ethylethoxy)ethoxy)pentyl)urea, AEPU.28 Similarly, 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea (TUPS) can block angiotensin-II induced cardiac hypertrophy.20 1,3-Disubstituted urea sEHi such as AUDA and AUDA-BE have demonstrated protective effects against ischemia reperfusion injury in the micromolar range.11,15 In the present study, t-AUCB exerted cardioprotective effects by inhibiting sEH activity at nanomolar concentrations. Apart from urea based sEHi, non-urea derivatives of potent sEHi have also been reported; however, no study has demonstrated in vivo effectiveness of these compounds.29,30
Cardioprotective effects towards ischemia reperfusion injury by sEHi compounds have only been demonstrated with AUDA and AUDA-BE.11,15 These sEHi compounds have several drawbacks which include limited oral bioavailability, instability due to rapid metabolism and poor physical properties such as low water solubility.18 With very careful formulation AUDA and its salts and esters can be orally administered for in vivo use, but the new generation of sEHi such as t-AUCB are dramatically easier to administer and have longer half lives.5,11,15,18,31 t-AUCB (IC50 = 1.3 nM) is a more potent inhibitor of sEH than AUDA (IC50 = 3 nM) and AUDA-nBE (IC50 = 7 nM), while it is equipotent to TPAU (IC50 = 1.1 nM) and c-AUCB (IC50 = 0.89 nM).26 Liu et al. compared AUDA-BE to t-AUCB for total epoxide and diols in plasma of LPS treated mice. Both, AUDA-BE and t-AUCB, significantly reduced DHET formation increasing the epoxide to diol ratio. However, t-AUCB was able to increase the EET/DHET ratio to similar levels of AUDA-BE at a 100 times lower dose.24 t-AUCB has better oral bioavailability and metabolic stability as higher area under concentration time curve (AUCt) and longer elimination half life (t1/2) (AUCt = 155 µM/L; t1/2 > 1400 min) compared to AUDA (AUCt = 24 µM/L; t1/2 = 575 min) and AUDA-BE (AUCt = 16 µM/L; t1/2 = 260 min).18,24,26,31 Similarly, pharmacokinetic studies done in canine model revealed that t-AUCB was found in the circulation for 1 day following oral administration.18 The improved physical and pharmacokinetic properties of t-AUCB make it an attractive therapeutic agent and experimental probe for increasing beneficial EETs.
Inhibition of sEH decreases metabolism of EET and thereby exerts protective effects against injury incurred following myocardial ischemia reperfusion and ischemic stroke.1,11,15,17 Increased EETs in the heart activate various cardioprotective pathways leading to improved contractile function and reduced damage to the heart.1,11,13 The cardioprotective effects of EETs and sEHi can be blocked by simultaneous treatment with putative EET receptor antagonist, 14,15-EEZE.1,9–11 Consistent with these observations, our data suggest EETs mediate the protective effects of t-AUCB, as the improved post ischemic functional recovery was abolished by co-perfusion with 14,15-EEZE. It was surprising that addition of EET to this system did not further increase cardiac function. This may be the result of sufficient amounts of EET, other omega-6 or 3 epoxides free or esterified in cardiac tissue, or the synthesis rate of epoxides is high enough that supplementation is not needed. Recent evidence suggests that EETs can activate PI3K/Akt signaling pathway which leads to inhibition of GSK-3β and reduces the damage to mitochondria.1,10 Dhanasekaran et al. reported EET-mediated activation of multiple antiapoptotic targets through PI3K/Akt survival signaling.31 Moreover, inhibition of PI3K pathway by wortmannin or LY294002, abolished the improved postischemic functional recovery of sEH null mice.1 Similarly, our results show significant reduction in postischemic functional recovery of hearts co-perfused with t-AUCB and PI3K inhibitor wortmannin or LY294003, which confirms the involvement of PI3K survival signaling.
CONCLUSION
In conclusion, we report improved postischemic contractile function and reduced infarct size by treatment with a sEHi, t-AUCB. Moreover, the cardioprotective effect was attributed to EETs and the PI3K survival pathway. The increased potency and water solubility of t-AUCB over other sEHi suggest this compound may serve as a potential therapeutic agent in myocardial ischemia reperfusion injury and potentially a valuable drug for treatment ischemic heart diseases.
Acknowledgments
FUNDING: JMS is the recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research. This work was supported by Canadian Institutes of Health Research Grant (JMS MOP79465). The work was supported in part by NIEHS 37 ES02710 (BDH) and R01 ES013933 (BDH).
Footnotes
CONFLICT OF INTEREST: BDH founded Arete Therapeutics to move sEH inhibitors to the clinic. SHH and BDH are authors of University of California patents in the area.
