Abstract
An imbalance in reducing and oxidizing (redox) systems favoring a more oxidative environment is present in asthma and linked to the pathophysiology of the defining symptoms and signs including airflow limitation, hyper-reactivity, and airway remodeling. High levels of hydrogen peroxide, nitric oxide (•NO), and 15-F2t-isoprostane in exhaled breath, and excessive oxidative protein products in lung epithelial lining fluid, peripheral blood, and urine provide abundant evidence for pathologic oxidizing processes in asthma. Parallel studies document loss of reducing potential by nonenzymatic and enzymatic antioxidants. The essential first line antioxidant enzymes superoxide dismutases (SOD) and catalase are reduced in asthma as compared to healthy individuals, with lowest levels in those patients with the most severe asthma. Loss of SOD and catalase activity is related to oxidative modifications of the enzymes, while other antioxidant gene polymorphisms are linked to susceptibility to develop asthma. Monitoring of exhaled •NO has entered clinical practice because it is useful to optimize asthma care, and a wide array of other biochemical oxidative and nitrative biomarkers are currently being evaluated for asthma monitoring and phenotyping. Novel therapeutic strategies that target correction of redox abnormalities show promise for the treatment of asthma. Antioxid. Redox Signal. 12, 93–124.
I. Introduction
Asthma is a chronic inflammatory disorder of the airways involving interaction of cells and mediators that ultimately result in high levels of reactive oxygen and nitrogen species (ROS, RNS) (92, 113, 131, 217). A wealth of studies identify that ROS and RNS and loss of antioxidant defenses participate in the pathogenesis of asthma. The measurement of one quantitative biomarker of RNS, nitric oxide (•NO), has entered clinical practice. In addition to elevated production of •NO, eosinophil-mediated oxidative tissue injury and bioactive lipid oxidation products are also characteristic features of asthma (239). Increased ROS and RNS lead to modifications of proteins and alterations in their function that are biologically relevant to the initiation and maintenance of inflammation, among which is the loss of antioxidant capacity of the superoxide dismutases (SOD) that catalyze the reaction of superoxide to hydrogen peroxide and catalase that catalyzes hydrogen peroxide to water. This review will chronicle the cumulative information gathered on redox abnormalities in asthma over the last three decades. Following an overview of redox and specific redox processes in the lung, redox changes in asthma and the consequences on molecular processes and protein chemistry are detailed. Finally, clinical use of biomarkers of redox state for asthma phenotyping and guiding standard therapy, and the potential for antioxidant therapeutics to reduce oxidative processes and/or their consequences is addressed.
II. Redox Reactions Form the Basis for Aerobic Life
Cellular respiration is the quintessential reduction–oxidation (redox) reaction in aerobic organisms. Cellular respiration takes place within the mitochondria and is fundamental for production of the energy that is required to maintain the ordered state of the cell. Hence, redox reactions form the basis for the most important physiologic process that takes place in healthy cells. Simply defined, oxidation is the loss of electrons and reduction is the gain of electrons. However, most oxidation reactions in cells are accomplished by the removal of hydrogen atoms. In cell respiration, glucose loses electrons in H atoms and serves as the electron donor, while oxygen is the terminal electron acceptor.
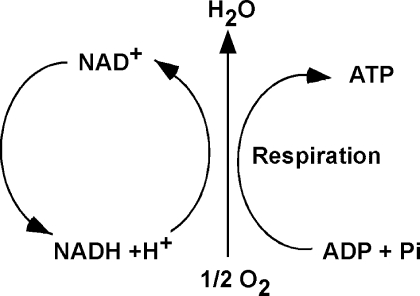
Generally, redox reactions are tightly regulated and occur in multiple steps, in which the electrons are shuttled by carriers, also called redox couples. Common redox couples include NAD+/NADH, NADP+/NADPH, and reduced to oxidized glutathione (GSH/GSSG) (Fig. 1). Chance et al. pioneered the study of oxidation and reduction states of proteins in the respiratory (electron transport) chain of various organs (50). Later, Bucher and co-workers developed experimental approaches to estimate the intracellular reduction potential by determining the ratio of NAD+/NADH and NADP+/NADP (32, 302). Subsequently, Buettner et al. suggested that the redox environment in cells, tissues, or in biological fluids might be defined by the reduction potential and reducing capacity of the redox couples present (33). In general, the ratio of the interconvertible oxidized and reduced form of a specific redox couple is used to define the redox environment in biologic systems (302).
FIG. 1.
Nicotinamide adenine dinucleotide (NAD+) functions in electron transfer reactions (redox) reactions. NAD+ acts as the oxidizing agent; it accepts electrons and become reduced to NADH. Subsequently, NADH serves as a reducing agent and donates electrons. Thus, NAD+ and NADH serve as a redox couple, as they accept and donate electrons in redox reactions, such as occur in cellular respiration. Multiple redox reactions constitute cellular respiration, in which oxygen is the terminal electron acceptor, and ATP is synthesized.
III. Redox Systems in the Lung
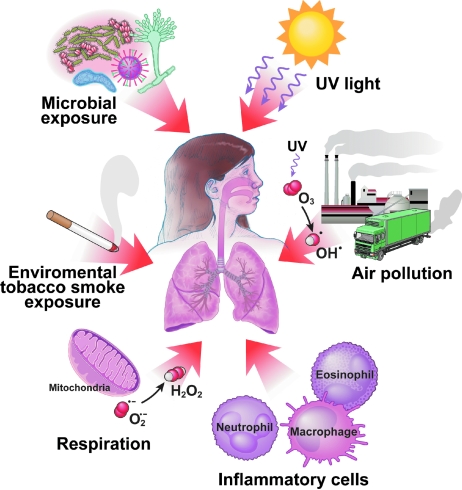
Oxygen is one of the most abundant elements in our world, constituting 21% of the air we breathe. The abundant supply of oxygen to aerobic organisms enables it to serve as a high capacitance acceptor for electrons. Furthermore, oxygen can damage cells by production of byproducts of respiration or by production of reactive nitrogen and oxygen species (RNS; ROS) (60). Thus, delivery of oxygen to human tissues is tightly regulated by the allosteric binding of oxygen to hemoglobin in red blood cells (113). However, the lungs are unique in having a vast moist mucosal epithelial surface area that is immediately and directly exposed to inhaled oxygen (and airborne reactive pollutants), which dissolve into the epithelial surface lining fluid. This makes the lungs particularly susceptible to environmental oxidant-mediated injury. Furthermore, the lung is exposed to a multitude of airborne microorganisms, and thus also endogenously generates high levels of RNS and ROS to maintain a remarkably sterile lower airway. Altogether, endogenous production of RNS and ROS by metabolic reactions (respiration, phagocytosis) and environmental exposures (air pollutants, cigarettes smoke. particulates) might be expected to produce an oxidizing lung environment (Fig. 2). However, redox state in the healthy lung is primarily reducing. This is attributed to the multiplicity and abundance of antioxidant systems available to the lung. The vast excess of reduced substances over oxidized ones is maintained by a rich array of antioxidant enzymatic and nonenzymatic effectors on the surface of, and within, the epithelial cells in the airways (176).
FIG. 2.
Sources of exogenous inhalational and endogenous reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the lung. Environmental sources leading to greater amounts of ROS and RNS in the lungs are ozone, air pollutants (particulates as from diesel fuel combustion), particulates containing metals, and cigarette smoke. Endogenous ROS are produced as byproducts of mitochondrial respiration. Inflammatory cells can produce high levels of ROS and RNS in response to allergens and microbial infections. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
A. ROS and RNS production in the lung
Many specific classes of lung cells have recently been compared for their capacity to generate ROS in the context of oxidant-induced lung injury, including tracheal epithelial cells, alveolar epithelial type I and type II cells, Clara cells, and vascular endothelial cells. While inflammatory cells such as neutrophils generate highest levels of ROS, alveolar macrophages and eosinophils are also high level producers of ROS.
1. Endogenous reactive oxygen species
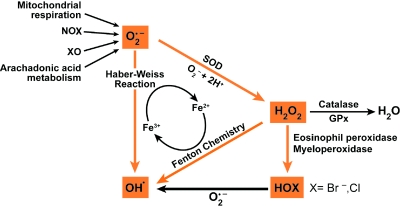
Reactive oxygen species include superoxide, hydrogen peroxide and hydroxyl radicals and can be generated by a number of metabolic pathways and are dangerous byproducts of oxygen consumption (Fig. 3).
FIG. 3.
Production of reactive oxygen species (ROS). Superoxide (O2•) reacts rapidly with itself, or is catalytically converted by superoxide dismutases (SOD), to form hydrogen peroxide (H2O2). Hydrogen peroxide is detoxified to water by catalase or glutathione peroxidase enzymes (GPx). Extremely toxic reactions of superoxide and hydrogen peroxide that form hydrogen radical occur via the Haber–Weiss and Fenton chemistry reactions in the presence of metal ions. Hydrogen peroxide is converted by myeloperoxide (MPO) or eosinophil peroxidase (EPO) to highly reactive halogenating acids, such as hypobromous acid (HOBr) or hypochlorous acid (HOCl), xanthine oxidase (XO). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
a. Superoxide
The tetravalent reduction of oxygen during mitochondrial electron transport is a safe process but also can result in formation of superoxide (O2•−) (60, 108, 132, 133, 272). Another source for intracellular generation of O2•− is the NADPH oxidase enzymatic system, which is found in neutrophils, monocytes, and macrophages (15, 51, 66, 81). O2•− can also be generated by mechanisms such as molybdenum hydroxylase reactions (including the xanthine, sulfite, and aldehyde oxidases) and arachidonic acid metabolism (60, 124). O2•− is unstable, with a half-life of milliseconds. Because it is charged, it does not easily cross cell membranes (21). O2•− will react, however, with proteins that contain transition-metal prosthetic groups, such as heme moieties or iron–sulfur clusters. These reactions may damage amino acids or cause protein/enzyme function to be lost (112, 356).
b. Hydrogen peroxide (H2O2)
Under biological conditions, the main reaction of superoxide is to react with itself to produce hydrogen peroxide and oxygen, a reaction known as “dismutation” (Reaction 1) (228). Superoxide dismutation can be spontaneous or can be catalyzed by the enzymes superoxide dismutases (SOD).
