Abstract
Mutations in either the mitochondrial or nuclear genomes can give rise to respiratory chain disease (RCD), a large class of devastating metabolic disorders. Their clinical management is challenging, in part because we lack facile and accurate biomarkers to aid in diagnosis and in the monitoring of disease progression. Here we introduce a sequential strategy that combines biochemical analysis of spent media from cell culture with analysis of patient plasma to identify disease biomarkers. First, we applied global metabolic profiling to spotlight 32 metabolites whose uptake or secretion kinetics were altered by chemical inhibition of the respiratory chain in cultured muscle . These metabolites span a wide range of pathways and include lactate and alanine, which are used clinically as biomarkers of RCD. We next measured the cell culture-defined metabolites in human plasma to discover that creatine is reproducibly elevated in two independent cohorts of RCD patients, exceeding lactate and alanine in magnitude of elevation and statistical significance. In cell culture extracellular creatine was inversely related to the intracellular phosphocreatine:creatine ratio suggesting that the elevation of plasma creatine in RCD patients signals a low energetic state of tissues using the phosphocreatine shuttle. Our study identifies plasma creatine as a potential biomarker of human mitochondrial dysfunction that could be clinically useful. More generally, we illustrate how spent media from cellular models of disease may provide a window into the biochemical derangements in human plasma, an approach that could, in principle, be extended to a range of complex diseases.
Keywords: biochemical genetics, biomarker, metabolomics, mitochondria
The respiratory chain (RC) of mammalian cells comprises a series of five enzymatic complexes embedded in the inner mitochondrial membrane and serves as the machinery for oxidative phosphorylation. Changes in RC activity influence key parameters such as the cellular energy charge and the NADH/NAD+ ratio and can impact the numerous metabolic pathways coupled to the RC directly or indirectly. In humans, inherited defects in mitochondrial (mt) or nuclear genes that encode RC proteins or factors necessary for its maturation and assembly lead to respiratory chain disease (RCD) (1). It is estimated that RCD affects at least 18.4 in 100,000 people (2) and represents the most common group of inborn errors of metabolism (3).
The clinical presentation of RCD is highly variable in severity, age of onset, and the combination of organ systems involved, and the factors contributing to this variability are poorly understood (1). Consequently, the diagnosis can be challenging, and there are very limited means of objectively monitoring disease progression. A number of diagnostic algorithms have been proposed (4 –6) that integrate clinical, biochemical, histological, and molecular findings. Abnormal concentrations of several metabolites in biological fluids, including the elevation of lactate, alanine, pyruvate, and the lactate/pyruvate ratio, represent a minimally invasive component of these algorithms. Altered levels of various tricarboxylic acid (TCA)-cycle intermediates, other organic acids, amino acids, and acylcarnitines also have been associated with RCD (7 –11), although these reports often are based on isolated cases and have not been reproduced widely enough to be incorporated into diagnostic algorithms. Lactate, the most widely used metabolic marker, unfortunately often is not elevated in RCD patients and may be elevated as the result of various other causes unrelated to RCD (8, 9). A reliable, simple-to-measure marker of RCD is not currently available (10). A systematic evaluation of metabolic alterations in RC dysfunction is needed to define a disease signature; such a signature could reveal the cellular pathways contingent on RC activity and provide new markers that increase the accuracy of RCD diagnosis and improve the monitoring of disease progression and therapeutic response.
Clinical investigation of rare disorders is limited by the relatively small patient population and the challenges of potential confounding factors. Disease models enable the study of well-defined defects and can focus the clinical investigation on fewer, more probable hypotheses. In particular, in cultured cells, the extracellular metabolic profile provides rich information on consumption and secretion of metabolites and has been used previously as an innovative method for rapidly classifying yeast mutants that were otherwise indistinguishable from wild type (12). Inspired by this approach, we hypothesized that the culture media profile of a cellular RCD model similarly could capture features of the metabolic derangements in the plasma of RCD patients. This hypothesis stems from the notion that the net sum of secretion and consumption by all body cells determines the concentration of metabolites in plasma. We recently have developed a targeted mass spectrometry (MS) method capable of measuring 191 metabolites in biological samples (13, 14). Importantly, this method spans a wide biochemical spectrum including amino acids, organic acids, nucleotides, and sugars, enabling simultaneous monitoring of multiple pathways. Here, we apply this technology to derive an extracellular profile of RC dysfunction from a cellular disease model and then examine this profile in plasma from individuals with RCD.
