Abstract
Human skeletal muscle fibers express five highly conserved type-II myosin heavy chain (MyHC) genes in distinct spatial and temporal patterns. In addition, the human genome contains an intact sixth gene, MyHC-IIb, which is thought under most circumstances not to be expressed. The physiological and biochemical properties of individual muscle fibers correlate with the predominantly expressed MyHC isoform, but a functional analysis of homogenous skeletal muscle myosin isoforms has not been possible. This is due to the difficulties of separating the multiple isoforms usually coexpressed in muscle fibers, as well as the lack of an expression system that produces active recombinant type II skeletal muscle myosin. In this study we describe a mammalian muscle cell expression system and the functional analysis of all six recombinant human type II skeletal muscle myosin isoforms. The diverse biochemical activities and actin-filament velocities of these myosins indicate that they likely have distinct functions in muscle. Our data also show that ATPase activity and motility are generally correlated for human skeletal muscle myosins. The exception, MyHC-IIb, encodes a protein that is high in ATPase activity but slow in motility; this is the first functional analysis of the protein from this gene. In addition, the developmental isoforms, hypothesized to have low ATPase activity, were indistinguishable from adult-fast MyHC-IIa and the specialized MyHC-Extraocular isoform, that was predicted to be the fastest of all six isoforms but was functionally similar to the slower isoforms.
Keywords: in vitro motility, recombinant myosins, skeletal myosin isoforms, actin-activated ATPase
Skeletal muscle fibers have long been classified according to their physiological, morphological, biochemical, and histological characteristics as either slow/type I or fast/type II. Within each group, there is a range of contractile velocities, and these velocities tend to correlate with myofibrillar ATPase activity and the myosin isoform predominantly expressed in the fiber (1–4). Eight distinct mammalian sarcomeric Myosin Heavy Chain (MyHC) genes have been identified (5–7), and molecular techniques have allowed the characterization of myosin isoform expression profiles in single muscle fibers (8–10). In mammals, slow/type I fibers express β-MyHC, while fast/type-II fibers express one or more of the six type II skeletal muscle myosin genes. These include the developmental MyHCs (Embryonic and Perinatal), the adult-fast MyHCs (IIa, IIb, and IId), and the specialized MyHC-Extraocular. The mammalian sarcomeric myosins are highly conserved, with between 78–98% amino acid identity (7). The highest identity is found among paralogous myosins (> 98%), while the six orthologous human skeletal fast myosins are 82–96% identical (5). Even though these genes are highly similar, they may not serve redundant functions, as suggested by the distinct phenotypes of MyHC-IIb and -IId null mice (11, 12).
The earliest expressed type II isoforms are MyHC-Embryonic and MyHC-Perinatal. They are first observed during early skeletal muscle development, and both decrease below detection levels shortly after birth (13, 14). However, these genes do continue to be expressed in some specialized muscles (15–17), and are reexpressed in regenerating and dystrophic muscles (18). Skeletal muscles in adult mammals predominantly express MyHC-IIa, -IId, -IIb, and β-MyHC, but expression patterns differ among species and are influenced by exercise, disease, and age. The MyHC-IIb isoform is highly expressed in the skeletal muscles of smaller mammals, but is not detectable in humans in which MyHC-IId predominates (19–21). The specialized MyHC-Extraocular isoform is expressed in the extraocular (15, 22, 23) and laryngeal muscles (24). The distinct expression profiles among highly specialized muscles, variable patterns of gene expression that correlate with scaling of body size between different species, and ability of muscle fibers to change isoform expression in response to disease and exercise, all suggest distinct functional roles for each isoform.
Myosin isotype expression has been linked to myofibrillar ATPase activity, contractile force, and unloaded contractile velocity (1, 3, 4, 25–30). This work has revealed that muscle fibers can display a wide range of biochemical and physiological properties, with their unloaded contractile velocities and ATPase activities increasing in order from Type-I, IIA, IID, to IIB. The ATPase activities of the developmental and the MyHC-Extraocular isoforms are less clear due to their heterogeneous expression with other isoforms. Preliminary work suggests the developmental myosins have low ATPase activities (31, 32), while MyHC-Extraocular is thought to be a very fast myosin (22, 29, 33). All of this evidence points to the conclusion that myosin isoform expression patterns are a likely mechanism for the specialization of muscles to perform specific tasks. Moreover, the biophysical and biochemical properties of the fast skeletal myosin isoforms are likely a primary determinant of muscle fiber contractile dynamics.
While previous work has described how myosin isoform expression relates to the performance of different muscle fiber types, the conclusions from these experiments come with several caveats. First, muscle fibers are isotyped by the myosin isoform they express, but quantification of MyHC mRNA demonstrated that transcripts for several isoforms are present in a fiber, and that both isoform mRNA and protein levels can vary longitudinally along a single fiber (34). While these isoforms may be undetectable at the resolution of SDS-PAGE, it is possible that a small percentage of another isoform may influence biochemical and biophysical experiments (35–37). The second caveat associated with whole fiber analysis is the number of variables that may exist among fibers beyond myosin isoform expression. Skeletal muscle fibers are among the largest and most complex vertebrate cells, and variation in expression levels of any number of regulatory proteins associated with muscle contraction could introduce variability in single-fiber experiments (20).
To address the lack of methodology to obtain pure isoforms from muscle, we undertook expression of the head region of myosin (subfragment-1, S1), which has been shown to be sufficient for the same F-actin-activated ATPase activity as full-length myosin and can direct in vitro motility of actin-filaments (38, 39). S1 is frequently used as a substitute for full-length myosin in biochemical and biophysical studies (40–42), but previous attempts to express skeletal myosin II S1 in traditional systems have yielded insoluble, nonfunctional protein, even though these systems have proven useful for expressing smooth muscle (43, 44) and nonmuscle myosins (45, 46).
A number of recent papers have shown that Caenorhabditis elegans unc-45 is a chaperone that interacts with myosin and is required for myosin folding and sarcomere assembly (47, 48). Two UNC-45 isoforms were later identified in mice and humans (49), and one of these,Skeletal Muscle UNC-45, is expressed specifically in cardiac and skeletal muscle. Based on these observations, we developed a muscle cell expression system that produces active recombinant human skeletal muscle myosin II S1. We then measured several kinetic and biophysical properties of these myosins that show significant functional differences among the highly related human skeletal muscle myosin II isoforms. These data demonstrate that some previous assumptions about enzymatic activities of myosin isoforms are not correct. For example, the developmental isoforms are not slower than all adult-fast isoforms, and the extraocular isoform is not faster.
