Abstract
Patients suffering from traumatic brain injury (TBI) have decreased markers of energy metabolism, including N-acetylaspartate (NAA) and ATP. In the nervous system, NAA-derived acetate provides acetyl-CoA required for myelin lipid synthesis. Acetate can also be oxidized in mitochondria for the derivation of metabolic energy. In the current study, using the controlled cortical impact model of TBI in rats, we investigated the effects of the hydrophobic acetate precursor, glyceryltriacetate (GTA), as a method of delivering metabolizable acetate to the injured brain. We found that GTA administration significantly increased the levels of both NAA and ATP in the injured hemisphere 4 and 6 days after injury, and also resulted in significantly improved motor performance in rats 3 days after injury.
Key words: ATP, glyceryltriacetate, myelin synthesis, N-acetylaspartate
Introduction
Traumatic brain injury (TBI) affects over 1.5 million people in the United States each year (Thurman et al., 1999). Providing immediate treatment to TBI victims can help prevent the development of secondary brain damage due to severe inflammation, edema, and delayed cell death. TBI is associated with substantial perturbations of metabolism, including a disruption of brain mitochondrial function, which contributes to the complex sequence of pathophysiological events that occurs after injury (Lee et al., 1999; Signoretti et al., 2001). Functional impairment of brain mitochondria after TBI results in reduced oxidative phosphorylation and decreased ATP levels (Singh et al., 2006). A substantial portion of brain ATP production is required to supply energy for maintaining steep ionic gradients across nerve-cell membranes through the action of ionic pumps. Reductions in mitochondrial energetics and brain energy stores resulting from TBI put extreme stress on brain metabolism to maintain these critical gradients.
Impaired brain energy metabolism resulting from TBI is a significant issue that remains to be addressed in the management of TBI (Lee et al., 1999; Vespa et al., 2005), and metabolic therapy provides an encouraging avenue for treatment. Disrupted activity of key mitochondrial enzymes, including mitochondrial pyruvate dehydrogenase complex, may be one consequence of TBI that can be addressed by metabolic approaches (Opii et al., 2007; Vagnozzi et al., 2007). Pyruvate dehydrogenase converts pyruvate to acetyl coenzyme A, which can then go on to participate in either mitochondrial energy production or cytosolic lipid synthesis, among other functions. It is possible to bypass insulin-mediated glucose uptake, glycolysis, and mitochondrial pyruvate dehydrogenase by providing acetate as an immediate source of metabolic energy in the form of a lipid-soluble acetate precursor. We have recently found that glyceryltriacetate (GTA), an FDA-approved food and drug additive, can be used as a dietary supplement to increase acetate levels in the brain by over 15-fold within 1 h of administration (Mathew et al., 2005). Due to its hydrophobic nature, GTA enters all cells in the brain rapidly, and is broken down to acetate and glycerol by the action of nonspecific esterases. The acetate is then converted to acetyl coenzyme A, which can be utilized for energy production, lipid synthesis, and other metabolic processes. As such, GTA represents a novel and promising candidate for rapidly improving brain mitochondrial function following TBI.
N-acetylaspartate (NAA) is a concentrated brain metabolite that is associated with brain energy metabolism (Clark, 1998; Moffett et al., 2007), and acts as a source of acetate for lipid synthesis and myelination (Madhavarao et al., 2005). NAA levels in the brain are substantially reduced along with ATP levels after TBI (Signoretti et al., 2001). In addition to the metabolic energy deficit, TBI results in diffuse axonal damage, which may necessitate increased lipid synthesis and remyelination. For these reasons, we investigated the effect of GTA-based acetate supplementation as a treatment strategy for TBI using the controlled cortical impact (CCI) model in rats.
Methods
Controlled cortical impact injury
Male Sprague-Dawley rats (200–250g) were obtained from Charles River Laboratories (Raleigh, NC). All procedures were performed in accordance with guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee. Animals were housed on a 12-h reversed light–dark cycle.
