Abstract
Bmp signaling has been shown to regulate early aspects of pancreas development, but its role in endocrine, and especially β-cell, differentiation remains unclear. Taking advantage of the ability in zebrafish embryos to cell-autonomously modulate Bmp signaling in single cells, we examined how Bmp signaling regulates the ability of individual endodermal cells to differentiate into β-cells. We find that specific temporal windows of Bmp signaling prevent β-cell differentiation. Thus, future dorsal bud-derived β-cells are sensitive to Bmp signaling specifically during gastrulation and early somitogenesis stages. In contrast, ventral pancreatic cells, which require an early Bmp signal to form, do not produce β-cells when exposed to Bmp signaling at 50 hpf, a stage when the ventral bud-derived extrapancreatic duct is the main source of new endocrine cells. Importantly, inhibiting Bmp signaling within endodermal cells via genetic means increased the number of β-cells, at early and late stages. Moreover, inhibition of Bmp signaling in the late stage embryo using dorsomorphin, a chemical inhibitor of Bmp receptors, significantly increased β-cell neogenesis near the extrapancreatic duct, demonstrating the feasibility of pharmacological approaches to increase β-cell numbers. Our in vivo single-cell analyses show that whereas Bmp signaling is necessary initially for formation of the ventral pancreas, differentiating endodermal cells need to be protected from exposure to Bmps during specific stages to permit β-cell differentiation. These results provide important unique insight into the intercellular signaling environment necessary for in vivo and in vitro generation of β-cells.
Keywords: Alk8/Acvr1/Alk2, β-cell, diabetes, endoderm, pancreas
The pancreas is a vital endodermal organ that consists of ductal, acinar, and endocrine cells. According to the hormones they produce, endocrine cells can be further divided into specific subtypes, including the Insulin-producing pancreatic β-cells. During embryogenesis, the pancreas is induced at specific positions along the developing gut and develops sharp boundaries with other endodermal organs, a process coordinated by a complex interplay of inductive and repressive signals (1). In this context, Bmp signaling has been implicated in inducing liver at the expense of Pdx1-expressing pancreas/intestine progenitors in fish and mouse, although sometimes with conflicting results (1–5). These discrepancies could stem from the inherent variability of in vitro studies and/or from the fact that the requirement for specific signaling pathways is very dynamic, as has been clearly shown for Wnt signaling in liver formation (6–8). In addition, because Pdx1-expressing progenitors give rise to intestine, stomach, and all pancreatic lineages, including ductal, acinar, and endocrine cells (9), the role of Bmp signaling during the formation of pancreatic β-cells, a small subset of the Pdx1-positive lineages, remains unclear (10). Bmp signaling also regulates dorsal-ventral (D-V) and anterior-posterior (A-P) patterning of all three germ layers (11–13), and Smad4 (a downstream component of the Tgf-β/activin/Bmp signaling pathways) has been implicated in the budding of the ventral pancreas (3). Therefore, to circumvent early patterning/morphological defects due to global reductions in Bmp signaling and to directly assess the role of Bmp signaling in pancreatic β-cell induction, one has to analyze single cells in mosaic embryos. Such insight is important because of the need to generate a large supply of Insulin-producing β-cells for therapeutic purposes and cannot come from analyzing the expression of general, or even cell-type-specific, pancreatic markers in tissue explants or mutant animals.
As in mammals (14), the pancreas in zebrafish forms from multiple buds and the endocrine cells derive from both the early-forming dorsal and the late-forming ventral buds (14, 15). In this study, we took advantage of the ability to transplant cells in zebrafish embryos to investigate the cell-autonomous roles of Bmp signaling in vivo in the induction of the dorsal and ventral bud-derived pancreatic endocrine cells, with a specific focus on β-cells. Our data show that whereas Bmp signaling is required for the outgrowth of the ventral pancreas, it needs to be suppressed cell-autonomously for the induction of β-cells.
Results
Activation of Bmp Signaling Cell-Autonomously Blocks the Induction of Dorsal Bud-Derived Endocrine Cells.
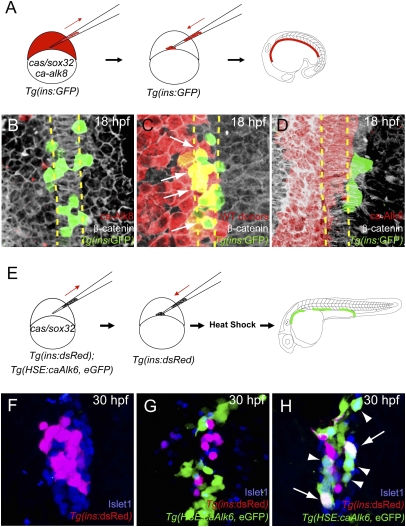
We performed cell transplantation experiments in the pregastrula embryo to determine the role of Bmp signaling in the induction of dorsal bud-derived pancreatic β-cells. To ensure incorporation of the transplanted cells into endoderm, we injected donor embryos with mRNA encoding Cas/Sox32, which directs cells to the endodermal lineage (16–18). Transgenic embryos expressing GFP under the control of the insulin promoter, Tg(ins:GFP), were injected with mRNA encoding Cas/Sox32 and a constitutively active form of the BMP receptor Alk8 (ca-Alk8), together with the lineage tracer rhodamine dextran, and transplanted into Tg(ins:GFP) hosts (Fig. 1A). After transplantation, we analyzed the hosts at 18 h postfertilization (hpf), a stage at which the endoderm still consists of a monolayer (17). Tg(ins:GFP)-expressing cells normally flank both sides of the notochord (17) (Fig. 1B) as was observed with transplanted control donor cells (Fig. 1C). However, donor cells expressing ca-Alk8 showed no Tg(ins:GFP) expression (Fig. 1D; n = 14). The absolute number of Tg(ins:GFP)-expressing cells was reduced from an average of 21 in control (n = 6) to 6 in host embryos in which ca-Alk8-expressing donor cells contributed to broad regions of the endodermal sheet (n = 4). All residual Tg(ins:GFP)-expressing cells were rhodamine dextran negative, indicating that ca-Alk8-expressing endodermal cells failed to differentiate into Tg(ins:GFP)-expressing cells.