Results
Protein Expression and Purification.
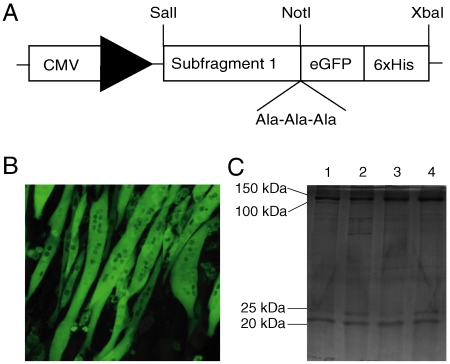
From published work concerning a muscle-specific myosin chaperone (47–52), we hypothesized that the expression of active human fast skeletal muscle myosin II S1 would require a muscle cell context that contained both known and unknown myosin folding cofactors. C2C12 myoblasts are transformed murine muscle cell precursors that can be differentiated into muscle fiber-like myotubes with functional sarcomeres (53, 54) and endogenously express all of the murine fast skeletal muscle myosin isoforms except for MyHC-Extraocular (55, 56). Taking cues from previous work with C2C12 cells to study myosin folding (51, 52), we constructed shuttle plasmids encoding S1 of the six human skeletal muscle myosins fused to enhanced GFP (eGFP) with a C-terminal 6xHis tag (Fig. 1A). The plasmids were used to produce replication-deficient adenoviruses, capable of infecting C2C12 cells and inducing expression of the recombinant proteins. Differentiated C2C12 myotubes infected with these adenoviruses exhibit bright eGFP fluorescence 72-h postinfection (Fig. 1B).
Fig. 1.
Expression and purification of the human skeletal myosin II isoforms. (A) Met1-Pro840 of the myosin was expressed under the control of the CMV promoter, fused to the gene encoding an enhanced GFP (eGFP) with a C-terminal 6xHistidine tag. This construct included a linker (Ala-Ala-Ala) between S1 and eGFP. (B) C2C12 cells expressing the recombinant myosins (MyHC-IId-eGFP-6xHis in this image) display bright eGFP fluorescence 72 h postinfection. (C) Coomassie Brilliant Blue stained SDS-PAGE gel with fractions corresponding to the 488 nm chromatographic peak (excitation peak for eGFP) during a linear salt gradient elution for MyHC-IIa-eGFP-6xHis (Lane 1), MyHC-IIb-eGFP-6xHis (Lane 2), MyHC-IId-eGFP-6xHis (Lane 3), and MyHC-Perinatal-eGFP-6xHis (Lane 4).
A three-step strategy was optimized for the purification of the recombinant S1-eGFP-6xHis proteins using low-salt precipitation of endogenous C2C12 myosin and both metal-affinity and ion-exchange chromatography. The resulting bands from purified samples subjected to SDS-PAGE correspond with the predicted molecular weight of S1-eGFP-6xHis of ∼125 kDa, and the molecular weights of several murine Myosin Light Chain (MLC) isoforms (20–25 kDa) that copurify with the recombinant myosin (Fig. 1C). MLCs were identified by mass spectrometry of purified MyHC-IId and MyHC-Perinatal samples (Table S1). These human adult-fast and developmental myosin isoforms copurify with a similar complement of MLCs, suggesting that the myosins overexpressed in this system may indiscriminately acquire endogenous C2C12 MLCs. This should actually allow for better comparison of the biochemical differences among these myosins, as we can attribute any differences to MyHC composition. All myosins copurified with myosin light chains as confirmed by SDS-PAGE (Fig. 1C), with the exception of MyHC-Embryonic and MyHC-Extraocular (Fig. S1).
F-Actin-Activated ATPase Assays.
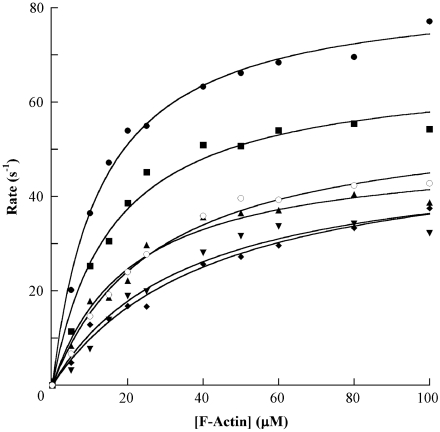
F-actin-activated ATPase assays were performed on all six human skeletal muscle myosin isoforms to determine their ATPase kinetics (Fig. 2) with chicken skeletal muscle actin. The average derived maximal F-actin-activated ATPase rates (Vmax) and Michaelis constants (KM) for each isoform were averaged and tabulated along with the basal Mg2+ ATPase activity (Table 1). The human skeletal muscle myosin isoforms display basal ATPase rates ≤ 0.1 s-1, and are activated 400- to 1000-fold with the addition of 100 μM F-actin (Fig. 2). We believe that the activation of the recombinant human fast skeletal myosin II isoforms is higher than previously published values because our assays were performed at physiological temperature (37 °C) with improved stirring techniques (38, 57, 58).
Fig. 2.
F-actin-activated ATPase saturation curves for the human IIb (•), IId (▪), IIa (⧫), Extraocular (▴), Embryonic (▾), and Perinatal (○) skeletal myosin II isoforms. Averaged data from F-actin-activated ATPase assays with the recombinant S1-eGFP-6xHis proteins are plotted. Each data point is the average ATPase rate from assays performed in duplicate on at least three different preparations of protein (except MyHC-Perinatal where n = 2) and is a reaction rate (s-1) calculated from the slope of a linear fit (r2 > 0.95 in most cases) of product (nMol Pi) versus time (seconds) over the course of the assay, corrected for the basal Mg2+ ATPase activity at 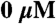 F-actin. The datasets for each preparation of an isoform across a range of 0–
F-actin. The datasets for each preparation of an isoform across a range of 0–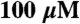 F-actin were fit to the Michaelis-Menten equation using KaleidaGraph.
F-actin were fit to the Michaelis-Menten equation using KaleidaGraph.
Table 1.