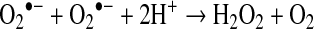 |
Reaction 1 |
H2O2 can also be produced by oxidase enzymes, including xanthine oxidase, monoamine, and amino acid oxidase (60). Once formed, the oxidizing potential of H2O2 may be amplified by eosinophil and neutrophil derived peroxidases, eosinophil peroxidase (EPO) and myeloperoxidase (MPO), respectively (103, 135, 184, 341) (Reaction 2).
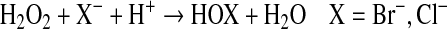 |
Reaction 2 |
The capacity to generate H2O2 varies among cell types. Kinnula et al. has shown that alveolar macrophages produce high levels of H2O2. Type II cells have the capacity to release an excessive amount of H2O2 whereas endothelial cells produce low amounts of H2O2 (178). Interestingly, the rate of inactivation of catalase via H2O2 production is the highest in Type II cells (178). This confirms that the generation of H2O2 depends upon resident and inflammatory cells in the lung.
c. Hydroxyl radical (•OH)
The hydroxyl radical is an extremely reactive oxidizing radical that will react to most biomolecules at diffusion controlled rates (54), which indicates that reactions occur nearly immediately with biomolecules. The hydroxyl radical is several orders of magnitude more reactive towards cellular constituents than superoxide radicals, and many orders more reactive than hydrogen peroxide. Much of the damage done by superoxide and H2O2 in vivo is due to their production of hydroxyl radicals (•OH) in a series of reactions catalyzed by traces of transition metal ions (60). In these reactions, superoxide acts as the reducing agent. The reduced metal catalyzes the breaking of the oxygen-oxygen bond of hydrogen peroxide to produce a hydroxyl radical (•OH) and a hydroxide ion (HO−). The classic example is the iron-catalyzed Haber–Weiss Reaction in which Fe3+ is reduced to Fe2+, followed by the Fenton Reaction in which the Fe2+ catalyzes the transformation of H2O2 into hydroxyl radical (•OH) (133).
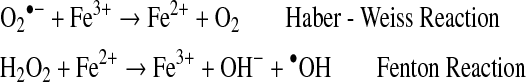 |
An alternative pathway for •OH formation in vivo may involve myeloperoxidase (MPO) and eosinophil peroxidase (EPO). Under physiological concentrations of halides, MPO produces hypochlorous acid (HOCl) and EPO produces hypobromous acid (HOBr). Studies of •OH with spin-trapping agents and chemical trap (138, 267) have demonstrated that hypohalous acids can generate •OH after reacting with O2•− (Reaction 3). •OH can react with different molecules such as protein (38), DNA, and lipids (111).
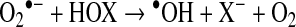 |
Reaction 3 |
d. Protein modifications by MPO and EPO
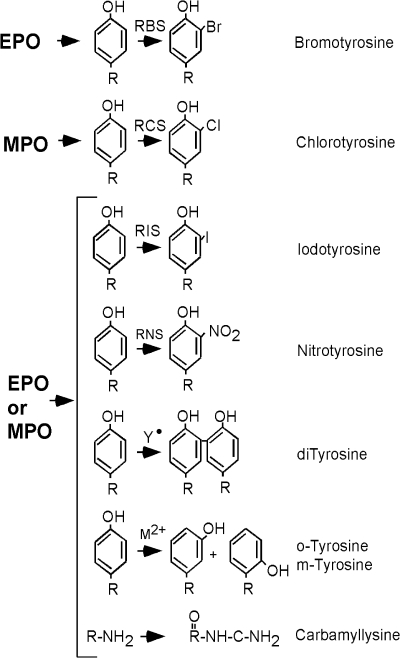
Influx of inflammatory cells, which contain, enzymatic systems such as EPO and MPO (Reaction 3) can produce ROS. EPO and MPO are enzymes that accelerate oxidative protein modifications. EPO selectively uses Br− (bromide) to form HOBr (hypobromous acid) (Reaction 2) (226, 341). EPO is the only human enzyme that selectively generates reactive brominating species, thus brominated products serve as fingerprints of atopic/eosinophilic inflammation. MPO is the most abundant protein stored in neutrophil granules, and secreted during cell activation (185). MPO selectively uses Cl− as substrate to generate HOCl (hypochlorous acid) (103, 341) (Reaction 2). These enzymes are secreted by inflammatory cells and produce protein oxidative damage through the production of reactive brominating species (RBS), reactive chlorinating species (RCS), and reactive nitrogen species (RNS). Specific brominated and chlorinated targets in plasma serve as signatures for EPO- and MPO-dependent, i.e. eosinophil- and neutrophil-dependent, oxidative injury (Fig. 4).
FIG. 4.
Amino acid oxidation products and cross-links formed by peroxidase enzymes. Protein oxidative damage mediated by EPO-generated reactive brominating species (RBS), MPO-generated reactive chlorinating species (RCS), reactive nitrating species (RNS), tyrosyl radical (oY), transition metal ions (M2+) that form hydroxyl radical, may be identified by stable products formed by each pathway. All are tyrosine derivatives, except the nonphysiologic o-tyrosine and m-tyrosine that form from the oxidation of phenylalanine.
2. Reactive nitrogen species
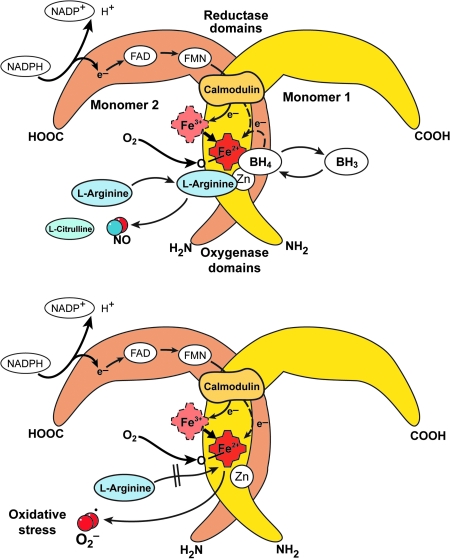
The RNS synthesized in the lung is nitric oxide (•NO) which is produced by nitric oxide synthases [NOS, EC 1.14.13.39] (321). All NOS convert L-arginine to NO and L-citrulline in a reaction that requires dimeric enzyme, oxygen, NADPH, and cofactors flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), tetrahydrobiopterin, calmodulin, and iron protoporphyrin IX. There are three forms of NOS, the inducible NOS (iNOS or NOS2), neuronal NOS (nNOS or NOS1), and endothelial NOS (eNOS or NOS3) (Table 1). Active NOS are dimeric, and each monomer is comprised of an N-terminus oxygenase domain that binds the heme, tetrahydrobiopterin, and substrate L-arginine. The carboxy terminus of NOS monomers bind the flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and NADPH (320). In general, nNOS and eNOS are constitutively expressed in neuronal and endothelial cells, respectively, dependent on increases in calcium to bind calmodulin that results in enzyme activation and picomolar levels of NO production (91, 318–321). Immunohistochemical studies reveal the presence of the three isoforms of NOS in the airway. NOS III is primarily localized to pulmonary endothelial cells, and NOS I in nonadrenergic, noncholinergic inhibitory neurons (19, 113). NOS II is continuously expressed in normal human airway epithelium at basal airway conditions (19, 113, 127, 128, 187, 320). NO is also produced by the upper respiratory tract epithelium within the nasopharynx and paranasal sinuses, most likely by NOS II (211). There is evidence that epithelial NOS II activity is a major determinant of NO present in exhaled breath (196). The iNOS is regulated at the level of transcription and mRNA stability, is calcium independent, and produces nanomolar levels of NO (320, 322). Regulation of iNOS expression varies in different cell types, but typically is increased by cytokines and pro-inflammatory factors, interferon gamma, TNF-alpha, and IL1-beta (126, 127). NO synthesis by iNOS is also regulated by availability of substrate arginine and cofactor tetrahydrobiopterin.
Table 1.
Different Forms of Nitric Oxide (NO) Synthesis
| Isoform | Chromosomal localization | Expression | Activity |
|---|---|---|---|
| nNOS | NOS1:12q24.2 | Constitutive | Ca2+ dependent |
| iNOS | NOS2:17cen-q12 | Inducible | Ca2+ independent |
| eNOS | NOS3:7q35-36 | Constitutive | Ca2+ dependent |
The carboxy-reductase domain transfers electrons to the heme iron of the oxygenase domain, which then binds oxygen and oxidizes arginine to generate NO and citrulline (320). Since the early 1990's, NOS have also been shown to generate superoxide by spin trapping/EPR spectroscopy and H2O2, which is presumed to derive from superoxide dismutation (271). This occurs when NADPH is oxidized by the enzyme in the absence of L-arginine (270). Thus, conditions that decrease arginine availability to NOS will lead to greater superoxide formation. Arginase, a critical enzyme in the urea cycle, converts arginine to ornithine and urea. There are two isoforms, arginase 1 and arginase 2, both of which play a regulatory role in •NO and superoxide synthesis by modulating the availability of arginine for NOS (151, 349) (Fig. 5).
FIG. 5.