Animals were given coded tail numbers, and assigned randomly to the treatment groups (4-day control, 4-day CCI-H2O, 4-day CCI-GTA, 6-day control, 6-day CCI-H2O, 6-day GTA). Anesthesia consisted of 4% isoflurane vaporized in O2, and was maintained with 2% isoflurane during surgery. The head was fixed in a stereotaxic frame (David Kopf instruments, Inc., Tujunga, CA), and after retracting the scalp, an 8-mm craniotomy was made over the left parietal cortex, 0.5 mm posterior to the coronal suture and 3 mm lateral to the sagittal suture. Care was taken to avoid injury to the dura, which was continuously bathed in sterile physiological saline during the procedure. Injury was produced by a single impact with a CCI device (Pittsburgh Precision Instruments, Inc., Pittsburgh, PA) using a 6-mm flat steel impact tip (5 m/s, 2.5 mm deformation). Impact velocity was measured for reproducibility. Immediately after the injury, the bone flap was replaced and sealed with dental acrylic cement, and the scalp and skin were closed with staples. Control animals were not surgically treated.
Glyceryltriacetate administration
Animals received the first dose of GTA intragastrically (7.5 g/kg) 10 min after the surgical procedure using a blunt-tipped intragastric feeding needle passed down the esophagus. Treated animals were given the same dose every 24 h until sacrifice. Control and untreated injured animals were given an equal volume of water intragastrically, instead of GTA. A total of 66 animals entered the study (24 for the 4-day group and 42 for the 6-day group), and all 66 completed the study. Animals that were sacrificed on day 4 for biochemical analyses were not pretrained or tested on the Rotarod (n = 8 per group), whereas animals that were sacrificed on day 6 for biochemical analyses were pretrained and tested on the Rotarod on days 1, 3, and 5 after injury (n = 14 per group).
Estimation of N-acetylaspartate
Animals were anesthetized with pentobarbital and killed by decapitation. Brains were removed rapidly (within approximately 1 min) and the left injured hemispheres were isolated and homogenized immediately in ice-cold PBS (10% wt/vol) to be used for the estimation of NAA and ATP. NAA concentrations were determined using methods described earlier (Arun et al., 2008). Briefly, 50 μl of the 10% homogenate made above was mixed with 500 μl of 90% methanol in water, mixed vigorously, and centrifuged at 10,000g for 10 min at 4°C. The supernatants were lyophilized, and residues were dissolved in water and passed through a cation exchange column (AG 50W-X8, Bio-Rad, Hercules, CA). The eluates and two column washings were pooled, pH adjusted between 7 and 8 with 1 mM KOH, and lyophilized. Aliquots of methanol (25 μl) were added to the residues, vortex mixed, and added to 100 μl of acetonitrile and 40 μl of p-bromophenazylbromide reagent. Samples were incubated at 80°C for 1 h. The solvent was then removed by lyophilization and the residues dissolved in acetonitrile:water (1:1) and used for high-performance liquid chromatography (HPLC) analysis. HPLC was performed isocratically using a Hewlett Packard system (series 1100; Agilent Technologies, Santa Clara, CA) connected with a reverse phase column (BIOAdvantage™ 100 C18, 5 μm, 250 × 4.6 mm; Thomson Instrument Company, Clear Brook, VA). The mobile phase used was acetonitrile:water (1:1) at 25°C with a flow rate of 1.0 ml/min with detection at 250 nm. Under these conditions, NAA had a retention time of 15.2 min and the quantitation was carried out using known concentrations of NAA standard.
Estimation of ATP
ATP levels were measured using the ATP lite assay kit (Perkin Elmer, Boston, MA). Briefly, 300 μl each of the 10% brain homogenates were mixed with 1 ml of methanol, and centrifuged at 10,000g for 10 min at 4°C. The supernatants were lyophilized, the residues dissolved in 50 μl of water, 50 μl of the ATP lite reagent added, and the luminescence measured using a SpectraMax Gemini XPS luminometer (Molecular Devices, Sunnyvale, CA). The data was analyzed using SoftMax® Pro 4.7.1 software (Molecular Devices, Sunnyvale, CA). Quantitation was carried out using standard ATP solutions.
