Abstract
During evolution, as organisms increased in complexity and function, the need for the ensheathment and insulation of axons by glia became vital for faster conductance of action potentials in nerves. Myelination, as the process is termed, facilitates the formation of discrete domains within the axolemma that are enriched in ion channels, and macromolecular complexes consisting of cell adhesion molecules and cytoskeletal regulators. While it is known that glia play a substantial role in the coordination and organization of these domains, the mechanisms involved and signals transduced between the axon and glia, as well as the proteins regulating axo–glial junction formation remain elusive. Emerging evidence has shed light on the processes regulating myelination and domain differentiation, and key molecules have been identified that are required for their assembly and maintenance. This review highlights these recent findings, and relates their significance to domain disorganization as seen in several demyelinating disorders and other neuropathies.
1 Introduction
One of the most critical processes of both the central and peripheral nervous systems is myelination, involving the ensheathment and insulation of axons by glial cell membranes. As the glial cells, comprising Schwann cells in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS), contact and continually wrap their membranes around axons, they create polarized domains (Bhat 2003; Salzer 2003). These domains include the node, paranode, juxtaparanode, and internode.
During development, axo–glial interactions mediate the restriction of ion channels into specific membrane domains; that is, potassium channels in the juxtaparanode and sodium channels in the node of Ranvier, which, in turn, allow for the rapid and efficient conduction of the nerve impulse. The exact mechanisms governing the segmentation of ion channels into specific domains is elusive, but evidence has shown that disruption of paranodal axo–glial junctions leads to severe impairments of saltatory conduction, motor coordination, and myelination (Bhat 2003; Salzer 2003; Salzer et al. 2008). These phenotypes are often seen in demyelinating disorders and other neuropathies, which exemplify the importance of axo–glial junctions to the steady state kinetics of the action potential and proper nervous system functioning. Recent findings have identified several critical molecules and signaling pathways mediating the formation of axo–glial junctions and the regional organization of ion channel domains in the axonal membrane. In this review, new advancements in our knowledge of myelination and the differentiation of four domains in myelinated fibers will be highlighted, in addition to discussing the mechanisms regulating their formation, maintenance during normal functioning, and disease onset and progression.
2 Myelination of Axons
Myelination is a process whereby specialized cells of the nervous system, termed glia, elaborate double membrane wrappings around axons, creating an insulating layer that promotes the fast conduction of nerve impulses. The many wrappings effectively increase total membrane resistance and decrease total membrane capacitance between nodes of Ranvier, which greatly reduces “leakage” of current across the internodal membrane. The “sparing” of axoplasmic current, in combination with very fast internodal electrotonic conduction, rapidly depolarize the downstream nodal membrane to threshold.
While myelination is required in both the PNS and CNS, there are distinct differences between the myelin forming cells with respect to the proteins and the signals required for myelination in these two systems. In the PNS, as Schwann cells differentiate, they will assume one of the two fates: they will either (1) form a 1:1 relationship with an axon and myelinate it or (2) extend multiple processes that will ensheath several axons (Jessen and Mirsky 2005). Oligodendrocytes, on the other hand, extend multiple processes that will contact and myelinate several axons, up to forty separate axons at a time (Simons and Trotter 2007). While there are several factors that affect glial cell differentiation, such as growth factors and the extracellular matrix (ECM), the most notable is the axon phenotype, which determines its diameter. Those axons greater than 1 μm in diameter will be myelinated; whereas those smaller than 1 μm will be ensheathed. Interestingly, axonal diameter also determines the length of the internode, the segment of myelin between two nodes, as well as the thickness of the myelin layer(s), but the exact mechanisms governing the detection of axonal thickness by Schwann cells and oligodendrocytes remains elusive.
Premyelinating Schwann cells are distinctly bipolar, with processes extending longitudinally along the length of an axon. This extension will ultimately determine the location of the nodes of Ranvier and the internodal length. Once the internode is defined, signals from the axon and the ECM induce Schwann cells to extend their membrane laterally and spiral inwardly around the axon. The continuous wrapping of the Schwann cell membrane facilitates the development of the adaxonal (i.e., adjacent to the axon) and abaxonal (i.e., abutting the ECM) membrane layers. On the abaxonal side, Schwann cells are surrounded by a specialized ECM known as the basal lamina. The basal lamina is unique to the PNS and is formed by the Schwann cells to assist with their maturation and differentiation into a myelinating phenotype (Chernousov and Carey 2000; Court et al. 2006). Another unique feature of peripheral Schwann cells is the formation of nodal microvilli. These structures are small protrusions that extend beyond the distal-most paranodal loop and contact the underlying node. These structures are believed to participate in the formation of the node and mediate communication between the axonal node and the adjacent Schwann cell (Gatto et al. 2003; Ichimura and Ellisman 1991; Melendez-Vasquez et al. 2001).
Oligodendrocytes, unlike Schwann cells, are multipolar cells that have numerous processes extending from their cell bodies. These processes mediate the defasciculation and seperation of axons, to which, eventually, the majority of the processes will attach to, and also myelinate several different axons. As mentioned earlier, one oligodendrocyte has the ability to myelinate as many as forty axons (Simons and Trotter 2007). The ensheathment of multiple axons by oligodendrocytes suggest that different signaling mechanisms govern their ability to identify neighboring cells and to distinguish the node and other axonal domains compared to Schwann cells. At this time, little is known about these mechanisms or the molecules involved. Additional distinctions between oligodendrocytes and Schwann cells are the absence of microvilli overlying the node and a basal lamina, which is absent in the parenchyma of the central nervous system (Hildebrand et al. 1993; Melendez-Vasquez et al. 2001).
Because of the absence of the nodal protrusions, it is unclear how oligodendrocytes mediate intercellular signaling during the formation of the node. There is, however, evidence that oligodendrocytes secrete specific factors that coordinate the clustering of nodal components preceding myelination (Kaplan et al. 2001, 1997). Additionally, the existence of perinodal astrocytes are hypothesized to interact with nodal components, and thus may provide signaling cues to adjacent myelinating oligodendrocytes (Black and Waxman 1988; Hildebrand et al. 1993). The absence of basal lamina from oligodendrocytes suggests that other ECM components or environmental factors may provide the binding sites for anchorage requisite for myelination, but at this time this remains an unresolved issue. Although substantial differences exist between the mechanisms of myelination between PNS Schwann cells and CNS oligodendrocytes, one common feature is the ability of both types of glia to potentiate the development of polarized axonal domains during myelination. The formation of these domains (viz. the node, paranode, juxtaparanode, and internode) is crucial for proper saltatory conduction of the action potential. Key molecules are involved with the formation of these domains and their absence results in grave consequences as discussed below (Fig. 1).
Fig. 1.
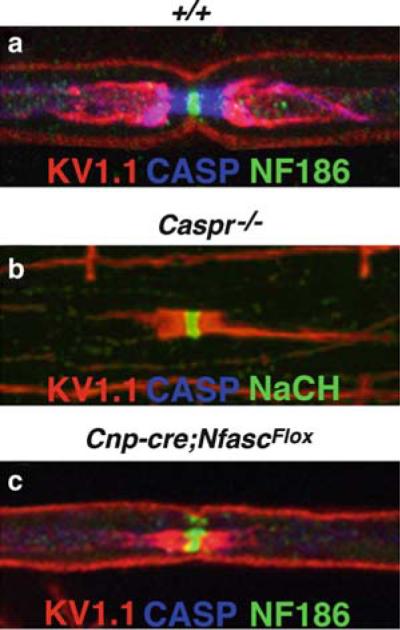
Domain organization in myelinated PNS nerve fibers. Teased sciatic nerve fibers from wild-type (+/+; a), Caspr null (Caspr-/- ;b), and NeurofascinNF155 (NF155) specific null mice (Cnpcre;NfascFlox; c) mice immunostained with antibodies against Kv1.1 (red), Caspr (blue), and Neurofascin 186 (NF186; green). In wild-type nerve fibers, localization of Kv1.1 fluorescence is restricted to the juxtaparanode. Caspr staining marks the paranode and NF186 is a marker of the nodal region. In Caspr null fibers, the lack of paranodal axo–glial junctions results in the diffusion of potassium channels into the paranode, as evident by the presence of Kv1.1 fluorescence adjacent to NF186 staining at the node (b). Loss of NF155 expression results in the lack of Caspr fluorescence at the paranode and the redistribution of potassium channels into the paranodal region, similar to Caspr mutants (c). In both mutants, the node remains unaltered as indicated by NF186 fluorescence
3 Axonal Domains of Myelinated Axons
3.1 The Node of Ranvier
The nodes of Ranvier are short, myelin-free segments of axonal membrane that are distributed at regular intervals along myelinated nerve fibers, in which the action potential is regenerated in a saltatory manner. These regions are enriched in voltage-gated sodium (Nav) ion channels, which occupy a density of approximately 1,500 μm−2 (Waxman and Ritchie 1993). Nav channels are heterotrimeric complexes comprised of a pore-forming α-subunit that regulates ion flow and one or more transmembrane-spanning β-subunits that mediate both extracellular and intracellular interactions (Isom 2002; Yu and Catterall 2003). During development, a transition between the α-subunits occurs, in which Nav1.2, present in immature nodes, is replaced by Nav1.6 in adult nodes (Boiko et al. 2001). While all mature nodes in the PNS express Nav1.6 exclusively, subsets of adult CNS nodes express Nav1.2 and Nav1.8 (Arroyo et al. 2002). The significance of this exchange in subunits is currently unknown, but may pertain to varying activity of the subunits. What is known is that the Nav channels are essential for the proper conduction of the nerve impulse, because loss of Nav1.6 causes a dramatic decrease in conduction velocities, accompanied with abnormal nodal and paranodal structure (Kearney et al. 2002).