Results
A Metabolic Signature of RC Dysfunction in the Media of Cultured Muscle Cells.
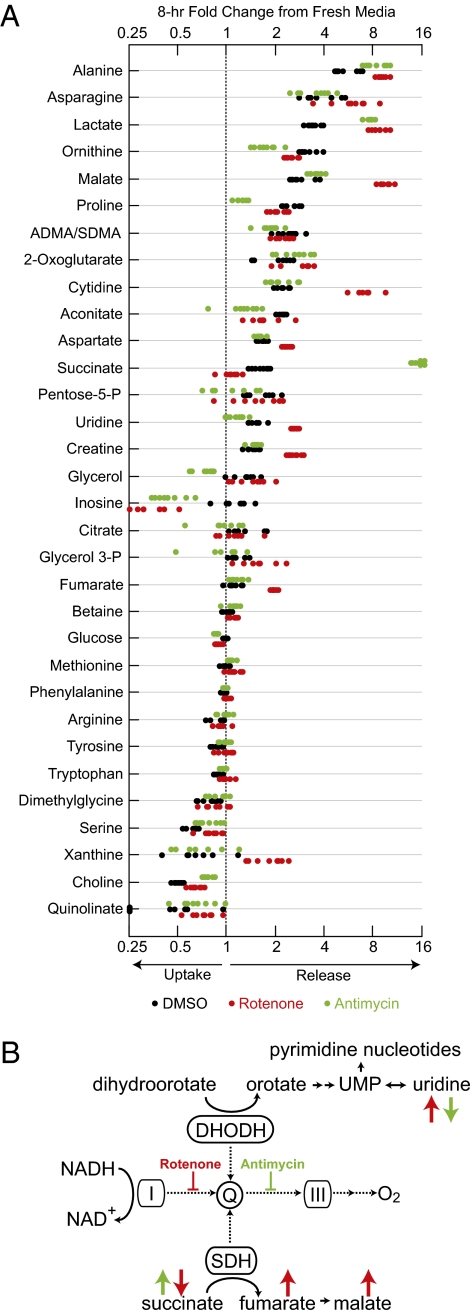
We induced RC dysfunction in differentiated myotubes using rotenone and antimycin, potent inhibitors of NADH dehydrogenase (complex I) and ubiquinol:cytochrome C oxidoreductase (complex III), respectively. We chose myotubes as a model system because of their high oxygen requirement and because skeletal muscle frequently is affected in RCD. The inhibitor concentrations we used were effective in blunting respiration, as is apparent from the decrease in the rate of oxygen consumption (Fig. S1), but did not decrease cell viability even after 24 h of drug treatment. We selected the incubation time (8 h), cell number (5 × 106), and media volume (10 mL) so as to produce at least a 2-fold elevation of lactate levels in the media compared with the vehicle control (DMSO). At the concentrations used, rotenone and antimycin elicited comparable effects on RC activity, as demonstrated by a similar decrease in oxygen consumption and a similar increase in lactate secretion (Fig. 1A).
Fig. 1.
Metabolic profile of RC dysfunction in culture media. (A) Thirty-two metabolites were altered significantly (P < 0.05) with rotenone, antimycin, or both inhibitors. Metabolites are ordered by the degree of change from fresh media in the control (DMSO) condition. Each condition was tested in 8 biological replicates. (B) Discordance between complex I and complex III profiles in pathways coupled to the RC. Dashed arrows represent electron flow. Red and green arrows denote trends with rotenone and antimycin, respectively. I, complex I; III, complex III; DHODH, dihydroorotate dehydrogenase; Q, coenzyme Q; UMP, uridine monophosphate.
In the absence of any inhibitors, the media levels of 25 metabolites changed significantly (P < 0.05) over 8 h, reflecting consumption and secretion by cells. We detected the consumption of nutrients provided in the media, including amino acids (serine, tryptophan, tyrosine, phenylalanine, and leucine/isoleucine) and choline. Changes in media levels of glucose and glutamine were not detected, probably because of their high concentration in media. We detected the secretion of a variety of metabolites, including amino acids that can be synthesized by mammalian cells (alanine, asparagine, ornithine, proline, aspartate, and glutamate), nucleosides (cytidine and uridine), and TCA-cycle intermediates (malate, 2-oxoglutarate, aconitate, succinate, and citrate), as well as creatine (Cr).