Fig. 1.
Activation of Bmp signaling cell-autonomously blocks the induction of dorsal bud-derived endocrine cells. (A) Schematic diagram of the cell transplantation protocol. Tg(ins:GFP) donors were injected with cas/sox32 and ca-alk8 RNA, along with rhodamine dextran, and the cells were transplanted into Tg(ins:GFP) hosts. (B–D) Ventral confocal images of Tg(ins:GFP) (green), β-catenin (white), and rhodamine dextran (red) at 18 hpf (the notochord is outlined by yellow dashed lines). (B and C) Tg(ins:GFP)-expressing cells are normally located close to the notochord in wild type (B) and hosts containing control donor cells (C). (D) Tg(ins:GFP) expression was absent in ca-Alk8-expressing endodermal cells. (E) Schematic diagram of the cell transplantation protocol. cas/sox32 overexpressing Tg(ins:dsRed); Tg(HSE:caAlk6, eGFP) donor cells were transplanted into Tg(ins:dsRed) hosts, and subsequently heat-shocked at various time points. (F–H) Confocal projection images of hosts with Tg(HSE:caAlk6, eGFP)-expressing cells (green) at 30 hpf after staining for dsRed (red) and Islet1 (blue), comparing control (F) and hosts [heat shock was applied at 9 (G) or 11 hpf (H)]. (G) When ca-alk6 expression was induced at 9 hpf, the number of Tg(ins:dsRed) and Islet1-expressing cells was reduced (compare F and G), and the Tg(HSE:caAlk6, eGFP)-expressing cells failed to express endocrine markers. (H) When ca-alk6 expression was induced at 11 hpf, several Tg(HSE:caAlk6, eGFP)-expressing cells expressed Islet1 (arrowheads) and some of them also coexpressed Tg(ins:dsRed) (arrows).
These data indicate that cell-autonomous activation of Bmp signaling is not compatible with differentiation of endodermal cells into pancreatic β-cells. To determine at which stage Bmp signaling needs to be suppressed to allow the induction of pancreatic endocrine cells including β-cells, we transplanted cells expressing ca-Alk6 under the control of a heat-shock promoter. [The Bmp receptors Alk3 (Bmpr1a), Alk6 (Bmpr1b), and Alk8 (Acvr1/Alk2) are thought to phosphorylate the same downstream components despite being activated by slightly different sets of ligands, meaning that they can be used interchangeably as constitutively active, but not dominant negative, receptors.] We transplanted cas/sox32-overexpressing Tg(HSE:caAlk6, eGFP) (19) donor cells, and heat-shocked the hosts to activate Bmp signaling at various stages during gastrulation (at 5.25, 8, or 9 hpf) or somitogenesis (at 10, 11, or 12 hpf) (Fig. 1E). Control donor cells incorporated into pancreatic endocrine cells, including Tg(ins:GFP)-expressing β-cells at 30 hpf (Fig. S1). In contrast, when hosts were heat-shocked at 5.25, 8, 9, or 10 hpf, the Tg(HSE:caAlk6, eGFP)-expressing donor cells failed to differentiate into Islet1-positive pancreatic endocrine cells or to express Tg(ins:dsRed) (Fig. 1G; n = 25). However, when the hosts were heat-shocked at 11 or 12 hpf, Tg(HSE:caAlk6, eGFP)-expressing donor cells could differentiate into Islet1-positive pancreatic endocrine cells and some of them coexpressed Tg(ins:dsRed) (Fig. 1H; n = 18). These data indicate that cell-autonomous activation of Bmp signaling in endodermal progenitors, when heat-shock induced at 10 hpf or earlier, blocks induction of dorsal bud-derived pancreatic endocrine cells.
Bmp Signaling Restricts the Number of Endodermal Progenitors that Retain Competence to Differentiate into Dorsal Bud-Derived β-Cells.
Several bmp genes, including bmp2b, bmp4, and bmp7, are known to be expressed on the ventral side of the gastrula, whereas genes encoding secreted Bmp antagonists, such as Noggin1, Chordin, and Gremlin1a, are expressed on the dorsal side (11, 20). This complementary expression pattern generates a Bmp signaling gradient along the D-V axis of the embryo (12). Fate-mapping data (21, 22) indicate that progenitors of dorsal bud-derived pancreatic endocrine cells are localized close to the dorsal organizer. After gastrulation, these endocrine progenitors remain close to the notochord (17, 22), a derivative of the dorsal organizer that continues to secrete Bmp inhibitors, and far from the lateral plate mesoderm, which expresses several Bmp genes (5). Given the origin of pancreatic endocrine cells and our transplantation data with activated Bmp receptors, we hypothesized that pancreatic endocrine progenitors need to be protected from Bmp ligands during gastrulation and early somitogenesis stages, and that continuous Bmp suppression is critical for inducing pancreatic endocrine cells.
