Abstract
Invariant natural killer T cells (iNKT cells) respond to CD1d-presented glycolipids from Borrelia burgdorferi, the causative agent of Lyme disease. Although mouse and human iNKT cells respond to different antigens based on subtle differences in their fatty acids, the mechanism by which fatty acid structure determines antigenic potency is not well understood. Here we show that the mouse and human CD1d present glycolipids having different fatty acids, based in part upon a difference at a single amino acid position that is involved in positioning the sugar epitope. CD1d also can bind nonantigenic lipids, however, but unexpectedly, mouse CD1d orients the two aliphatic chains of a nonantigenic lipid rotated 180°, causing a dramatic repositioning of the exposed sugar. Therefore, our data reveal the biochemical basis for the high degree of antigenic specificity of iNKT cells for certain fatty acids, and they suggest how microbes could alter fatty acid biosynthesis as an immune evasion mechanism.
Keywords: microbial glycolipids, immune evasion, lyme disease
CD1 proteins constitute a family of antigen-presenting molecules (1), similar in structure to MHC class I antigen-presenting molecules. CD1 molecules recycle through intracellular vesicular compartments, where they sample different lipid-containing antigens for cell-surface presentation to reactive T cells (2 –4). Humans express five family members (CD1a–e) that can be grouped into three groups based on sequence similarity: group 1 (CD1a–c), group 2 (CD1d), and group 3 (CD1e) (5, 6). Mice express only CD1d. Crystal structures of human CD1a, b, and d, as well as mouse CD1d, without loading specific antigens, or in complex with different glycolipids or lipopeptides, have been extensively reviewed elsewhere (7 –10). A common feature revealed by these structures is a CD1 hydrophobic binding groove that is formed between two antiparallel helices that sit above an antiparallel β-sheet platform. However, the size, number, and connection of the individual binding pockets (A′, C′, F′, and T′) within the grooves differ among the CD1 family members, which in turn affects the size and shape of the lipid antigens that each member can present (7). Generally, however, the glycolipids or lipopeptides bind to CD1 with their lipid backbone tucked into the deeply buried hydrophobic pockets, whereas the head group is exposed at the CD1 surface for T cell recognition (9).
A specialized subset of glycolipid and CD1d-restricted T lymphocytes are known as type I or semi-invariant (i) natural killer T (NKT) cells. These cells express an invariant Vα14-Jα18 chain in mouse, and in humans they have an orthologous Vα24 segment also rearranged with Jα18. The invariant TCR α chain is paired with a limited number of β chains; mainly Vβ8.2, Vβ7, and Vβ2 in mouse, and Vβ11 in humans (11, 12). iNKT cells respond to glycosphingolipids (GSLs) with α-linked sugars, such as the model synthetic antigen α-galactosylceramide (α-GalCer), or microbial antigens such as α-galacturonosylceramide (GalA-GSL) from Sphingomonas spp. bacteria (13, 14).
Recently, a different category of glycolipids, α-galactosyl diacylglycerolipids (α-GalDAG), have been identified as novel iNKT cell antigens. These compounds were derived from Borrelia burgdorferi (B. burgdorferi), the causative agent of Lyme disease, and a direct role for iNKT cells in host defense, and clearance of these microbes recently has been demonstrated (15, 16). Naturally occurring B. burgdorferi α-galactosyl diacylglycerolipids (α-GalDAG) are composed of two fatty acids, esterified to the sn-1 and sn-2 hydroxyls of glycerol, whereas the galactose is attached to the sn-3 position with an α-glycosidic bond (17). The fatty acids in the purified material, referred to as B. burgdorferi glycolipid-2 (BbGL-2), varied in length from C14 to C18 (methylene units) and can either be fully saturated or mono- or di-unsaturated. The most abundant natural fatty acids in the purified material included palmitic (C16:0), stearic (C18:0), oleic (C18:1), and linoleic (C18:2) acids. Because it was not possible to separate different compounds in the purified mixture based only on the fatty acid composition, we synthesized a panel of different α-GalDAG species with distinct fatty acid compositions representing the likely major species. In a previous report, it was demonstrated that, surprisingly, not all B. burgdorferi α-GalDAG antigens activated mouse and human iNKT cells (16), despite the fact that they differ in a subtle fashion only for the fatty acids, which would be buried in the CD1d groove and therefore not in direct contact with the TCR. Furthermore, there was a dichotomy in the response of human and mouse iNKT cells to the synthetic BbGL-2 antigens, with those antigens having more unsaturated fatty acids best at activating only human iNKT cells (16).
Here we have used a variety of methods to elucidate the biochemical and structural mechanisms underlying the unexpected and highly selective glycolipid antigen recognition of microbial antigens based on differences in the fatty acids. We also identify a key residue in CD1d that is in part responsible for the different specificities for α-GalDAG antigens when mouse and human iNKT cells are compared.
Results
Diacylglycerol-Containing Antigens Are Relatively Weak Agonists.