Measured and calculated kinetic data for the six human fast skeletal myosin II isoforms
| Basal Mg2+ATPase (s-1) | Vmax (s-1) | KM (μM) | In vitro Motility Velocity (μm s-1) | |
| IIb-eGFP | 0.08 ± .02 | 86.1 ± 8.4* | 14.0 ± 3.2‡ | 0.69 ± 0.08 |
| IId-eGFP | 0.11 ± .01 | 65.7 ± 2.1† | 15.7 ± 1.3§ | 1.4 ± 0.2 |
| IIa-eGFP | 0.08 ± .01 | 52.8 ± 4.4 | 44.5 ± 5.6 | 1.1 ± 0.1 |
| Embryonic-eGFP | 0.08 ± .01 | 41.2 ± 3.7 | 35.1 ± 1.8 | 0.67 ± 0.07 |
| Perinatal-eGFP | 0.04 ± .03 | 59.7 ± 4.9 | 41.2 ± 3.7 | 1.1 ± 0.1 |
| Extraocular-eGFP | 0.13 ± .02 | 59.2 ± 5.7 | 37.2 ± 4.5 | n.d. |
The Basal Mg2+ ATPase rate, maximal F-actin-activated ATPase rate (Vmax), associated constant of myosin for F-actin (KM) derived from the fit of ATPase saturation curves (Fig. 2) to the Michaelis-Menten equation using KaleidaGraph, and the maximal in vitro velocity of actin-filaments directed by the recombinant myosins. ATPase associated values are quoted as the mean ± s.e.m. for assays run in duplicate for at least three separate protein preparations except for Perinatal-eGFP where n = 2. Motility velocities are quoted as the mean filament velocity ± s.d.. Statistical significance (p < 0.05 by one-way ANOVA with Student-Newman-Keuls post hoc test) from
*IId-eGFP, IIa-eGFP, Embryonic-eGFP, Perinatal-eGFP, and Extraocular-eGFP
†Embryonic-eGFP
‡IIa-eGFP, Embryonic-eGFP, Perinatal-eGFP, and Extraocular-eGFP
§IIa-eGFP, Embryonic-eGFP, Extraocular-eGFP, and Perinatal-eGFP.
When the F-actin-activated ATPase saturation curves for all six of the human fast skeletal muscle myosin II isoforms are overlaid (Fig. 2), they display a wide range of actin-activated ATPase profiles, and the derived maximal F-actin-activated ATPase rates show a range of values from 41 to 86 s-1 (Table 1). MyHC-IIb displays the highest maximal actin-activated ATPase rate, and the rates for the adult-fast isoforms follow the order from fastest to slowest: MyHC-IIb > MyHC-IId > MyHC-IIa.
The developmental isoforms display ATPase turnover rates at the lower end of the spectrum of the six fast isoforms, but not significantly lower than adult-fast MyHC-IIa. MyHC-Embryonic has a Vmax of 41.2 ± 3.7 s-1 (compared to 52.8 ± 4.4 s-1 for MyHC-IIa), and MyHC-Perinatal displays a slightly higher ATPase profile than MyHC-Embryonic and MyHC-IIa, and has a Vmax of 59.7 ± 4.9 s-1. MyHC-Extraocular has an ATPase saturation curve intermediate between the MyHC-IId and MyHC-IIa isoforms with a Vmax of 59.2 ± 5.7 s-1.
The Michaelis constants calculated from the ATPase assays fitted to the Michaelis-Menten equation also show differences among the isoforms. The six human fast skeletal muscle myosin isoforms display a range of KM values (Table 1), and interestingly, the isoforms with higher Vmax rates tend to have a lower KM. The three adult-fast isoforms show a relationship of Vmaxof MyHC-IIb > MyHC-IId > MyHC-IIa while the relationship between KM is reversed with MyHC-IIa > MyHC-IId≥MyHC-IIb. The developmental isoforms and MyHC-Extraocular have KM values close to that of MyHC-IIa, as might be expected with their lower Vmax rates, if one assumes that actin-affinity is a primary determinant of myosin kinetics.
In Vitro Motility Assays.
Motor activity of the recombinant myosin isoforms was measured using an in vitro motility assay. We used this assay to determine the maximal myosin-mediated motility velocities of fluorescent actin-filaments on a slide surface. All of the recombinant myosin isoforms were capable of directing motility of actin-filaments except for MyHC-Extraocular. The observed motility of actin-filaments in the in vitro assays was variably discontinuous, with actin-filaments undergoing periods of motion irregularly interrupted by stalls. This activity was dependent on the myosin isoform observed, which historically is not always the case in this type of assay. This result may be due to factors unique to skeletal myosin function, and better in vitro assays that address these factors need to be developed. The average maximal in vitro actin-filament velocities are reported (Table 1).
Measured actin-filament velocities for all but one of the recombinant human skeletal muscle myosin isoforms parallel the maximal actin-activated ATPase activities (Vmax) defined in the steady-state kinetic assays. For the adult-fast isoforms, MyHC-IId has a faster maximal filament velocity (1.4 ± 0.2 μm/s) than MyHC-IIa (1.1 ± 0.1 μm/s), following their ATPase relationship. These velocity values are similar to those measured for the S1 forms of skeletal myosins previously reported (59), and our major conclusion is that we have indeed succeeded in expressing functional motor domains with normal motility. While further experiments are required to make detailed comparisons, these first results suggest that MyHC-IIb, which has the highest maximal ATPase activity of the isoforms in this study, exhibits much slower maximal motility (0.69 ± 0.08 μm/s) than all but the developmental MyHC-Embryonic isoform, which has the lowest Vmax of the human skeletal muscle myosin isoforms as well as the slowest filament velocity (0.67 ± 0.07 μm/s). The maximal velocity of filaments driven by MyHC-Perinatal (1.1 ± 0.1 μm/s) is similar to that of MyHC-IIa, and correlates well with their maximal actin-activated ATPase activities. We did not detect motile filaments with MyHC-Extraocular, and believe this is due to the complete lack of light chains associated with the isoform. MyHC-Embryonic was also determined not to copurify with detectable associated light chains, but was capable of directing actin-filament motility, albeit at a slower velocity than would be expected from its Vmax. Historically, embryonic myosins fractionated from muscle sources do copurify with associated light chains (31), and we hypothesize that these myosins may only associate with specific myosin light chains not present in C2C12 cells. It was shown previously that skeletal muscle myosin with no light chains had 10-fold reduced motility without significant effect on ATPase activity (60), and this is an issue we are currently working to resolve.