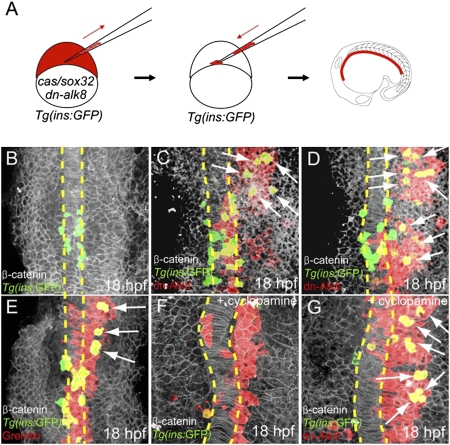
To further test this hypothesis and investigate whether Bmp suppression was sufficient for pancreatic endocrine induction, we transplanted cells expressing dominant-negative Alk8 (dn-Alk8) (23). Tg(ins:GFP) donor cells injected with cas/sox32 and dn-alk8 mRNA, together with the lineage tracer rhodamine dextran, were transplanted into Tg(ins:GFP) hosts (Fig. 2A). The dn-Alk8-expressing cells in many cases turned on Tg(ins:GFP) expression (Fig. 2 C and D, n = 31), even when they were located in the lateral part of the anterior endodermal sheet (which normally gives rise to liver, intestine, and exocrine pancreas) (5), and were clearly distinct from the medial pancreatic endocrine cells (compare Fig. 2 B, C, and D). The number of Tg(ins:GFP)-expressing cells increased from an average of 21 in control embryos (n = 6) to 37 in host embryos in which dn-Alk8-expressing donor cells contributed to broad regions of the endodermal sheet (n = 5). During somitogenesis, gremlin1a (20) is also expressed in adaxial cells (24), which constitute the most medial compartment of the somites and are closest to the region containing the pancreatic endocrine progenitors (17). Therefore, we investigated whether overexpression of Gremlin1a could also induce the formation of ectopic pancreatic endocrine cells. We found that Gremlin1a overexpression in the endoderm also led to ectopic pancreatic β-cells (Fig. 2E; n = 7), albeit with less efficiency than dn-Alk8 expression. The number of Tg(ins:GFP)-expressing cells increased from an average of 21 cells in control embryos (n = 6) to 30 in host embryos in which Gremlin1a-overexpressing donor cells contributed to broad regions of the endodermal sheet (n = 4). Together, these data indicate that suppression of Bmp signaling is sufficient to induce pancreatic β-cells in a cell-autonomous manner.
Fig. 2.
Suppression of Bmp signaling is sufficient to induce ectopic dorsal bud-derived pancreatic β-cells cell-autonomously. (A) Schematic diagram of the cell transplantation protocol. Tg(ins:GFP) donors were injected with cas/sox32 and dn-alk8 RNA along with rhodamine dextran (red), and the cells were transplanted into Tg(ins:GFP) hosts. (B–E) Ventral confocal images of Tg(ins:GFP) (green), β-catenin (white), and rhodamine dextran (red) at 18 hpf (the notochord is outlined by yellow dashed lines). (B) In control embryos, Tg(ins:GFP)-expressing cells are located close to the notochord. (C and D) Ectopic Tg(ins:GFP)-expressing cells (arrows) were found in lateral and anterior endodermal regions where dn-Alk8-expressing cells had incorporated, and all these ectopic cells were donor derived. (E) Gremlin1a overexpression in the endoderm also resulted in the ectopic formation of Tg(ins:GFP)-expressing cells (arrows). (F) When Hedgehog signaling was blocked with cyclopamine, Tg(ins:GFP)-expressing cells were almost absent in hosts containing control donor cells. (G) dn-Alk8 expression still induced the formation of Tg(ins:GFP)-expressing cells (arrows) even after cyclopamine treatment, and it did so cell-autonomously.
We previously reported that, in zebrafish, hedgehog (Hh) ligands secreted from the dorsal organizer and the notochord are required for inducing pancreatic endocrine cells via an unknown relay mechanism (17). Interestingly, gremlin1a expression has been shown to be regulated by Hh ligands such that it is absent in sonic hedgehog a mutants (20). Therefore, we hypothesized that Hh ligands from the dorsal organizer and its derivatives induce gremlin1a expression in neighboring cells, thereby protecting β-cell progenitors from Bmp signals. To test this hypothesis, we determined the epistasis between Hh signaling and Bmp suppression during pancreatic β-cell formation. After transplanting dn-Alk8-expressing cells to the endoderm, we blocked the Hh signaling pathway with the Smoothened inhibitor cyclopamine starting at 5.25 hpf. In control embryos, the number of Tg(ins:GFP)-expressing cells was severely reduced after cyclopamine treatment (Fig. 2F), as shown previously (17, 25). However, in experimental embryos, Tg(ins:GFP) was expressed ectopically by dn-Alk8-expressing donor-derived cells that had incorporated into the anterior endoderm [Fig. 2G; n = 13, note that Tg(ins:GFP) expression was nearly absent in host cells]. These data indicate that Hh signaling promotes formation of pancreatic endocrine cells at least in part by suppressing Bmp signaling. Together, these data lead us to propose that for the dorsal pancreas, Bmp signaling restricts the number of endodermal progenitors that retain competence to differentiate into pancreatic endocrine cells.