We next determined which uptake or release patterns were influenced by RC inhibition. We found a significant difference (P < 0.05) in the levels of 32 metabolites compared with DMSO (Fig. 1A). Cultures treated with RC inhibitors exhibited greater secretion of lactate and alanine, known markers of human RCD. Glucose consumption was higher, together with the lactate pattern reflecting acceleration of nonoxidative glucose metabolism. In contrast, the uptake of multiple amino acids and choline was reduced, suggesting less biosynthesis of proteins and phospholipids, respectively. Six TCA-cycle intermediates exhibited altered secretion (citrate, aconitate, 2-oxoglutarate, succinate, fumarate, and malate); citrate and aconitate were secreted more slowly with both inhibitors, and the other four metabolites were secreted faster with one or both inhibitors. RC inhibition altered the media patterns of multiple other metabolites, including amino acids that can be synthesized (aspartate, asparagine, ornithine, and proline), purines (xanthine and inosine), pyrimidines (cytidine and uridine), and Cr.
We found multiple differences between the rotenone and the antimycin treatments (Fig. 1A). Two metabolites, succinate and uridine, exhibited a particularly striking discordance, being significantly altered by both treatments but in opposite directions (Fig. 1A). Succinate secretion was strongly enhanced with antimycin but blunted with rotenone. The succinate pattern, as well as the higher secretion of fumarate and malate with rotenone than with antimycin, suggest that the two inhibitors have opposite effects on succinate dehydrogenase (SDH or complex II) activity: Whereas antimycin blocks all enzymes transferring electrons to ubiquinone by preventing its reoxidation, rotenone disables only complex I (Fig. 1B). It has been observed previously that complex I inhibition enhances SDH activity, potentially by making a larger part of the coenzyme Q pool available (15). Modulation of SDH activity thus can account for the patterns of succinate, fumarate, and malate. Dihydroorotate dehydrogenase, a key enzyme in de novo pyrimidine biosynthesis, interacts with coenzyme Q similarly to SDH (16, 17) and therefore is expected to be affected by antimycin and rotenone in the same fashion. Indeed, the secretion of uridine, a product of this pathway, was elevated with rotenone but decreased with antimycin. Higher dependence on uridine salvage for pyrimidine nucleotide synthesis also might contribute to the slower uridine secretion with antimycin. Consistent with media uridine levels, intracellular levels of pyrimidine nucleotides, but not of purine nucleotides, were decreased significantly following antimycin treatment (Fig. S2). These findings demonstrate that altered extracellular levels of metabolites in RC-coupled pathways can reflect the location of enzymatic defects within the RC.
Cell Culture-Derived Metabolites Are Altered in Plasma of RCD Patients.
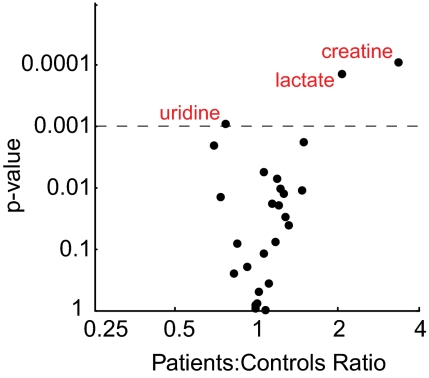
To identify biochemical alterations in human RCD, we performed metabolic profiling on plasma from 16 patients (cohort 1 in Table S1) and 25 healthy controls. All RCD patients had a pathogenic mutation (seven patients) or an abnormally low RC enzyme activity in muscle (nine patients) and had skeletal muscle involvement in their clinical presentation. The cohort included four patients with the mitochondrial encephalomyopathy, lactic acidosis, and stroke mutation (MELAS, mt A3243G), one patient with the myoclonus, epilepsy, and ragged-red fibers mutation (MERRF, mt A8344G), two patients with mtDNA deletions, and one patient with mtDNA depletion. Plasma was obtained during a routine outpatient visit. We detected 68 metabolites in plasma, including 26 of the 32 metabolites altered in culture media (cytidine, inosine, pentose-phosphate, aconitate, glycerol-phosphate, and quinolinate were not detected). The magnitude and significance of the difference between the patient group and the control group was calculated for each of the 26 culture-derived metabolites (Fig. 2). Lactate and alanine, the two most commonly used plasma markers of RCD, were both elevated in patients (difference between patient and control group medians: +107% and +46%, respectively), with a statistically significant difference (P < 0.001) in lactate. Two other metabolites were significantly altered in patients compared with controls: Cr (+233%) and uridine (−24%). The three metabolites reaching statistical significance trended in the same direction as observed in cell culture media (Fig. 1A) with one of the inhibitors (elevated Cr secretion with rotenone and decreased uridine secretion with antimycin) or with both (elevated lactate secretion). The difference in Cr levels was calculated after five patients who were taking a Cr supplement at the time of the test were excluded from the analysis.