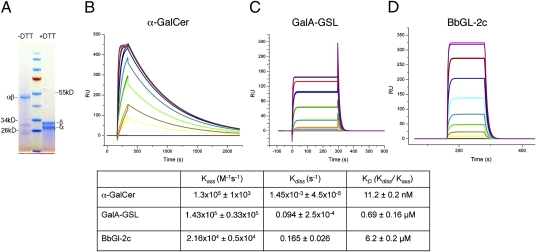
Two types of microbial glycolipid antigens have been identified for iNKT cells: GSLs from Sphingomonas bacteria and GalDAG antigens from B. burgdorferi. The results from immune assays suggest that the GalDAG antigens are weaker antigens, despite containing α-linked galactose similar to α-GalCer. This difference in antigenic potency could reflect a number of factors in addition to the strength of interaction with the TCR, including solubility, intracellular distribution, and stability of CD1d binding. Therefore, to achieve a quantitative comparison of the TCR interactions with the two different types of microbial antigens, we determined the equilibrium binding kinetics of a refolded Vα14Vβ8.2 TCR from an iNKT cell hybridoma with mouse CD1d (mCD1d) molecules loaded with α-GalCer, GalA-GSL, or BbGL-2c (Fig. 1), which is the most potent α-GalDAG antigen that we have identified for mouse iNKT cells. In surface plasmon resonance studies, when CD1d-glycolipid complexes were immobilized on the sensor chip, our TCR construct exhibited highly specific binding with α-GalCer (KD = 11.2 nM), calculated on the basis of the kinetic parameters (Fig. 1B). Although this is in agreement with earlier measurements showing that the invariant TCR has an extraordinarily high affinity for mCD1d−α-GalCer complexes, the binding previously was reported to be weaker, in the 70- to 300-nM range (18 –20). This discrepancy could be the result of the different constructs used and/or the different CDR3β sequences in the TCRs analyzed. The binding affinity of the same TCR for the Sphingomonas GalA-GSL antigen bound to mCD1d was weaker (KD = 0.69 μM), consistent with the results of functional assays (Fig. 1C). This reflects the combined influences of the galacturonic acid in GalA-GSL, which is not as potent as galactose, and the absence of the 4′ hydroxyl of the sphingosine base in GalA-GSL (13, 21–23). To assess the binding of weak antigens, such as BbGl-2c, we immobilized the TCR on the sensor chip while passing over CD1d-glycolipid complexes (Fig. 1D). As a control we also assessed the binding of the TCR for mCD1d-α-GalCer (KD = 12.5 nM), which was almost identical to the earlier measurements in which CD1d was immobilized. The TCR binding to BbGL-2c-mCD1d complexes is likewise consistent with immune response–based studies, with an even weaker KD than GalA-GSL, in the low micromolar range (6.2 μM), or approximately three orders of magnitude less than TCR binding to α-GalCer complexes with mCD1d. The TCR binding affinity for BbGL-2c-mCD1d complexes is comparable, however, to the binding of TCRs from conventional T lymphocytes to peptide-MHCI complexes (24).
Fig. 1.
TCR refolding and binding kinetics. (A) Reducing (+DTT) and nonreducing (–DTT) SDS/PAGE of refolded and purified TCR. (B and D) Mean kinetic equilibrium binding data of the TCR for mCD1d-glycolipid complexes from two to three independent experiments. Binding response of Vα14Vβ8.2 TCR to mCD1d loaded with α-GalCer (B), GalA-GSL (C), or BbGL-2c (D). Each colored curve depicts a different concentration of injected TCR (B and C) or mCD1d-BbGL-2c (D) (details in Methods). One representative sensorgram is shown for each glycolipid.
CD1d Defines Antigenic Fatty Acids in Borrelia Glycolipids.
In general, the specificity of mouse and human iNKT cells is highly conserved (25), reflecting conservation of the key CD1d and Vα TCR amino acids. Despite this, mouse and human iNKT cells respond with surprising selectivity to α-GalDAG glycolipids that differ with regard to their fatty acids. Mouse iNKT cells are best activated by BbGL-2c, the lipid backbone of which consists of a sn-1–linked oleic acid (18:1) and a sn-2–linked palmitic acid (16:0). By contrast, human iNKT cells react best to antigens with more unsaturated fatty acids, particularly BbGL-2f with sn-1–linked linoleic acid (18:2) and an sn-2–linked oleic acid (16). This difference could reflect the preferential binding of lipid antigens with certain fatty acids. Amino acids of human and mouse CD1d are approximately only 60% identical in the α1 and α2 domains; as a result, the lipid binding properties of these homologs might not be equivalent. Alternatively, the different α-GalDAG antigens might bind well to either mouse or human CD1d, but the length and degree of unsaturation of the fatty acids might alter the position of the exposed hydrophilic head group or the conformation of CD1d. In this case, sequence divergence in the CD1d molecules and/or in the TCRs might define the observed species-specific reactivity.