Nitric oxide synthases (NOS). General schematic for all active NOS shows that the dimeric enzymes are comprised of two monomers. The oxygenase domains of two subunits interact to form the homodimer. NOS convert L-arginine to NO and L-citrulline in a reaction that requires oxygen, NADPH, and cofactors FAD, FMN, tetrahydrobiopterin, calmodulin, and iron protoporphyrin IX. The N-terminus oxygenase domain of each monomer binds the heme, tetrahydrobiopterin, and substrate L-arginine. The carboxy terminus of each monomer binds the FAD, FMN, and NADPH. The carboxy-reductase domain of one monomer transfers electrons from NADPH to FAD to FMN and ultimately to the oxygenase domain ferric heme iron of the other monomer, which then binds oxygen and oxidizes L-arginine to generate NO and citrulline. NO synthesis is regulated by availability of substrate L-arginine and cofactor tetrahydrobiopterin. In ‘coupled’ NOS, tetrahydrobiopterin enables electrons from NADPH to be used for NO synthesis. In ‘uncoupled’ NOS, oxygen reduction occurs but results in superoxide or H202 release instead of NO.
The unpaired electron of •NO makes it highly reactive (311). Since •NO is freely diffusible, consumption of •NO can occur at different sites within the cell, extracellular fluids, and intravascular compartments (195). The diffusion of NO may be most limited by its many possible chemical reactions (87, 342) (Fig. 6). When metabolized, •NO gives rise to a group of compounds collectively known as the reactive nitrogen species (RNS) that possess their own unique characteristics. In biologic systems, up to 40% of the NO synthesized may be consumed by chemical reactions (87, 343). Autooxidation of •NO with O2 results in the formation of nitrite (NO2−). NO2− is also a substrate for hemeperoxidases such as MPO and EPO. Peroxidase-catalyzed oxidation of NO2− results in the formation of nitrogen dioxide radical (NO2•) or related molecules (1–3, 29). These substances contribute to the nitration of phenolic compounds, such as tyrosine, to form dimerized (dityrosine) and nitrated (3-nitrotyrosine) products, which are stable.
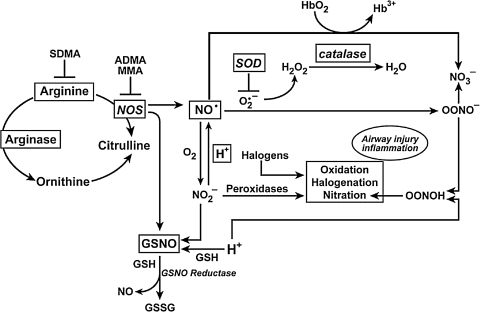
FIG. 6.
Redox chemistry in the lung. Levels of NO and other nitrogen oxides, superoxide, and other reactive oxygen species, are regulated both enzymatically and by nonenzymatic reactions. Arginase enzymes serve as a metabolic branch point controlling the flow of L-arginine to protein synthesis, NO synthesis, and ornithine and urea cycle. Arginase activity is increased in asthmatic lungs. Ornithine is a precursor for polyamines and proline for cell proliferation and collagen synthesis, respectively, critical components of airway remodeling. Once formed, NO may react rapidly with O2•− yielding ONOO-. Following ONOO- protonation, ONOOH can nitrate tyrosine (Tyr-NO2) or convert to NO3-. NO2- formation from NO is slow. Rather, NO2− protonation to form NO is favored in the increased acidity that is present in the asthmatic airway. NO3− is present at higher than normal levels in the oxidizing acidic environment of the asthmatic lung, but NO2*#x2212; is similar in asthmatic and control lungs. NO2- is also consumed in leukocyte peroxidase (EPO and MPO) catalyzed reactions, which also generate halogenating reactive species. Nitrosoglutathione (GSNO) is a beneficial endogenous bronchodilator that is catabolized by GSNO reductase to release NO.
Nitrite was previously considered an end-product of NO, but studies now indicate that nitrite can be recycled to generate bioactive NO. Nitrite reduction to NO occurs in blood and tissues by many mechanisms, such as by xanthine oxidases or reaction with hydrogen ions. Thus, nitrite can serve as a storage pool for NO production at times when NO synthases may be unable to function. •NO is also rapidly oxidized by reaction with oxyhemoglobin (HbO2), resulting in formation of methemoglobin (Hb3+) and NO3− (8). The rapid reaction of •NO with free radicals (radical–radical reaction) has emerged as one of the major routes to the formation of RNS (8). •NO reacts with superoxide to form peroxynitrite (ONOO−). ONOO− can nitrate tyrosine residues and alter levels or function of enzymes, structural and signaling proteins (13, 120, 224). Tyrosine nitration can cause either gain or loss of protein function (13). On average, proteins are composed of 4% tyrosine residues, but chemical nitration of isolated proteins modifies only a subset of tyrosine residues, and the basis for this selectivity is not fully understood. This suggests that an innate property of the target protein or its location may predispose it toward nitration (13). In acid environments, ONOO− can be protonated to yield peroxynitrous acid (ONOOH), which rapidly decomposes to NO3− via the intermediate formation of OH• and NO2-like species. ONOOH can also react with thiol residues to form S-nitrosothiols (SNO), which have been proposed as a potential unique signaling mechanism induced by nitrosative stress (149). The exact mechanism by which S-nitrosation occurs in vivo is still unclear, but it involves the formation of •NO-derived intermediates with the redox equivalence of NO+ (the primary candidates are N2O3 and ONOOH) and (di)nitrosyl iron complex (113, 273).
3. Environmental exposures
Because the lung interfaces with the external environment, it is frequently exposed to airborne oxidant gases and particulates, and thus prone to oxidant-mediated cellular damage.
a. Atmospheric ozone (O3) and particulate matter pollution
Ozone, a component of photochemical air pollution, is formed from volatile hydrocarbons, halogenated organics, and oxides of nitrogen in the presence of sunlight (244). Ambient ozone levels usually vary between 20 and 40 parts per billion (ppb); moderate elevations in levels are usually 70–120 ppb (335). There is a great deal of evidence which shows that high concentrations of ozone can be harmful to the lung (73, 165, 190, 239, 242, 244, 261). Ozone can react directly with unsaturated fatty acids and cell membranes to produce lipid ozonation products, which are small, diffusible, and relatively stable (169, 170, 202). Particulate matter pollution is one of the most serious air pollution problems in urban environments (56). The size of the particle is very important since it will determine where the particle will come to rest in the respiratory tract when inhaled (56). One of the most dangerous forms of particulate matter pollution is diesel exhaust particle. Diesel exhaust particles are a polyaromatic hydrocarbon, a hydrophobic molecule that can diffuse easily through cell membranes. Diesel exhaust particles may therefore modify cell growth and differentiation (56).
b. Cigarette smoke and environmental tobacco smoke
Environmental tobacco smoke or secondhand smoke is a complex mixture of gases and particles that include smoke from the burning cigarette (sidestream smoke) and exhaled mainstream smoke. Environmental tobacco smoke contains a large number of components, and many of them are toxic to epithelial cells. Cigarette smoke contains >4,000 chemicals and poisons, including 50 that are known to cause cancer. Some of the chemicals in cigarette smoke are carbon monoxide, cyanide, arsenic, mercury, and NO. Furthermore, cigarette smoke generates or contains ∼1014 oxidative molecules per puff such as hydrogen peroxide and superoxide. Furthermore, environmental tobacco smoke leads to activation of phagocytes augmenting release of free radicals. Because free radicals cause oxidative damage to macromolecules such as DNA, lipids, and protein, they are believed to be involved in the pathogenesis of many diseases (333).
4. Oxidative processes in biology
The formation of ROS and RNS is an essential prerequisite for neutrophils, monocytes, macrophages, and eosinophils to kill certain bacteria. These phagocytic cells use NADPH oxidase enzymatic systems to generate O2•− directly as part of their armamentarium against invading microorganisms (15, 51, 66, 81). They can also form HOCl through myeloperoxidase-catalyzed oxidation of the Cl− ion by H2O2 (21). •NO is also involved in mononuclear cell-mediated killing of Mycobacterium tuberculosis and other pathogens in rodents and is toxic to tumor cell lines in vitro (252). In the upper respiratory tract of humans, NO appears to be important in maintaining ciliary function and may have a role in sterilizing the mucosa. The heme protein cytochrome P450 catalyzes a series of reactions that detoxify lipid-soluble drugs and toxic metabolic byproducts. This enzyme uses high-energy electrons transferred from NADPH to add hydroxyl groups to potentially harmful hydrophobic hydrocarbons dissolved in the lipid bilayer (88). Such reactions convert water-insoluble drugs or metabolites that would otherwise accumulate in cell membranes into water-soluble compounds, which then diffuse out of the cell and are excreted in the urine. Cytochrome P450 also exploits the reactivity of the iron–oxygen complex to catalyze oxidation of a number of endogenous compounds and xenobiotics (21). These examples show that ROS and RNS play important physiologic functions and yet can also cause extensive damage. Tissue health is maintained under physiologic conditions by antioxidants.
B. Antioxidants in the lung
The balance between physiologic functions and damage is determined by the relative rates of formation and the removal of ROS and RNS, and free radicals. All aerobic organisms use a series of primary antioxidant defenses to protect against oxidative damage. An antioxidant is most simply defined as a molecule capable of slowing down or preventing redox changes in the cell.
The lungs have developed several endogenous antioxidant systems to deal with the production of free radicals. These systems may be divided into enzymatic and nonenzymatic groups. The enzymatic antioxidants include superoxide dismutases (SOD), catalase, glutathione peroxidases, heme oxygenase, glutaredoxin, thioredoxin, and peroxiredoxin. These antioxidant enzymes usually require trace metal cofactors (109). SOD, for example, consists of proteins co-factored with copper, zinc, or manganese (109). Iron is required as a co-factor for catalase (218). The most well-researched nonenzymatic antioxidants include lipid-soluble vitamin E (tocopherol), vitamin A, and carotenoids (including beta-carotene), and water-soluble vitamin C and glutathione (GSH). Glutathione, which is synthesized intracellularly from amino acids cysteine, glycine, and glutamate, is capable of scavenging free radicals either directly or enzymatically via glutathione peroxidase. In addition, GSH is crucial to the maintenance of enzymes and other cellular components in a reduced state (59–62).