Discussion
We have developed a skeletal muscle myosin overexpression system in a murine muscle tissue culture cell line, which produces active recombinant human skeletal muscle myosin II. We also determined some of the biochemical and biophysical characteristics of the six human fast skeletal muscle myosin isoforms. Our hypothesis was that the ATPase activities and motility velocities of purified individual isoforms would correlate with muscle fiber contractile velocities. Until now, much research had been done characterizing the contractile velocities of individual muscle fibers (3, 8, 10, 25), and total myosin from single fibers (3, 26, 61, 62). These experiments are difficult to interpret, though, as single muscle fibers do not express solely one isoform. The system we developed overcomes that problem, in that we can purify the S1 fragment of a single isoform in quantities useful for biochemical and biophysical characterization.
Our initial work with the six human isoforms measured their F-actin-activated ATPase kinetics. At the sequence level, the six human fast skeletal muscle myosins are quite similar, but when comparing the ATPase saturation curves for these isoforms, one observes a range of activities. The developmental MyHC-Embryonic and MyHC-Perinatal isoforms were thought to be slower isoforms due to lower in vitro motility velocity and ATPase activity of embryonic chicken myosin as compared to that from four month old chickens (31). We show that indeed the developmental isoforms are among the slower of the human skeletal muscle myosin II isoforms in terms of Vmax but do not seem to have a reduced functionality, as their Vmax values are both very similar to that of MyHC-IIa, one of the major MyHCs expressed in adult human skeletal muscle. These results were not altogether unexpected, as motor domain mutations in both developmental isoforms cause severe developmental diseases (63–65). They are also consistent with the presumed role of these myosins in the adaptation of neonatal muscles to demanding physiological functions including breathing (66). MyHC-Perinatal displays a slightly higher ATPase profile than MyHC-Embryonic, which correlates well the increased enzymatic activity in the developing muscles of chickens and rats when MyHC-Perinatal replaces MyHC-Embryonic (32).
The F-actin-activated ATPase assays also reveal that the three adult-fast isoforms display a range of maximal actin-activated ATPase rates that are close, but point to distinct physiological functions. These fall in the order MyHC-IIb > MyHC-IId > MyHc-IIa from fastest to slowest. This order is the same as that seen for the contractile velocities of fibers predominantly expressing these isoforms (1, 2, 25, 26, 62). Previous studies have shown a 30% difference in ATPase activity (26) between human muscle fibers predominantly expressing MyHC-IIa and MyHC-IId and this correlates extremely well with our results. Rat muscle fibers, which unlike human fibers, do express MyHC-IIb, show a 6% difference in ATPase activity between MyHC-IIa and MyHC-IId fibers, and 30% from MyHC-IId to MyHC-IIb fibers. This relationship is accompanied by a 7% and 35% difference in unloaded shortening velocity respectively (3). We observed a 31% difference in maximal actin-activated ATPase activity between human MyHC-IId and MyHC-IIb, which compares well, but overall, we see a higher range of differences between MyHC-IIa and MyHC-IIb. This may be due to evolutionary differences between rats and humans brought about by the differential requirements for muscle function, as well as the loss of expression of MyHC-IIb in human skeletal muscle. Overall, the range of ATPase activities displayed by the three adult isoforms, and the differences among them, support the prevalent theory that these proteins are not redundant (11, 12) and likely have specialized roles and distinct functions in determining whole muscle fiber kinetics and contractile dynamics.
Finally, MyHC-Extraocular, which is only expressed in some specialized muscle fibers and usually in combination with other isoforms, has a Vmax close to that of the developmental and MyHC-IIa isoforms. The fact that extraocular myosin does not have light chains should not have an effect on ATPase activity based on experiments on skeletal muscle myosin (60). Several studies have shown that fibers expressing MyHC-Extraocular exhibit isometric contraction twitch times twice as fast as those from conventional skeletal muscles (29, 67). The unique requirements of extraocular muscles, which contract very rapidly, repetitively, and for prolonged periods against very low load to move the eye and laryngeal muscles may indicate a specialized role for this isoform. It is possible that MyHC-Extraocular is responsible for some of these unique properties, but its relatively low ATPase activity suggests that some other biochemical or biophysical component of the motor, or another component of these fibers, may be responsible for their fast contraction. The low tension output of MyHC-Extraocular fibers (67) may provide a clue as to what this is, and further biophysical studies on this protein may reveal differences in the properties of the swinging crossbridge and force output under different loads, which give these fibers rapid twitch contraction without the motor having elevated ATPase activity.
In addition to Vmax, one can also calculate a Michaelis constant from the fit of the actin-activated curves to the Michaelis-Menten equation. In the case of these assays, the KM value is not a true Michaelis constant or dissociation constant for substrate as it is with most enzymes because these assays vary the concentration of F-actin, which is not a true substrate in the reaction. This constant is typically called the ‘associated constant of myosin for actin’ and represents the concentration of F-actin required for half-maximal activation of myosin at saturating ATP. It is considered to be indicative of the association constant of myosin for actin (KA), thus, the higher the KM, the weaker the interaction between myosin and actin, and vice versa.
The human skeletal muscle myosins show an interesting relationship between Vmax and KM. For the adult-fast isoforms, the decrease in Vmax from MyHC-IIb → MyHC-IId → MyHC-IIa is loosely correlated with an increase in KM. The developmental MyHC-Embryonic and MyHC-Perinatal isoforms, as well as MyHC-Extraocular all have Vmax rates at the lower end of the spectrum, similar to MyHC-IIa, and all have relatively high KM value. These data point to the role that the actin-myosin interaction may play in regulating ATPase kinetics for these isoforms, and we hypothesize the modulation of actin-affinity is a possible mechanism for kinetic tuning of the human fast skeletal muscle myosins.