Fig. 2.
Differences between patient and control groups in plasma metabolites derived from the cellular model. Significance and magnitude of differences between plasma levels of patients and controls in cohort 1, for all 26 metabolites detected in human plasma out of the 32 metabolites altered in cell culture media (Fig. 1A). Metabolites marked in red exhibited a significant difference (P < 0.001).
Creatine Is Reproducibly Elevated in Plasma of Individuals with RCD.
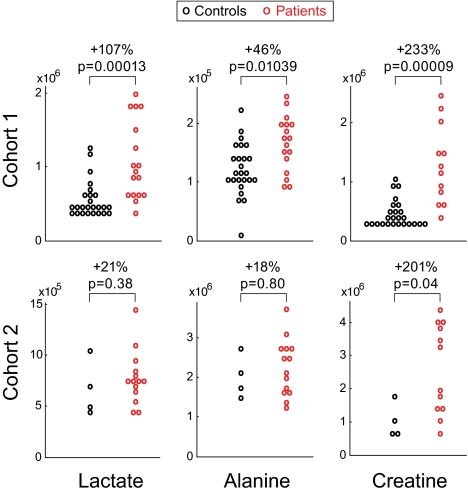
To assess rigorously the reproducibility of plasma metabolite differences, we evaluated a second, independent human cohort consisting of 14 RCD patients selected using the same inclusion criteria (cohort 2 in Table S1) and 4 healthy controls. Metabolite measurement in this cohort was done using complementary chromatographic methods (SI Materials and Methods). We found Cr levels to be significantly higher (P < 0.05) in the patient group (+201%), whereas the difference in lactate and uridine did not replicate significantly (P > 0.05). As in cohort 1, two patients in cohort 2 who were taking a Cr supplement at the time of the test were excluded from the analysis. It is notable that in comparison with lactate and alanine, which currently are used as markers of RCD, Cr exhibited a larger and more statistically significant difference between patients and controls in both cohorts (Fig. 3).
Fig. 3.
Plasma levels of lactate, alanine, and Cr in two human cohorts. Levels are expressed as chromatographic peak area (arbitrary units). The magnitude (in percentage) and significance (denoted “p”) of the difference between the patient and control groups are shown for each metabolite. Magnitude is calculated based on patient group median relative to control group median.
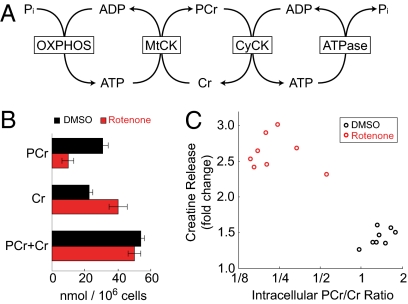
These metabolic profiling studies reveal that extracellular Cr is elevated in response to RC inhibition in cultured cells and in human plasma of individuals with RCD. To examine the relation of the extracellular Cr level to the cellular energetic state, we treated cultured myotubes with the RC inhibitor rotenone, which we previously had found to increase Cr secretion (Fig. 1A), and extracted intracellular metabolites after 8 h. The concentration of Cr is linked to the concentration of phosphocreatine (PCr) through the Cr kinase reaction, whose kinetics are influenced by the balance between mitochondrial oxidative phosphorylation activity and ATP demand (Fig. 4A). The PCr/Cr ratio thus reflects the cellular energetic state. Proton NMR spectroscopy was used to measure absolute concentrations of Cr and PCr in the myotube extract (Fig. 4B and SI Materials and Methods). In DMSO-treated cultures, PCr was slightly higher than Cr, comprising 58% of total Cr (PCr + Cr). This proportion is similar to previously reported measurements in skeletal muscle of healthy individuals at rest (18). In the rotenone-treated cultures the size of the total Cr pool was similar, but PCr accounted for only 19% of the total, and Cr was significantly elevated (P = 0.0002). Cr release into the media was inversely correlated with the intracellular PCr/Cr ratio (Fig. 4C).