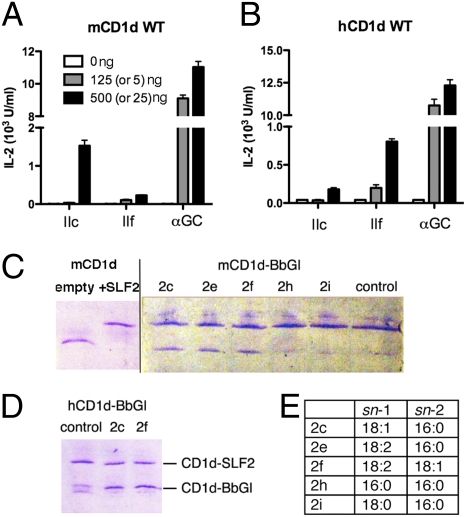
To determine whether CD1d structural differences between species select for the presentation of certain glycolipid antigens, the mouse iNKT cell hybridomas 1.2 and 2C12 were tested for their ability to respond to plates coated with CD1d molecules that had been incubated with different glycolipid antigens. In agreement with previous results, plates coated with mCD1d presented BbGL-2c but not BbGL-2f (Fig. 2A). By contrast, when tested with plates coated with human CD1d, the same iNKT cell hybridomas responded to BbGL-2f but not BbGL-2c (Fig. 2B). These results indicate that differences in the CD1d sequences between the two species, and not TCR sequence differences, are the primary factor influencing the selective recognition of certain α-GalDAG antigens by mouse iNKT cells. Therefore, the CD1d polypeptide sequences, presumably sequences in the binding grooves, determine which fatty acids are required for antigenic activity.
Fig. 2.
Vα14i NKT cell response to B. burgdorferi α-galactosyl diacylglycerolipids. Response of Vα14i NKT cell hybridoma 1.2 to mCD1d (A) and human CD1d-coated plates (B) loaded with the indicated amount of BbGL-2c, -2f, and α-GalCer. Lower amount in parentheses refers to α-GalCer concentration. IL-2 levels were measured in culture supernatants by ELISA, after overnight incubation with the iNKT cell hybridoma. All data are mean ± SD for triplicate wells and represent two independent experiments. Response of 2C12 hybridoma to borrelia antigens was comparable and is not depicted here. Glycolipid loading to mouse (C) and human (D) CD1d molecules assessed by native IEF gel electrophoresis. (C) Purified “empty” CD1d was loaded with di-sulfatide (SLF2) and purified from unbound lipid (Left). CD1d-SLF2 complexes were incubated with the indicated BbGL-2 species and, upon successful lipid exchange, resulted in a gel shift to the lower (noncharged) position. (E) Fatty acid composition of synthetic BbGL-2 compounds used in the lipid-loading studies. Control lane: CD1d-SLF2 complexes were incubated only with buffer. No lipid is exchanged, and SLF2 remains stably bound.
Borrelia Glycolipids with Different Fatty Acids Bind to CD1d.
It is possible that the selective presentation of certain α-GalDAG antigens is due simply to their selective ability to bind to CD1d molecules. The naturally occurring fatty acids in the B. burgdorferi antigens are characterized not only by differences in aliphatic chain length, but also by the presence of unsaturated bonds in the cis configuration. As a result, the alkyl chain is kinked at the position of the unsaturated bond. To identify whether the differential recognition of borrelial diacylglycerolipids is correlated with differences in the binding capacity of human and mouse CD1d molecules, we carried out CD1d–lipid exchange experiments. As BbGL-2 is uncharged, loading to CD1d cannot directly be visualized by native isoelectic focusing gel electrophoresis (IEF). Therefore, we first loaded CD1d polypeptides with a sulfatide molecule bearing two negative charges (SLF2), purified the CD1d-SLF2 complexes from excess lipids, and incubated the CD1d-SLF2 complexes with the different borrelial glycolipids (Fig. 2 C and D). This incubation was done in the absence of detergent, as Tween 20 alone has the potential to replace lipids from the CD1d binding groove. Successful loading of BbGL-2 results in a shift back to the original migration position of CD1d on the IEF gel. Our results demonstrate that there is no qualitative difference in the binding capabilities of mouse and human CD1d for either BbGL-2c or BbGL-2f (Fig. 2 C and D). However, binding was not observed if replacement of SLF2 was attempted with one of the fully saturated BbGL-2 ligands, either BbGL-2h or -2i, possibly because of the reduced solubility and/or flexibility of the saturated alkyl chains. Also it appears that SLF2, which is a sphingolipid, binds more tightly to mCD1d than the borrelial diacylglycerolipids. Although the shift of CD1d on the IEF gels indicates SLF2 could completely replace the endogenous glycolipid loaded into CD1d in insect cells, reported to be phosphatidylcholine (26), a 6-fold excess of the α-GalDAG antigens could not completely replace SLF2 (Fig. 2). This is consistent with the notion that sphingolipids such as SLF2 bind more tightly to CD1d than the diacylglycerolipids, owing perhaps to the intricate hydrogen bonding network between sphingolipids and residues of CD1d (8, 9).
Critical Amino Acid Position Determines Antigenicity of Borrelia Glycolipids.