Rotarod balance test
An accelerated Rotarod task was used with an Accuscan rat Rotarod system (Accuscan Electronics, Columbus, OH). Rats in the 6-day groups were pretrained for 2 days, three times per day for 5 min each, prior to the start of the study. Each animal was tested three times on the device at each time period during the study. The speed of rotation was increased linearly from 3 rpm to 30 rpm over the course of the 5-min trials (setting 7 on the accelerated Accuscan Rotarod protocol). Animals had to pace forward and adjust their gait continuously to remain on the rotating drum. The mean duration that the animals stayed on the device, expressed in seconds, was recorded for each trial.
Results
CCI injury
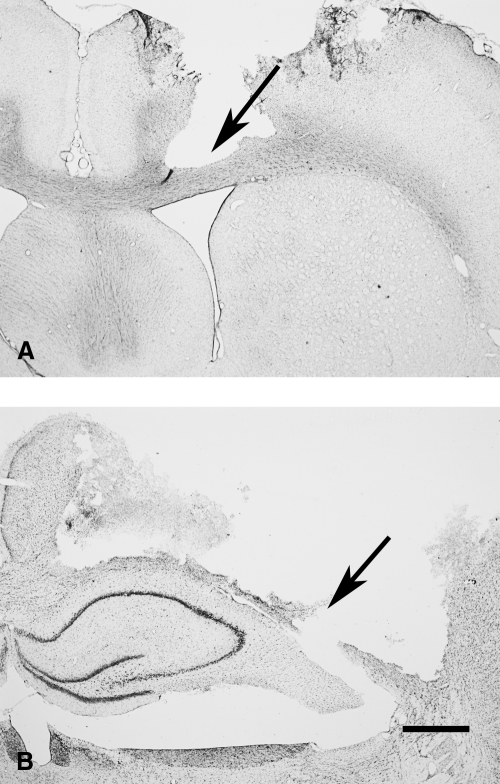
Cresyl violet acetate stained sections of a typical CCI injury from a rat on post-injury day 3 are shown in Figure 1. The rostral edge of the injury is shown in A, with substantial loss of tissue extending through all cortical layers, but with the corpus callosum remaining mostly intact (arrow in Fig. 1A). The approximate center of the injury is shown in B, with extensive cortical tissue loss extending through the corpus callosum (arrow in Fig. 1B), and approximately 6 mm in the horizontal dimension.
FIG. 1.
Cresyl violet acetate stained forebrain sections showing CCI injury to the left hemisphere at the level of the septal nucleus (A) and the dorsal hippocampus (B). At the periphery of the injury, the corpus callosum remained intact (arrow in A), but at the core of the injury, the corpus callosum was completely transected (arrow in B). Bar = 1 mm.
N-acetylaspartate levels in the injured hemisphere
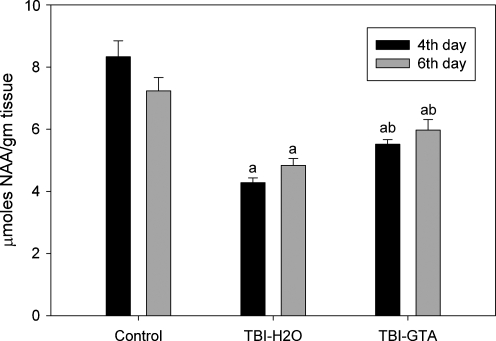
NAA levels examined in animals on the 4th and 6th days after CCI injury were analyzed with analysis of variance (ANOVA). There were significant differences in NAA levels on post-injury day 4 [F (2, 21) = 41.532, p < 0.001] and post-injury day 6 [F (2, 37) = 13.126, p < 0.001] Tukey's post-hoc tests showed significant differences between all groups on both post-injury days. NAA levels were decreased an average of 49% in the damaged hemisphere on day 4 after injury in animals treated with water (Fig. 2). GTA treatment increased NAA levels in the injured hemisphere by approximately 29% compared with water-treated controls. In the animals examined 6 days after CCI injury, NAA levels were decreased an average of 33% in the water-treated animals. GTA treatment increased NAA levels in the injured hemisphere by an average of 23% relative to water-treated animals at this time point.