Fig. 4.
Correlation of Cr release with the intracellular energetic state in cultured muscle cells. (A) The creatine phosphate energy shuttle. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CyCK, cytosolic creatine kinase; MtCK, mitochondrial creatine kinase; OXPHOS, oxidative phosphorylation. (B) Mean PCr, Cr, and total Cr concentrations in C2C12 myotube extract 8 h after treatment with rotenone or DMSO (control). Each condition was measured in eight biological replicates. Error bars indicate SD. (C) Cr release as a function of the intracellular PCr/Cr ratio. Each circle represents an individual culture plate treated with rotenone or DMSO.
Discussion
In the current study we derived an extracellular metabolic profile of RC dysfunction from a simple cellular disease model and used the profile to identify a reproducible plasma signature of human RCD. The use of a cellular model allowed us to study well-defined defects in two key enzymes of the RC. Concordance of metabolite trends in plasma with their trend in culture media supports a functional link to the RC. Our findings suggest that consumption and secretion patterns in cell culture can capture, at least in part, the altered metabolic state in plasma of individuals with RCD. This approach could be applicable to other metabolic diseases that can be modeled adequately in cell culture.
Characterizing the spectrum of metabolic alterations in RC dysfunction could reveal the pathways contingent on RC activity and holds potential for better understanding the pathogenesis of human RCD. A number of recent studies have each evaluated a single family of metabolites, such as amino acids or TCA-cycle intermediates, in biological fluids of individuals with RCD (7, 19) or in animal models of the disease (20). In the current study we targeted multiple biochemical groups, enabling the discovery of a rich metabolic signature spanning amino acids and other nitrogenous compounds, glycolysis, the TCA cycle, purines, and pyrimidines (Fig. 1A). Our decision to probe two key components of the RC, complex I and complex III, uncovered a wealth of metabolic differences between these two inhibition modes. Some of these differences can be attributed to the different effects rotenone and antimycin exert on RC-linked enzymes such as succinate dehydrogenase and dihydroorotate dehydrogenase (Fig. 1B). For other differences, such as the greater elevation of Cr secretion with rotenone than with antimycin, the reason is not as clear. Importantly, combining a global profiling approach with probing of multiple RC complexes enabled us to highlight similarities between two different RC-coupled pathways: pyrimidine biosynthesis and the TCA cycle (Fig. 1B). These findings enhance our understanding of the metabolic consequences of RC dysfunction and provide clues for the identification of specific enzymatic defects.
The pathogenesis of RCD has been associated with a low cellular energetic state. 31P magnetic resonance spectroscopy (31P-MRS), which can measure the concentration of ATP, PCr, and inorganic phosphate (Pi) noninvasively, has been used extensively to study the energetic state of muscle in this patient population. In muscle of individuals with RCD and myopathy, PCr and the PCr/Pi ratio are decreased at rest, and the recovery of PCr after exercise is slow (21, 22). 31P-MRS also has detected low PCr in brain of individuals with RCD (21, 23). Although 31P-MRS offers direct and noninvasive measurement of the cellular energetic state, it is not in routine clinical use because it is technically difficult to perform, inaccessible to most clinical centers, and lacks diagnostic sensitivity for RCD (24). In the current study, we demonstrate a correlation between extracellular Cr levels and intracellular PCr depletion in RC-inhibited myotubes (Fig. 4C). Consistent with this cellular model, plasma Cr was elevated in individuals with RCD in two independent cohorts using two different measurement techniques (Fig. 3). While numerous previous studies have demonstrated a PCr decrease in the muscle and brain of patients with RCD, the current study demonstrates that the plasma Cr level is elevated in these individuals and hence may reflect the intracellular energetic state. The effect of the intracellular energetic state on extracellular Cr levels may be mediated through the Cr transporter; indeed, accumulation of intracellular Cr was shown previously to inhibit uptake through this transporter (25). In both the human cohorts studied here, the elevation in plasma Cr was larger in magnitude and statistically more significant than the elevation of lactate and alanine, the most commonly used markers of RCD (Fig. 3). If our results are corroborated in future independent cohorts, measurement of plasma Cr could improve the accuracy of minimally invasive diagnosis of RCD and, importantly, provide a means for monitoring disease progression. Oral Cr supplementation often is prescribed to individuals with RCD and myopathy with the goal of improving muscle function (26, 27); baseline plasma Cr measurement may be informative for predicting whether Cr supplementation is likely to be effective.