Activation of Bmp Signaling Cell-Autonomously Blocks the Induction of Ventral Bud-Derived Endocrine Cells.
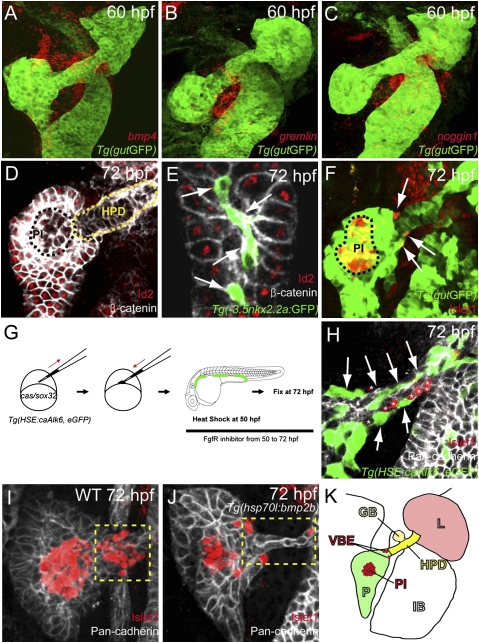
Because the ventral bud-derived pancreatic β-cells arise at a later developmental stage than the dorsal bud-derived β-cells (15) and may be induced through different mechanisms, we also tested the role of Bmp signaling in their formation. We first characterized the expression pattern of several bmp genes. Among those tested, bmp2b, bmp4, and bmp6 were expressed in the mesenchymal cells immediately adjacent to the foregut endoderm starting at the stage at which the ventral pancreas forms (Fig. S2). At later stages, bmp2b was expressed in mesenchymal cells surrounding the intestinal bulb (Fig. S2 A–C), and bmp4 (Fig. 3A and Fig. S2 D–F) and bmp6 (Fig. S2L) were expressed in mesenchymal cells directly adjacent to the developing ventral pancreas and subsequently in mesenchymal cells surrounding the hepatopancreatic duct, which in zebrafish is a source of pancreatic endocrine cells (26).
Fig. 3.
Activation of Bmp signaling cell-autonomously blocks the induction of ventral bud-derived endocrine cells. (A–C) Confocal images of Tg(gutGFP) (green) with mRNA expression (red) of bmp4 (A), gremlin1a (B), and noggin1 (C) at 60 hpf. (D and E) Confocal images of wild-type endoderm showing β-catenin (white) and Id2 (red) expression at 72 hpf. (D) Id2 expression is excluded from the hepatopancreatic duct cells (HPD; yellow dashed line) and appears to be downregulated or excluded from the pancreatic endocrine cells (PI, principal islet; black dashed line). (E) Id2 expression is also excluded from the intrapancreatic duct cells, labeled by Tg(-3.5nkx2.2a:GFP) expression (41) (green; arrows). (F) Confocal image of wild-type endoderm showing Tg(gutGFP) (green) and Islet1 (red) expression at 72 hpf. The ventral bud-derived endocrine cells (arrows) can be found at the junction between the pancreas and hepatopancreatic duct [the principal islet (PI) is outlined by black dashed line]. (G) Schematic diagram of the cell transplantation protocol. cas/sox32-overexpressing Tg(HSE:caAlk6, eGFP) donor cells were transplanted into wild-type hosts. Hosts were heat-shocked at 50 hpf and fixed at 72 hpf. After applying heat shock, hosts were treated with the Fgf receptor inhibitor SU5402, which induces ectopic Islet1-positive cells in the hepatopancreatic duct. (H) Single plane image of embryo stained for GFP (green), Pan-cadherin (white), and Islet1 (red) showing that Tg(HSE:caAlk6, eGFP)-expressing cells (arrows) fail to express Islet1 and display clear segregation from the ectopic Islet1-expressing cells (asterisks). (I and J) Effect of cell-nonautonomous activation of Bmp signaling by heat shock of Tg(hsp70l:bmp2b) at 50 hpf and subsequent treatment with SU5402 until fixation. Confocal projections of embryos stained for Pan-cadherin (white) and Islet1 (red) comparing control (I) and experimental (J) embryos. (I) Upon SU5402 treatment, ectopic Islet1-positive endocrine cells appeared in the hepatopancreatic duct (yellow dashed area), an effect that can be partially blocked by overexpression of Bmp2b (J). (K) Diagram of the endodermal organs at 72 hpf showing the liver (L; light red), pancreas (P; green), hepatopancreatic duct (HPD; yellow), gall bladder (GB; light yellow), and intestinal bulb (IB), as well as the ventral bud-derived endocrine cells (VBE; red) and principal islet (PI; red).
Because several Bmp ligands are expressed near the ventral pancreas and hepatopancreatic duct, we examined the endodermal expression of Inhibitor of DNA binding (Id) proteins, which are cell-autonomous markers of Bmp signaling activity (27). id genes are immediate targets of Bmp signaling (27) and have been implicated in promoting proliferation of pancreatic epithelial cells (28). Id proteins can also block the function of Neurod and Ngn3, basic helix–loop–helix transcription factors (27) critical for pancreatic endocrine development. We found that at 72 hpf, Id2 was expressed in almost all endodermal cells except for the cells of the endocrine pancreas, hepatopancreatic duct (Fig. 3D), and intrapancreatic ducts (Fig. 3E and Fig. S3). These data indicate that Bmp signaling is specifically blocked in the intrapancreatic and hepatopancreatic ducts, tissues that retain the potential to form endocrine cells. In support of this notion, we found that at 50 hpf and onwards, the Bmp antagonist genes, gremlin1a and noggin1 were expressed in the mesenchymal cells surrounding the hepatopancreatic duct (Fig. 3 B and C and Fig. S2 G–K).