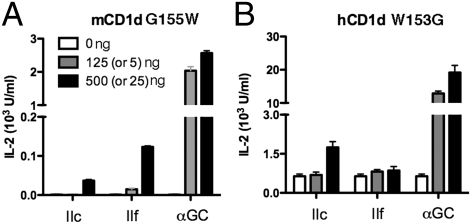
Although the binding grooves of mouse and human CD1d are only ≈60% identical, their overall structural features are generally conserved. A prominent difference is found, however, at glycine 155 in mouse CD1d, equivalent to tryptophan 153 in human CD1d, which is located on the α2-helix at the entrance to the binding groove. We considered it likely that this nonconservative substitution would be important for the species-specific presentation of α-GalDAG antigens by mouse and human CD1d. We therefore prepared soluble CD1d molecules in which these positions were substituted. Gly155 of mouse CD1d was replaced with tryptophan G155(mCD1d)W, thereby humanizing a soluble mouse CD1d protein at this position. Similarly, position 153 tryptophan in human CD1d was replaced with glycine, creating the variant designated as W153(hCD1d)G. The soluble CD1d molecules were tested for their ability to activate two mouse iNKT cell hybridomas. It is striking that the humanized G155(mCD1d)W molecule lost the ability to present BbGL-2c (Fig. 3A). By contrast, the antigen-stimulated IL-2 production by BbGL-2f remained low, but it was increased slightly compared with wild type mCD1d (Fig. 2A). Overall, therefore, the G155(mCD1d)W molecule had a preferential response to BbGL-2f, similar to human CD1d. The lower response compared to a fully human CD1d molecule suggests other amino acids also might contribute to the selectivity for more unsaturated fatty acids. The results from antigen presentation assays carried out with W153(hCD1d)G, with tryptophan 153 of human CD1d mutated to glycine, confirm the importance of this site. In the response to plates coated with this human CD1d mutant, the mouse iNKT cell hybridomas exhibited a greatly increased response to BbGL-2c but not to BbGL-2f, yielding a preference pattern similar to the response to wild-type mouse CD1d molecules (Fig. 3B), although the background response to W153(hCD1d)G without antigen was generally higher. In summary, the species-specific pattern of preferential responses based on the DAG antigen fatty acid composition could be recapitulated by substituting a single α2 helix amino acid position. Although this substitution had only a mild effect on the presentation of BbGL-2f, the amino acid at this position had a very strong effect on the presentation of BbGL-2c.
Fig. 3.
Vα14i NKT cell response to B. burgdorferi α-galactosyl diacylglycerolipids, presented by CD1d mutants. (A) “Humanized” mCD1d G155W mutant preferentially stimulates an iNKT cell hybridoma with BbGL-2f, whereas “mouse-like” humanCD1d W153G mutant (B) activates the same hybridoma when presenting BbGL-2c (details in Fig. 2 legend).
Crystal Structure Determination of mCD1d with BbGL-2c and BbGL-2f.
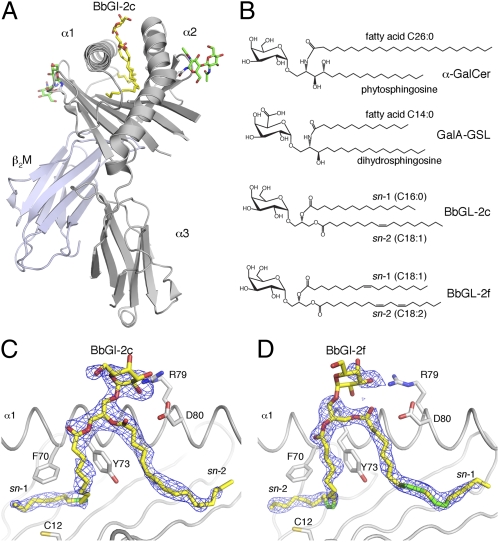
To elucidate the structural basis for BbGL-2c recognition by mouse iNKT cells, and to address why BbGL-2f when presented by mCD1d is not recognized, we have determined the crystal structures of both BbGL-2c and BbGL-2f bound to mCD1d at resolutions of 2.05Å and 1.85Å, respectively (Fig. 4 and Table S1). BbGL-2c is bound by mCD1d with the sn-1–linked oleic acid (18:1) inside the A′ pocket, where the cis-unsaturation facilitates the encircling of the A′ pole, formed by Cys12 and Phe70, in a counterclockwise orientation, when looked at from the top of the CD1d-binding groove. The sn-2–linked palmitic acid (16:0) is inserted in an almost straight conformation into the F′ pocket, whereas the sn-3 linked galactose is exposed above the center of the binding groove between the A′ and F′ pocket for recognition by iNKT cells (Fig. 4 A-C).
Fig. 4.
BbGL-2 binding to mCD1d. (A) Overview of mCD1d-BbGL-2c crystal structure. N-linked glycosylation of mCD1d is shown as green sticks. (B) Structures of α-galactosyl containing sphingolipids (α-GalCer and GalA-GSL) and diacylglycerolipid (BbGL-2c and -2f) iNKT cell antigens. Orientations of BbGL-2c (C) and BbGL-2f (D) in mCD1d binding groove are depicted. View of final 2FoFc map drawn as a blue mesh around BbGL-2 antigens (yellow) and contoured at 1.0σ contour level. The α2-helix is removed for clarity. Individual cis-unsaturations of alkyl chains are indicated in green. Note that electron density is fairly weak for BbGL-2f headgroup, reflecting less than full occupancy or increased binding flexibility, whereas lipid backbone binding is very well defined. Also note how sn-1 and sn-2 alkyl chains of BbGL-2c and -2f are bound in opposite orientations inside mCD1d groove.