The potential influence of confounding factors is a major challenge in the clinical investigation of rare disorders, in which cohort sizes are relatively small. With respect to plasma Cr, dietary intake of Cr as a supplement or in food is an important potential confounder. We were careful to exclude from our analysis patients who were taking an oral Cr supplement. Although our first human cohort was not balanced with respect to recent food intake, replication of the Cr elevation in a second cohort, which was balanced in that respect, has increased our confidence that the elevation observed in the first cohort was not caused by dietary Cr. In addition, we have reported previously on the effect of glucose ingestion on the plasma levels of metabolites (14); although Cr was measured in plasma, glucose ingestion did not affect Cr plasma levels. We also considered as potential confounders age and gender, which were balanced in one of two cohorts (Materials and Methods). Future well-powered studies are needed to validate the elevation of plasma Cr in RCD patients. Furthermore, human in vivo studies will be required to explore the relationship between circulating Cr levels and the intracellular energetic state.
Metabolic profiling holds tremendous potential for understanding of human disease, improving diagnostics, and proposing therapies. In the current study we combined a cellular model with clinical investigation to gain insight into RCD, using metabolic profiling to explore a broad biochemical spectrum, and detected a rich profile implicating multiple pathways. Future work applying this approach to genetic disease models could reveal further how cells and organisms adapt to RC dysfunction and provide additional insight into RCD pathogenesis. This study offers proof of concept that extracellular profiling in culture may provide a window into the metabolic derangements in plasma, an approach that could be extended to complex human diseases.
Materials and Methods
RC Inhibition in Cell Culture.
Mouse C2C12 myoblasts were obtained from ATCC and grown in DMEM (Sigma) containing 4.5 g/mL glucose with 10% FBS in 10-cm plates. To induce myotube differentiation, FBS was replaced with 2% horse serum (Sigma) for 4 days. On the day of the experiment, cultures were placed in 10 mL fresh media in the presence of 0.1 μM rotenone (Sigma; R8875), 0.5 μM antimycin (Sigma; A8674), or DMSO, and after 8 h of incubation spent media were sampled for metabolic profiling. The experiment was done in 8 biological replicates, and 8 samples of fresh media also obtained for comparison. To prepare media samples for LC-MS/MS analysis, 100 μL of media was mixed with 100 μL of ethanol solution (80% ethanol, 19.9% water, and 0.1% formic acid). After 20 min at 4 °C, the samples were centrifuged at 15,000 × g for 15 min, and 180 μL of the supernatant was extracted and evaporated under nitrogen gas. Samples were reconstituted in 60 μL HPLC-grade water and analyzed.
To measure intracellular metabolite levels, cell contents were extracted using 1 mL of a methanol solution (80% methanol, 20% water) at dry ice temperature, after aspirating all media and quickly washing samples with ice-cold PBS. The cell suspension was centrifuged at 14,000 × g for 10 min, and 100 μL of the supernatant was removed for analysis by NMR and liquid chromatography coupled with tandem MS (LC-MS/MS). To prepare for LC-MS/MS analysis, the supernatant was evaporated under nitrogen gas and reconstituted in 60 μL HPLC-grade water.
To evaluate the efficacy of RC inhibitors at the concentrations used, the oxygen consumption rate was measured few minutes before and after the addition of inhibitors or DMSO using a dedicated instrument (Seahorse Bioscience) as previously described (28). The change in oxygen consumption was calculated from the ratio of the two measurements.
Cell viability was measured with a calcein assay (C34852; Invitrogen) after 24-h incubation with RC inhibitors or DMSO.
Human Subjects.
The criterion for inclusion of RCD patients in the study was a confirmed diagnosis based either on the presence of a pathogenic mutation or on reduced RC activity in muscle and muscle involvement in the clinical presentation. Patients were tested during an outpatient visit to the mitochondrial clinic at Massachusetts General Hospital.