1. Nonenzymatic lung antioxidants
The nonenzymatic antioxidants can be classified depending whether they are hydrophilic or hydrophobic. In general, hydrophilic antioxidants react with oxidants in the cell cytosol and/or bloodstream, whereas the hydrophobic antioxidants protect the cell membranes from lipid peroxidation. Nonenzymatic antioxidants react directly with the oxidants. Such antioxidants are said to be ‘scavengers;’ their roles are unavoidably suicidal.
a. Vitamin E (alpha-tocopherol)
Vitamin E is an important hydrophilic antioxidant. It protects the cell membrane from oxidation by reacting with lipid radicals, such as lipid peroxyl radicals (LOO•) that are produced during lipid peroxidation reactions (233, 336). Alpha-tocopherol is the predominant form of vitamin E in tissues and the primary form in supplements. However, gamma-tocopherol is the major form of vitamin E in plant seeds and in the US diet, yet has drawn little attention compared with alpha-tocopherol. Recent studies indicate that gamma-tocopherol may be important to human health. Gamma-tocopherol appears to be a more effective trap for lipophilic electrophiles than is alpha-tocopherol (162).
b. Vitamin C (ascorbic acid)
Vitamin C is a hydrophilic vitamin that can directly scavenge O2•− and •OH by forming the semidehydroascorbate free radical that subsequently is reduced by GSH (227). Vitamin C, however, is usually not considered a major antioxidant because it also has pro-oxidant properties. It is probably the only cellular reducing agent other than O2•−capable of converting Fe3+ to Fe2+, which then reacts with H2O2 to form •OH (291). Whether the pro-oxidant or antioxidant properties of vitamin C prevail in any particular tissue is determined by the extent of available iron stores; iron overload favors excess oxidant generation (21, 291).
c. Glutathione
Glutathione (GSH) is the predominant nonprotein thiol in the cells and is important for maintenance of the cellular redox (302). GSH is a cysteine-containing peptide found in most forms of aerobic life, and is present in high concentration in blood and lung (39–41, 58, 62). Independent of the GSH system (see later), free GSH can function as a water-soluble antioxidant by interacting directly with radical intermediates in nonenzymatic catalyzed reactions. Lung epithelial lining fluid contains up to 300 micromolar concentration of GSH (290), and >90% of the GSH is maintained in the reduced form. Scavenging of O2•− by GSH leads via several steps to the formation of thiyl radicals (GS•) and H2O2, which is a radical propagation reaction (21, 113). Increased intracellular GSH is a response to oxidative stress (59, 278), and a critical determinant of cellular tolerance to oxidizing environments (277). Reactive oxygen species increase GSH through induction of γ-glutamyl cysteine synthetase, the rate-limiting enzyme of GSH biosynthesis (281). Uptake of GSH into cells (84, 230), and export of the oxidized form to overcome an accumulation of GSSG within the cytosol occurs rapidly in conditions of oxidative stress (59).
Other nonenzymatic antioxidants include β-carotene (scavenger of superoxide anions and peroxyl radicals), uric acid (hydroxyl radical, superoxide, peroxyl radical scavenger), bilirubin (lipid peroxyl radical scavenger), taurine (hypochlorous acid quencher), albumin (transition metal binding, glutathione precursor and hydrogen peroxide scavenger), and cysteine and cysteamine (donators of sulfhydryl groups).
2. Enzymatic lung antioxidants
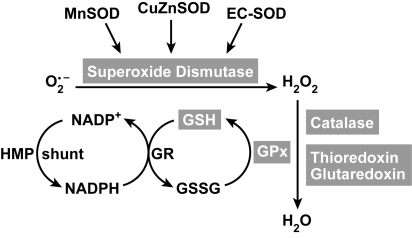
The detoxification pathway of superoxide to water is a result of multiple enzymatic antioxidants (Fig. 7). The major enzymatic antioxidants are discussed below.
FIG. 7.
Antioxidants in redox reactions. Superoxide can be detoxified by superoxide dismutases (SOD). There are three forms: an intracellular CuZnSOD, mitochondrial MnSOD, and an extracellular EC-SOD. Hydrogen peroxide (H202) can be further detoxified to water by catalase, thioredoxin (TRX), glutoredoxin (GRX) and/or by the glutathione peroxidase (GPx). TRX, GRX and GPx use glutathione as a cofactor. The oxidized glutathione (GSSG) is subsequently returned to GSH by glutathione reductase, an intracellular enzyme that uses NADPH generated from the hexose monophosphate shunt system (HMP shunt) as an electron donor.
a. Superoxide dismutases (SOD)
Superoxide dismutases (EC 1.15.1.11) are ubiquitous enzymes with an essential function in protecting aerobic cells against oxidative stress and are essentially present in every cell in the human body. They catalyze the reaction of superoxide radicals to hydrogen peroxide. Superoxide dismutase enzymes contain metal ion cofactors that, depending on the isozyme, can be copper–zinc, manganese, or iron. Human lung epithelium expresses three forms of eukaryotic SODs that are located on three different chromosomes (Table 2). The distribution of the three SOD isoforms in the lung has been reviewed previously (177), with CuZnSOD expression in bronchial epithelium, alveolar epithelium, mesenchymal cells, fibroblasts, arterioles, and capillary endothelilal cells (98, 193, 266). MnSOD is expressed in the airways, especially in the septal tips of alveolar duct and arterioles near the airways (57). Furthermore, MnSOD is also moderately or highly expressed in respiratory epithelium, alveolar type II epithelial cells, and alveolar macrophages (65, 194). EC-SOD is found in bronchial epithelium, alveolar epithelium, epithelial cells lining intrapulmonary airways, alveolar macrophages, and endothelial cells lining both arteries and veins (256, 257).
Table 2.
Superoxide Dismutases in Human Cells
| Enzyme | Chromosomal localization | Catalytic metal ion | Localization | Inhibitors |
|---|---|---|---|---|
| CuZnSOD | SOD1:21q22.1 | Cu2+ | Cytosol | H2O2 cyanide |
| MnSOD | SOD2:6Q25.3 | Mn2+ | Mitochondria | – |
| ECSOD | SOD3:4p15.3-p15.1 | Cu2+ | Extracellular | H2O2 cyanide |
The copper–zinc superoxide dismutase (CuZnSOD) protein constitutes up to 80–90% of the intracellular SOD activity and is mainly found in the cytosol, although it also is present at low levels in lysosomes, peroxisomes, nucleus, and intermembrane space of the mitochondria (72). CuZnSOD is expressed in lung cells, such as bronchial epithelial, alveolar macrophages, and capillary endothelium of the lung (53, 63, 82). The gene located on chromosome 21q22.1 gives rise to a 16 kDa protein, each containing a catalytic Cu2+ metal ion which bridges via a histidine residue to a Zn+ ion (20, 109). Active CuZnSOD is a homodimeric protein and accelerates the spontaneous dismutation of superoxide radical by >40-fold through the cyclic oxidation–reduction of its Cu2+ metal ion (109). The reactions are very fast, and do not require reducing equivalents, enabling the reaction to proceed in the absence of any energy input.
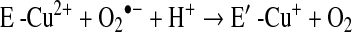 |
Reaction 4 |
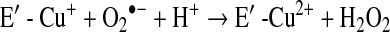 |
Reaction 5 |
In addition to this reaction, CuZnSOD may have peroxidase activity (310). At high levels, hydrogen peroxide reduces the Cu2+ to produce Cu+-O or Cu2+-OH, which either can oxidize the adjacent histidine residue in the monomer, inactivating itself, or oxidize residues in other proteins (7, 125, 355). CuZnSOD may also nitrate tyrosine in proteins via a reaction involving peroxynitrite (24, 74), and it is also reported to catalyze the release of NO from nitrosothiols (166). Over 90 genetic polymorphisms of the CuZnSOD have been described in the causation of the neurodegenerative disease amyotrophic lateral sclerosis (20). However, the lack of abnormalities in genetic deletion of CuZnSOD in mice (283) has led to the belief that pathologic consequences of mutations are due to gain of function of the enzyme's alternate peroxidase or nitration reactions, and are not due to loss of superoxide dismutase activity.
The Mn superoxide dismutase (MnSOD) protein constitutes up to 10% of the intracellular SOD activity and is mainly expressed in the matrix of the mitochondria. The MnSOD gene is on chromosome 6q25.3, and its sequence has no homology to CuZnSOD. The 25 kDa protein is expressed in the cytosol and imported into the mitochondria where the mitochondrial targeting sequence is cleaved to yield a protein of 22 kDa (324, 344). Each monomer contains a Mn and Zn metal ion, and the functional enzyme is a homotetramer (107). The Mn ion is held in place by the nitrogen of three histidines and the oxygen of one aspartate (20). Superoxide dismutation by MnSOD proceeds through the following reactions:
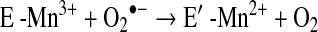 |
Reaction 6 |
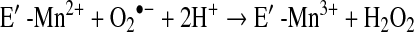 |
Reaction 7 |
Unlike CuZnSOD, the MnSOD does not have peroxidase or nitration ability. In fact, MnSOD is inactivated by nitration of the tyrosine 34 residue, which is required for enzyme catalytic activity (65, 216, 351). Further differences include that MnSOD is not inactivated by hydrogen peroxide or cyanide, and this allows distinction among the intracellular SODs on native gels (20, 82). Oxidative stress can strongly upregulate MnSOD gene expression (345). A recent report by Yeh et al. demonstrated that CuZnSOD expression can be upregulated via Nrf2 in rats treated with phenolic acids (352). Mitochondria consume large amounts of oxygen in the cell; MnSOD is the primary protection from the superoxide produced as an intermediary of cellular respiration. As might be expected, genetic deletion of this critical enzyme in mice is inconsistent with life, with death occurring due to mitochondrial pathology and oxidative damage to DNA shortly after birth when animals are exposed to ambient oxygen concentrations (206).