Several proteins expressed in the node are known either to interact with and/or mediate Nav channel function, including the cytoskeletal proteins ankyrin G (AnkG), βIV spectrin, αII spectrin, and the cell adhesion molecules (CAMs) neurofascin (NF186), and NrCAM (Salzer 2003). AnkG belongs to a family of scaffolding proteins that function to stabilize membrane-associated proteins by linking them to the actin–spectrin cytoskeleton within specialized domains (Bennett and Lambert 1999). It is expressed in both the axon initial segment (AIS) and the nodes of neurons, where it interacts with Nav channels through either their α-, or β-subunit(s) (Bouzidi et al. 2002; Kordeli et al. 1995; Lemaillet et al. 2003; Malhotra et al. 2002). This interaction is essential for the targeting of Nav channels to the AIS, as mice deficient in a cerebellar-specific AnkG show a loss of Nav channel clustering at the AIS of Purkinje neurons and the inability to fire action potentials (Zhou et al. 1998a). Presumably, the loss of AnkG within the nodes may result in a similar loss of Nav channels; however, while the AIS and nodes have many similarities in molecular composition, their functions may be differentially regulated.
AnkG associates with the cytoskeleton through its interaction with b-spectrins; specifically, bIV spectrin, which is also localized to the nodes of Ranvier and AISs (Berghs et al. 2000; Komada and Soriano 2002). This interaction is critical for the clustering of AnkG, and in turn, Nav channels to the node inasmuch as loss of bIV spectrin in mice results in reduced levels of these proteins in nodes and AISs, increased nodal axonal diameter, and severe tremors and impaired nerve conduction (Komada and Soriano 2002; Lacas-Gervais et al. 2004). Concomitantly, AnkG null Purkinje neurons show loss of βIV spectrin in the AIS, revealing a codependent relationship between AnkG and βIV spectrin and their localization to these critical areas of action potential propagation (Jenkins and Bennett 2001).
Recently, another spectrin, αII spectrin, was identified at the nodes, and proposed to have physiological significance to the nodal architecture. Normally, αII spectrin is associated with the paranode, but new findings have indicated the presence of aII spectrin in immature nodes (Garcia-Fresco et al. 2006; Ogawa et al. 2006). Initially, αII spectrin is expressed in both the nodes and the paranodes in developing nerves, but gradually becomes restricted to the paranode as myelination progresses. Although its final site of expression resides at the paranode, αII spectrin was proposed to play a role in the assembly of the nodes and the clustering of Nav channels, since loss of its expression in the neurons of zebra fish resulted in abnormal nodal dimensions (Voas et al. 2007). Although electrophysiological data from these mutants could not be assessed, it is probable that the increase in nodal length observed may perturb the propagation of the action potential and slow down nerve conduction. Interestingly, the progressive restriction of aII spectrin during myelination may suggest that βIV spectrin replaces it in mature nodes. Further analysis of the significance of αII spectrin to the node or the paranode may prove to be important to our understanding of how these domains are initially constructed.
Neurofascin (NF186) and NrCAM are members of the L1 subfamily of immunoglobulin (Ig) cell adhesion molecules (CAMs) that mediate cell–cell, and cell–matrix interactions (Grumet 1997; Volkmer et al. 1992). Both proteins are expressed in the nodes and AISs and interact with AnkG through a conserved region present in the cytoplasmic domain of each protein (Davis et al. 1996; Lustig et al. 2001; Zhang et al. 1998). The association of AnkG with NF186 is mediated by tyrosine phosphorylation. The unphosphorylated form of NF186 is able to associate with AnkG at the nodes, while phosphorylation perturbs the interaction of NF186 with AnkG (Garver et al. 1997; Zhang et al. 1998). Through their interaction with AnkG, both NrCAM and NF186 are thought to coordinate Nav channel clustering and node formation, because accumulation of these CAMs occurs prior to the presence of both AnkG and Nav channels in PNS nodes (Custer et al. 2003; Lambert et al. 1997; Lustig et al. 2001). Additionally, experiments utilizing function-blocking antibodies against the CAMs revealed that both Nav channels and AnkG failed to accumulate at the nodes in in vitro cocultures (Lustig et al. 2001). Furthermore, mice deficient in NrCAM expression resulted in delayed aggregation of Nav channels and AnkG to the nodes, although they did eventually cluster and the nodes functioned normally (Custer et al. 2003). Conversely, other findings suggest that AnkG is responsible for the initial assembling of Nav channels and CAMs to CNS nodes (Jenkins and Bennett 2002).
At this time, no mutational analysis of NF186 alone has been conducted, but a conventional null mutant lacking both isoforms of neurofascin, NF186, and the glial neurofascin (NF155) expressed in the paranode exhibited complete loss of nodal and paranodal formation (Sherman et al. 2005). These mice died at postnatal day 6 (P6), which prevented further characterization of their functions in axo–glial domain formation and maintenance. However, recent findings by the same group revealed that reexpression of NF186 in the mutant axons resulted in the relocalization of AnkG and Nav channels to the node, suggesting that NF186 coordinates the formation of the node and the clustering of the Nav channels (Zonta et al. 2008). These results are very compelling and further analysis of a true NF186 knockout would greatly contribute to our future understanding of its role in nodal development and organization.
A unique set of nodal proteins exists that is expressed specifically in the PNS. These proteins, which reside within the Schwann cell microvilli that extend from the outermost paranodal loop of myelin, contact the node and are proposed to function in nodal development and formation. An array of proteins are expressed within these small protrusions, including gliomedin, ERM (ezrin/radixin/moesin) proteins, EBP-50 (ezrin binding protein 50), dystroglycan, RhoA-GTPase, and syndecans (Eshed et al. 2005; Gatto et al. 2003; Goutebroze et al. 2003; Melendez-Vasquez et al. 2004, 2001; Saito et al. 2003). Of particular interest is Gliomedin, which was shown to interact with NF186 and NrCAM in the nodal axolemma. This interaction was proposed to coordinate the clustering of these proteins into the PNS node (Eshed et al. 2005). Similarly, ablation of dystroglycan, a laminin receptor, in myelinating Schwann cells resulted in reduced Nav channel clustering at the nodes and disrupted nodal microvilli formation (Saito et al. 2003). These findings indicate that Schwann cells may function to coordinate the initial formation and clustering of nodal components, most likely through their interactions with NF186 and NrCAM, and further exemplify the importance of glial signals to nodal development, particularly in the PNS. While we have yet to discover the exact mechanisms regulating nodal development, it is evident that all the proteins discussed above play significant roles in nodal domain formation, maintenance, and function. Further studies to elucidate their mode of action may provide insight into the mechanisms regulating these processes in disease.
3.2 The Paranode
3.2.1 The Function of the Vertebrate Paranodal Region
The paranode is a region in myelinated nerve fibers where the terminal myelin loops form specialized septate-like junctions with the axolemma. These axo–glial junctions are directly contiguous to the nodes of Ranvier and are thought to act as a barrier or molecular sieve, which impedes free diffusion between the nodal space and juxtaparanodal periaxonal space (Pedraza et al. 2001). As myelination progresses, the internodal myelin layers are compacted. This compaction forces cytoplasm to redistribute outwardly towards the paranode, and results in the formation of the characteristic paranodal loops. These paranodal loops, representing the initial wraps of myelin, also function as an anchorage point to stabilize the glial cell as myelination proceeds. Accordingly, the appearance of transverse bands, or the septate-like junctions, is first observed at the distal-most loop of the preforming paranode. Formation of the bands then progresses inwardly towards the juxtaparanode. The continual wrapping of myelin and the formation of these “septae” serve to cluster the juxtaparanodal potassium (Kv) channels and separate them from the nodal Nav channels. Thus, formation of the paranodal axo–glial junctions is crucial to the demarcation and segmentation of axonal domains in nerve fibers that allow for proper conduction of the nerve impulse (see below).
3.2.2 Functional Relevance of Invertebrate Septate Junctions to Vertebrate Paranodal Axo–Glial Junctions
Septate junctions (SJs) are one of the most widely and diversely expressed junctions in invertebrates. These junctions play critical roles in governing cell polarity, cell adhesion, and the diffusion of molecules between cells (Banerjee et al. 2008, 2006a, b; Baumgartner et al. 1996). During epithelial cell development, SJs form in a region known as the apico-lateral domain, an area just basal to the apical hemiadherens junctions, and zonula adherens. These junctions form in a circumferential pattern and function to maintain epithelial cell polarization and integrity by sustaining a constant distance of 15 nm between adjoining cells. Additionally, SJs were found to act as a diffusion or paracellular barrier to restrict the movement of molecules between the apical and basolateral surfaces of epithelia (Banerjee et al. 2008, 2006a; Carlson et al. 2000; Tepass et al. 2001). Similarly, vertebrate paranodal axo–glial junctions function by providing a periaxonal barrier to ionic diffusion between regional longitudinal axonal domains of myelinated fibers and thus are considered orthologous to invertebrate SJs (Banerjee and Bhat, 2007; Bhat 2003; Salzer 2003). Perhaps the most relevant example of invertebrate SJs to vertebrate paranodal axo–glial junctions is found in the nervous system of Drosophila (Fig. 2). Similar to oligodendrocytes in the mammalian CNS, Drosophila glial cells encompass several axons with their membrane, but instead of myelinating the axons, they simply ensheath them (Banerjee and Bhat 2008). A separate layer of perineurial cells develops around the glia, similar to the layer of fibroblasts that form the perineurium in vertebrates, which surrounds myelinated nerve fasciculi in the PNS (Hildebrand et al. 1993; Jessen and Mirsky 2005). To limit the flow of ions from the axons, septate junctions are formed between glial cells in a homotypic fashion and heterotypically between the glial and perineurial cells. As is the case with epithelial cells, these SJs function as a paracellular barrier to regulate or prevent the diffusion of ions and molecules from the hemolymph (Banerjee and Bhat 2007; Tepass et al. 2001). Similarly, vertebrate paranodal axo–glial junctions behave as diffusion barriers between Nav channels in the node and Kv channels in the juxtaparanode. By maintaining the segregation of ion channels into their respective domains, the paranode facilitates proper saltatory conduction, while also ensuring repolarization of the action potential.