In striking contrast, BbGL-2f is bound in an opposite orientation. Here, the oleic acid is again bound in the A′ pocket; but because oleic acid is at the sn-2 position of the glycerol, the complete lipid backbone is rotated 180° in the binding groove (Fig. 4D). The sn-1–linked linoleic acid (18:2) is bound in the F′ pocket in a rather extended conformation. Consequently, the α-linked galactose is exposed at the CD1d surface but is oriented in a different position, as discussed below. Thus, the fatty acid composition, especially the number and position of cis-unsaturations within the fatty acids of the diacylglycerolipid backbone, directly dictates the glycolipid orientation in the CD1d binding groove. This contrasts with the binding of sphingolipids, where the orientation of the ceramide backbone is conserved with the sphingosine base in the A′ pocket, even when the alkyl chains are truncated to a length that would allow sphingolipids to bind in opposite orientations. This conserved orientation of GSL binding is manifest by comparing the orientation of α-GalCer binding with the binding by GSLs with shorter fatty acids, including GalA-GSL, with a C14 fatty acid, and PBS-25, with a C8 fatty acid (23, 27, 28). The fixed orientation for GSL binding to mCD1d is likely due to the good fit of the planar N-amide linkage of the ceramide backbone within the CD1d groove, combined with specific hydrogen bond interactions between CD1d and the hydroxyls of the sphingosine.
Comparison α-GalCer vs. BbGL-2 mCD1d Complexes.
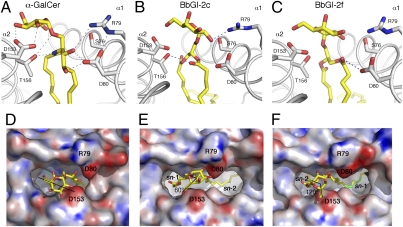
Although BbGL-2c has an α-anomeric galactose, identical to α-GalCer, the epitope it forms for TCR recognition is clearly different as a result of differences in lipid binding (Fig. 5). The glycerol moiety of BbGL-2c is tilted toward the α1 helix, whereas α-GalCer sits more perpendicular inside the mCD1d groove. Consequently, the galactose of BbGL-2c is pointed upward and away from the α2 helix, thereby losing crucial hydrogen bond (H-bond) interactions with Asp153. This interaction is known to stabilize α-GalCer and to give rise to very well–defined electron density (27, 28). Furthermore, the galactose headgroup rotates toward the α1-helix and forms a direct H-bond with Arg79 of CD1d that has not been observed in any structure of a mCD1d bound to an α-linked GSL antigen (23, 27, 28). In comparison with α-GalCer, BbGL-2c is less stabilized by H-bond interactions. BbGL-2c forms only one H-bond between the 2′-OH of galactose and Arg79, in contrast to the 2′- and 3′-OH of galactose that interact with Asp153 when α-GalCer is bound. In addition, one H-bond is formed between the oxygen atom of the sn-2 linked fatty acid with threonine 156, whereas α-GalCer has four stabilizing H-bonds to bind the ceramide backbone in a conserved orientation (Fig. 5 A and B).
Fig. 5.
Comparison of α-GalCer, BbGL-2c, and -2f binding to mCD1d. (A–C) H-bond interactions between glycolipids and mCD1d are indicated with blue dashed lines. (D–F) Glycolipid ligand presentation shown in “TCR view” from top. Electrostatic surface potentials were calculated using APBS program (36). Red is electronegative, and blue is electropositive (−30 to +30 kT/e). Important residues lining entrance to groove are labeled. Note how galactose of BbGL-2c and -2f loses binding to α2-helix of mCD1d but forms new contacts with α1-helix (mostly Arg79). Also, the lipid backbone of BbGL-2 is less stabilized by H-bond interactions compared with that of α-GalCer. BbGL-2c headgroup is rotated by 60° compared with α-GalCer and shifted to C-terminal end of α1-helix (to the right), whereas the nonantigentic BbGL-2f superimposes well with α-GalCer but is rotated 120° counterclockwise.
Binding of nonantigenic BbGL-2f is even more divergent from binding of the prototypical GSL antigen α-GalCer. As the lipid backbone is bound 180° rotated inside the CD1d groove compared with BbGL-2c, because of the differences in the fatty acid structures, the lipid backbone is now tilted more toward the α2 helix, whereas the galactose of BbGL-2f is further rotated toward the α1-helix (Fig. 5C). However, as the electron density for the headgroup of BbGL-2f is not well defined, a detailed analysis of the contacts between the galactose of BbGL-2f and CD1d would be speculative, and potential H-bonds are therefore not shown. Overall, the orientation of the galactose appears to be more flexible and dynamic in the crystal. Similar to BbGL-2c, only one H-bond is formed between the lipid backbone oxygen of sn1-linked linoleic acid and mCD1d; but in the case of BbGL-2f, this bond is formed with aspartic acid 80 in the α1 helix, instead of the H-bond with threonine 156 in the α2 helix observed with binding of BbGL-2c (Fig. 5B).