Extracellular superoxide dismutase (EC-SOD), a secretory, tetrameric hydrophobic glycoprotein, is the major extracellular SOD in the interstitial spaces of the lungs (100, 219, 220, 258). Each 24 kDa subunit contains a Cu and Zn ion and the active site is similar to the CuZnSOD. The CuZnSOD and EC-SOD have 50% similarity in amino acid sequence. An important characteristic of EC-SOD is that it contains a heparin/matrix binding domain consisting of positively charged arginines and lysines, which is located in the C-terminal region of EC-SOD (171). It is through interaction with heparin and heparan sulfate proteoglycans on cell surfaces and in the extracellular matrix that the extracellular localization of EC-SOD is maintained (100). The heparin/matrix-binding domain is sensitive to proteolysis, which can lead to release of EC-SOD from tissue matrix and sequentially alter oxidant/antioxidant balance. Recent study showed that EC-SOD protects the oxidative fragmentation of heparin/heparan suflate/syndecan-1 (186). The localization of EC-SOD in the lungs is primarily within the smooth muscle region surrounding blood vessels and airways (110). EC-SOD may have an important role in a number of lung diseases, where it modulates oxidant injury, inflammation, hyperoxia-induced lung injury, and pulmonary fibrosis. Polymorphisms are found in EC-SOD; the Arg 213-gly polymorphism (R213G) is frequently found in the human population (4–6%) and is associated with patient outcomes in chronic obstructive pulmonary disease (COPD) and lung injury (10).
b. Catalase
Catalase is a metalloprotein oxidoreductase enzyme (EC 1.11.1.6) and the principal scavenger of hydrogen peroxide when the latter is present at very high concentrations. Catalase is relatively limited in cellular distribution (e.g., peroxisomes and a few other locations). Glutathione peroxidase and peroxiredoxin systems, as classes, are of comparable, if not potentially greater, importance than catalase. The tetrameric hemoprotein undergoes alternate divalent oxidation and reduction at its active site, which contains the porphyrin ring and iron, in the presence of H2O2 (83, 285). The iron is held in place by the four nitrogen atoms of the porphyrin; the fifth valence position is coordinated to tyrosine 358 of catalase, and the sixth valence left free for interaction with substrate. The reaction mechanism proceeds through two steps. First, Fe(3+) reacts with hydrogen peroxide that results in cleavage of the O–O bond in H2O2, and the oxygen remains bound to the sixth valence position of Fe(+5), leading to formation of compound I. Compound I may oxidize a second peroxide molecule to oxygen, while the oxygen bound to the iron is released as water (20). Alternatively, Compound I may undergo inactivation by reduction to Compound II [Fe(4+)] by oxidants, or from itself by formation of a tyrosyl radical (tyrosine 370) under prolonged oxidative stress. Catalase has appreciable reductive activity for small molecules such as H2O2 and methyl or ethyl hydroperoxide (83, 285), but is unable to metabolize large molecular peroxides such as lipid hydroperoxide products of lipid peroxidation. Catalase is effective in the presence of high H2O2 concentrations (43), but under prolonged oxidative stress with oxidation of NADPH, catalase activity drops (181). NADPH binds to the enzyme and stabilizes the structure, and protects catalase from inactivation apparently by reversing accumulation of Compound II (181). The catalase gene located on chromosome11p13 is not generally inducible by oxidant stress (353). Enzyme activity can be regulated by post-translational processes. Under oxidative stress, the Abl family of receptor tyrosine kinases lead to phosphorylation of catalase at tyrosine 231 and tyrosine 386, which results in greater activity and lower cellular H2O2 levels (44). On the other hand, oxidation of tyrosine residues, in particular tyrosine 358, has been linked to loss of catalase activity under oxidative stress, for example, in asthma (119).
c. Glutathione system
The glutathione system consists of reduced (GSH), oxidized (GSSG) and GPx (Fig. 7). It is considered to be the major thiol–disulfide redox buffer of the cell. It is a central mechanism for reducing H2O2. It complements catalase as a reducing system for H2O2 but exceeds catalase in its capacity to eliminate additional varieties of toxic peroxides. Other metabolized substrate species include large molecule lipid peroxides, formed by free radical attack on polyunsaturated lipid membranes and products of lipo-oxygenase-catalyzed reactions (139). The key enzyme in the glutathione system responsible for the reduction of H2O2 are the glutathione peroxidases (GPx, EC 1.11.1.9). The reducing capacity of glutathione peroxidase enzymes are based on high levels of GSH (L-γ-glutamyl-L-cysteinylglycine). Glutathione peroxidases reduce hydrogen peroxide to water by oxidizing glutathione to oxidized/disulfide form (GSSG). The glutathione disulfide (GSSG) that is formed in the course of the reaction is subsequently reduced back to GSH by glutathione reductase, an intracellular enzyme that uses NADPH generated from the hexose monophosphate shunt system as an electron donor (133). Subsequently, GSSG breaks down to its amino acid components for cellular uptake and recycling. The capacity to recycle GSH makes the glutathione system pivotal to the antioxidant defense mechanism of a cell and prevents the depletion of cellular thiols. Four GPx have been described, all selenium enzymes: (a) the classic cytosolic form (cGPx), found in all cells; (b) a membrane-associated glutathione peroxidase phospholipid hydrogen peroxide GPx (90) (PHGPx); (c) another cytoplasmic enzyme, gastrointestinal GPx (giGPx), which was first found in cells of the gastrointestinal tract; and (d) an extracellular glutathione peroxidase (eGPx), first identified as a distinct enzyme in human plasma (354). All members of this family of enzymes can be oxidized by organic hydroperoxides, hydroperoxide, or both, and can subsequently be reduced by glutathione. The existence of multiple forms of GPx is due to the expression of four different gene products (354). All GPx contain a selenium atom in the active site in the form of selenocysteine (SeCys). The alveolar epithelial lining fluid contains a very high amount of both extra and intracellular glutathione peroxidase and micromolar levels of GSH (59–62). Previous reports have shown that S-nitrosoglutathione (GSNO) is an equivalent effective co-substrate of GPx (106, 146). Glutathione peroxidase use of GSNO leads to release of •NO and reduction of the GSNO storage form (106, 146). GNSO induces eGPx gene expression (59, 64) while overexpression of SOD prevents the induction of eGPx (59).
d. Thioredoxin system
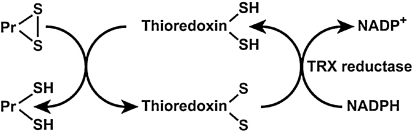
Thioredoxins (TRX) are oxidoreductase enzymes containing a dithiol–disulfide active site (-Cys-Gly-Pro-Cys-) (145). The cysteine residues reverse from a dithiol (-SH HS-) group to a disulfide bridge (-S-S-). The oxidized TRX is a disulfide with one bridge between two cysteines whereas the reduced TRX is a dithiol with two cysteines (145). TRXs are kept in the reduced state by flavoenzyme thioredoxin reductase, via an NADPH-dependent reaction (Fig. 8). Thioredoxin reductases in human are closely related to glutathione reductases. There are two thioredoxins, 1 and 2, with different cellular locations, and there are two thioredoxin reductases, with locations corresponding to the intracellular thioredoxins 1 and 2. The strong reducing activity of the sequence results from the cysteine residues acting as proton donors and cleaving disulfide (S-S) bonds in the target protein (145). Overall, TRXs can reduce protein disulfides (Pr-SH) and protein sulfenic acid (Pr-SO3H) intermediates by cysteine thiol–disulfide exchanges (79). Thioredoxins in human are closely related to glutathione reductase. There are two types of thioredoxins. Thioredoxin 1 is found in the cytoplasm and Thioredoxin 2 in the mitochondria (12). Thioredoxin 1 is a strong scavenger of ROS (142, 245, 246) and inhibits H2O2 in cooperation with the TRX-dependent peroxidase peroxiredoxin (288). Thioredoxin 1 augments gene expression of other antioxidants, such as MnSOD (80). The importance of thioredoxin has been identified in signal transduction, inflammatory response, and other biological functions such as apoptosis, cell growth, and proliferation (153, 247, 250). Specific protein disulfide targets for reduction by thioreoxin are ribonucleotide reductase (284), protein disulfide isomerase (212), and several transcription factors including p53, NF-κB, and AP-1 (102). This small multifunctional protein refolds oxidized proteins and activates transcription factors by reducing cysteine in the DNA binding site (102). Thioredoxins are expressed in bronchial epithelial cells and alveolar macrophages, metaplastic alveolar epithelial cells, and chondrocytes of the bronchus (314).
FIG. 8.
Thioredoxin redox system. Thioredoxins [Thioredoxin-(SH)2] act as proton donors and cleave disulfide (S–S) bonds in target proteins [P-(S–S)]. Thioredoxin reductase is responsible for reconstitution of the reduced thioredoxin from the oxidized form [thioredoxin-(S–S)].
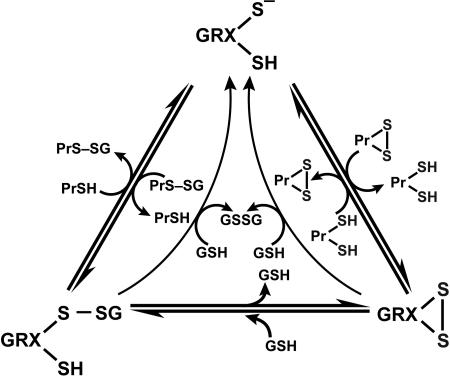
e. Glutaredoxin system
Glutaredoxins (GRX) are thiol–disulfide oxidoreductases that use glutathione as a cofactor and catalyze the reversible exchange of GSH with protein thiol groups (P-SH) (Fig. 9). There are two groups of glutaredoxins (Grxs), dithiol GRXs, which contain the Cys-Pro-Tyr-Cys active site motive and the monothiol GRXs lacking the C-terminal active site thiol in its Cys-Gly-Phe-Ser active site (207). Glutaredoxins uniquely also reduce mixed disulfides (-S-S-) with glutathione via a monothiol mechanism (deglutathionylation) where only an N-terminal low pKa Cys residue is required (79, 207) (Fig. 9). It is of note that GRX are dependent on GSH/GSSG concentrations. The human cell contains four GRXs, two dithiol (GRX 1 and GRX 2), one multiple monothiol (GRX 3), and one monothiol (GRX 4) (207). Glutaredoxin also catalyzes the formation of protein disulfide of certain proteins in the presence of a GS-radical generating system (79, 316). The formation of protein–SG mixed disulfide (glutathionylation) by glutaredoxin through a monothiol mechanism may play an important role in protecting against more drastic irreversible modifications of protein thiols, particularly when the redox state of the cytoplasm becomes more oxidizing, as under conditions of oxidative stress (79, 101).