Fig. 2.
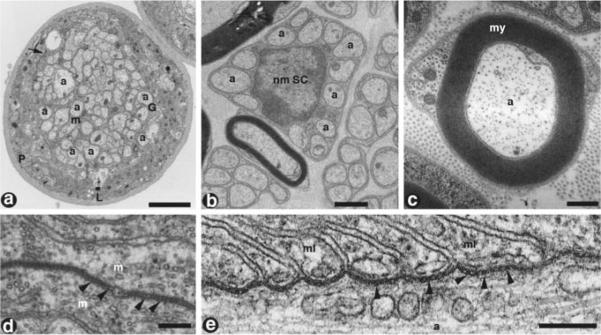
Comparative ultrastructure of Drosophila and mouse unmyelinated and myelinated nerve fibers. (a) Cross-sections of peripheral nerve fibers from Drosophila show the inner glia (G) ensheathing axons (a). Electron dense, ladder-like structures (arrow) known as septate junctions form between the outer perineurial (P) and the inner ensheathing glial cell (G) membranes (m) of Drosophila (arrowheads, D). Electron micrograph cross-section of a Remak bundle in the mouse peripheral nerve (b). Remak bundles consist of several small diameter axons (a) that are ensheathed by a single nonmyelinating Schwann cell. These fibers do not acquire myelination, and are similar in structure to Drosophila nerve fibers. Ultrastructure of a single myelinated mouse peripheral nerve fiber in cross-section (c). The continual wrapping of the Schwann cell myelin membrane (my) forms a multilamellar layer that is electron dense. A longitudinal section of the paranodal region of a myelinated axon (a) shows the septate-like junctions that form between the myelin loops (ml) and the underlying axolemma. Scale bars: (a) 2 μm; (b) 1 μm; (c) 0.5 μm; (d, e) 0.2 μm
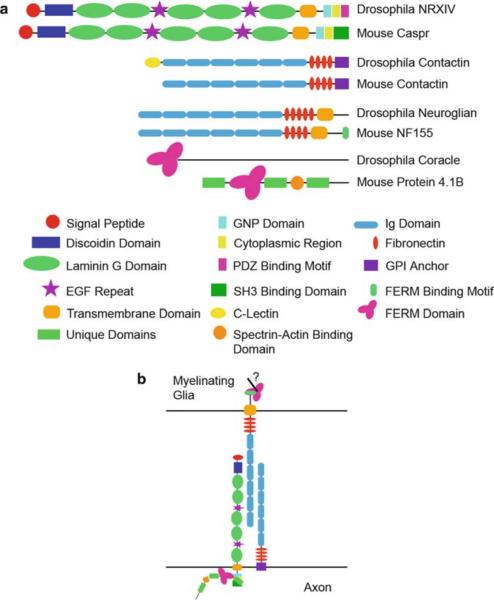
The identification of Drosophila SJs and the proteins involved in their formation and stabilization has lead to the elucidation of several homologues expressed in vertebrate paranodes (Fig. 3). Of note are the Drosophila cell adhesion molecules (CAMs) neurexin IV, contactin, and neuroglian, and the cytoskeletal protein, coracle (Banerjee et al. 2006b; Faivre-Sarrailh et al. 2004). Loss of expression of these genes has devastating effects on septate junction formation, and the stabilization of the paracellular barrier (Banerjee et al. 2006a; Baumgartner et al. 1996). The vertebrate counterparts of these molecules are contactin-associated protein (Caspr), contactin, the 155 kDa isoform of neurofascin (NF155), and protein 4.1B, respectively (Bhat et al. 2001; Boyle et al. 2001; Peles et al. 1997; Tait et al. 2000). All these proteins localize to the paranode, and through genetic ablation and biochemical analysis we have begun to understand their importance to the formation of the paranode and the maintenance and segregation of axonal domains.
Fig. 3.
Major components of septate junctions in Drosophila and mouse. The domain structure of Drosophila Nrx IV, Contactin, Neuroglian, and Coracle, and their vertebrate counterparts in mouse, Caspr, Contactin, NF155, and Protein 4.1B reveals significant homology between these proteins (a). Schematic representation of the proteins involved in the formation of the paranodal axo–glial septate junctions in mouse (b). NF155 is expressed strictly in the myelinating glial within the paranodal loops. The presence of a FERM binding domain within NF155 predicts an interaction with a FERM protein, which may mediate signaling to the glial cytoskeleton. NF155 is hypothesized to bind to either Caspr or Contactin, but the exact mechanisms are yet unknown
3.2.3 Key Regulators of Paranodal Formation and Stability
Initial studies aimed towards elucidating the proteins involved in the formation of the paranodal axo–glial junctions were focused around the Neurexin/Caspr/Paranodin (NCP) family of cell recognition molecules (Bellen et al. 1998). This superfamily is composed of five vertebrate homologues, that is, Caspr–Caspr5 (Spiegel et al. 2002). Of these isoforms, only Caspr is expressed at the paranodes, where it becomes enriched in the axolemma when myelination arrests (Arroyo et al. 1999; Bhat et al. 2001; Einheber et al. 1997; Menegoz et al. 1997). Caspr (aka Paranodin) is a Type 1 transmembrane protein that is comprised of a large expansive extracellular domain and a short intracellular domain. The extracellular domain of Caspr contains an array of subdomains implicated in cell–cell and cell–matrix interactions, including discoidin, EGF (epidermal growth factor), laminin G, and fibrinogen-like domains (Bellen et al. 1998; Bhat 2003; Denisenko-Nehrbass et al. 2002). While the specific function of each individual domain remains elusive, it is known that the extracellular domain of Caspr is vital for the formation of transverse septae, as evidenced by the absence of these junctions in Caspr-deficient mice (Bhat et al. 2001). The periaxonal space of the Caspr mutants was often invaded by astrocytic process in the CNS, Schwann cell microvilli in the PNS, and extracellular matrix components. The lack of stability resulted in the eversion of the paranodal loops, in which severe ataxia and reduced conduction velocity were also noted in these mice. Additionally, the juxtaparanodal rectifying Shaker-like potassium channels, Kv1.1 and 1.2, were frequently mislocalized to the paranodal region, while the nodal Nav channels remained unchanged. This alteration is likely to be mediated by the short intracellular domain of Caspr, which includes proline rich and glycophorin C domains that are known to interact with the actin cytoskeleton and coordinate it (Denisenko-Nehrbass et al. 2003a, b; Gollan et al. 2002; Menegoz et al. 1997). Thus, these findings exemplified the importance of Caspr to the formation of the axo–glial junctions and to the distribution, segregation, and organization of ion channels within axonal domains.
Support for the role of Caspr in the organization of axonal domains and the formation and stabilization of paranodes came with the discovery of its protein binding partners and regulators. On the axonal side, Caspr associates with contactin, a glycosylphosphatidylinositol (GPI)-anchored protein belonging to the Ig superfamily (Brummendorf and Rathjen 1996; Falk et al. 2002). Contactin is also enriched at the paranodes of myelinated fibers and forms a cis interaction with Caspr in the axolemma (Peles et al. 1997; Reid et al. 1994). This interaction is mediated by the extracellular domain of Caspr and the fibronectin III domains of contactin (Bonnon et al. 2003; Faivre-Sarrailh et al. 2000). Genetic ablation of contactin in mice results in an analogous phenotype as Caspr mutants, with the loss of transverse bands and attendant paranodal disorganization (Boyle et al. 2001). In addition, Caspr fails to localize to the plasma membrane, suggesting that contactin may function to transport and/or stabilize Caspr to the paranodal axonal membrane. Indeed, further characterization of the interaction between contactin and Caspr reveals that a mutually exclusive relationship exists between the two proteins. Without contactin, Caspr is retained in the endoplasmic reticulum and fails to traffic to the paranodal axolemma (Bonnon et al. 2003; Faivre-Sarrailh et al. 2000). Concomitantly, contactin cannot stably localize to the paranode in the absence of Caspr, but rather is found in the nodes in the CNS (Bhat et al. 2001; Rios et al. 2000).