FIG. 2.
NAA concentrations in the injured hemisphere 4 and 6 days after injury, with and without GTA treatment. Statistical analysis was carried out using one-way ANOVA and Tukey's post-hoc test by HSD multiple comparisons (n = 8 for 4 days, n = 14 for 6 days). aValues (mean ± SEM) of the uninjured (control) group are compared to those of injured groups (p < 0.001). bValues (mean ± SEM) of water-treated injured group are compared to those of GTA-treated injured group (p < 0.001).
ATP levels in the injured hemisphere
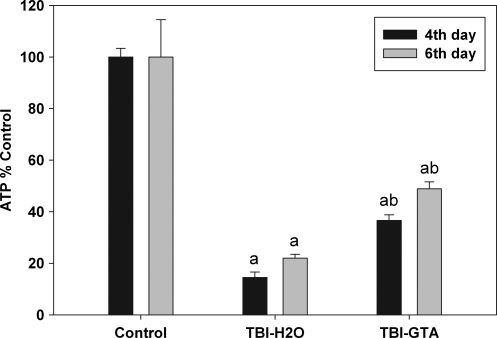
ATP levels examined in animals on post-injury days 4 and 6 were analyzed with analysis of variance (ANOVA). There were significant differences in ATP levels on post-injury day 4 [F (2, 21) = 281.88, p < 0.001) and post-injury day 6 [F (2, 37) = 25.04, p < 0.001]. Tukey's post-hoc tests revealed that significant differences occurred between all groups on these two post-injury days. ATP levels decreased sharply in the injured hemisphere after CCI injury on post-injury days 4 and 6 (Fig. 3). In the case of the water-treated injured animals, the decreases were about 85% and 78% on days 4 and 6 respectively. After GTA treatment, the ATP levels in the injured hemisphere increased more than two-fold compared to water-treated injured animals (a 2.5-fold increase on day 4, and 2.2-fold increase on day 6).
FIG. 3.
Percent change in ATP concentrations in the injured hemisphere 4 and 6 days after injury with and without GTA treatment. Statistical analysis was carried out using one-way ANOVA and Tukey's post-hoc test by HSD multiple comparisons (n = 8 for 4 days, and n = 14 for 6 days). aValues (% control ± SEM) of uninjured (control) group are compared to those of injured groups (p < 0.001). bValues (% control ± SEM) of water treated injured group are compared to those of GTA treated injured group (p < 0.001).
Rotarod performance
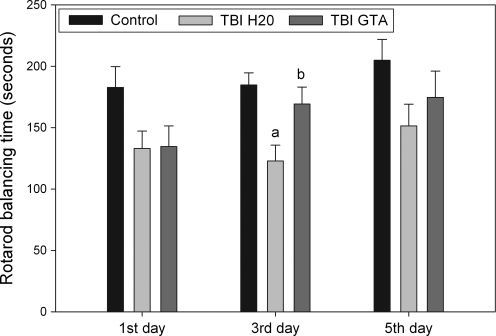
Behavioral data from the Rotarod balance test were analyzed by analyses of variance (ANOVA) on days 1, 3, and 5 after CCI injury (Fig. 4). There was a significant difference in motor performance on the third post-injury day [F (2, 37) = 6.57, p < 0.01]. Balancing time on the Rotarod after injury showed decreases compared to uninjured animals at all three time points, but the decreases were statistically significant only on the 3rd post-injury day (p < 0.05). At this time point, the GTA-treated animals showed significant improvement (average of 38% increase) in balancing time compared to the water-treated animals. Balancing time on the 3rd post-injury day for the GTA-treated injured group (average of 169 ± 52 sec) approached the balancing time for uninjured controls (average of 185 ± 34 sec). This compares favorably with an average balancing time for water-treated injured animals of 123 ± 48 sec.