As a secondary measure of the functionality of the myosins produced using the unique overexpression system, we completed an analysis of a biophysical component of myosin II using an in vitro motility assay. Importantly, this assay shows that the myosin produced using our expression system is capable of moving actin-filaments at rates expected from earlier studies, which demonstrates motor functionality. These early results also suggest differences among the isoforms at a biophysical level in an assay that is suggested to be a corollary to unloaded shortening velocity. Previous studies have shown a 41–77% difference in maximum shortening velocity between human muscle fibers predominantly expressing MyHC-IIa and MyHC-IId depending on the fiber source (25). MyHC-IId has a 29% higher maximal motility velocity that MyHC-IIa, following their ATPase and fiber contractile relationship, and the developmental MyHC-Perinatal, with a similar Vmax as MyHC-IIa, has a very similar maximal motility velocity. MyHC-Embryonic on the other hand, has the slowest motility of the isoforms with about a twofold lower maximal motility velocity than MyHC-IIa, MyHC-IId, and MyHC-Perinatal, which seems to be disconnected from any major reduction in Vmax compared to MyHC-IIa and MyHC-Perinatal. This twofold reduction in motility compares well though with a twofold reduction in filament velocity observed in myosin purified from embryonic chickens compared to that of 12-d posthatch chickens (31), but it must be noted that MyHC-Embryonic does not copurify with detectable myosin light chains, which can significantly affect motility (60). This result will be further investigated in future studies.
MyHC-IIb, the ‘fastest’ of the isoforms at a biochemical level, unexpectedly has very low motility activity. This is not a problem of lack of association of light chains; this isoform shows similar amounts of copurifying light chains as the other isoforms (Fig. 1C), which have higher motility activity. Functionally, at a biochemical level, it seems the human isoform has not lost any activity, and this compares well to mouse muscle fiber contractile data showing that MyHC-IIb expressing fibers have the fastest contractile velocity of the adult-fast isoforms. Humans though, do not express MyHC-IIb normally, and it is possible that accumulated mutations, which have no physiological effect on human muscle due to the isoform not being expressed, have reduced the motility function of this isoform without affecting its ATPase activity.
Unfortunately, we were not able to measure motility for MyHC-Extraocular, and we observed that MyHC-Extraocular does not copurify with any light chains. This suggests that muscle fibers expressing MyHC-Extraocular may express unique light chains that associate with the neck regions of MyHC-Extraocular better than those endogenously expressed in the murine C2C12 cell line, and confer biophysical functionality to this unique myosin. Associated myosin light chains have been shown to be required for motility and full force production of myosins (60, 68), and we believe that the lack of motility displayed by MyHC-Extraocular is not due to a functional problem with the motor, as it has quite high actin-activated ATPase activity, but due to a yet unresolved lack of associated light chains.
The motor domains of the human fast skeletal muscle myosin II isoforms are highly conserved but show divergent motor functionality. A closer analysis of sequence variation among the isoforms reveals factors that may determine the kinetic properties of these isoforms and regulate the differences among them. The loop regions connecting the three major domains of the myosin motor show a higher divergence of amino acid sequence among the striated muscle myosin isoforms than the rest of the molecule (7), and previous studies on the regulation of myosin II motor kinetics have shown that the loops are an important determinant in the ‘tuning’ of a myosin II motor (for review, see (69)). Their proximity to the nucleotide- and actin-binding sites of myosin also implies that their sequence may have the ability to affect binding constants for ATP, ADP, and F-actin. The roles played by the loops in regulating Dictyostelium myosin have been well characterized, but when the Dictyostelium data are compared to those from smooth muscle myosin, scallop myosins, and mammalian cardiac myosins, it becomes evident that the regulation of myosin kinetics by the loops can have variable roles depending on the myosin type. Future analysis of the affinity of the six human fast skeletal muscle myosin II myosin isoforms for F-actin as well as pre-steady-state kinetic studies should reveal more information about the functional differences among the isoforms. Studies on the modulation of these parameters by loop length and sequence, as well as studies on disease-causing mutations in the human skeletal myosins, will also help us to learn more about how the myosin motor works.
Materials and Methods
Adenovirus Production.
For expression of recombinant human skeletal myosin II-eGFP chimeras, NotI and XbaI restriction sites and a C-terminal 6xHistidine tag were added to the gene encoding eGFP (Clonetech) using PCR amplification. The product was ligated into pShuttle-CMV (Qbiogene) downstream of the cytomegalovirus (CMV) promoter. Subfragment-1 (conserved residues Met1-Pro840 corresponding to the chicken skeletal myosin sequence) of the six human skeletal muscle myosin II isoforms had SalI and NotI restriction sites added by PCR amplification, and these products were individually ligated upstream of eGFP-6xHis. Replication-deficient recombinant adenoviruses were produced, amplified, and purified with these plasmids using the pAdEasy kit (Qbiogene) with modifications (70).
Cell Culture and Protein Expression.
Murine C2C12 myoblasts (American Type Tissue Collection) were cultured in Dulbecco’s Modified Eagle Media (DMEM) (Gibco) supplemented 1.5 g/L sodium bicarbonate (Sigma-Aldrich) and 20% FBS (Hyclone). Cells were grown to ∼100% confluence and the medium was replaced with differentiation medium (DMEM supplemented with 1.5 g/L sodium bicarbonate and 2% horse serum (Gibco-BRL)). 48 h postdifferentiation into myotubes, cells were infected with 1 × 106–1 × 108 plaque forming units of adenovirus per 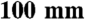 plate. 48-hours postinfection the medium was switched back to growth medium, and the cells were incubated for 3–5 more days before harvesting.
plate. 48-hours postinfection the medium was switched back to growth medium, and the cells were incubated for 3–5 more days before harvesting.
Protein Purification.
Myosin: The cell monolayer was washed once with cold PBS, and was collected in 250 μL of lysis buffer [50 mM Tris-HCl pH 7.0, 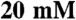 Imidazole,
Imidazole, 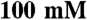 NaCl, 0.5% Tween-20,
NaCl, 0.5% Tween-20, 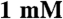 DTT, and 1x protease inhibitor cocktail (Roche)] per plate using a cell scraper. The cells were lysed in a dounce homogenizer and cleared twice by centrifugation at 50,000 × g for 15 min. The cleared lysate was brought to 0.5 M NaCl and fractionated on an AKTApurifier (Amersham) with a 1 mL HisTrap HP nickel-sepharose column (Amersham) using a step-imidazole gradient. The elution peak fractions were pooled and dialyzed overnight at 4 °C into a low-salt buffer [
DTT, and 1x protease inhibitor cocktail (Roche)] per plate using a cell scraper. The cells were lysed in a dounce homogenizer and cleared twice by centrifugation at 50,000 × g for 15 min. The cleared lysate was brought to 0.5 M NaCl and fractionated on an AKTApurifier (Amersham) with a 1 mL HisTrap HP nickel-sepharose column (Amersham) using a step-imidazole gradient. The elution peak fractions were pooled and dialyzed overnight at 4 °C into a low-salt buffer [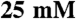 imidazole pH 7.0,
imidazole pH 7.0, 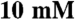 KCl,
KCl, 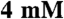 MgCl2,
MgCl2, 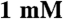 DTT]. The recombinant myosin was then purified on a
DTT]. The recombinant myosin was then purified on a 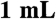 HiTrap Q HP sepharose anion-exchange column (Amersham) using a linear NaCl gradient from 0 to
HiTrap Q HP sepharose anion-exchange column (Amersham) using a linear NaCl gradient from 0 to 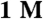 . The peak elution fractions were dialyzed overnight into an assay-appropriate buffer, and concentration was determined by the absorbance of eGFP at its excitation peak of 488 nm, assuming an extinction coefficient of 61,000 cm-1 M-1. Actin: F-Actin was prepared from fresh chicken breast muscle as previously described (71). For in vitro motility assays, F-actin was incubated overnight with a 2-fold molar excess of TRITC phalloidin (Sigma).