A structural comparison of α-GalCer presentation by mouse and human CD1d molecules clearly illustrates the impact of the tryptophan side chain on the galactose positioning (Fig. S1A). Furthermore, a model illustrating the glycine vs. tryptophan swap in mCD1d indicates that the current orientation of the galactose of both BbGL-2c and BbGL-2f will be affected by the bulky tryptophan side chain, which in turn would explain why BbGL-2c loses its antigenicity when presented by the mCD1dG155W mutant, whereas BbGl-2f remains a relatively weak antigen (Fig. S1B).
The most striking difference in the display of the galactose moiety to the TCR is the increased rotation of the galactose headgroup above the mCD1d groove. In addition, the galactose of BbGL-2f emerges 2.7Å closer to the N-terminal end of the α1 helix, compared with that of BbGL-2c (Figs. 5 E and F). When the CD1d-glycolipid complexes are viewed from the top (TCR view), BbGL-2c and BbGL-2f are rotated counterclockwise compared with α-GalCer by ∼60° and 120°, respectively. This rotation moves the OH-groups of the galactose by one (BbGL-2c) or two positions (BbGL-2f). That means that the 3′-OH group of BbGL-2c corresponds in structure to the disposition of the 2′OH group of α-GalCer, at least when placed in context with other OH groups. However, the galactose does not occupy the same lateral position in all three structures. It is not known what the role of the TCR is in positioning the galactose of BbGL-2c, as current models show a steric clash with CDR3α of the TCR (Fig. S2). Similar to iGb3, which we proposed is squashed flat by the TCR (29), we also postulate a repositioning of the galactose of BbGL-2c, at least slightly, which in turn could explain the overall weaker potency of BbGL-2c, compared even with GalA-GSL.
Discussion
Although the lipid portions of antigens are buried in the CD1 groove away from the TCR, there are several reports suggesting that the composition of the fatty acids, including the length, the degree of unsaturation, and the presence of other modifications, such as cyclopropyl groups (30), can strongly influence antigenic potency. For example, considering just iNKT cell recognition of CD1d molecules, an earlier report indicated that subtle changes in the lipid structure could profoundly affect the recognition of self-antigens, such as phosphatidylethanolamine, by the mouse 24.8.A iNKT cell hybridoma. Also, we previously reported that mouse and human iNKT cells are highly selective for the recognition of different, closely related α-GalDAG antigens, with the discrimination based entirely on fatty acid composition. The underlying molecular mechanism for this degree of specificity has remained elusive (31), although in principle it could be due to the selective ability of CD1d molecules to bind certain lipids. Alternatively, CD1d binding to compounds with different fatty acids might be relatively promiscuous, but the type of binding might be critical, with the fatty acid determining the orientation of the hydrophilic head group or required induced conformational changes in CD1d.
In this study we report the molecular basis of how the lipid composition of natural DAG lipids from the pathogen B. burgdorferi affects their antigenic potency. We show that CD1d binding of the borrelial α-GalDAG antigens is relatively promiscuous, in that it included some nonantigenic compounds, although α-GalDAG antigens without any unsaturated bonds did not bind under our assay conditions. Furthermore, we show that differences in the structure of CD1d rather than the TCR are likely the major determinant of fine specificity based on lipid composition in this system of natural antigens, as mouse iNKT cell TCRs could recognize the human-specific antigen BbGL-2f, when it was presented by heterologous human CD1d. Surprisingly, and unlike GSL antigens, borrelial α-GalDAG antigens can bind in two orientations, with the sn-1 acyl chain buried in either the A′ pocket, or rotated 180° and bound in the F′ pocket of CD1d. The binding orientation of the borrelial α-GalDAG antigens appears to depend exclusively on the nature of the fatty acid, especially the length and number of cis-unsaturations. Mouse CD1d clearly favors oleic acid in the A′ pocket, with fully unsaturated palmitic acid (BbGL-2c) or linoleic acid (BbGL-2f) bound in the F′ pocket. The shape of the mCD1d F′ pocket suggests that it is less restrictive and broader within the second half of the pocket (28) and, as such, presumably better suited for binding more diverse fatty acids including those with more than one cis-unsaturation. In the acidic environment of the lysosome where CD1d antigen loading occurs and where lipid exchange proteins also are found (32), it is possible that antigens undergo cycles of rebinding until they achieve the best fit with CD1d, which, in the case of mouse CD1d, would apparently favor oleic acid in the A′ pocket. Furthermore, depending on the fatty acid composition, it is possible that some antigens have a mixture of the two binding modes with the sn-1 fatty acid in the A′ pocket or the F′ pocket.