To formally test the hypothesis that blocking Bmp signaling is required for the formation of ventral bud-derived endocrine cells, we performed cell transplantation experiments with the Tg(HSE:caAlk6, eGFP) line as a donor and induced ca-alk6 expression at 50 hpf, at which time the ventral pancreas has budded out and fused with the dorsal pancreas (Fig. 3G). In wild-type animals, most of the ventral bud-derived endocrine cells are found at the junction between the pancreas and hepatopancreatic duct (Fig. 3 F and K). Because there are normally very few ventral bud-derived endocrine cells at 72 hpf, we treated the hosts with the Fgf receptor inhibitor SU5402, starting at 50 hpf, which increases the number of newly formed endocrine cells in the hepatopancreatic duct (26), and thereby facilitates the analysis of ca-Alk6 function in endocrine cell differentiation. When ca-alk6 was induced in the donor cells at 50 hpf, the Tg(HSE:caAlk6, eGFP)-expressing cells in the hepatopancreatic duct never coexpressed Islet1 and showed a clear segregation from the endocrine cells (Fig. 3H; n = 16). To examine whether global activation of Bmp signaling could decrease the number of Islet1-expressing cells originating from the hepatopancreatic duct, we used Tg(hsp70l:bmp2b) embryos treated with SU5402 starting at 50 hpf and also heat-shocked at 50 hpf. We found that the number of Islet1-positive cells in or adjacent to the hepatopancreatic duct decreased in Bmp2b-overexpressing embryos (Fig. 3J; average 9 cells, n = 8) compared to control embryos (Fig. 3I; average 16.2 cells, n = 20, P = 0.0028). Altogether, these data clearly show that activating Bmp signaling can also block induction of ventral bud-derived endocrine cells cell-autonomously.
Differential Requirement for Bmp Signaling During Ventral Pancreas Outgrowth and Subsequent Endocrine Differentiation.
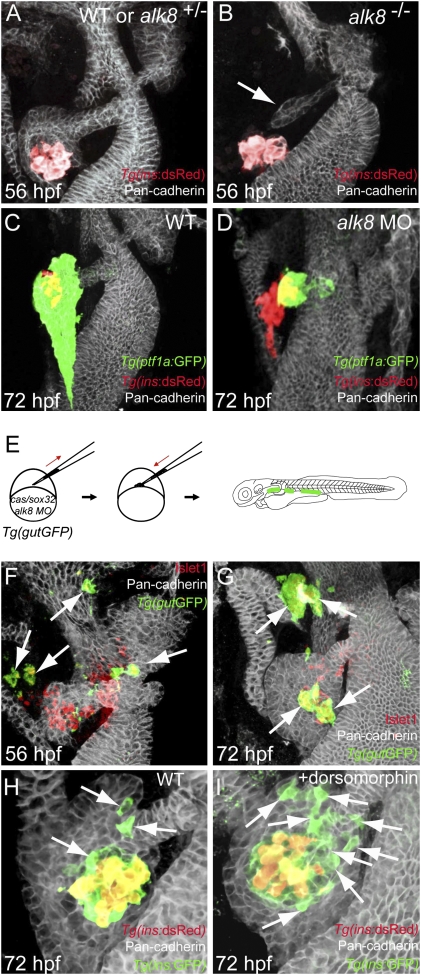
To investigate the role of endogenous Bmp signaling during formation of the ventral pancreas, we analyzed alk8 mutant embryos. These mutants have previously been shown to have a small liver (29), and we found that the outgrowth of the ventral pancreas was also severely affected (Fig. 4B) compared to wild type (Fig. 4A). Most mutants had no ventral pancreatic bud or a very small one that was not in contact with the islet at 56 hpf. Injection of an alk8 splice-blocking morpholino (MO) into Tg(ptf1a:GFP);Tg(ins:dsRed) embryos induced a similar phenotype (Fig. 4D) at 72 hpf (for verification of knockdown by the morpholino see Fig. S4). The hypoplastic ventral pancreatic bud marked by Tg(ptf1a:GFP) expression did not expand or engulf the principal islet at 72 hpf as in wild type, indicating that, in zebrafish as in mouse (3), Bmp signaling is necessary for outgrowth of the ventral pancreas. Because maternal Alk8 is not affected in the zygotic alk8 mutant or by the splice-blocking MO, alk8 mutants and alk8 MO-injected embryos did not show any defects in formation of the dorsal pancreas (Fig. 4 A–D). We further examined the cell-autonomous role of alk8 during formation of ventral bud-derived endocrine cells using alk8 MO-injected embryos as donors for transplantation experiments. When we transplanted alk8 MO-injected cells into wild-type embryos, the donor cells did not give rise to ectopic Tg(ins:GFP)-expressing cells at 18 hpf (Fig. S5; n = 6), most likely owing to the function of maternal Alk8. When we examined the hosts at 56 hpf (Fig. 4F; n = 13), we found that most of the donor cells expressed Islet1 and that these ectopic endocrine cells often were located in nonpancreatic tissues, such as the intestine and pharyngeal endoderm. At 72 hpf, the donor cells had migrated to the principal islet or sometimes delaminated from the endoderm to form ectopic pancreatic endocrine clusters (Fig. 4G; n = 13). Some of the ectopic endocrine cells that were located in nonpancreatic tissues sent out long protrusions toward the principal islet (Fig. S6). The alk8 MO-injected donor cells mainly gave rise to Insulin and Somatostatin-expressing cells but rarely to Glucagon-expressing cells (Fig. S7; n = 21), possibly due to a requirement for Bmp signaling during the differentiation of α-cells, as suggested previously (30). To further determine the stage at which the ectopic pancreatic endocrine cells were formed from alk8 MO-injected donor cells, we injected the MO and lineage tracer into Tg(ins:GFP);Tg(ins:dsRed) donors and transplanted cells into Tg(ins:GFP);Tg(ins:dsRed) hosts (Fig. S8A). Because dsRed takes ≈24 h longer than GFP to mature (31), it is possible to distinguish ventral bud-derived endocrine cells (positive only for GFP) from dorsal bud-derived endocrine cells (positive for both GFP