. The peak elution fractions were dialyzed overnight into an assay-appropriate buffer, and concentration was determined by the absorbance of eGFP at its excitation peak of 488 nm, assuming an extinction coefficient of 61,000 cm-1 M-1. Actin: F-Actin was prepared from fresh chicken breast muscle as previously described (71). For in vitro motility assays, F-actin was incubated overnight with a 2-fold molar excess of TRITC phalloidin (Sigma).
F-Actin-Activated ATPase Assays.
F-actin-activated myosin ATPase assays were performed as previously described (72) with some modifications. S1-eGFP-6xHis was diluted to a final concentration of 5 μg/mL (25 μg/mL in the 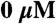 F-actin basal ATPase control to amplify signal) with varying concentrations of F-actin (0–
F-actin basal ATPase control to amplify signal) with varying concentrations of F-actin (0–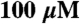 ) and saturating Na2ATP (
) and saturating Na2ATP (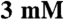 ). The reactions were performed in a low-salt buffer (
). The reactions were performed in a low-salt buffer (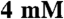 imidazole pH 7.0,
imidazole pH 7.0, 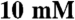 KCl,
KCl, 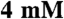 MgCl2,
MgCl2, 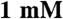 NaN3,
NaN3, 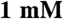 DTT) at 37 °C in a Reacti-Therm heated stirring module (Pierce). Assays were allowed to equilibrate to temperature, and started with the addition of Na2ATP. Aliquots were taken at four time points and quenched with an equal volume of stop solution (
DTT) at 37 °C in a Reacti-Therm heated stirring module (Pierce). Assays were allowed to equilibrate to temperature, and started with the addition of Na2ATP. Aliquots were taken at four time points and quenched with an equal volume of stop solution (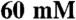 EDTA, pH 6.5, 6.6% SDS). Appropriate time courses were determined for each isoform to establish conditions that would be within detection limits and would not significantly alter substrate or product concentration. Inorganic phosphate was quantified at each time point using established methods (72). These data were plotted versus time to determine a rate for the reaction, and rates were plotted versus [F-Actin] using KaleidaGraph v4.0 (Synergy Software) to generate a saturation curve, which was fitted to the Michaelis-Menten equation to determine maximal actin-activate ATPase rate (Vmax) and the associated constant of myosin for actin or Michaelis constant (KM). These assays were run in duplicate at each F-actin concentration, and performed on at least three individual preparations of each isoform. Statistics were run using KaleidaGraph, differences were considered significant with a p-value < 0.05 with a one-way ANOVA with a Student-Newman-Keuls post hoc test.
EDTA, pH 6.5, 6.6% SDS). Appropriate time courses were determined for each isoform to establish conditions that would be within detection limits and would not significantly alter substrate or product concentration. Inorganic phosphate was quantified at each time point using established methods (72). These data were plotted versus time to determine a rate for the reaction, and rates were plotted versus [F-Actin] using KaleidaGraph v4.0 (Synergy Software) to generate a saturation curve, which was fitted to the Michaelis-Menten equation to determine maximal actin-activate ATPase rate (Vmax) and the associated constant of myosin for actin or Michaelis constant (KM). These assays were run in duplicate at each F-actin concentration, and performed on at least three individual preparations of each isoform. Statistics were run using KaleidaGraph, differences were considered significant with a p-value < 0.05 with a one-way ANOVA with a Student-Newman-Keuls post hoc test.
In Vitro Motility Assays.
The myosin-dependent motility of actin-filaments was measured using a standard in vitro motility assay at 24 °C (73), which was modified as follows. Prior to the addition of myosin to the flow cell, the nitrocellulose-coated cover slip was incubated with an antiGFP antibody (3E6, Invitrogen), then blocked with bovine serum albumin. Images were obtained at 0.1 sec /frame using an iXon camera (Andor), and velocities were determined by collecting displacements at 0.3–2 sec intervals using ImageJ (National Institutes of Health). At least 50 filaments were examined from two independent preparations for each recombinant myosin isoform. Motility could sometimes be improved by pretreating the myosin sample with an incubation with F-actin (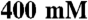 ) and ATP (
) and ATP (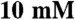 ) followed by centrifugation @ 400 K × g for 15 min to remove inactive myosin motors. Greater than 300 intervals were collected for each myosin isoform and the calculated filament velocities had a wide distribution. Discontinuous filament motion results in lower velocities being overrepresented in the pool of observations; therefore, the velocities of the fastest 15% of the pool were averaged to determine the maximal filament velocity for each isoform.
) followed by centrifugation @ 400 K × g for 15 min to remove inactive myosin motors. Greater than 300 intervals were collected for each myosin isoform and the calculated filament velocities had a wide distribution. Discontinuous filament motion results in lower velocities being overrepresented in the pool of observations; therefore, the velocities of the fastest 15% of the pool were averaged to determine the maximal filament velocity for each isoform.
Supplementary Material
Acknowledgments.
We thank Ann Robinson, Nancy Rice, Lisa Sampson, and Steve Langer for help with cloning and viral preparation, and Robert Thompson for assistance with assay development. This work was supported by National Institutes of Health/CU Molecular Biophysics Training Grant T32 GM65013 (to D.I.R. and J.C.D.), National Institutes of Health Grant GM29090 (to L.A.L.), and National Institutes of Health Grant GM33289 (to J.A.S).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0913527107/DCSupplemental.