Cohort 1 included 16 patients (Table S1), with one patient (#1080) tested on two separate visits 6 months apart, and 25 controls who were healthy adults participating in an epidemiologic study at Massachusetts Institute of Technology. The gender composition was similar in the patient group (56% females) and the control group (60% females). The patient and control groups were not balanced with respect to age or recent food intake: age was significantly (P < 0.05) lower in the control group (24 ± 4 years) than in the patient group (44 ± 13 years), and all control subjects were tested after overnight fast, whereas most patients were not fasting.
Cohort 2 included 14 patients (Table S1) and 4 controls who were healthy family members of patients visiting the mitochondrial clinic. The gender composition was not balanced between patients (50% females) and controls (0% females). The age of the patient group (31 ± 21 years) was lower than in the control group (49 ± 6 years), but the difference was not statistically significant (P > 0.05). The patient and control groups were balanced with respect to recent food intake.
The study was approved by the Institutional Review Boards of Massachusetts General Hospital and Massachusetts Institute of Technology, and informed consent was obtained from all participants.
Plasma extraction and preparation for LC-MS/MS analysis was done as previously described (14).
LC-MS/MS Metabolic Profiling.
Metabolite levels were measured by LC-MS/MS in culture media and in cohort 1 plasma as previously described (14). Data were acquired using a 4000 QTRAP triple quadrupole mass spectrometer (Applied Biosystems/Sciex) equipped with an HTS PAL autosampler (Leap Technologies) and an Agilent 1200 Series binary HPLC pump. Multiple reaction monitoring was used to acquire targeted MS data for specific metabolites. Declustering potentials and collision energies were optimized for each metabolite by infusion of reference standards before sample analyses. MultiQuant software version 1.1 (Applied Biosystems/Sciex) was used for automated peak integration, and metabolite peaks were reviewed manually for quality of integration and compared against known standards to confirm identity. Measurement of plasma metabolites in cohort 2 was done using LC-MS/MS with the same equipment but different liquid chromatography methods (SI Materials and Methods).
Statistical Analysis.
The significance of differences in metabolite levels between cell culture conditions or between patients and controls was evaluated using the Wilcoxon rank sum test. To correct for multiple hypotheses testing in cohort 1, a significance threshold of P = 0.001 was used.
Supplementary Material
Acknowledgments
We thank R. Wei and E. Yang for assistance with MS; H. Vu and W. Schaefer for assistance with NMR spectroscopy; and V. Gohil, M. Jain, T. Kitami, R. Nilsson, S. Vafai, and R. Haller for valuable discussions and comments on the manuscript. O.S. was supported by a training grant for bioinformatics and integrative genomics from the National Human Genome Research Institute and a fellowship from the Eli and Dorothy Berman Fund. V.K.M. was supported by an Early Career Physician-Scientist Award from the Howard Hughes Medical Institute. Clinical studies were conducted in part at the Clinical Research Center at the Massachusetts Institute of Technology funded by Grant RR-01066 from the National Center for Research Resources, National Institutes of Health. This work was supported by a grant from the Broad Institute Scientific Planning and Allocation of Resources Committee and by Grant R01DK081457 from the National Institutes of Health to V.K.M.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906039107/DCSupplemental.