Several scaffolding and cytoskeletal components reside at the paranodes, and emerging evidence suggests a critical role for these proteins in the maintenance of axo–glial junctions. Protein 4.1B belongs to the Band 4.1 superfamily of membrane cytoskeletal linking proteins (Hoover and Bryant 2000; Parra et al. 2000; Sun et al. 2002). It is present in the paranodes, and distributed diffusely in the juxtaparanodes of axons; to date, it is the only known protein 4.1 isoform localized to the axo–glial junctions (Ohara et al. 2000). Protein 4.1B contains a conserved FERM (four point one/ezrin/radixin/moesin) domain that mediates its binding with several transmembrane receptors, including Caspr at the paranodes and Caspr2 at the juxtaparanodes (Denisenko-Nehrbass et al. 2003b; Garcia-Fresco et al. 2006; Gollan et al. 2002). The conserved cytoplasmic GNP (glycophorin C/Neurexin IV/paranodin) domain of Caspr mediates its association with protein 4.1B (Gollan et al. 2002; Sousa and Bhat, 2007). Loss of the GNP domain resulted in the internalization of the Caspr–contactin complex from the axonal plasma membrane, suggesting an important role for Protein 4.1B in the stabilization of this complex and axo–glial junctions at the paranode (Gollan et al. 2002). It remains to be seen what effect the loss of protein 4.1B might have on the formation and maintenance of the paranode; however, studies of the Drosophila orthologue, coracle, suggest a key role for the FERM domain-containing proteins in the organization and stabilization of septate junctions (Baumgartner et al. 1996; Laval et al. 2008; Ward et al. 1998). Recent work has identified the presence of a macromolecular complex consisting of protein 4.1B, Ankyrin B (AnkB), and the aII and bII spectrins that associates with Caspr and contactin at the paranodal junctions (Garcia-Fresco et al. 2006; Ogawa et al. 2006). Loss of Caspr resulted in the absence of AnkB, or its diffusion out of the paranodes, which reflects the importance of Caspr in orchestrating the assembly of the underlying paranodal cytoskeleton (Garcia-Fresco et al. 2006; Pillai et al. 2007; Sousa and Bhat, 2007). Given that the junctional specialization of the paranode functions as a “fence” in regulating the diffusion of ions associated with functional activity in the axon, it will be interesting to see how future studies may elucidate the roles of these cytoskeletal proteins in segregating the axonal domains.
The interaction(s) between glia and axons is important in regulating myelination and the formation of axonal domains. As oligodendrocytes and Schwann cells associate with the axon, they initiate the clustering of the paranodal components in the axolemma. While several axonal proteins have been identified at the paranode, only one glial protein is known to localize to the paranodal axo–glial junctions, the 155 kDa isoform of neurofascin, NF155 (Collinson et al. 1998; Moscoso and Sanes 1995; Tait et al. 2000). NF155 is a CAM that belongs to the L1 subgroup of the Ig superfamily (Davis and Bennett 1993; Holm et al. 1996; Volkmer et al. 1992). It differs from the axonally expressed NF186, in that it lacks the mucin-like domain and contains an extra fibronectin type III domain (Davis et al. 1996). The exact mechanisms governing their varied expression pattern is not clear, but over 50 alternatively spliced isoforms exist that appear to be developmentally regulated (Hassel et al. 1997). Initial mutational analysis in mice resulted in the loss of both isoforms of neurofascin, NF186 and NF155 (Sherman et al. 2005). These mice were unable to form either the nodal complex or the paranodal axo–glial junctions and died at postnatal day 6. Caspr and contactin were diffused throughout the axon in these mutants. Interestingly, upon reexpression of NF155 in glia, the paranodes reorganized and Caspr and contactin relocalized to the axolemma. This coincided with previous reports that expression of NF155 colocalized with Caspr during the clustering of the paranodal loops during myelination (Tait et al. 2000). Further analysis of the proposed interaction(s) revealed that the extracellular domain of NF155 could associate with the Caspr–contactin complex in vitro (Charles et al. 2002). These results are somewhat controversial, however, as other studies employing similar in vitro techniques find that NF155 binds contactin and that the presence of Caspr perturbs this interaction (Gollan et al. 2003). Although it seems unclear as to how these proteins associate, these results do suggest a role for NF155 in paranodal organization, possibly through interactions with Caspr and/or contactin.
Recent findings utilizing Cre-loxP conditional knockout strategies has provided new insights into the specific role of NF155 in axo–glial formation and maintenance. Using a Cre recombinase driven by the CNPase promoter, which is specific for glia, it was shown that loss of NF155 expression alone results in the loss of septate-like junctions, paranodal disorganization, and the failure to segregate axonal domains (Pillai et al. 2009). Because of the strong correlation of phenotype with those of Caspr and contactin mutants, it was shown that both of these axonally expressed proteins were absent from the paranodal axolemma in NF155 mutant nerves. This suggested that NF155 and Caspr and/or contactin interact with the glial NF155 protein, and that this interaction mediates the formation and stability of the Caspr–contactin complex to the paranodal-domain of the axonal plasma membrane. The ability of NF155 to direct the formation of the paranode and stabilize the complex formation suggests that it may interact with cytoskeletal proteins. Although no protein-binding partners have been identified, it has been shown that the intracellular domain of NF155 contains a FERM domain-binding motif (Gunn-Moore et al. 2006). Given that FERM domain-containing proteins are cytoskeletal linkers, NF155 may coordinate signals originating from the axon that direct changes in the glial cytoskeleton prior to and during myelination; whereby, axonal domains are partitioned.
3.2.4 The Importance of Lipids to Paranodal Formation
As noted previously, myelin is the elaborated glial membrane sheath that circumferentially wraps axons, which becomes compacted to form a multilamellar layer of insulation. The major constituents of myelin are lipids, which make up approximately 70–85% of the dry weight (Morell et al. 1994). Nearly one-third of the lipid mass is comprised of gylcosphingolipids, galactosylceramide (GalC), and its sulfated derivative, sulfatide (Coetzee et al. 1996; Norton and Cammer 1984). These lipids were shown to have a significant role in oligodendrocyte development, the initiation of myelination, and the stabilization of the compacted myelin layers (Marcus and Popko 2002). Studies aimed towards elucidating the function of these lipids during myelination resulted in the development of knockout mice deficient in CGT (UDP-galactose:ceramide galactosyltransferase), the enzyme that synthesizes galactosylceramide (Coetzee et al. 1996). Surprisingly, these mice formed myelin, but displayed severe ataxia, tremors, and slowed nerve conduction. Further examination of these mice revealed the appearance of disorganized and everted paranodal loops and the absence of axo–glial junctions (Dupree et al. 1998).
Similar phenotypes were also observed in CST (cerebroside sulfotransferase) null mice (Honke et al. 2002; Ishibashi et al. 2002). These mice differ from CGT, in that they are unable to synthesize only sulfatide, whereas galactosylceramide is still produced. These findings implied that glial sulphosphingolipids were essential to the formation and/or maintenance of the paranode. The exact mechanisms in which these lipids function to promote axo–glial junction formation are unknown, but it is hypothesized that they are responsible for creating lipid microdomains, where paranodal components may reside and function. In this context, it is noteworthy that studies have indicated that NF155, Caspr, and contactin are present in lipid-rich microdomains (Bonnon et al. 2003; Schafer et al. 2004). Furthermore, NF155, Caspr, and contactin are mislocalized outside of the paranode in CGT mice (Dupree et al. 1999; Hoshi et al. 2007; Poliak et al. 2001). Alternatively, it may be that an unidentified protein(s) may be localized in the domain to interact with these lipids, either in cis or trans relation, and may thereby direct the localization of Caspr, contactin, and NF155 to the paranode.
3.3 The Juxtaparanodal Region
The juxtaparanode is a potassium channel-rich region that lies underneath the compact myelin sheath, just proximal to the paranodes. It has been proposed that the significance of localizing potassium channels in this domain is concerned with repolarization of the action potential, as well as counteracting instability of excitability, especially in the transition zone between myelinated and unmyelinated portions of distal motor nerve fibers (Chiu et al. 1999; Rasband et al. 1998; Zhou et al. 1998b). Specifically, two rectifying Shaker-like potassium channels, Kv1.1 and Kv1.2, occupy a majority of the domain, along with the CAMs, Caspr2, and TAG-1 (Arroyo et al. 1999; Mi and Berkowitz 1995; Rasband et al. 1998; Wang et al. 1993). Caspr2 is expressed strictly in the axon and is functionally related to Caspr, except that it contains a PDZ binding motif in its intracellular domain that Caspr lacks (Poliak et al. 1999). Caspr2 was shown to be critical to the maintenance of Kv channels in the juxtaparanode, as its absence in mice resulted in the diffusion of these channels throughout the internode (Poliak et al. 1999). Subsequently, it was found that the PDZ binding domain links Caspr2 to Kv1.1 and Kv1.2 (Poliak et al. 2003). Initial reports predicted that PSD-95, a PDZ-containing protein found within the juxtaparanode, might be a potential candidate for this interaction, as it has been shown to associate with Kvβ2; however, later studies reported that PSD-95 and Caspr2 do not interact (Baba et al. 1999; Poliak et al. 1999). Furthermore, Kv1.1 and Kv1.2 remained clustered at the juxtaparanodes of PSD-95 deficient mice; therefore, it remains to be elucidated what PDZ protein(s) links Caspr2 and Kv channels in the juxtaparanode (Rasband et al. 2002).