References
- 1.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil. 1994;15:413–419. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- 2.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478(Pt 2):341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottinelli R, Canepari M, Reggiani C, Stienen GJ. Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. J Physiol. 1994;481(Pt 3):663–675. doi: 10.1113/jphysiol.1994.sp020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- 5.Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- 6.Weiss A, Mayer DC, Leinwand LA. Diversity of myosin-based motility: Multiple genes and functions. Soc Gen Phy . 1994;49:159–171. [PubMed] [Google Scholar]
- 7.Weiss A, Schiaffino S, Leinwand LA. Comparative sequence analysis of the complete human sarcomeric myosin heavy chain family: implications for functional diversity. J Mol Biol. 1999;290:61–75. doi: 10.1006/jmbi.1999.2865. [DOI] [PubMed] [Google Scholar]
- 8.Nyitrai M, et al. What limits the velocity of fast-skeletal muscle contraction in mammals? J Mol Biol. 2006;355:432–442. doi: 10.1016/j.jmb.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Weiss S, Chizhov I, Geeves MA. A flash photolysis fluorescence/light scattering apparatus for use with sub microgram quantities of muscle proteins. J Muscle Res Cell Motil. 2000;21:423–432. doi: 10.1023/a:1005690106951. [DOI] [PubMed] [Google Scholar]
- 10.Weiss S, Rossi R, Pellegrino MA, Bottinelli R, Geeves MA. Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. J Biol Chem. 2001;276:45902–45908. doi: 10.1074/jbc.M107434200. [DOI] [PubMed] [Google Scholar]
- 11.Acakpo-Satchivi LJ, et al. Growth and muscle defects in mice lacking adult myosin heavy chain genes. J Cell Biol. 1997;139:1219–1229. doi: 10.1083/jcb.139.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen DL, Harrison BC, Leinwand LA. Inactivation of myosin heavy chain genes in the mouse: Diverse and unexpected phenotypes. Microsc Res Tech. 2000;50:492–499. doi: 10.1002/1097-0029(20000915)50:6<492::AID-JEMT6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Lyons GE, Ontell M, Cox R, Sassoon D, Buckingham M. The expression of myosin genes in developing skeletal muscle in the mouse embryo. J Cell Biol. 1990;111:1465–1476. doi: 10.1083/jcb.111.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen DL, Leinwand LA. Postnatal myosin heavy chain isoform expression in normal mice and mice null for IIb or IId myosin heavy chains. Dev Biol. 2001;229:383–395. doi: 10.1006/dbio.2000.9974. [DOI] [PubMed] [Google Scholar]
- 15.Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler-Browne GS, Eriksson PO, Laurent C, Thornell LE. Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle Nerve. 1988;11:610–620. doi: 10.1002/mus.880110614. [DOI] [PubMed] [Google Scholar]
- 17.d’Albis A, Janmot C, Bechet JJ. Comparison of myosins from the masseter muscle of adult rat, mouse and guinea-pig. Persistence of neonatal-type isoforms in the murine muscle. Eur J Biochem. 1986;156:291–296. doi: 10.1111/j.1432-1033.1986.tb09580.x. [DOI] [PubMed] [Google Scholar]
- 18.Bandman E. Continued expression of neonatal myosin heavy chain in adult dystrophic skeletal muscle. Science. 1985;227:780–782. doi: 10.1126/science.3969567. [DOI] [PubMed] [Google Scholar]
- 19.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 20.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 21.Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- 22.Sartore S, et al. Fibre types in extraocular muscles: A new myosin isoform in the fast fibres. J Muscle Res Cell Motil. 1987;8:161–172. doi: 10.1007/BF01753992. [DOI] [PubMed] [Google Scholar]
- 23.Brueckner JK, Itkis O, Porter JD. Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil. 1996;17:297–312. doi: 10.1007/BF00240928. [DOI] [PubMed] [Google Scholar]
- 24.Lucas CA, Rughani A, Hoh JF. Expression of extraocular myosin heavy chain in rabbit laryngeal muscle. J Muscle Res Cell Motil. 1995;16:368–378. doi: 10.1007/BF00114502. [DOI] [PubMed] [Google Scholar]
- 25.Harridge SD, et al. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Archiv. 1996;432:913–920. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- 26.Stienen GJ, Kiers JL, Bottinelli R, Reggiani C. Myofibrillar ATPase activity in skinned human skeletal muscle fibres: Fibre type and temperature dependence. J Physiol. 1996;493(Pt 2):299–307. doi: 10.1113/jphysiol.1996.sp021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edman KA, Reggiani C, Schiaffino S, te Kronnie G. Maximum velocity of shortening related to myosin isoform composition in frog skeletal muscle fibres. J Physiol. 1988;395:679–694. doi: 10.1113/jphysiol.1988.sp016941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sciote JJ, Morris TJ, Brandon CA, Horton MJ, Rosen C. Unloaded shortening velocity and myosin heavy chain variations in human laryngeal muscle fibers. Ann Otol Rhinol Laryn. 2002;111:120–127. doi: 10.1177/000348940211100203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reggiani C, Bottinelli R, Stienen GJ. Sarcomeric myosin isoforms: Fine tuning of a molecular motor. News Physiol Sci. 2000;15:26–33. doi: 10.1152/physiologyonline.2000.15.1.26. [DOI] [PubMed] [Google Scholar]
- 31.Lowey S, Waller GS, Trybus KM. Function of skeletal muscle myosin heavy and light chain isoforms by an in vitro motility assay. J Biol Chem. 1993;268:20414–20418. [PubMed] [Google Scholar]
- 32.Drachman DB, Johnston DM. Development of a mammalian fast muscle: Dynamic and biochemical properties correlated. J Physiol. 1973;234:29–42. doi: 10.1113/jphysiol.1973.sp010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper S, Eccles JC. The isometric responses of mammalian muscles. J Physiol. 1930;69:377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staron RS, Pette D. Nonuniform myosin expression along single fibers of chronically stimulated and contralateral rabbit tibialis anterior muscles. Pflugers Archiv. 1987;409:67–73. doi: 10.1007/BF00584751. [DOI] [PubMed] [Google Scholar]
- 35.Peuker H, Pette D. Quantitative analyses of myosin heavy-chain mRNA and protein isoforms in single fibers reveal a pronounced fiber heterogeneity in normal rabbit muscles. Eur J Biochem. 1997;247:30–36. doi: 10.1111/j.1432-1033.1997.00030.x. [DOI] [PubMed] [Google Scholar]