FIG. 4.
Rotarod performance 1, 3, and 5 days after CCI injury with and without GTA treatment. Statistical analysis was carried out using one-way ANOVA and Tukey's post-hoc test by HSD multiple comparisons (n = 14 per group). aValues (means ± SEM) of uninjured (control) group are compared to those of injured groups (p < 0.01). bValues (means ± SEM) of water-treated injured group are compared to those of GTA-treated injured group (p < 0.01).
Discussion
NAA and ATP levels are reduced immediately after brain injury and remain depressed for days or weeks, reflecting both metabolic impairment and depletion of brain energy stores (Signoretti et al., 2001; Tavazzi et al., 2005; Vagnozzi et al., 2005). In the current studies, we found that GTA administration after unilateral experimental TBI in rats significantly improved NAA and ATP levels in the injured hemisphere 4 days and 6 days after injury. These results indicate that the decreases in brain energy stores resulting from TBI can be partly reversed by the administration of GTA. Improvements in motor performance on a treadmill balance test were also significant 3 days after injury, suggesting that GTA administration increases recovery rates after TBI. In fact, on post-injury day 3, the GTA-treated injured rats' performance was not significantly different from that of uninjured controls. There are a number of mechanisms that might account for the efficacy of GTA treatment.
The NAA increases we observed in the injured hemisphere are noteworthy because in earlier studies we have found that high doses of GTA given over time to control mice do not increase the normal levels of NAA in the brain (Mathew et al., 2005). The results in the current study are likely to be a consequence of the significant drop in brain acetyl coenzyme A levels associated with TBI (Vagnozzi et al., 2007), which would affect both ATP and NAA synthesis rates. Increasing acetate availability after TBI via GTA administration would compensate for the reduced availability of acetyl coenzyme A, and improve both ATP and NAA synthesis.
Reductions in NAA and ATP are positively correlated with the severity of the brain injury (Signoretti et al., 2001). In a recent series of experimental studies on TBI, the relationships between NAA, acetyl coenzyme A, and energy metabolism have been examined in detail. Vagnozzi and colleagues (2007) found that a number of indicators of mitochondrial energy production were significantly decreased after two successive mild TBI in rats, including decreases in ATP, the ATP/ADP ratio, NAA, oxidized and reduced NAD, and acetyl coenzyme A. They proposed using antioxidants to protect pyruvate dehydrogenase from oxidative damage, thus enhancing mitochondrial energy production from glucose. We note that providing acetate directly in the form of GTA would obviate the need to protect pyruvate dehydrogenase. GTA bypasses pyruvate dehydrogenase and directly provides a key cellular metabolite, acetate, for enhancing oxidative phosphorylation.
The brain derives most of its energy from glucose, but the brain can also make use of alternate energy sources including ketone bodies, lactate, and acetate. Alternate energy sources have been used to improve energy status after TBI, including lactate and β-hydroxybutyrate. Intravenous lactate infusion was found to improve cognitive performance as assessed by the Morris water maze test in rats subjected to fluid percussion brain injury (Rice et al., 2002). ATP levels in the injured hemisphere were also improved compared with saline-treated controls. In another study, ATP reductions in the ipsilateral cortex resulting from CCI injury were reversed by a 3-h infusion of the ketone body β-hydroxybutyrate (Prins et al., 2004). In the current study, we have found similar improvements in ipsilateral ATP levels with GTA administration after unilateral controlled CCI.
Brain damage is associated with perturbations of lipid metabolism,and degradation of mitochondrial phospholipids (Adibhatla et al., 2006). Damage to subcortical white matter is also observed after TBI, and delayed axonal degeneration is seen distant to the immediate injury zone (Hall et al., 2005; Park et al., 2008). Because NAA-derived acetate is a key building block for myelin lipid synthesis in the brain, we have pursued the concept that GTA could be used to increase myelin lipid synthesis in neurological disorders that involve hypomyelination or demyelination (Madhavarao et al., 2005; Mathew et al., 2005). It is possible that GTA administration following TBI could contribute to increased lipid synthesis and remyelination.