and dsRed) until at least 60 hpf (32). We found that, whereas control donor cells showed no preference (Fig. S8 B and B′), many alk8 MO-injected donor cells gave rise to GFP-only-positive β-cells at 60 hpf (Fig. S8 C–D′; n = 10), indicating that they were induced later than the dorsal bud-derived endocrine cells. To further test this model, we treated Tg(ins:GFP);Tg(ins:dsRed) embryos with dorsomorphin (a selective inhibitor of the BMP type I receptors Alk3, Alk6, and Alk8, see ref. 39) between 44 and 72 hpf to inhibit Bmp signaling specifically during the formation of ventral bud-derived endocrine cells (this time period was chosen to allow budding of the ventral pancreas, but inhibit Bmp signaling during subsequent ventral bud development). We found that the number of GFP-only-positive β-cells adjacent to the extrapancreatic duct was increased in the dorsomorphin-treated embryos (Fig. 4I; average 18 cells, n = 9) compared to control (Fig. 4H; average 9 cells, n = 7, P = 0.0162). Thus, suppression of Bmp signaling in mosaic embryos results in ectopic formation of pancreatic endocrine cells in the gut, and both global suppression of Bmp signaling by dorsomorphin and knockdown of alk8 in mosaic embryos increases neogenesis of β-cells adjacent to the extrapancreatic duct. We therefore conclude that Bmp signaling needs to be suppressed in both the dorsal and ventral pancreatic buds, albeit at very different times, for β-cells to form.
Fig. 4.
Suppression of Bmp signaling in a cell-autonomous fashion leads to an increase in ventral bud-derived endocrine cells. (A and B) Confocal projection of control (A) and alk8 mutant (B) endoderm at 56 hpf. Tg(ins:dsRed) embryos were stained for Pan-cadherin (white). (B) alk8 mutants exhibit a very small ventral pancreatic bud (arrow). (C and D) Confocal projections of control (C) and alk8 morphant (D) Tg(ptf1a:GFP);Tg(ins:dsRed) embryos stained for Pan-cadherin (white). In alk8 morphants (D), the ventral pancreas buds out but fails to expand and engulf the principal islet. (E) Schematic diagram of the cell transplantation protocol. Tg(gutGFP) donors were injected with cas/sox32 mRNA and alk8 splice-blocking MO, and the donor cells were transplanted into wild-type hosts. (F and G) Confocal projections of hosts containing alk8 morphant donor cells (green) stained for Pan-cadherin (white) and Islet1 (red) at 56 (F) and 72 (G) hpf. Most alk8 morphant donor cells gave rise to Islet1-expressing endocrine cells (arrows). (H and I) Confocal projections of Tg(ins:GFP);Tg(ins:dsRed) embryos stained for Pan-cadherin (white) comparing control (H) and dorsomorphin-treated (I) embryos. Compared with control embryos (H), dorsomorphin-treated embryos showed an increased number of GFP-only-positive β-cells (arrows) (I).
Discussion
We (5) and others (2, 33) have previously shown that overexpression of Bmp ligands (2, 5, 33) or ca-Bmp receptors (33) restricts expression of Pdx1 and formation of pancreas/duodenum tissue in zebrafish (5), Xenopus (33), and mouse (2). However, these studies did not analyze the function of Bmp signaling during pancreatic β-cell formation. The data presented here clearly show that cell-autonomous suppression of Bmp signaling is required, and in some settings sufficient, for pancreatic β-cell induction. Interestingly, in vitro differentiation of human embryonic stem cells into pancreatic β-cells appears to be promoted by addition of Noggin (34), an effect that is consistent with our in vivo findings. Furthermore, our studies indicate that blocking Bmp signaling from the earliest stages of differentiation could result in a more effective differentiation toward the β-cell lineage.
Previous reports on the effects of Bmp signaling on pancreas development are conflicting. However, some of these disparities may simply reflect different temporal effects of Bmp signaling on pancreatic development. It is also important that the specificity with which different Bmp ligands interact with their corresponding receptors be taken into account. Overexpression of dn-Alk3, which presumably blocks Bmp2 and -4 but not Bmp6 and -7, does not affect endocrine development in the mouse (35), in contrast to our findings from experiments using dn-Alk8. Likewise, Pdx1:Bmp4 transgenic mice do not exhibit a developmental phenotype (35), whereas Pdx1:Bmp6 mice exhibit pancreas agenesis (36), an observation that may be explained by the fact that Bmp6 binds more efficiently to Alk2 (the murine Alk8 ortholog) than to Alk3 (37). Therefore, it would be interesting to examine whether a conditional deletion of Alk2 in the mouse, using a pan-endodermal Cre driver, results in pancreatic growth defects and/or ectopic formation of endocrine cells.