Our results further suggest that, in combination with the lipid binding orientation, a key residue, tryptophan 153 in human CD1d, equivalent to glycine 155 in mouse CD1d, influences the species-specific ability of CD1d molecules to present certain α-GalDAG antigens so that a stimulatory TCR epitope is formed, apparently in mouse CD1d by influencing the position of the exposed sugar. In the absence of a set of structures of human CD1d bound to the different α-GalDAG antigens, we remain less certain about the reason(s) for the preferential presentation of BbGL-2f by human CD1d or exactly why a human CD1d molecule with glycine at position 155 can present the Bb-GL2c antigen. Based on our findings, however, we propose a model predicting that for human CD1d the orientations of the two BbGL-2 DAG compounds would also differ by 180°, but that they would exhibit the opposite pattern, with the sn-1 fatty acid of BbGL-2f, but not BbGL-2c, loaded in the A′ pocket. This would suggest that binding of oleic acid in the A′ pocket of human CD1d is somewhat disfavored. Consistent with this model, we propose that the increased presentation of BbGL-2c by the human CD1d mutant with a glycine at position 155 might also reflect a reversal in the lipid binding orientation. Although we can only speculate as to how a CD1d α helical amino acid affects lipid loading, such an influence is at least possible, however, considering the movements of the helices that must be required for opening up the CD1d groove for lipid binding.
In conclusion, in this study we have identified a mechanism determining the chemical bases for the fine specificity of glycolipid antigen recognition based on differences in the CD1d-buried lipid, and shedding light onto the conundrum as to why only a few glycolipids within a naturally occurring, larger family of α-GalDAG compounds are recognized by iNKT cells. Furthermore, we have uncovered how one α-helical amino acid can influence the species-specific responses to antigens, based at least in part by influencing the position of the sugar. It is intriguing to speculate that the remarkable and unexpected differences in the binding orientation of DAG antigens could be used as an immune evasion strategy by certain microbes, in which predominantly nonantigenic DAG antigens could be produced under some circumstances to avoid iNKT cell activation.
Methods
Protein Expression and Purification.
Mouse and human CD1d-β2-microglobulin (β2M) heterodimeric proteins were expressed and purified essentially as reported previously for mCD1d (33).
Vα14-Vβ8.2 TCR Refolding.
TCR refolding was carried out in analogy to the published method for the human Vα24Vβ11 TCR (34) but with modifications, detailed in SI Text. The refolded TCR was either centrifuged for 30,000 x g for 10 min or directly filtered through 0.22-μM filter. DEAE Sepharose beads (GE Healthcare, 5 mL settled resin) were added to the dialyzed refolding mix and stirred for 2–4 h at 4 °C. DEAE beads were collected with 40–60 μM Buchner funnel, washed with 10 mM Tris-HCl, pH 8.0 and transferred to an Econo column (Biorad). The refolded TCR was eluted with 100 mM NaCl in 10 mM Tris-HCl, pH 8.0, diluted 4-fold with 10 mM Tris-HCl, pH 8.0, and injected into a MonoQ 5/50 GL (GE Healthcare). The TCR was eluted using a linear NaCl gradient. TCR-containing fractions were pooled, concentrated, and further purified by SEC using a Superdex S200 10/300 GL (GE Healthcare).
BirA-tagged Vα14-Vβ8.2 TCR Refolding.
We prepared a C-terminally biotinylated Vα14/Vβ8.2 TCR for surface plasmon resonance (SPR) studies (details in SI Text). Refolding was carried out similarly to the native TCR, except that an equimolar amount of α and β chains were diluted with the refolding buffer. The refolded and purified TCR was biotinylated using a commercial biotinylation kit (Avidity) and purified from free biotin by SEC on Superdex S200.
Glycolipid Loading.
Borrelia burgdorferi diacylglycerolipids (BbGL) were dissolved in 0.5% Tween, 0.9% NaCl at a concentration of 0.22 mg/mL. For crystallographic studies, BbGL-2c and BbGL-2f were dissolved in DMSO at 2 mg/mL. mCD1d was incubated overnight with 3–6 molar excess of glycolipids and subsequently purified from aggregated mCD1d and free glycolipids by gel filtration on Superdex S200. For native IEF gel electrophoresis, CD1d molecules were preloaded with a 3-fold molar excess of 3-,6-di-O-lauroyl-sulfatide (SLF2). CD1d-SLF2 complexes were purified from excess glycolipids by SEC, before incubation with a 6-fold molar excess of the individual BbGL-2 glycolipids. Exchange of SLF2 with BbGL-2 lipids was monitored on native IEF gels. Details of the synthesis of the BbGL-2 series glycolipid antigens will be reported elsewhere.
Cell-free antigen presentation assay.
Antigen presentation experiments using wild-type or point-mutated CD1d molecules and Vα14/Vβ8.2 iNKT cell hybridoma (1.2) were carried out as reported previously (16).
Surface Plasmon Resonance Studies.
SPR studies were performed using a Biacore 3000 (Biacore) according to published methods (34) but with modifications as detailed in SI Text. Biotinylated mCD1d was immobilized on a streptavidin sensor chip to measure the TCR bining kinetics toward α-GalCer and GalA-GSL, whereas biotinylated TCR was immobilized to measure the weak binding affinity toward mCD1d-BbGL-2c.
Crystallization and Structure Determination.
Both mCD1d-BbGL-2c and mCD1d-BbGL-2f complexes were buffer exchanged against 10 mM Hepes, pH7.5, 30 mM NaCl, and concentrated to 6.5 mg/mL Crystals were grown at 22.3 °C by sitting drop vapor diffusion by mixing 1 μl protein with 1 μl precipitate (17% polyethylene glycol 3350, 150 mM ammonium citrate dibasic for mCD1d-BbGL-2c or 20% polyethylene glycol 4000, 100 mM sodium citrate, pH5.5, 10% n-propanol for mCD1d-BbGL-2f).