REFERENCES
- 1.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006 Aug;99(4):442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007 Jan;82(1–4):50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007 Mar;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 4.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004 Jan;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 5.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007 Sept;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 6.Gauthier KM, Yang W, Gross GJ, Campbell WB. Roles of epoxyeicosatrienoic acids in vascular regulation and cardiac preconditioning. J Cardiovasc Pharmacol. 2007 Dec;50(6):601–608. doi: 10.1097/FJC.0b013e318159cbe3. [DOI] [PubMed] [Google Scholar]
- 7.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007 Jun;71(11):1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 8.Pratt PF, Medhora M, Harder DR. Mechanisms regulating cerebral blood flow as therapeutic targets. Curr Opin Investig Drugs. 2004 Sept;5(9):952–956. [PubMed] [Google Scholar]
- 9.Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009 Jan;46(1):67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary KR, Batchu SN, Das D, Suresh MR, Falck JR, Graves JP, Zeldin DC, Seubert JM. Role of B-type Natriuretic Peptide in Epoxyeicosatrienoic Acid Mediated Improved Postischemic Recovery of Heart Contractile Function. Cardiovasc Res. 2009 May; doi: 10.1093/cvr/cvp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008 Jun;294(6):H2838–H2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katragadda D, Batchu SN, Cho WJ, Chaudhary KR, Falck JR, Seubert JM. Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol. 2009 Mar; doi: 10.1016/j.yjmcc.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004 Sept;95(5):506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 14.Wang YX, Zeng XJ, Lu LQ, Ma LQ, Jiang DQ, Mu J, Wang XY, Zhang LK, Tang CS, Hao G. [Effects of 11, 12-epoxyeicosatrienoic acid preconditioning and postconditioning on Ca(2+)- handling proteins in myocardial ischemia/reperfusion injury in rats] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007 Dec;29(6):787–791. [PubMed] [Google Scholar]
- 15.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005 Feb;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007 Dec;27(12):1931–1940. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris TR, Li N, Chiamvimonvat N, Hammock BD. The potential of soluble epoxide hydrolase inhibition in the treatment of cardiac hypertrophy. Congest Heart Fail. 2008 Jul–Aug;14(4):219–224. doi: 10.1111/j.1751-7133.2008.08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, Gosele C, Heuser A, Fischer R, Schmidt C, Schirdewan A, Gross V, Hummel O, Maatz H, Patone G, Saar K, Vingron M, Weldon SM, Lindpaintner K, Hammock BD, Rohde K, Dietz R, Cook SA, Schunck WH, Luft FC, Hubner N. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008 May;40(5):529–537. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, Zhu Y. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009 Jan;106(2):564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Carroll MA, Chander PN, Falck JR, Sangras B, Stier CT. Soluble epoxide hydrolase inhibitor, AUDA, prevents early salt-sensitive hypertension. Front Biosci. 2008 May;13:3480–3487. doi: 10.2741/2942. [DOI] [PubMed] [Google Scholar]
- 22.Morisseau C, Du G, Newman JW, Hammock BD. Mechanism of mammalian soluble epoxide hydrolase inhibition by chalcone oxide derivatives. Arch Biochem Biophys. 1998 Aug;356(2):214–228. doi: 10.1006/abbi.1998.0756. [DOI] [PubMed] [Google Scholar]
- 23.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, Sanborn JR, Hammock BD. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci U S A. 1999 Aug;96(16):8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009 Jan;156(2):284–296. doi: 10.1111/j.1476-5381.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem. 2004 Apr;47(8):2110–2122. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007 Aug;50(16):3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revermann M, Barbosa-Sicard E, Dony E, Schermuly RT, Morisseau C, Geisslinger G, Fleming I, Hammock BD, Brandes RP. Inhibition of the soluble epoxide hydrolase attenuates monocrotaline-induced pulmonary hypertension in rats. J Hypertens. 2009 Feb;27(2):322–331. doi: 10.1097/hjh.0b013e32831aedfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu D, Li N, He Y, Timofeyev V, Lu L, Tsai HJ, Kim IH, Tuteja D, Mateo RK, Singapuri A, Davis BB, Low R, Hammock BD, Chiamvimonvat N. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006 Dec;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anandan SK, Do ZN, Webb HK, Patel DV, Gless RD. Non-urea functionality as the primary pharmacophore in soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2009 Feb;19(4):1066–1070. doi: 10.1016/j.bmcl.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Xie Y, Liu Y, Gong G, Smith DH, Yan F, Rinderspacher A, Feng Y, Zhu Z, Li X, Deng SX, Branden L, Vidovic D, Chung C, Schurer S, Morisseau C, Hammock BD, Landry DW. Discovery of potent non-urea inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett. 2009 Apr;19(8):2354–2359. doi: 10.1016/j.bmcl.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, Patel PK, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H724–H735. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]