Another critical component of the juxtaparanode is the GPI-anchored adhesion molecule, TAG-1 (Furley et al. 1990; Karagogeos et al. 1991; Traka et al. 2002). TAG-1 belongs to the Ig superfamily and shares 50% sequence homology with contactin; it is expressed in both neurons and myelinating glia (Traka et al. 2002). Like Caspr and contactin in the paranode, TAG-1 and Caspr2 associate and form a cis complex within the juxtaparanodal axolemma. This interaction is mediated by the Ig-domains of TAG-1 (Traka et al. 2003; Tzimourakas et al. 2007). Interestingly, the axonal cis complex of TAG-1/Caspr2 binds with TAG-1 expressed in glia by homophilic interactions between the TAG-1 molecules (Traka et al. 2003). Mutational analysis in mice revealed that a codependent relationship between TAG-1 and Caspr2 exists, similar to that of Caspr and contactin, where the accumulation of either protein to the juxtaparanode relies on the expression of the other (Poliak et al. 2003; Traka et al. 2003). Deletion of TAG-1 in mice resulted in the exclusion of Kv1.1 and Kv1.2 channels from the underlying juxtaparanodal axonal membrane concomitant to Caspr2 null mice (Traka et al. 2003). Although the Kv channels were absent from the membrane, no change in conduction velocity was observed in TAG-1 mutant nerves (Traka et al. 2003). Accordingly, Caspr2 mutant mice displayed normal conduction (Poliak et al. 2003). While no electrophysiological abnormalities were observed in TAG-1 or Caspr2 mutant mice, the effects of extended loss of these proteins to action potential propagation and resting potential are unknown. Examining these effects may be of particular interest to disease pathology because Kv1.1 mutant mice display backfiring of the action potential and extended hyperexcitability (Zhou et al. 1999). Additionally, TAG-1 function has been recently implicated in learning and cognition and suggests an important role for these proteins in long-term plasticity (Savvaki et al. 2008).
3.4 The Internodal Region
The internode comprises the area between the juxtaparanodes, and accounts for 99% of the total length of a myelinated nerve segment (Salzer 2003). As previously mentioned, the length of this region is determined by the axonal diameter and represents the most extended region involving axo–glial interactions. Very little is known about the mechanisms regulating the ability of glia to detect the axonal diameter to determine the final internodal length. Additionally, as animals grow, this region lengthens to compensate for the extension of limbs (Abe et al. 2004). A handful of proteins have been found to localize to the adaxonal membrane at the inner-most lip of the myelin membrane. These proteins include the nectin-like proteins, Necl1 and Necl4, the polarity protein Par-3, and the myelin associated glycoprotein (MAG). The nectin-like proteins (Necl) are cell adhesion molecules that belong to the Ig superfamily (Takai et al. 2003). They are related to the nectin cell adhesion proteins, but differ by their inability to bind to afadin. Necls are often associated with tight junctions and contain PDZ binding motifs that may facilitate their interaction with cytoskeletal scaffolding proteins. Recent findings have implicated Necl1 and Necl4 in myelination. These proteins were expressed in a polarized fashion at the inner mesaxon or at the initial contact of the glial myelin membrane with axons (Maurel et al. 2007). Heterophilic interactions between Necl1 on axons and Necl4 on glia mediate the initial attachment and wrapping of the glial membrane (Maurel et al. 2007). Perturbation of Necl4 by use of RNAi in Schwann cell-DRG neuron cocultures inhibited myelination (Maurel et al. 2007). Similar results were observed in mutant mice deficient in the axonally expressed Necl1 (Park et al. 2008). These results indicate the importance of these proteins to myelination and axo–glial recognition and adhesion.
Par-3 is a PDZ-containing adaptor protein, which regulates polarity in many cell types (Ohno 2001). The Drosophila homolog of Par-3, Bazooka, functions to establish cell polarity in epithelial cells through its restricted expression at the apical surface (Pinheiro and Montell 2004). Like Bazooka, the vertebrate Par-3 is a polarized protein that is expressed asymmetrically in glia at the junction of the inner mesaxon or the adaxonal layer. It is proposed to facilitate the adhesion of glia to axons (Chan et al. 2006). Disruption of Par-3 function in Schwann cells resulted in the inability of Schwann cells to adhere to and myelinate axons (Chan et al. 2006). Further analysis determined that Par-3 is associated with p75NTR, a BDNF receptor that promotes myelination through its PDZ1 domain. While other binding partners of Par-3 are unknown, it is likely that it interacts with many cell adhesion receptors that contain PDZ binding motifs. Of particular interest is the possible association of Necl4 and Par-3. Given their colocalization to the inner mesaxon, the presence of a PDZ binding motif in Necl4, and the observation in mice that an association of Par-3 and nectins occurs, these proteins may interact to coordinate axo–glial adhesion prior to myelination (Takekuni et al. 2003).
4 Mechanisms Regulating Axonal Domain Formation and Maintenance
A key question regarding the assembly of axonal domains remains elusive; namely, what comes first, the node or the paranode? Is formation of the nodes dependent on or independent of paranodal formation? Furthermore, does the long-term maintenance of the node require intact paranodes? A central theme has emerged from the numerous studies on axo–glial domains, which is that distinct differences exist between the mechanisms regulating the partitioning of axonal domains in the PNS vs. the CNS. This is, to a degree, to be expected as myelination proceeds in different fashions between oligodendrocytes and Schwann cells. Later we attempt to interpret recent findings to shed light on the organization of axonal domains and their dependence on adjacent domains for long-term stability and maintenance.
4.1 Nodal Formation in the PNS and CNS: Extrinsic vs. Intrinsic; Dependent vs. Independent Mechanisms
In the PNS, Schwann cell development and myelination are intimately linked to axonal and ECM stimuli and adhesion. The maturation of Schwann cells into a myelinating phenotype and the segmentation of axonal domains rely on forming this intimate relationship. Studies have shown that PNS domain organization begins with the formation of the node and proceeds inwardly towards the internode (Melendez-Vasquez et al. 2001; Poliak et al. 2001). An extrinsic pattern of nodal assembly is suggested for the PNS as the presence of NF186 and NrCAM precedes that of Nav channels and the cytoskeletal components, AnkG and βIV spectrin (Peles and Salzer 2000; Salzer 2003). Additionally, the appearance of these nodal components is observed before the paranodal proteins, Caspr and contactin, which suggests that PNS nodes form prior to and independently of the paranodes (Melendez-Vasquez et al. 2001).
Schwann cell distal microvilli are believed to coordinate the formation of nodes through their association with NF186 and/or NrCAM. This association may occur through gliomedin, as it is expressed in the nodal microvilli and interacts with both NF186 and NrCAM (Eshed et al. 2005). Disruption of gliomedin by RNAi resulted in the absence of Nav channels at the node and suggests that glia facilitate the initial assembly of the node by mediating NF186 localization. This follows an extrinsic pattern of node formation, in which extracellular signals initiate node formation and also transmit signaling to the axonal cytoskeleton to stabilize the complex once formed. Interestingly, gliomedin can be secreted, resulting in soluble forms that are accumulated and incorporated into the surrounding basal lamina in the perinodal space (Eshed et al. 2007, 2005). In the absence of Schwann cells, axons exposed to the soluble form of gliomedin formed nodes, further supporting a role for glia in the formation of the node extrinsically. Dystroglycan, a laminin receptor expressed in Schwann cell nodal microvilli, may also mediate the extrinsic formation of PNS nodes, as genetic ablation of its gene in mice results in severe conduction blockade, dysmyelination, and the loss of nodal Nav channel clustering (Occhi et al. 2005; Saito et al. 2003). Additionally, these mice form normal axo–glial junctions at the paranodes, suggesting that paranodal formation does not require the formation of the node. It may also indicate that the formation of paranodes alone is not sufficient to cluster the nodal complex, although further studies in these mice revealed that AnkG and NF186 are still clustered at the node in the absence of Nav channels. This suggests that dystroglycan may simply act to stabilize these ion channels to the node instead of coordinating the organization of the entire node. However, the presence of NF186 at these nodes still suggests that an extrinsic mechanism of assembly may occur albeit through alternative mechanisms.
Nodal formation in the CNS is considered to behave quite differently than that of the PNS. It is hypothesized that instead of an extrinsic glial mediated assembly, a more intrinsic form of coordination occurs, beginning with the cortical axonal cytoskeleton and assembling in association with the axolemma. One of the major factors contributing to this model of an intrinsic assembly is that oligodendrocytes do not extend nodal microvilli (Melendez-Vasquez et al. 2001). Additionally, NF186, NrCAM, and Nav channels were recruited to the node subsequent to the appearance of AnkG in intermediate nodes (Jenkins and Bennett 2002). An attempt to disrupt AnkG resulted in the generation of a cerebellum specific knockout (Zhou et al. 1998a). Aberrant Nav channel clustering was observed in the AISs of Purkinje neurons, yet AnkG was still present in the nodes of these mutants; therefore, the effects of loss of AnkG on CNS node formation could not be evaluated (Jenkins and Bennett 2001; Zhou et al. 1998a). While it is evident from these findings that AnkG expression is important for Nav channel clustering at AIS, they also present evidence that several isoforms of AnkG may exist that differentially localize to separate areas of Nav channel clustering. Although oligodendrocytes lack nodal microvilli, they still retain the ability to cluster Nav channels through the secretion of soluble factors (Kaplan et al. 1997). This mechanism of clustering is similar to the aggregation of Nav channels to PNS nodes by secreted gliomedin, and suggests that oligodendrocytes may, in fact, assemble nodes without direct contact (Eshed et al. 2007, 2005). Further examination revealed that oligodendrocyte conditioned medium clustered Nav1.2, the immature Nav channel isoform; whereas, Nav1.6 present in mature nodes required myelination (Kaplan et al. 2001). Additionally, the early assembly required an intact axonal cytoskeleton, and suggests that certain intrinsic signals may still be required for CNS nodal formation and maturation. The identification of the soluble factor(s) released by oligodendrocytes will certainly be critical to the future analysis and elucidation of the mechanisms governing CNS node formation.