FIG. 9.
Glutaredoxin system. Glutaredoxins (GRX) are thiol–disulfide oxidoreductases that catalyze the reversible exchange of GSH with protein thiol groups (PrSH). Dithiol GRXs contain Cys-Pro-Tyr-Cys active site motif and monothiol GRXs have Cys-Gly-Phe-Ser active sites. Modifed from Hurd et al. (150).
f. The role of protein thiolation (Pr-SH); S-glutathionylation in redox signaling
Maintaining the optimal GSH/GSSG ratio in the cell is critical to cell survival and is important in regulating the redox state of protein thiols. Changes in the cellular redox status, mainly due to decrease in GSH/GSSG ratio, initiates a series of redox-dependent modifications of proteins, lipids, and nucleic acids. With respect to proteins, cysteinyl residues are of particular interest, because their thiol group (Pr-SH) is susceptible to a number of oxidative modifications (30, 96, 122). Dominic et al. showed that cells may resist oxidative stress by protein thiolation (86). Proteins containing cysteine (Cys-SH) residues in the thiolate form (S-) are very likely to undergo oxidative modifications, which can interfere with biological functions. Protein sulfhydryl groups can be present as reduced thiols (Pr-SH), or oxidized to sulfenic (Pr-SOH), sulfinic (Pr-SO2H), or sulfonic acid (Pr-SO3H). Mild sulfhydryl oxidation produces disulfides and sulfenic acids, which are easily converted to disulfides by reaction with an adjacent sulfhydryl reside. Sulfenic acid may also be progressively oxidized to sulfinic acid and then to sulfonic acid. Disulfides and sulfenic acids may be reduced back to the sulfhydryl stage by TRX or GRX or other thiol reductases under high reducing potential. Recent reports have shown that sulfinic acid can also be reduced to the sulfhydryl stage although the reaction requires ATP and, hence, is not a simple reduction reaction (27). Sulfonic acid is not reversibly reduced to sulfhydryl under physiological conditions. It is difficult to accurately evaluate generation of oxidation of sulfhydryls because they are highly reactive and in a dynamic equilibrium. In general, they can be found as intra-or intermolecular disulfides (Pr-S-S-Pr) or mixed disulfides (Pr-S-S-X) with X as a low molecular mass thiol, such as cysteine or glutathione [i.e., S-thiolated proteins (150)]. Since GSH is widely distributed in cell compartments such as in cytoplasm, (1–10 mM of GSH) and mitochondria (5–10 mM GSH) as well as in the extracellular compartments such as epithelial lining fluid of the lung (100 μM GSH), S-glutathionyated (Pr-S-S-G) proteins are likely the main mixed disulfides in the lung (328).
Protein S-glutathionylation is a post-translational modification resulting in the formation of mixed disulfides between glutathione and protein sulfhydryl groups (78, 79). Protein S-glutathionylation can occur by several mechanisms [see recent review by Dall–Donne, (79)]. S-glutathionylation can occur not only during oxidative stress, but also under basal conditions (49, 208, 286). S-glutathionylation is involved in numerous physiological processes such as growth, differentiation, cell cycle progression, transcriptional activity, and metabolism. This suggests that S-glutathionylation is a widespread mechanism of redox regulation and important to basic cell function. The small amount of proteins that are S-glutathionylated in the cell under basal conditions can increase up to 50% under oxidative stress, and is accompanied by decrease of GSH (78, 79). The role for S-glutathionylation of proteins might be storage for GSH or as a protection of protein sulfhydryl integrity against more irreversible modifications and protein damage in response to higher levels of oxidative stress (78, 79). The reaction of GSH with protein thiols occurs by thiol–disulphide exchange and is catalyzed by GRX, enabling protein thiols to respond to a wide range of redox changes (i.e., GSH/GSSG ratio) during oxidative stress and redox signaling [see recent review by Dall–Donne, (79, 150)]. The main feature that makes S-glutahtionylation an attractive mechanism in the cell is its easy reversibility. Deglutathionylation is the process for removal of GSH from the protein mixed disulfides. This occurs when the redox environment becomes more reduced and can happen in an enzyme-dependent or -independent manner (79) (Fig. 9). Thus, S-glutathionylation serves the dual purposes of redox signaling in physiological conditions and protecting proteins from irreversible oxidative modifications during mild oxidative stress (78).
g. Peroxiredoxins
Peroxidredoxins (Prxs, EC 1.11.1.15) have received considerable attention in recent years as a new family of nonseleno peroxidases. Prxs exert their protective antioxidant effects through their broad spectrum of peroxidase activity, whereby hydrogen peroxide, peroxynitrite, and a wide range of organic hydroperoxides (ROOH) are reduced and detoxified. The antioxidant function of Prxs is dependent on redox-active cysteines. Prxs also modulate cytokine-induced hydrogen peroxide levels, which have been shown to mediate signaling cascades leading to cell proliferation, differentiation, and apoptosis. There are at least four different peroxiredoxins, with varying hydrogen peroxide-, lipid hydroperoxide-, and/or phospholipid hydroperoxide-substrate specificities and intracellular locations. Six different types of Prxs have been characterized in human lung (179). The bronchial epithelium showed moderate to high expression of Prxs I, III, V, and VI, the alveolar epithelium expressed mainly Prxs V and VI, and alveolar macrophages expressed mainly Prxs I and III (179).
h. Heme oxygenase
Heme oxygenases are members of the heat-shock family of proteins that play a protective role in inflammation and oxidative stress. These enzymes catalyze the degradation of heme molecules into biliverdin, bile pigments, and generate carbon monoxide and iron. Carbon monoxide and biliverdin have been attributed antioxidant properties (55). Consistent with this role, heme oxygenase-1 knockout mice are more susceptible to oxidative stress (268). Furthermore, induction of heme oxygenase by the repeated administration of hemin suppresses inflammation in the airway in ovalbumin-challenged guinea pigs, a model of asthma (46). Heme oxygenases are expressed in lung inflammatory cells of rats exposed to hypoxia. Recently, heme oxygenase-1 has been reported in human airways during asthma (46); levels in sputum of asthma patients are higher than in controls. Carbon monoxide concentrations are higher in exhaled breath of asthmatics as compared to healthy controls, which also suggests heme oxygenases are increased in human asthma. There are three forms of heme oxygenases. Heme oxygenase-1 is inducible, whereas heme oxygenase-2 and - 3 are constitutive (279). Heme oxygenase is expressed in airway epithelial cells, alveolar macrophages, bronchial epithelial cells, and inflammatory cells of the lungs (279).
IV. The Role of Redox in Asthma
A. Pathophysiology of asthma
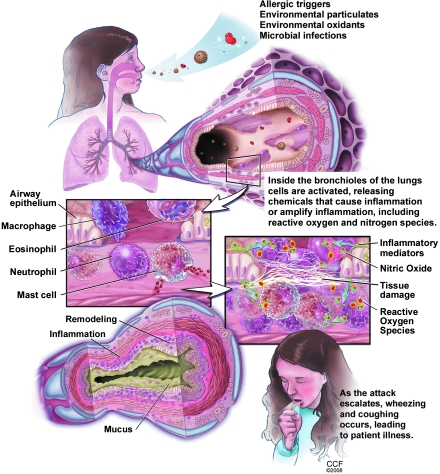
Asthma is a chronic inflammatory disease of the lower airways, characterized clinically by reversible airway obstruction and airway hyperresponsiveness. The characteristic feature of asthma is airway inflammation that results in epithelial cell desquamation, mucus production, and airway remodeling. Inflammatory cells in the airway include mast cells, eosinophils, lymphocytes, and activated monocytes, macrophages, and neutrophils (Fig. 10). Research has revealed that a complex interaction of cells and numerous biological active proinflammatory mediators are responsible for the pathogenesis of asthma. Among these mediators, there is overwhelming evidence that endogenous reactive oxygen and nitrogen species are responsible for the airway inflammation of asthma, and that the disequilibrium of the airway reducing state is a determinant of asthma severity (13, 21, 37, 58, 59, 65, 92, 119, 269, 294, 305, 357).
FIG. 10.
Pathophysiology of the inflammation and redox abnormalities in asthma. 137 × 177 mm (300 × 300 DPI).
B. Production of ROS in asthma
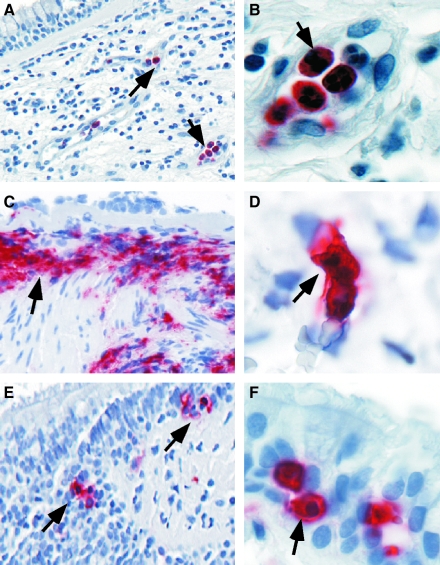
Enhanced levels of oxidant production are abundantly documented in asthma. Inflammatory cells are increased in asthmatics (Fig. 11) and produce more ROS as compared to control subjects. (21, 35–37, 145, 159, 160, 269, 305). Airway antigen (Ag) challenge in atopic individuals has been used as an experimental model to study mechanisms/mediators that lead to asthmatic responses and airway inflammation (37, 58, 92). Exposure of asthmatic individuals to appropriate Ag results in both an immediate asthmatic response occurring within minutes and a similar but prolonged late response after many hours. Asthma attacks and experimental Ag challenge are both associated with immediate formation of O2•− that persists throughout the late asthmatic response (300). As early as 10 min following local instillation of antigen into airways of atopic individuals, over twofold increase in O2•− generation is noted (37). Reports of O2•− generation by airspace cells range from 4 × 106 nmol/5 × 105 cells/h (37), with production of superoxide being high at sites of Ag challenge (300). Spontaneous and experimental allergen-induced asthma attacks lead to leukocyte (eosinophil, neutrophil) activation, during which NADPH oxidase is activated and ROS such as O2•− and its dismutation product, H2O2 are rapidly formed (16, 183).