Severe TBI in humans is associated with hypermetabolism, hypercatabolism, increased nitrogen excretion, and an increase in daily caloric requirements (Pepe and Barba, 1999). Increased nutritional support immediately following TBI can reduce the negative nitrogen balance resulting from increased protein catabolism, but the effects of improved early nutrition on long-term outcomes are uncertain. Ketogenic diets have been shown to have neuroprotective effects in animal models of TBI, but these protective effects are restricted to TBI in young animals (Hu et al., 2009). Younger animals have a greater capacity to produce ketone bodies, have higher expression of ketone metabolizing enzymes, and greater enzymatic capacity for ketone metabolism than adults (Appelberg et al., 2009). Acetate is not technically a ketone body because it is metabolized by different enzymes than acetoacetate or β-hydroxybutyrate. However, like ketone bodies, it can be used for both energy derivation and fatty-acid synthesis. Free acetate is metabolized to acetyl coenzyme A by acetyl coenzyme A synthases, but the cellular distribution of these enzymes in the brain is currently unknown.
GTA is a hydrophobic molecule that crosses the blood–brain barrier and enters all cell types in the brain. This high degree of bioavailability may be an important factor in the treatment of TBI due to compromised cerebral circulation, which can limit the distribution of water-soluble compounds. Once internalized in neurons and glial cells, GTA is rapidly broken down by nonspecific esterases to generate free acetate and glycerol. Acetate can be converted to acetyl coenzyme A by the action of acetyl coenzyme A synthetase (Nikolau et al., 2000). Two forms of acetyl coenzyme A synthetase exist in cells: a mitochondrial form involved in energy metabolism (Fujino et al., 2001), and a nuclear-cytosolic form involved in lipid synthesis (Luong et al., 2000). Therefore, the acetyl coenzyme A produced from GTA can go on to be oxidized in the TCA cycle for energy production in mitochondria, or can enter lipid synthetic pathways for the production of membrane and myelin lipids in the cytoplasm.
One recently discovered function of acetyl coenzyme A synthase type 1, the nuclear-cytosolic form of the enzyme, is to provide acetyl coenzyme A for nuclear histone acetyltransferase enzymes involved in global gene regulation (Takahashi et al., 2006). In order for gene transcription to occur, histone protein acetylation is required to dissociate histones from DNA, allowing transcription-associated enzymes such as RNA polymerase access to the appropriate DNA strands. This fact has recently been exploited to improve learning and memory in rats following TBI by the use of a histone deacetylase inhibitor. Histone deacetylase enzymes (HDACs) act to complex histone proteins with DNA, and shut off gene transcription. Inhibiting HDACs permits transcription to continue. Dash and colleagues (2009) have shown that sodium butyrate, an HDAC inhibitor, improves learning and memory in rats following experimental TBI. Inhibiting histone deacetylation may enhance gene transcription for cellular repair mechanisms and neuronal plasticity. Acetyl coenzyme A levels are reduced after TBI (Vagnozzi et al., 2007), which could limit the ability of cells to maintain proper transcription levels due to reduced histone acetylation. We have found that acetyl coenzyme A synthase type 1 is strongly upregulated in neuronal cell nuclei after TBI (unpublished observations), suggesting increased enzymatic capacity to synthesize acetyl coenzyme A from free acetate in response to TBI. Nuclear localized acetyl coenzyme A synthase-1 can make use of GTA-derived acetate for the production of acetyl coenzyme A, which then could be used for histone acetylation reactions, thus maintaining gene transcription levels required for both cellular repair mechanisms and neuronal plasticity.