An alternative method of CNS assembly may involve signaling from perinodal astrocytes that contact the nodal domain. The relationship of these astrocytes to nodal function is not well characterized, but interestingly, these cells were shown to express the cytoskeletal mediating ERM protein ezrin (Melendez-Vasquez et al. 2001). As mentioned earlier, ERM proteins are also present in the nodal microvilli of Schwann cells, and their expression at these domains is coordinated with the extrinsic formation of the node in the PNS. Taken together, it is possible that perinodal astrocytes may compensate for the lack of nodal microvilli in oligodendrocytes and provide nodal assembly cues in a similar manner as Schwann cell microvilli. It would be interesting to see if these perinodal astrocytes express gliomedin, or a derivative, as well as dystroglycan.
Yet another possible mechanism of CNS node development is the idea that nodes are formed by the sequestration of nodal components from the axolemma by the formation of the paranodes. In support of this idea is the finding that Caspr expression in the forming paranode preceded the expression of Nav channels in the nodes of optic nerves (Rasband et al. 1999). Since oligodendrocyte myelination does not depend on the demarcation of the internode prior to myelination, as it does with Schwann cells, it is possible that oligodendrocytes begin to wrap their membrane while progressively extending processes along the axon. In this manner, the oligodendrocytes create a barrier that will induce the aggregation of the nodal proteins. In support of this mechanism, it was found in mice that reexpression of NF155 alone, in a complete neurofascin null background, resulted in the clustering of Nav channels (Zonta et al. 2008). These findings implicate a paranodal dependent formation of nodes in the CNS, but these mice also did not live past the complete knockout mice, and no assessment of conduction velocities was performed in these animals. Furthermore, mice deficient in Caspr expression form nodes in the CNS and PNS in the absence of intact axo–glial junctions, suggesting that paranodal formation is not a prerequisite for CNS node formation (Bhat et al. 2001). Concomitantly, NF155-specific knockout mice also form nodes independently of paranodal formation, further supporting the hypothesis that nodes form through separate autonomous mechanisms than paranodes (Pillai et al. 2009). Taken together, it appears that nodal stability may rely on paranodal domains, but the initial assembly of the node forms independently of other axonal domains.
New evidence has emerged regarding the requirement of paranodal axo–glial junctions in the maintenance and long-term stability of the node. Recently, Pillai et al. (2009) tested this hypothesis by knocking out NF155 in adult mice through the use of tamoxifen inducible Cre recombinase, driven by the proteolipid protein (PLP) promoter (Doerflinger et al. 2003). They allowed the mice to reach adult stage (P23) and subsequently treated the mice with tamoxifen to induce glial-specific genetic ablation of neurofascin (NF155). They found that axo–glial junctions dissembled, and essentially unraveled, from the juxtaparanodal side distally outward towards the node. The progressive unraveling of the paranodes resulted in the invasion of the paranodal space with Kv channels. While the node remained unchanged initially, it was found that with extended time (up to 1 year posttreatment), NF186, Nav channels, and AnkG diffused out of the node (CT and MB, unpublished observation). Upon further examination, these same proteins were diffused out of the AIS, further revealing a progressive loss from the external axonal nodes sequentially upwards towards the AIS at the neuron cell body. This suggests that through development and maturation, the paranodes not only function to sequester ion channels to specific domains, but that they maintain segregation by stabilizing these components. Therefore, even though nodes may form independently of paranodes during development, their long-term integrity may rely upon paranodal axo–glial junctions. As intriguing as these results may be, further experimentation will need to be conducted to elucidate the signaling involved. Additionally, the use of this inducible system may help with future studies to elucidate the effects of progressive myelin loss on axonal domains, much like that seen in multiple sclerosis.
4.2 Disease Manifestation in the Absence of Segmented Axonal Domains
Several human neuropathies and disorders, such as multiple sclerosis and Charcot–Marie Tooth disease, result in the progressive demyelination of nerves, leading to axonal degeneration. There are varied causes to the development of these diseases, be it autoimmune attack or genetic predisposition, but the dissolution of axonal domain organization appears to be a central indicator of the development and progression of these disorders (Berger et al. 2006; Lubetzki et al. 2005; Nave et al. 2007; Oguievetskaia et al. 2005; Shy 2006; Trapp and Nave 2008). While NF155, NF186, Caspr, and contactin have not been directly associated with disease predisposition and onset, they have been shown to be disrupted in disease pathology (Coman et al. 2006; Howell et al. 2006; Mathey et al. 2007; Wolswijk and Balesar 2003). Emerging evidence has revealed that NF155 is targeted in multiple sclerosis (MS) (Howell et al. 2006; Maier et al. 2007, 2005; Mathey et al. 2007). Initially, it was found that NF155 expression was reduced in the paranodes of MS patients, and was considered an early marker for the ensuing demyelination of the nerve tracts (Howell et al. 2006; Maier et al. 2005). Further analysis revealed that NF155 had decreased association with lipid rafts in MS, and therefore, axo–glial junction stability was compromised (Maier et al. 2007). The mislocalization of Kv channels to the paranodes confirmed the dissolution of axo–glial junctions in the diseased lesions (Howell et al. 2006). A recent report revealed the presence of autoantibodies to NF155 and NF186 in patients with MS (Mathey et al. 2007). Interestingly, increasing amounts of autoantibodies were found in patients with chronic progressive MS compared to those with relapsing MS, suggesting that as the disease becomes more severe it targets these critical proteins, preventing the reformation of the paranodes and nodes. The inability of new oligodendrocytes to myelinate the affected lesions and organize axonal domains, due to the lack of both Neurofascins, would promote the axonal degeneration observed in chronic MS individuals. In support of the degeneration of paranodes in MS lesions, it was also found that loss of Caspr expression precedes demyelination in MS patients (Wolswijk and Balesar 2003). Other compelling evidence to support the role of intact axo–glial junctions in the prevention of axonal degeneration is found in Caspr mutants, in which loss of Caspr expression in mice resulted in the presence of axonal swellings and cytoskeletal abnormalities in Purkinje neurons of the cerebellum (Garcia-Fresco et al. 2006). These abnormalities precede the degeneration of axons, as seen in multiple neuropathies (Fabrizi et al. 2007; Lappe-Siefke et al. 2003; Rodriguez and Scheithauer 1994). In addition to their importance to axonal domain organization, the juxtaparanodal proteins, Caspr2 and Tag-1, have been implicated in autism, language impairment, as well as learning and cognition disorders, respectively, suggesting other roles for these CAMs in nervous system function (Alarcon et al. 2008; Bakkaloglu et al. 2008; Savvaki et al. 2008; Vernes et al. 2008). While genetic ablation of axonal domain proteins has not been identified as the causative agent for disease manifestation, it is clear that these proteins are critical in preventing the progression of demyelinating neuropathies, and serve as possible therapeutic targets for the future treatment of these devastating disorders.
5 Concluding Remarks
To date, significant advancements through the development of mouse models have contributed greatly to our knowledge of the proteins involved in myelination and the segregation of axonal domains. While many of the proteins involved have been identified and characterized, future studies may provide more insight into the signaling mechanisms involved in the formation and stabilization of each domain. The future efforts are likely to be centered on cytoskeletal scaffolding proteins and the signaling mechanisms governing their function, expression, and localization to specific axonal as well as glial domains. Additionally, emerging advances in animal model systems are sure to facilitate research directed towards studying the effects of extended loss of axo–glial junctions. These studies will certainly shed light on the processes, proteins, and functions misregulated during disease, and may reveal new roles for many of the axo–glial junctional proteins in demyelinating disorders, axonopathies, and other neuropathies.
Acknowledgments
The work in our laboratory is supported by the grants from NIGMS and NINDS of the National Institutes of Health, National Multiple Sclerosis Society, and funds from the State of North Carolina.