FIG. 11.
Increased Inflammatory cells in asthmatic airways. Immunohistochemistry of endobronchial biopsies obtained from asthmatic lungs show the presence of increased numbers of polymorphonuclear cells (A, B), eosinophils (C, D) and mast cells (E, F) infiltrating throughout the mucosa and submucosa. Other remodeling changes seen in the biopsies include thickened basement membrane and sloughing of the surface epithelium (seen in C), increased vascularity (identifiable in A), and hypertrophy of the smooth muscle cells and layer (seen in C and E).
It is widely agreed that a link exists between increase of reactive species and asthma severity. For example, ROS production by asthmatics' neutrophils correlates with severity of reactivity of airways (37, 269, 299). Significant increase in neutrophils have been observed in the late-phase reaction after antigen challenge, in many cases of fatal asthma (189, 238), nocturnal asthma (222), in long-standing asthma even during periods of remission (104), and in patients with steroid responsive intractable asthma (326).
Oxidative modifications are characteristics of asthma (171, 301). Increased levels of eosinophil peroxidase and myeloperoxidase parallel numbers of eosinophils and neutrophils, respectively, and are found at higher than normal levels in asthmatic peripheral blood, induced sputum, and bronchoalveolar lavage fluid. Biomarkers of eosinophil activation include release of granule proteins including EPO (28, 45, 71, 143, 234, 254, 255, 293, 327) and major basic protein (MBP) (123, 134, 144, 340), which are readily found at high levels in blood, sputum, bronchoalveolar lavage and bronchial tissues of asthmatics (Fig. 11). Eosinophils, or MBP, in bronchial biopsies or induced sputa have been traditionally used to judge inflammation and the response, or lack of response, to therapies (8). However, activation of eosinophils and EPO generation of brominating oxidants is more accurately detected by oxidatively modified amino acids, among which 3-bromotyrosine is a unique product of EPO and eosinophils. Increased levels of 3-bromotyrosine are found in asthmatics bronchoalveolar lavage as compared to controls subjects (347). The levels of 3-bromotyrosine are increased further when asthmatics are exposed to experimental segmental antigen challenge (347). Consistent with a pathogenic link of free radicals and asthma severity, 3-bromotyrosine in airways of individuals with severe asthma admitted to the Intensive Care Unit with respiratory failure are elevated ∼100-fold over individuals in the Intensive Care Unit for nonasthma causes (217). Recent studies indicate that urinary bromotyrosine is elevated in asthmatics as compared to healthy controls, and may further increase during exacerbations, highlighting a potential role as a systemic noninvasive biomarker (141, 236).
MPO-mediated oxidant modifications also contribute to the pathophysiology of severe asthma (161). Significant (two- to threefold) elevations in chlorotyrosine are recovered from allergen challenged subsegments from asthmatic subjects undergoing segmental allergen challenge (347). Malondialdehyde and thiobarbituric acid reactive products have also been detected in urine, plasma, sputum, and bronchoalveolar lavage fluid that relate to the severity of asthma. Furthermore, 8-isoprostane, a biomarker of lipid peroxidation, is also elevated in exhaled breath condensate in adults and children with asthma (94, 237, 240, 346).
Perhaps most impressive is the striking increase of numbers and amounts of specific proteins that undergo nitration modifications in vivo in the experimental allergen-induced murine model of asthma (119). In murine and human allergen challenge studies, tyrosine nitration increases following allergen exposure of sensitized mice or atopic asthmatic humans (8, 92, 137, 152). The temporal sequence of events and airway localization of nitrotyrosine (13, 92), clearly support a link between eosinophilic infiltration and oxidation events and suggest that eosinophils may contribute to the generation of large number of oxidant products in asthma (29, 119).
C. Inhalation of exogenous ROS or RNS: Contribution to asthma severity
Recent studies have suggested that ozone and diesel exhaust particles have an additive effect on airway hyperreactivity and inflammation in asthma. Ozone increases hyperreactivity, induces IL-5 and granulocyte-macrophage-colony stimulating factor (GM-CSF) in bronchoalveolar lavage, which recruits and enhances the longevity of eosinophils in a mouse model of allergic asthma (175). Ozone also leads to oxidative modification of surfactant proteins, such as SP-A, which causes the lung to be more susceptible to lipid peroxidation and inflammation, and results in reduction of phagocytosis (235). Exposure of human airway epithelial cells to lipid ozonation products in vitro leads to activation of eicosanoid metabolism, phospholipases A2, C, and D, as well as induction inflammatory mediators such as IL-6, IL-8, and prostaglandin E2 (169, 170, 202). This provides evidence of a direct link between lipid ozonation products produced by ozone exposure and ozone-induced inflammation and cell damage (56).
Diesel exhaust particles and their components have been demonstrated to enhance airway hyperreactivity in a murine model of asthma. A recent study by McCreanor et al. demonstrated that adult asthmatics, walking for 2 h in a street with only diesel-powered vehicles, had significant reduction in lung function. These changes were accompanied by increased myeloperoxidase and 8-isoprostane in sputum and exhaled breath condensate, suggesting endogenous production of oxidants in response to the inhaled particulate materials (229, 276).
Tobacco smoke, a mixture of gases and particles that include smoke from the burning cigarette and exhaled mainstream smoke (333), contains >1014 oxidative molecules per puff of smoke, including superoxide and hydrogen peroxide. Active cigarette smoking has been associated in some studies with the development of asthma. Smoking asthmatics have an increased in morbidity and mortality as compared to nonsmoking asthmatics. Furthermore, smoking has a marked detrimental effect on lung function in asthmatic subjects and it increases the risk of severe asthma exacerbation. Cigarette smoke also influences the efficiency of inhaled corticiosteroid treatment in asthma.
Environmental tobacco smoke or second-hand smoke is also related to asthma [i.e., the association between environmental tobacco smoke exposure and pulmonary function is well documented (6, 97, 157, 198, 232)]. A recent report shows that lung function in bartenders improved after legislative ban of smoking in public places (5), and the cohort with preexisting asthma or rhinitis had the greatest increase of lung function after ban of smoking (232). This indicates that those individuals with airway inflammation have the greatest effect from inhalation of ambient free radical species. Etiological studies of the effect of environmental tobacco smoke on adults have found an increased risk of asthma, dose-dependent relationship to wheezing, and a greater risk for more severe airflow obstruction (147, 156, 163, 164, 191, 204, 329). The importance of environmental tobacco smoke in the etiology of asthma in children has been established. Environmental tobacco smoke exposure of children related to parental smoking is associated with poorer lung functions in asthmatic children, and the relative risk of asthma is greater in children exposed to cigarette smoking by both parents compared with smoking of neither parent (67, 147, 156, 158, 337).
Taken together, these data indicate that exogenous oxidant species contribute to asthma severity and asthma pathogenesis.
D. Nitric oxide in the lungs: Relation to oxidative modifications
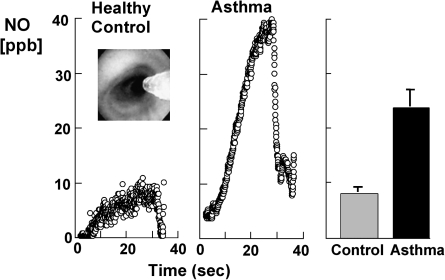
Evidence supporting increased •NO in asthma is substantial (92, 113, 126, 172, 264). •NO is increased in the lower airway and in the exhaled breath of asthmatics (92, 113, 126, 172, 264) (Fig. 12). Exhaled NO is clinically used as a noninvasive biomarker of asthma and therapeutic responsiveness (113, 308) but some studies suggest limitations of its value (243).
FIG. 12.
High levels of nitric oxide production and nitrotyrosine in asthma. Kinetics of NO accumulation in the gas phase in the airway lumen (left panel) are shown over time of a breath hold of a healthy control and an asthmatic individual. Individuals underwent bronchoscopy with a flexible fiberoptic bronchoscope and the levels of NO measured at a segmental bronchus with a collection Teflon catheter adapted to the working channel of the flexible bronchoscope (inset picture shows the catheter in the airway lumen). Sampling is performed in bronchioles between 5–7 mm in diameter. Individuals perform breath-hold (20 sec) and the accumulation of NO recorded in the absence of airflow. This type of evaluation yields a plot of NO (ppb) versus time (sec). During the breath-hold, bronchiolar gases accumulate NO quickly to a plateau. At the end of expiratory breath-hold, individuals exhale completely and this is accompanied by a rapid drop of NO as alveolar gases, which do not accumulate NO, are delivered to the sampling catheter. Levels of NO are measured with chemiluminescent analyser (NOA 280 Sievers) adapted for on-line data recording of NO concentration [methods as in Dweik et al. (90)]. Asthmatics generate levels of NO in the airway that are higher than healthy controls. Nitrotyrosine immunostaining of asthmatic and healthy control bronchial mucosa is shown in the right panel. Healthy control bronchial mucosa has pseudostratified columnar epithelium, with nitrotyrosine (red) staining present in apical portions of cells. Asthmatic bronchial mucosa has marked increase in immunoreactivity for nitrotyrosine in the epithelial cells. There are increased numbers of goblet cells in the biopsy, which are seen as cells with clear, nonstaining intracellular areas. Figures are modified from Dweik et al. (92).