- 36.Andersen JL, Schiaffino S. Mismatch between myosin heavy chain mRNA and protein distribution in human skeletal muscle fibers. Am J Physiol. 1997;272:C1881–1889. doi: 10.1152/ajpcell.1997.272.6.C1881. [DOI] [PubMed] [Google Scholar]
- 37.Eizema K, et al. Differential expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J Histochem Cytochem. 2003;51:1207–1216. doi: 10.1177/002215540305100911. [DOI] [PubMed] [Google Scholar]
- 38.Toyoshima YY, et al. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987;328:536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- 39.Weeds AG, Taylor RS. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975;257:54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- 40.Patterson B, Ruppel KM, Wu Y, Spudich JA. Cold-sensitive mutants G680V and G691C of Dictyostelium myosin II confer dramatically different biochemical defects. J Biol Chem. 1997;272:27612–27617. doi: 10.1074/jbc.272.44.27612. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney HL, et al. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CT, Spudich JA. The sequence of the myosin 50–20 K loop affects Myosin’s affinity for actin throughout the actin-myosin ATPase cycle and its maximum ATPase activity. Biochemistry. 1999;38:3785–3792. doi: 10.1021/bi9826815. [DOI] [PubMed] [Google Scholar]
- 43.Lauzon AM, et al. A 7-amino-acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil. 1998;19:825–837. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- 44.Rovner AS, Freyzon Y, Trybus KM. Chimeric substitutions of the actin-binding loop activate dephosphorylated but not phosphorylated smooth muscle heavy meromyosin. J Biol Chem. 1995;270:30260–30263. doi: 10.1074/jbc.270.51.30260. [DOI] [PubMed] [Google Scholar]
- 45.De La Cruz EM, Wells AL, Rosenfeld SS, Ostap EM, Sweeney HL. The kinetic mechanism of myosin V. Proc Natl Acad Sci U S A. 1999;96:13726–13731. doi: 10.1073/pnas.96.24.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rock RS, et al. MyosinVI is a processive motor with a large step size. Proc Natl Acad Sci U S A. 2001;98:13655–13659. doi: 10.1073/pnas.191512398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barral JM, Bauer CC, Ortiz I, Epstein HF. Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly. J Cell Biol. 1998;143:1215–1225. doi: 10.1083/jcb.143.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- 49.Price MG, Landsverk ML, Barral JM, Epstein HF. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J Cell Sci. 2002;115:4013–4023. doi: 10.1242/jcs.00108. [DOI] [PubMed] [Google Scholar]
- 50.Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–27273. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- 51.Chow D, Srikakulam R, Chen Y, Winkelmann DA. Folding of the striated muscle myosin motor domain. J Biol Chem. 2002;277:36799–36807. doi: 10.1074/jbc.M204101200. [DOI] [PubMed] [Google Scholar]
- 52.Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004;117:641–652. doi: 10.1242/jcs.00899. [DOI] [PubMed] [Google Scholar]
- 53.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 55.Beylkin DH, Allen DL, Leinwand LA. MyoD, Myf5, and the calcineurin pathway activate the developmental myosin heavy chain genes. Dev Biol. 2006;294:541–553. doi: 10.1016/j.ydbio.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 56.Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001;276:43524–43533. doi: 10.1074/jbc.M108017200. [DOI] [PubMed] [Google Scholar]
- 57.Murphy CT, Rock RS, Spudich JA. A myosin II mutation uncouples ATPase activity from motility and shortens step size. Nat Cell Biol. 2001;3:311–315. doi: 10.1038/35060110. [DOI] [PubMed] [Google Scholar]
- 58.Sweeney HL, Straceski AJ, Leinwand LA, Tikunov BA, Faust L. Heterologous expression of a cardiomyopathic myosin that is defective in its actin interaction. J Biol Chem. 1994;269:1603–1605. [PubMed] [Google Scholar]
- 59.Sellers JR. Myosins. Oxford; New York: Oxford Univ Press; 1999. [Google Scholar]
- 60.Lowey S, Waller GS, Trybus KM. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993;365:454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- 61.Reggiani C, et al. Chemo-mechanical energy transduction in relation to myosin isoform composition in skeletal muscle fibres of the rat. J Physiol. 1997;502(Pt 2):449–460. doi: 10.1111/j.1469-7793.1997.449bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol. 2003;94:2188–2196. doi: 10.1152/japplphysiol.00618.2002. [DOI] [PubMed] [Google Scholar]
- 63.Toydemir RM, et al. Mutations in embryonic myosin heavy chain (MYH3) cause Freeman-Sheldon syndrome and Sheldon-Hall syndrome. Nat Genet. 2006;38:561–565. doi: 10.1038/ng1775. [DOI] [PubMed] [Google Scholar]
- 64.Veugelers M, et al. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. N Engl J Med. 2004;351:460–469. doi: 10.1056/NEJMoa040584. [DOI] [PubMed] [Google Scholar]
- 65.Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol. 2005;6:501–508. doi: 10.1016/S1470-2045(05)70244-8. [DOI] [PubMed] [Google Scholar]
- 66.Agbulut O, Noirez P, Beaumont F, Butler-Browne G. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell. 2003;95:399–406. doi: 10.1016/s0248-4900(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 67.Close RI, Luff AR. Dynamic properties of inferior rectus muscle of the rat. J Physiol. 1974;236:259–270. doi: 10.1113/jphysiol.1974.sp010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.VanBuren P, et al. The essential light chain is required for full force production by skeletal muscle myosin. Proc Natl Acad Sci U S A. 1994;91:12403–12407. doi: 10.1073/pnas.91.26.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy CT, Spudich JA. Variable surface loops and myosin activity: Accessories to a motor. J Muscle Res Cell Motil. 2000;21:139–151. doi: 10.1023/a:1005610007209. [DOI] [PubMed] [Google Scholar]
- 70.He T-C, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85(Pt B):164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- 72.Trybus KM. Biochemical studies of myosin. Methods. 2000;22:327–335. doi: 10.1006/meth.2000.1085. [DOI] [PubMed] [Google Scholar]
- 73.Sellers JR. In: In Current Protocols in Cell Biol. Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada JM, editors. Bethesda: Wiley; 1998. pp. 1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.