Our single-cell analyses in mosaic zebrafish embryos have uncovered a unique role for Bmp signaling in restricting widespread induction of pancreatic β-cells in endodermal progenitors. We have also shown that Bmp signaling plays multiple roles during pancreatic development: it is required for the budding of the ventral pancreas, as in mouse, but needs to be suppressed for pancreatic β-cell induction. Altogether, these data clarify the role of Bmp signaling in pancreatic β-cell induction and should help optimize in vitro and in vivo differentiation protocols of pancreatic progenitors into β-cells, or even the transdifferentiation of various cell types into β-cells, for the treatment of type I diabetes.
Materials and Methods
Zebrafish Strains.
Embryos and adult fish were raised and maintained under standard laboratory conditions. We used the following mutant and transgenic lines: laf/alk8tm110 (23), Tg(ins:GFP)zf5 (38), Tg(ins:dsRed)m1018 (from W. Driever, Freiburg), Tg(HSE:caAlk6, eGFP)w64 (19), Tg(gutGFP)s854 (also known as Tg(XlEef1a1:GFP)s854) (15), Tg(hsp70l:bmp2b)f13 (39), Tg(ptf1a:GFP)jh1 (40), and Tg(-3.5nkx2.2a:GFP)ia3/ia3 (41).
Embryo Microinjection and Transplantation.
Sense-strand-capped cas/sox32 (16), dn-alk8 (23), ca-alk8 (23), and gremlin1a (20) mRNA were synthesized with mMESSAGE mMACHINE (Ambion). For mRNA overexpression, embryos were injected with 200 pg of cas/sox32 mRNA in combination with 200 pg of dn-alk8, 50 pg of ca-alk8, or 100 pg of gremlin1a mRNA. Knockdown of Alk8 was performed via injection of 6 ng of an alk8 splice-blocking MO (5′–3′) ATTAAAAGTCTTACTTGTGAGCAGC (Open Biosystems). For transplantation, donor embryos were injected with a 2.5% solution of lineage tracer (tetramethylrhodamine dextran, 70,000 MW or Alexa Fluor 647 dextran, 10,000 MW; Invitrogen) along with cas/sox32 and other mRNA/or alk8 MO. After injection, we performed transplantations as described previously (17).
Chemical Treatment.
For experiments with cyclopamine (EMD Chemicals), host embryos containing dn-Alk8-expressing donor cells were raised on agarose-coated plates. Embryos were treated with 100 μM cyclopamine (in 1% DMSO in egg water) between blastula stage and 18 hpf. For experiments with the Fgf receptor inhibitor (gift from B. Jungblut, Bad-Nauheim, Germany), host embryos containing Tg(HSE:caAlk6, eGFP) donor cells were heat-shocked at 50 hpf and subsequently treated with 3 μM SU5402 solution (in 0.1% DMSO in egg water) until 72 hpf. Treatment of embryos with dorsomorphin (42) (EMD Chemicals) was carried out with 30 μM dorsomorphin (in 0.6% DMSO in egg water) between 44 and 72 hpf.
Heat-Shock Conditions.
Host embryos containing Tg(HSE:caAlk6, eGFP) cells were heat-shocked during gastrulation at 5.25, 8, or 9 hpf or early somitogenesis stages at 10, 11, or 12 hpf for analyses of the dorsal pancreas, and both Tg(HSE:caAlk6, eGFP) and Tg(hsp70l:bmp2b) were heat-shocked at 50 hpf for analyses of the ventral pancreas. Embryos were transferred into egg water prewarmed at 40 °C. After a 30-min heat shock in the 40 °C incubator, the plate containing the embryos was transferred into a 28 °C incubator and the embryos harvested at various stages.
In Situ Hybridization and Histochemical Methods.
Whole-mount in situ hybridization was performed as described previously (43), using the following probes: bmp2b (23), bmp4 (44), and noggin1 (45). The bmp6 probe was made from a 567-bp-long template, which was amplified by PCR with the following primers (5′–3′) GCCATCACAGCTGCAGAAT and GTTTGCTGCGTAGCCTTCA, and cloned into pCRII. To detect mRNA transcript along with Tg(gutGFP)s854 expression, we fluorescently labeled mRNA expression using Fast Red (Roche) (46). We used the following antibodies: polyclonal chicken anti-GFP (1:1,000; Aves Labs), polyclonal rabbit anti-dsRed (1:200; Clontech), monoclonal mouse anti-Islet1 (1:15; Developmental Studies Hybridoma Bank, clone 39.4D5), mouse IgG anti-β-catenin (1:100; BD Transduction Laboratory), polyclonal rabbit anti-Id2 (I-16) (1:50; Santa Cruz Biotechnology), polyclonal guinea pig anti-Insulin (1:500; Biomeda), polyclonal rabbit anti-pan-cadherin (1:5,000; Sigma), polyclonal rabbit anti-Somatostatin (1:100; AbD Serotec), mouse anti-Glucagon (1:100; Sigma), and fluorescently conjugated Alexa antibodies from Molecular Probes.
Supplementary Material
Acknowledgments
We thank Ana Ayala for expert help with the fish; Ryan Anderson for discussions; and Philipp Gut, Tamsin Lindstrom, and Dan Hesselson for critical reading of the manuscript. W.-S.C. was supported in part by a fellowship from the California Institute for Regenerative Medicine. O.A. is supported in part by fellowships from the Swedish Research Council and the Wenner-Gren Fellows program. This work was supported in part by grants from the National Institutes of Health (DK075032) (to D.Y.R.S.) and the Packard Foundation (D.Y.R.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0910205107/DCSupplemental.