References
- Abe I, Ochiai N, Ichimura H, Tsujino A, Sun J, Hara Y. Internodes can nearly double in length with gradual elongation of the adult rat sciatic nerve. J Orthop Res. 2004;22:571–577. doi: 10.1016/j.orthres.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Xu YT, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS. Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvbeta2, and Caspr. J Neurocytol. 1999;28:333–347. doi: 10.1023/a:1007009613484. [DOI] [PubMed] [Google Scholar]
- Arroyo EJ, Xu T, Grinspan J, Lambert S, Levinson SR, Brophy PJ, Peles E, Scherer SS. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci. 2002;22:1726–1737. doi: 10.1523/JNEUROSCI.22-05-01726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K+ channel clustering. J Neurosci Res. 1999;58:752–764. [PubMed] [Google Scholar]
- Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Glial ensheathment of peripheral axons in Drosophila. J Neurosci Res. 2008;86:1189–1198. doi: 10.1002/jnr.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006a;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006b;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bainton RJ, Mayer N, Beckstead R, Bhat MA. Septate junctions are required for ommatidial integrity and blood-eye barrier function in Drosophila. Dev Biol. 2008;317:585–599. doi: 10.1016/j.ydbio.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin-novel members of the neurexin family: encounters of axons andglia. Trends Neurosci. 1998;21:444–449. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bennett V, Lambert S. Physiological roles of axonal ankyrins in survival of premyelinated axons and localization of voltage-gated sodium channels. J Neurocytol. 1999;28:303–318. doi: 10.1023/a:1007005528505. [DOI] [PubMed] [Google Scholar]
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T. βIV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA. Molecular organization of axo–glial junctions. Curr Opin Neurobiol. 2003;13:552–559. doi: 10.1016/j.conb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin M, St, Li J, Einheber S, Chesler M, Rosenbluth J. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1:169–183. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Bonnon C, Goutebroze L, Denisenko-Nehrbass N, Girault JA, Faivre-Sarrailh C. The paranodal complex of F3/contactin and caspr/paranodin traffics to the cell surface via a nonconventional pathway. J Biol Chem. 2003;278:48339–48347. doi: 10.1074/jbc.M309120200. [DOI] [PubMed] [Google Scholar]
- Bouzidi M, Tricaud N, Giraud P, Kordeli E, Caillol G, Deleuze C, Couraud F, Alcaraz G. Interaction of the Nav1.2a subunit of the voltage-dependent sodium channel with nodal ankyrinG. In vitro mapping of the interacting domains and association in synaptosomes. J Biol Chem. 2002;277:28996–29004. doi: 10.1074/jbc.M201760200. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobio. 1996;16:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the in sect. Annu Rev Entomol. 2000;45:151–174. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Chan JR, Jolicoeur C, Yamauchi J, Elliott J, Fawcett JP, Ng BK, Cayouette M. The polarity protein Par-3 directly interacts with p75NTR to regulate myelination. Science. 2006;314:832–836. doi: 10.1126/science.1134069. [DOI] [PubMed] [Google Scholar]
- Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Zhou L, Zhang CL, Messing A. Analysis of potassium channel functions In mammalian axons by gene knockouts. J Neurocytol. 1999;28:349–364. doi: 10.1023/a:1007013731231. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Marshall D, Gillespie CS, Brophy PJ. Transient expression of neurofascin by oligodendrocytes at the onset of myelinogenesis: implications for mechanisms of axon-glial interaction. Glia. 1998;23:11–23. doi: 10.1002/(sici)1098-1136(199805)23:1<11::aid-glia2>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Coman I, Aigrot MS, Seilhean D, Reynolds R, Girault JA, Zalc B, Lubetzki C. Nodal, paranodal and juxtaparanodal axonal proteins during demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3186–3195. doi: 10.1093/brain/awl144. [DOI] [PubMed] [Google Scholar]
- Court FA, Wrabetz L, Feltri ML. Basal lamina: Schwann cells wrap to the rhythm of space-time. Curr Opin Neurobiol. 2006;16:501–507. doi: 10.1016/j.conb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Custer AW, Kazarinova-Noyes K, Sakurai T, Xu X, Simon W, Grumet M, Shrager P. The role of the ankyrin-binding protein NrCAM in node of Ranvier formation. J Neurosci. 2003;23:10032–10039. doi: 10.1523/JNEUROSCI.23-31-10032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JQ, Bennett V. Ankyrin-binding activity of nervous system cell adhesion molecules expressed in adult brain. J Cell Sci Suppl. 1993;17:109–117. doi: 10.1242/jcs.1993.supplement_17.16. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Faivre-Sarrailh C, Goutebroze L, Girault JA. A molecular view on paranodal junctions of myelinated fibers. J Physiol. 2002;96:99–103. doi: 10.1016/s0928-4257(01)00085-7. [DOI] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Goutebroze L, Galvez T, Bonnon C, Stankoff B, Ezan P, Giovannini M, Faivre-Sarrailh C, Girault JA. Associationof Caspr/paranodin with tumour suppressor schwannomin/merlin and 1 integrin in the central nervous system. J Neurochem. 2003a;84:209–221. doi: 10.1046/j.1471-4159.2003.01503.x. [DOI] [PubMed] [Google Scholar]
- Denisenko-Nehrbass N, Oguievetskaia K, Goutebroze L, Galvez T, Yamakawa H, Ohara O, Carnaud M, Girault JA. Protein 4.1B associates with both Caspr/paranodin and Caspr2 at paranodes and juxtaparanodes of myelinated fibres. Eur J Neurosci. 2003b;17:411–416. doi: 10.1046/j.1460-9568.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Suzuki K, Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. J Neurocytol. 1998;27:649–659. doi: 10.1023/a:1006908013972. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo–glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Poliak S, Sabanay H, Sarig-Nadir O, Spiegel I, Bermingham JR, Jr, Peles E. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron. 2005;47:215–229. doi: 10.1016/j.neuron.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Carey DJ, Peles E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol. 2007;177:551–562. doi: 10.1083/jcb.200612139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi GM, Ferrarini M, Cavallaro T, Cabrini I, Cerini R, Bertolasi L, Rizzuto N. Two novel mutations in dynamin-2 cause axonal Charcot-Marie-Tooth disease. Neurol. 2007;69:291–295. doi: 10.1212/01.wnl.0000265820.51075.61. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Gauthier F, Denisenko-Nehrbass N, Le Bivic A, Rougon G, Girault JA. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr) J Cell Biol. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Falk J, Bonnon C, Girault JA, Faivre-Sarrailh C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol Cell. 2002;94:327–334. doi: 10.1016/s0248-4900(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glyco-protein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Fresco GP, Sousa AD, Pillai AM, Moy SS, Crawley JN, Tessarollo L, Dupree JL, Bhat MA. Disruption of axo–glial junctions causes cytoskeletal disorganization and degeneration of Purkinje neuron axons. Proc Natl Acad Sci U S A. 2006;103:5137–5142. doi: 10.1073/pnas.0601082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol. 1997;137:703–714. doi: 10.1083/jcb.137.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Walker BJ, Lambert S. Local ERM activation and dynamic growth cones at Schwann cell tips implicated in efficient formation of nodes of Ranvier. J Cell Biol. 2003;162:489–498. doi: 10.1083/jcb.200303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol. 2002;157:1247–1256. doi: 10.1083/jcb.200203050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutebroze L, Carnaud M, Denisenko N, Boutterin MC, Girault JA. Syndecan-3 and syndecan-4 are enriched in Schwann cell perinodal processes. BMC Neurosci. 2003;4:29. doi: 10.1186/1471-2202-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M. Nr-CAM: a cell adhesion molecule with ligand and receptor functions. Cell Tissue Res. 1997;290:423–428. doi: 10.1007/s004410050949. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore FJ, Hill M, Davey F, Herron LR, Tait S, Sherman D, Brophy PJ. A functional FERM domain binding motif in neurofascin. Mol Cell Neurosci. 2006;33:441–446. doi: 10.1016/j.mcn.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Hassel B, Rathjen FG, Volkmer H. Organization of the neurofascin gene and analysis of developmentally regulated alternative splicing. J Biol Chem. 1997;272:28742–28749. doi: 10.1074/jbc.272.45.28742. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40:319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, Montag D, Schachner M. Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur J Neurosci. 1996;8:1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci U S A. 2002;99:4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover KB, Bryant PJ. The genetics of the protein 4.1 family: organizers of the membrane andcytoskeleton. Curr Opin Cell Biol. 2000;12:229–234. doi: 10.1016/s0955-0674(99)00080-0. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Suzuki A, Hayashi S, Tohyama K, Hayashi A, Yamaguchi Y, Takeuchi K, Baba H. Nodal protrusions, increased Schmidt-Lanterman incisures, and paranodal disorganization are characteristic featuresof sulfatide-deficient peripheral nerves. Glia. 2007;55:584–594. doi: 10.1002/glia.20487. [DOI] [PubMed] [Google Scholar]
- Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, Vora AJ, Brophy PJ, Reynolds R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3173–3185. doi: 10.1093/brain/awl290. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Ellisman MH. Three-dimensional fine structure of cytoskeletal- membrane interactions at nodes of Ranvier. J Neurocytol. 1991;20:667–681. doi: 10.1007/BF01187068. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci. 2002;22:6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL. The role of sodium channels in cell adhesion. Front Biosci. 2002;7:12–23. doi: 10.2741/isom. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Developing nodes of Ranvier are defined by ankyrin-G clustering and are independent of paranodal axoglial adhesion. Proc Natl Acad Sci U S A. 2002;99:2303–2308. doi: 10.1073/pnas.042601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Cho MH, Ullian EM, Isom LL, Levinson SR, Barres BA. Differential control of clustering of the sodium channels Na(v)1.2 and Na(v)1.6 at developing CNS nodes of Ranvier. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Karagogeos D, Morton SB, Casano F, Dodd J, Jessell TM. Developmental expression of the axonal glycoprotein TAG-1: differential regulation by central and peripheral neurons in vitro. Development. 1991;112:51–67. doi: 10.1242/dev.112.1.51. [DOI] [PubMed] [Google Scholar]