The tripartite effects of GTA make this compound uniquely suited for the treatment of TBI, wherein it provides: (1) an immediate energy source for mitochondria that bypasses insulin-mediated glucose uptake, glycolysis, and pyruvate oxidation; (2) a cytosolic acetate source for membrane and myelin lipid synthesis; and (3) a source of acetate in cell nuclei for histone acetylation reactions required to maintain enhanced gene transcription levels during repair and recovery. For all of these reasons, it is possible that GTA administration, both immediately after injury and throughout post-injury therapy, could be effective in improving neurological outcomes.
Conclusion
GTA is an FDA-approved food additive that is useful as both an oral and parenteral nutrient. It has been found to be safe when given intravenously to dogs at hypercaloric rates (1.5 × resting energy expenditure) (Bailey et al., 1991), and we have found it to be safe when given orally to mice and rats at a dose of 5.8 g/kg (Madhavarao et al., 2009; Mathew et al., 2005). As such, GTA can be used intravenously when TBI patients arrive at a medical facility, and can be given orally when appropriate. Our data are consistent with previous studies showing that impaired energy metabolism is a potential target for TBI therapy. Bypassing both glucose and NAA metabolism in TBI patients by directly providing acetate for brain energy production would help compensate for the substantial reductions in NAA and ATP levels observed after TBI. Providing additional acetate for nuclear histone acetylation reactions would help maintain transcriptional control of gene expression during cellular repair and recovery. Prolonged treatment with GTA after TBI could also contribute to remyelination of injured white matter and improve long-term outcomes.
Acknowledgments
This work was supported by grants from the NIH (NS RO1/R56; 039387) and the Samueli Institute (G170VR – under Award No. W81XWH-06-2-0009 from the U.S. Army Medical Research and Materiel Command).
Author Disclosure Statement
No competing financial interests exist.
References
- Adibhatla R.M. Hatcher J.F. Dempsey R.J. Lipids and lipidomics in brain injury and diseases. AAPS J. 2006;8:E314–E321. doi: 10.1007/BF02854902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg K.S. Hovda D.A. Prins M.L. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J. Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P. Madhavarao C.N. Moffett J.R. Namboodiri A.M. Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J. Neurochem. 2008;106:1669–1680. doi: 10.1111/j.1471-4159.2008.05524.x. [DOI] [PubMed] [Google Scholar]
- Bailey J.W. Haymond M.W. Miles J.M. Triacetin: A potential parenteral nutrient. JPEN J. Parenter. Enteral Nutr. 1991;15:32–36. doi: 10.1177/014860719101500132. [DOI] [PubMed] [Google Scholar]
- Clark J.B. N-acetylaspartate: A marker for neuronal loss or mitochondrial dysfunction. Dev. Neurosci. 1998;20:271–276. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- Dash P.K. Orsi S.A. Moore A.N. HDAC inhibition combined with behavioral therapy enhances learning and memory following traumatic brain injury. Neuroscience. 2009;163:1–8. doi: 10.1016/j.neuroscience.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T. Kondo J. Ishikawa M. Morikawa K. Yamamoto T.T. Acetyl-CoA synthetase 2, a mitochondrial matrix enzyme involved in the oxidation of acetate. J. Biol. Chem. 2001;276:11420–11426. doi: 10.1074/jbc.M008782200. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Sullivan P.G. Gibson T.R. Pavel K.M. Thompson B.M. Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: More than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hu Z.G. Wang H.D. Jin W. Yin H.X. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann. Clin. Lab. Sci. 2009;39:76–83. [PubMed] [Google Scholar]