References
- 1.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 2.Uusimaa J, et al. Prevalence, segregation, and phenotype of the mitochondrial DNA 3243A>G mutation in children. Ann Neurol. 2007;62:278–287. doi: 10.1002/ana.21196. [DOI] [PubMed] [Google Scholar]
- 3.Thorburn DR. Mitochondrial disorders: Prevalence, myths and advances. J Inherit Metab Dis. 2004;27:349–362. doi: 10.1023/B:BOLI.0000031098.41409.55. [DOI] [PubMed] [Google Scholar]
- 4.Bernier FP, et al. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology. 2002;59:1406–1411. doi: 10.1212/01.wnl.0000033795.17156.00. [DOI] [PubMed] [Google Scholar]
- 5.Morava E, et al. Mitochondrial disease criteria: Diagnostic applications in children. Neurology. 2006;67:1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 6.Walker UA, Collins S, Byrne E. Respiratory chain encephalomyopathies: A diagnostic classification. Eur Neurol. 1996;36:260–267. doi: 10.1159/000117269. [DOI] [PubMed] [Google Scholar]
- 7.Barshop BA. Metabolomic approaches to mitochondrial disease: Correlation of urine organic acids. Mitochondrion. 2004;4:521–527. doi: 10.1016/j.mito.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Haas RH, et al. Mitochondrial disease: A practical approach for primary care physicians. Pediatrics. 2007;120:1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 9.Haas RH Mitochondrial Medicine Society's Committee on Diagnosis. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab. 2008;94:16–37. doi: 10.1016/j.ymgme.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancuso M, et al. Diagnostic approach to mitochondrial disorders: The need for a reliable biomarker. Curr Mol Med. 2009;9(9):1095–1107. doi: 10.2174/156652409789839099. [DOI] [PubMed] [Google Scholar]
- 11.Shoffner JM. Oxidative phosphorylation disease diagnosis. Semin Neurol. 1999;19:341–351. doi: 10.1055/s-2008-1040849. [DOI] [PubMed] [Google Scholar]
- 12.Allen J, et al. High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat Biotechnol. 2003;21:692–696. doi: 10.1038/nbt823. [DOI] [PubMed] [Google Scholar]
- 13.Lewis GD, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–3512. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaham O, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:1–9. doi: 10.1038/msb.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutman M, Silman N. Mutual inhibition between NADH oxidase and succinoxidase activities in respiring submitochondrial particles. FEBS Lett. 1972;26:207–210. doi: 10.1016/0014-5793(72)80574-x. [DOI] [PubMed] [Google Scholar]
- 16.Jones ME. Pyrimidine nucleotide biosynthesis in animals: Genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- 17.Löffler M, Jöckel J, Schuster G, Becker C. Dihydroorotate-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol Cell Biochem. 1997;174:125–129. [PubMed] [Google Scholar]
- 18.Tarnopolsky MA, Parise G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve. 1999;22:1228–1233. doi: 10.1002/(sici)1097-4598(199909)22:9<1228::aid-mus9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Atkuri KR, et al. Inherited disorders affecting mitochondrial function are associated with glutathione deficiency and hypocitrullinemia. Proc Natl Acad Sci USA. 2009;106:3941–3945. doi: 10.1073/pnas.0813409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falk MJ, et al. Metabolic pathway profiling of mitochondrial respiratory chain mutants in C. elegans . Mol Genet Metab. 2008;93:388–397. doi: 10.1016/j.ymgme.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Argov Z. Functional evaluation techniques in mitochondrial disorders. Eur Neurol. 1998;39:65–71. doi: 10.1159/000007912. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DL, Taylor DJ, Radda GK. Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol. 1985;18:189–196. doi: 10.1002/ana.410180205. [DOI] [PubMed] [Google Scholar]
- 23.Eleff SM, et al. Phosphorus magnetic resonance spectroscopy of patients with mitochondrial cytopathies demonstrates decreased levels of brain phosphocreatine. Ann Neurol. 1990;27:626–630. doi: 10.1002/ana.410270607. [DOI] [PubMed] [Google Scholar]
- 24.Jeppesen TD, Quistorff B, Wibrand F, Vissing J. 31P-MRS of skeletal muscle is not a sensitive diagnostic test for mitochondrial myopathy. J Neurol. 2007;254:29–37. doi: 10.1007/s00415-006-0229-5. [DOI] [PubMed] [Google Scholar]
- 25.Dodd JR, Zheng T, Christie DL. Creatine accumulation and exchange by HEK293 cells stably expressing high levels of a creatine transporter. Biochim Biophys Acta. 1999;1472:128–136. doi: 10.1016/s0304-4165(99)00113-0. [DOI] [PubMed] [Google Scholar]
- 26.Klopstock T, et al. A placebo-controlled crossover trial of creatine in mitochondrial diseases. Neurology. 2000;55:1748–1751. doi: 10.1212/wnl.55.11.1748. [DOI] [PubMed] [Google Scholar]
- 27.Tarnopolsky MA. Clinical use of creatine in neuromuscular and neurometabolic disorders. Subcell Biochem. 2007;46:183–204. doi: 10.1007/978-1-4020-6486-9_10. [DOI] [PubMed] [Google Scholar]
- 28.Wu M, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–C136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.