- Kearney JA, Buchner DA, De Haan G, Adamska M, Levin SI, Furay AR, Albin RL, Jones JM, Montal M, Stevens MJ. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6) Hum Mol Genet. 2002;11:2765–2775. doi: 10.1093/hmg/11.22.2765. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. BetaIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. AnkyrinG. A new ankyrin gene with neural- specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Lacas-Gervais S, Guo J, Strenzke N, Scarfone E, Kolpe M, Jahkel M, De Camilli P, Moser T, Rasband MN, Solimena M. BetaIVSigma1 spectrin stabilizes the nodes of Ranvier and axon initial segments. J Cell Biol. 2004;166:983–990. doi: 10.1083/jcb.200408007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S, Davis JQ, Bennett V. Morphogenesis of the node of Ranvier: co- clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Laval M, Bel C, Faivre-Sarrailh C. The lateral mobility of cell adhesion molecules is highly restricted at septate junctions in Drosophila. BMC Cell Biol. 2008;9:38. doi: 10.1186/1471-2121-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel alpha subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Williams A, Stankoff B. Promoting repair in multiple sclerosis: problems and prospects. Curr Opin Neurol. 2005;18:237–244. doi: 10.1097/01.wco.0000169739.83793.e0. [DOI] [PubMed] [Google Scholar]
- Lustig M, Zanazzi G, Sakurai T, Blanco C, Levinson SR, Lambert S, Grumet M, Salzer JL. Nr-CAM and neurofascin interactions regulate ankyrin G and sodium channel clustering at the node of Ranvier. Curr Biol. 2001;11:1864–1869. doi: 10.1016/s0960-9822(01)00586-3. [DOI] [PubMed] [Google Scholar]
- Maier O, van der Heide T, van Dam AM, Baron W, de Vries H, Hoekstra D. Alteration of the extracellular matrix interferes with raft association of neurofascin in oligodendrocytes. Potential significance for multiple sclerosis? Mol Cell Neurosci. 2005;28:390–401. doi: 10.1016/j.mcn.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maier O, Baron W, Hoekstra D. Reduced raft-association of NF155 in active MS-lesions is accompanied by the disruption of the paranodal junction. Glia. 2007;55:885–895. doi: 10.1002/glia.20510. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel β1 subunits with ankyrin. J Biol Chem. 2002;277:26681–26688. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo–glial organization. Biochim Biophys Acta. 2002;1573:406–413. doi: 10.1016/s0304-4165(02)00410-5. [DOI] [PubMed] [Google Scholar]
- Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P, Einheber S, Galinska J, Thaker P, Lam I, Rubin MB, Scherer SS, Murakami Y, Gutmann DH, Salzer JL. Nectin-like proteins mediate axon Schwann cell interactions along the internode and are essential for myelination. J Cell Biol. 2007;178:861–874. doi: 10.1083/jcb.200705132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Rios JC, Zanazzi G, Lambert S, Bretscher A, Salzer JL. Nodes of Ranvier form in association with ezrin-radixin-moesin (ERM)-positive Schwann cell processes. Proc Natl Acad Sci U S A. 2001;98:1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates schwann cell myelination and formation of associated axonal domains. J Neurosci. 2004;24:3953–3963. doi: 10.1523/JNEUROSCI.4920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Mi F, Berkowitz GA. Development of a K+-channel probe and its use for identification of an intracellular plant membrane K+ channel. Proc Natl Acad Sci U S A. 1995;92:3386–3390. doi: 10.1073/pnas.92.8.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Toews AD, Wagner M, Goodrum JF. Gene expression during tellurium- induced primary demyelination. Neurotoxicology. 1994;15:171–180. [PubMed] [Google Scholar]
- Moscoso LM, Sanes JR. Expression of four immunoglobulin superfamily adhesion molecules (L1, Nr-CAM/Bravo, neurofascin/ABGP, and N-CAM) in the developing mouse spinal cord. J Comp Neurol. 1995;352:321–334. doi: 10.1002/cne.903520302. [DOI] [PubMed] [Google Scholar]
- Nave KA, Sereda MW, Ehrenreich H. Mechanisms of disease: inherited demyelinating neuropathies-from basic to clinical research. Nat Clin Pract Neurol. 2007;3:453–464. doi: 10.1038/ncpneuro0583. [DOI] [PubMed] [Google Scholar]
- Norton WT, Cammer W. In: Isolation and characterization of myelin. Morell P, editor. Myelin. Plenum; New York: 1984. pp. 147–195. [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci. 2005;25:9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Schafer DP, Horresh I, Bar V, Hales K, Yang Y, Susuki K, Peles E, Stankewich MC, Rasband M. Spectrins and ankyrinB constitute a specialized paranodal cytoskeleton. J Neurosci. 2006;26:5230–5239. doi: 10.1523/JNEUROSCI.0425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguievetskaia K, Cifuentes-Diaz C, Girault JA, Goutebroze L. Cellular contacts in myelinated fibers of the peripheral nervous system. Med Sci. 2005;21:162–169. doi: 10.1051/medsci/2005212162. [DOI] [PubMed] [Google Scholar]
- Ohara R, Yamakawa H, Nakayama M, Ohara O. Type II brain 4.1 (4.1B/KIAA0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Brain Res Mol Brain Res. 2000;85:41–52. doi: 10.1016/s0169-328x(00)00233-3. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Park J, Liu B, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra M, Gascard P, Walensky LD, Gimm JA, Blackshaw S, Chan N, Takakuwa Y, Berger T, Lee G, Chasis JA. Molecular and functional characterization of protein 4.1B, a novel member of the protein 4.1 family with high level, focal expression in brain. J Biol Chem. 2000;275:3247–3255. doi: 10.1074/jbc.275.5.3247. [DOI] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Garcia-Fresco GP, Sousa AD, Dupree JL, Philpot BD, Bhat MA. No effect of genetic deletion of contactin-associated protein (CASPR) on axonal orientation and synaptic plasticity. J Neurosci Res. 2007;85:2318–2331. doi: 10.1002/jnr.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AM, Thaxton C, Pribisko AL, Cheng J-G, Dupree JL, Bhat MA. Spatio- temporal ablation of myelinating glia-specific neurofascin (nfascnf155) in mice reveals gradual loss of paranodal axo–glial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009 Jan 30; doi: 10.1002/jnr.22015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–5251. doi: 10.1242/dev.01412. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Salomon D, Berglund EO, Ohara R, Ranscht B, Peles E. Localization of Caspr2 in myelinated nerves depends on axon-glia interactions and the generation of barriers along the axon. J Neurosci. 2001;21:7568–7575. doi: 10.1523/JNEUROSCI.21-19-07568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Zhen D, Arbuckle MI, Poliak S, Peles E, Grant SG, Trimmer JS. Clustering of neuronal potassium channels is independent of their interaction with PSD-95. J Cell Biol. 2002;159:663–672. doi: 10.1083/jcb.200206024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RA, Bronson DD, Young KM, Hemperly JJ. Identification and characterization of the human cell adhesion molecule contactin. Brain Res Mol Brain Res. 1994;21:1–8. doi: 10.1016/0169-328x(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Scheithauer B. Ultrastructure of multiple sclerosis. Ultrastruct Pathol. 1994;18:3–13. doi: 10.3109/01913129409016267. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Brophy PJ, Peles E. Molecular domains of myelinated axons in the peripheral nervous system. Glia. 2008;56:1532–1540. doi: 10.1002/glia.20750. [DOI] [PubMed] [Google Scholar]
- Savvaki M, Panagiotaropoulos T, Stamatakis A, Sargiannidou I, Karatzioula P, Watanabe K, Stylianopoulou F, Karagogeos D, Kleopa KA. Impairment of learning and memory in TAG-1 deficient mice associated with shorter CNS internodes and disrupted juxtaparanodes. Mol Cell Neurosci. 2008;39:478–490. doi: 10.1016/j.mcn.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci. 2004;24:3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Shy ME. Peripheral neuropathies caused by mutations in the myelin protein zero. J Neurol Sci. 2006;242:55–66. doi: 10.1016/j.jns.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Sousa AD, Bhat MA. Cytoskeletal transition at the paranodes: the Achilles' heel of myelinated axons. Neuron Glia Biol. 2007;3:169–178. doi: 10.1017/S1740925X07000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Salomon D, Erne B, Schaeren-Wiemers N, Peles E. Caspr3 and caspr4, two novel members of the caspr family are expressed in the nervous system and interact with PDZ domains. Mol Cell Neurosci. 2002;20:283–297. doi: 10.1006/mcne.2002.1110. [DOI] [PubMed] [Google Scholar]
- Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;115:3991–4000. doi: 10.1242/jcs.00094. [DOI] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo–glial junction. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 2003;94:655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–5500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Tzimourakas A, Giasemi S, Mouratidou M, Karagogeos D. Structure-function analysis of protein complexes involved in the molecular architecture of juxtaparanodal regions of myelinated fibers. Biotechnol J. 2007;2:577–583. doi: 10.1002/biot.200700023. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voas MG, Lyons DA, Naylor SG, Arana N, Rasband MN, Talbot WS. αII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol. 2007;17:562–568. doi: 10.1016/j.cub.2007.01.071. [DOI] [PubMed] [Google Scholar]
- Volkmer H, Hassel B, Wolff JM, Frank R, Rathjen FG. Structure of the axonal surface recognition molecule neurofascin and its relationship to a neural subgroup of the immunoglobulin superfamily. J Cell Biol. 1992;118:149–161. doi: 10.1083/jcb.118.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Ward RE, Lamb RS, Fehon RG. A conserved functional domain of Drosophila coracle is required for localization at the septate junction and has membrane- organizing activity. J Cell Biol. 1998;140:1463–1473. doi: 10.1083/jcb.140.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Davis JQ, Carpenter S, Bennett V. Structural requirements for association of neurofascin with ankyrin. J Biol Chem. 1998;273:30785–30794. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998a;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang CL, Messing A, Chiu SY. Temperature-sensitive neuromuscular transmission in Kv1.1 null mice: role of potassium channels under the myelin sheath in young nerves. J Neurosci. 1998b;1:7200–7215. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Messing A, Chiu SY. Determinants of excitability at transition zones in Kv1.1-deficient myelinated nerves. J Neurosci. 1999;19:5768–5781. doi: 10.1523/JNEUROSCI.19-14-05768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. J Cell Biol. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]