- Lee S.M. Wong M.D. Samii A. Hovda D.A. Evidence for energy failure following irreversible traumatic brain injury. Ann. N. Y. Acad. Sci. 1999;893:337–340. doi: 10.1111/j.1749-6632.1999.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Luong A. Hannah V.C. Brown M.S. Goldstein J.L. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 2000;275:26458–26466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- Madhavarao C.N. Arun P. Anikster Y. Mog S.R. Staretz-Chacham O. Moffett J.R. Grunberg N.E. Gahl W.A. Namboodiri A.M. Glyceryl triacetate for Canavan disease: A low-dose trial in infants and evaluation of a higher dose for toxicity in the tremor rat model. J. Inherit. Metab. Dis. 2009;32:640–650. doi: 10.1007/s10545-009-1155-3. [DOI] [PubMed] [Google Scholar]
- Madhavarao C.N. Arun P. Moffett J.R. Szucs S. Surendran S. Matalon R. Garbern J. Hristova D. Johnson A. Jiang W. Namboodiri M.A. Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease. Proc. Natl. Acad. Sci. USA. 2005;102:5221–5226. doi: 10.1073/pnas.0409184102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. Arun P. Madhavarao C. Moffett J. Namboodiri A. Progress toward acetate supplementation therapy for Canavan disease: Glyceryl triacetate administration increases acetate, but not N-acetylaspartate levels in brain. J. Pharmacol. Exp. Ther. 2005;315:297–303. doi: 10.1124/jpet.105.087536. [DOI] [PubMed] [Google Scholar]
- Moffett J.R. Ross B. Arun P. Madhavarao C.N. Namboodiri A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolau B.J. Oliver D.J. Schnable P.S. Wurtele E.S. Molecular biology of acetyl-CoA metabolism. Biochem. Soc. Trans. 2000;28:591–593. [PubMed] [Google Scholar]
- Opii W.O. Nukala V.N. Sultana R. Pandya J.D. Day K.M. Merchant M.L. Klein J.B. Sullivan P.G. Butterfield D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Park E. Bell J.D. Baker A.J. Traumatic brain injury: Can the consequences be stopped? CMAJ. 2008;178:1163–1170. doi: 10.1503/cmaj.080282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe J.L. Barba C.A. The metabolic response to acute traumatic brain injury and implications for nutritional support. J. Head Trauma Rehabil. 1999;14:462–474. doi: 10.1097/00001199-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Prins M.L. Lee S.M. Fujima L.S. Hovda D.A. Increased cerebral uptake and oxidation of exogenous betaHB improves ATP following traumatic brain injury in adult rats. J. Neurochem. 2004;90:666–672. doi: 10.1111/j.1471-4159.2004.02542.x. [DOI] [PubMed] [Google Scholar]
- Rice A.C. Zsoldos R. Chen T. Wilson M.S. Alessandri B. Hamm R.J. Bullock M.R. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. 2002;928:156–159. doi: 10.1016/s0006-8993(01)03299-1. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Marmarou A. Tavazzi B. Lazzarino G. Beaumont A. Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J. Neurotrauma. 2001;18:977–991. doi: 10.1089/08977150152693683. [DOI] [PubMed] [Google Scholar]
- Singh I.N. Sullivan P.G. Deng Y. Mbye L.H. Hall E.D. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: Implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Takahashi H. McCaffery J.M. Irizarry R.A. Boeke J.D. Nucleocytosolic acetyl-coenzyme a synthetase is required for histone acetylation and global transcription. Mol. Cell. 2006;23:207–217. doi: 10.1016/j.molcel.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Tavazzi B. Signoretti S. Lazzarino G. Amorini A.M. Delfini R. Cimatti M. Marmarou A. Vagnozzi R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery. 2005;56:582–589. doi: 10.1227/01.neu.0000156715.04900.e6. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Alverson C. Dunn K.A. Guerro J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Cimatti M. Amorini A.M. Donzelli S. Delfini R. Lazzarino G. Hypothesis of the postconcussive vulnerable brain: Experimental evidence of its metabolic occurrence. Neurosurgery. 2005;57:164–171. doi: 10.1227/01.neu.0000163413.90259.85. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Tavazzi B. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: Mitochondrial-related impairment – Part I. Neurosurgery. 2007;61:379–388. doi: 10.1227/01.NEU.0000280002.41696.D8. [DOI] [PubMed] [Google Scholar]
- Vespa P. Bergsneider M. Hattori N. Wu H. M. Huang S.C. Martin N.A. Glenn T. C. McArthur D.L. Hovda D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]