Details for the x-ray data collection and structure determination can be found in SI Text. The mCD1d-BbGL-2c structure has a final Rcryst = 20.8% and Rfree = 25.1%, whereas the mCD1d-BbGL-2f structure has a final Rcryst = 20.5% and Rfree = 23.3%. The quality of both models (Table S1) was excellent, as assessed with the program Molprobity (35).
Supplementary Material
Acknowledgments
This work is supported in part by grants from the National Institute of Health AI 074952 (to D.M.Z.) and AI 45053, 69296 (to M.K.) and the Foundation for Research Science and Technology C08X0808 (to G.P.). D.M.Z. is recipient of an investigator award from the Cancer Research Institute, and Y.K. was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute. We thank the Stanford Synchrotron Radiation Laboratory, especially support staff for beamlines 7.1 and 9.2, for kind assistance and access to remote data collection.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Coordinates and structure factors for the mouse CD1d-BbGL-2c and mouse CD1d-BbGL-2f complexes have been deposited in the Protein Data Bank under accession codes 3ILQ and 3ILP, respectively.
This article contains supporting information online at www.pnas.org/cgi/content/full/0909479107/DCSupplemental.
References
- 1.Calabi F, Milstein C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986;323:540–543. doi: 10.1038/323540a0. [DOI] [PubMed] [Google Scholar]
- 2.Moody DB, et al. T cell activation by lipopeptide antigens. Science. 2004;303:527–531. doi: 10.1126/science.1089353. [DOI] [PubMed] [Google Scholar]
- 3.Brigl M, Brenner MB. CD1: Antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 4.Van Rhijn I, et al. CD1c bypasses lysosomes to present a lipopeptide antigen with 12 amino acids. J Exp Med. 2009;206:1409–1422. doi: 10.1084/jem.20082480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 6.de la Salle H, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 7.Moody DB, Zajonc DM, Wilson IA. Anatomy of CD1-lipid antigen complexes. Nat Rev Immunol. 2005;5:387–399. doi: 10.1038/nri1605. [DOI] [PubMed] [Google Scholar]
- 8.Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zajonc DM, Wilson IA. Architecture of CD1 proteins. Curr Top Microbiol Immunol. 2007;314:27–50. doi: 10.1007/978-3-540-69511-0_2. [DOI] [PubMed] [Google Scholar]
- 10.Zajonc DM, Kronenberg M. Carbohydrate specificity of the recognition of diverse glycolipids by natural killer T cells. Immunol Rev. 2009;230:188–200. doi: 10.1111/j.1600-065X.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 11.Bendelac A. Mouse NK1+ T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 12.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 13.Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 14.Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 15.Tupin E, et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci USA. 2008;105:19863–19868. doi: 10.1073/pnas.0810519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinjo Y, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi . Proc Natl Acad Sci USA. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidobre S, et al. The V α 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J Immunol. 2002;169:1340–1348. doi: 10.4049/jimmunol.169.3.1340. [DOI] [PubMed] [Google Scholar]
- 19.Cantu C, 3rd, Benlagha K, Savage PB, Bendelac A, Teyton L. The paradox of immune molecular recognition of α-galactosylceramide: Low affinity, low specificity for CD1d, high affinity for α β TCRs. J Immunol. 2003;170:4673–4682. doi: 10.4049/jimmunol.170.9.4673. [DOI] [PubMed] [Google Scholar]
- 20.Pellicci DG, et al. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity . 2009;31(1):47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 22.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 23.Wu D, et al. Design of natural killer T cell activators: Structure and function of a microbial glycosphingolipid bound to mouse CD1d. Proc Natl Acad Sci USA. 2006;103:3972–3977. doi: 10.1073/pnas.0600285103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 25.Brossay L, Kronenberg M. Highly conserved antigen-presenting function of CD1d molecules. Immunogenetics. 1999;50:146–151. doi: 10.1007/s002510050590. [DOI] [PubMed] [Google Scholar]
- 26.Giabbai B, et al. Crystal structure of mouse CD1d bound to the self ligand phosphatidylcholine: A molecular basis for NKT cell activation. J Immunol. 2005;175:977–984. doi: 10.4049/jimmunol.175.2.977. [DOI] [PubMed] [Google Scholar]
- 27.Koch M, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. 2005;8:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 28.Zajonc DM, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;8:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zajonc DM, Savage PB, Bendelac A, Wilson IA, Teyton L. Crystal structures of mouse CD1d-iGb3 complex and its cognate Valpha14 T cell receptor suggest a model for dual recognition of foreign and self glycolipids. J Mol Biol. 2008;377:1104–1116. doi: 10.1016/j.jmb.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinjo Y, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rauch J, et al. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: Stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu Rev Cell Dev Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- 33.Zajonc DM, et al. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjer-Nielsen L, et al. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–673. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovell SC, et al. Structure validation by Calpha geometry: ϕ,ψ and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 36.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.