References
- 1.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wandzioch E, Zaret K. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324(5935):1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, et al. Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development. 2008;135(19):3209–3218. doi: 10.1242/dev.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 7.Goessling W, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 8.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 9.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 10.Gittes GK. Developmental biology of the pancreas: A comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Kimelman D, Pyati UJ. Bmp signaling: Turning a half into a whole. Cell. 2005;123:982–984. doi: 10.1016/j.cell.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 12.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- 14.Edlund H. Pancreatic organogenesis—developmental mechanisms and implications for therapy. Nat Rev Genet. 2002;3:524–532. doi: 10.1038/nrg841. [DOI] [PubMed] [Google Scholar]
- 15.Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol. 2003;261:197–208. doi: 10.1016/s0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi Y, et al. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001;15:1493–1505. doi: 10.1101/gad.892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung WS, Stainier DY. Intra-endodermal interactions are required for pancreatic beta cell induction. Dev Cell. 2008;14:582–593. doi: 10.1016/j.devcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stafford D, et al. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- 19.Row RH, Kimelman D. Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Dev Biol. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicoli S, Gilardelli CN, Pozzoli O, Presta M, Cotelli F. Regulated expression pattern of gremlin during zebrafish development. Gene Expr Patterns. 2005;5:539–544. doi: 10.1016/j.modgep.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Warga RM, Nüsslein-Volhard C. Origin and development of the zebrafish endoderm. Development. 1999;126:827–838. doi: 10.1242/dev.126.4.827. [DOI] [PubMed] [Google Scholar]
- 22.Ward AB, Warga RM, Prince VE. Origin of the zebrafish endocrine and exocrine pancreas. Dev Dyn. 2007;236:1558–1569. doi: 10.1002/dvdy.21168. [DOI] [PubMed] [Google Scholar]
- 23.Bauer H, Lele Z, Rauch GJ, Geisler R, Hammerschmidt M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development. 2001;128:849–858. doi: 10.1242/dev.128.6.849. [DOI] [PubMed] [Google Scholar]
- 24.Yin C, Solnica-Krezel L. Convergence and extension movements mediate the specification and fate maintenance of zebrafish slow muscle precursors. Dev Biol. 2007;304:141–155. doi: 10.1016/j.ydbio.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 25.diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog is required early in pancreatic islet development. Dev Biol. 2002;244:75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- 26.Dong PD, et al. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet. 2007;39:397–402. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- 27.Miyazono K, Miyazawa K. Id: A target of BMP signaling. Sci STKE. 2002;2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 28.Hua H, et al. BMP4 regulates pancreatic progenitor cell expansion through Id2. J Biol Chem. 2006;281:13574–13580. doi: 10.1074/jbc.M600526200. [DOI] [PubMed] [Google Scholar]
- 29.Shin D, et al. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- 30.Hua H, Sarvetnick N. Expression of Id1 in adult, regenerating and developing pancreas. Endocrine. 2007;32:280–286. doi: 10.1007/s12020-008-9036-3. [DOI] [PubMed] [Google Scholar]
- 31.Verkhusha VV, et al. An enhanced mutant of red fluorescent protein DsRed for double labeling and developmental timer of neural fiber bundle formation. J Biol Chem. 2001;276:29621–29624. doi: 10.1074/jbc.C100200200. [DOI] [PubMed] [Google Scholar]
- 32.Hesselson D, Anderson RM, Beinat M, Stainier DY. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc Natl Acad Sci USA. 2009;106:14896–14901. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spagnoli FM, Brivanlou AH. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development. 2008;135:451–461. doi: 10.1242/dev.008458. [DOI] [PubMed] [Google Scholar]
- 34.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 35.Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207–219. doi: 10.1016/j.cmet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Dichmann DS, Miller CP, Jensen J, Scott Heller R, Serup P. Expression and misexpression of members of the FGF and TGFbeta families of growth factors in the developing mouse pancreas. Dev Dyn. 2003;226:663–674. doi: 10.1002/dvdy.10270. [DOI] [PubMed] [Google Scholar]
- 37.Ebisawa T, et al. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Vogel SS, Liu N, Melton DA, Lin S. Analysis of pancreatic development in living transgenic zebrafish embryos. Mol Cell Endocrinol. 2001;177:117–124. doi: 10.1016/s0303-7207(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 39.Chocron S, Verhoeven M, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Godinho L, et al. Targeting of amacrine cell neurites to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- 41.Pauls S, Zecchin E, Tiso N, Bortolussi M, Argenton F. Function and regulation of zebrafish nkx2.2a during development of pancreatic islet and ducts. Dev Biol. 2007;304:875–890. doi: 10.1016/j.ydbio.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Yu PB, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander J, Stainier DY, Yelon D. Screening mosaic F1 females for mutations affecting zebrafish heart induction and patterning. Dev Genet. 1998;22:288–299. doi: 10.1002/(SICI)1520-6408(1998)22:3<288::AID-DVG10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen JN, et al. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- 45.Fürthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 46.Novak AE, Ribera AB. Immunocytochemistry as a tool for zebrafish developmental neurobiology. Methods Cell Sci. 2003;25:79–83. doi: 10.1023/B:MICS.0000006894.43940.b1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.