Exhaled •NO in asthmatics increases after allergen challenge during the late asthmatic response (92, 174). It is increasingly suggested that high-output synthesis of •NO is a marker of, and/or contributes to, the airway inflammation that defines asthma. Multiple mechanisms function together to support high level •NO synthesis in the asthmatic airway. Individuals with asthma have 3-fold higher than normal •NO concentrations, and increased NOS2 mRNA and protein in airway epithelial cells (126, 127). This is principally observed in steroid-naïve patients with atopic asthma, and the inter-individual variation in exhaled nitric oxide concentrations can be significant. The increase in •NO concentrations is due to increased transcriptional activation of the NOS2 gene and a greater catabolic breakdown of storage pools of GSNO in the lung related to alterations in the redox state (113, 115, 273).
The biological effects of •NO have been attributed to its binding to guanyl cyclase, but its byproducts also have a biologic role. The biochemistry of NO oxidation products is critical in the balance of beneficial and adverse effects associated with •NO. For example, NO synthesis under oxidative and acidic conditions causes injury, in part because •NO oxidation in weak acid yields ONOOH and HONO (92, 113, 115). The dynamics of •NO metabolism in the asthmatic airway during an experimentally provoked asthmatic response to Ag reveal multiple and sequential reactions, and suggest a multifunctional role for •NO in the airway. In comparison to healthy controls, mild well-controlled atopic asthmatics tend to have increased •NO, NO3−, and nitrotyrosine but undetectable S-nitrosothiols (SNO) in the lower airways. Within minutes of Ag-induced asthmatic response, NO3− increases markedly in all asthmatics, while NO2− or SNO do not change, and •NO tends to decrease. Decreasing •NO and increasing NO3− suggests that •NO may be reacting with O2•− to yield ONOO−, which subsequently decays to NO3− or leads to nitrotyrosine formation (92). NO3− may also be formed as a product of peroxidase generated RNS (29, 92). In the late asthmatic response, nitration of thiols may occur by ONOOCO2− mediated thiol oxidation and nitration, or by free radical events such as formation of thiyl radicals (Fig. 7). Despite notable changes in asthmatic airways, healthy control individuals have no changes in levels of •NO or NO reaction products, even after challenge with aerosolized allergen.
The content of nitrotyrosine in airway proteins recovered from patients with severe asthma are an order of magnitude higher than those in healthy controls (217). It has been postulated that increased levels of HOBr production may result in increased peroxynitrite formation by interaction of HOBr with •NO, which favors nitration. Levels of nitrotyrosine have been found to be elevated in exhaled breath of asthmatics, and immunoreactivity to nitrotyrosine has also been shown to present in airway epithelial cells of asthmatics. Furthermore, increased nitration is found during an asthma exacerbation (92, 217, 347) and S-nitrosothiols concentrations are elevated in exhaled breath condensate in patients with asthma (69). Persistently increased ROS and NO in asthma leads to RNS formation, and subsequent oxidation and nitration of proteins, which may cause alterations in protein function that are biologically relevant to airway injury/inflammation. The measurement of nitration of tyrosine residues, which form from a reaction product of superoxide and NO, provides a stable and quantitative marker of tissue oxidative stress.
On the other hand, NO synthesis can also decrease airway resistance, an effect mediated in part by formation of the endogenous •NO oxidation product and bronchodilator, S-nitrosoglutathione (GSNO) (113, 115). GSNOR, glutathione-dependent formaldehyde dehydrogenase (FALDH; EC 1.2.1.1) is a ubiquitous enzyme known as a class III alcohol dehydrogenase. FALDH catalyzes the NAD+-dependent formation of S-formylglutathione from S-hydroxymethylglutathione, which forms spontaneously by condensation between formaldehyde and glutathione. Recently, it has been demonstrated that FALDH is very active in reduction of GSNO, which leads to generation of NO (48, 85). Unfortunately, airway activity of GSNO reductase (GSNOR) is increased in asthma (114). In fact, GSNOR substrate, GSNO, is undetectable in the human airway during asthmatic respiratory failure (114, 273). GSNOR-deficient mice, which cannot break down GSNO, are completely protected from methacholine hyper-reactivity following allergen sensitization and challenge (273).
GSNO inhalation increases exhaled NO in humans in part because GSNOR reduces GSNO to hydroxylamine which is converted to NO by catalase (105). Thus, increased airway GSNOR activity can lead to increased exhaled NO and methacholine hyper-responsiveness.
E. Redox imbalance in asthma
1. Oxidative stress
Homeostasis of cellular functions during oxidative stress depends on the rapid induction of protective antioxidant enzymes (240). For example, detectable concentrations of 8-isoprostane in EBC in healthy subjects are reported and suggest “physiological” levels of oxidative processes (240). Naturally occurring antioxidants exist to protect cells and tissue against the continuous production of ROS/RNS during normal metabolism (139). However, high levels of reactive species may overwhelm the antioxidant defenses, resulting in oxidant-mediated injury or cell death (15, 42). The terms “oxidant stress” or “oxidative stress” are often used to refer to this effect (132). Studies suggest that oxidant stress plays a crucial role in the initiation and progression of asthma.
2. Antioxidant deficiency in asthma
Both enzymatic and nonezymatic antioxidants are employed with the lung. The lung epithelial surface lining fluid contains several nonenzymatic antioxidants, such as glutathione, ascorbic acid, albumin, and alpha-tocopherol. Enzymatic antioxidants defenses are present in the epithelial lining fluid as well as in plasma and epithelial cells. Asthma is characterized by loss of antioxidant activities.
a. SOD deficiency
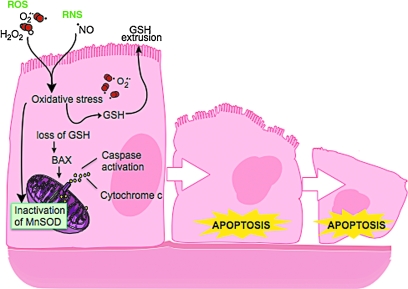
In asthma, SOD activity is significantly lower in epithelial lining fluid and airway epithelial cells as compared to healthy controls. Loss of SOD activity occurs within minutes of an acute asthmatic response to segmental antigen instillation into the lung of individuals with atopic asthma. This rapid decrease in SOD activity occurs in relation to a twofold increase in O2•− generation after antigen instillation into airways of atopic individuals (37). DeRaeve et al. and Smith et al. initially showed a correlation between the degree of airway reactivity and SOD activity levels (82, 313). Later studies in large populations confirmed that airway reactivity is inversely related to SOD activity (58, 63, 65). Together, these findings support a link between SOD activity and physiologic parameters of asthma severity. Murine models of asthma also provide evidence of a link between antioxidants and airway hyper-responsiveness. For example, transgenic mice that overexpress SOD have decreased allergen-induced physiologic changes in the airway in comparison to controls (197). Studies indicate that the lower SOD activity in asthma is a consequence of the increased oxidative and nitrative stress in the asthmatic airway, and thus serves as a sensitive marker of airway redox and asthma severity. Reduction in SOD activity can also contribute to oxidative stress and perhaps asthma severity. Oxidatively modified and nitrated MnSOD is present in epithelial cells recovered during bronchoscopy from asthmatic airways (65, 119). Stable isotope dilution tandem mass spectrometry of MnSOD isolated from human asthmatic airways reveals the presence of oxidation of phenylalanine and tyrosine residues. Dominant modifications include nitration of tyrosine, nonphysiologic tyrosine isomers [m-Tyr (meta-tyrosine) and o-Tyr (ortho-tyrosine)] that typically occur with exposure to hydroxyl radical-like oxidants, chlorination of tyrosine (a specific molecular marker for myeloperoxidase-catalyzed halogenation), and oxidative cross-linking of tyrosine as monitored by dityrosine (a product of tyrosyl radical) (63, 119, 129, 217). This pattern of oxidative modification is consistent with MnSOD exposure to Fenton/Haber–Weiss reaction mechanisms in asthmatic airways. The presence of a diverse array of distinct oxidative modifications indicates functional impairment of activity due to oxidative processes. Generation of reactive oxygen and nitrogen species is greatly increased during acute asthma attacks (37, 217, 347). Thus, loss of SOD contributes to oxidative stress during acute asthma exacerbations (37, 58, 217, 347). Other reports have shown that MnSOD is a target for tyrosine nitration and oxidation (213, 215), which leads to loss of enzyme function, and tissue injury (214, 215). Based on the reported quantitative data on MnSOD oxidation and nitration in human asthmatic lungs, up to 10% of MnSOD recovered from asthmatic airway epithelial cells possess at least 1 oxidative modification (65) (Fig. 13) (Table 3). Although it is unclear whether this average amount of modification of MnSOD can affect redox and cell functions in vivo, oxidative modification/inhibition of MnSOD triggers apoptosis in airway epithelial cells in vitro. Cleavage fragments of caspase-9 (35 kDa) and PARP (85 kDa) are present in asthmatic epithelial cells and are correlated with airflow in asthma. Apoptosis and shedding of epithelial cells are also observed in asthmatic patients (31, 89, 253, 331, 332) (Fig. 14). Thus, the redox modifications of SOD may contribute to a major component of asthmatic airway remodeling, airway epithelial apoptosis, which leads to denudation of the airway surface and predisposes to greater airway hyperreactivity. Recent studies also report a loss of circulating SOD activity in asthmatics. However, the isoform of SOD responsible for the loss is not known. The intracellular enzymes CuZnSOD and MnSOD are released to the circulation during normal turnover of cells and account for serum SOD activity. Although EC-SOD is found in extracellular matrix space, it is bound to heparan sulfate proteoglycans of endothelial cell surfaces and <1% of EC SOD is found in the serum (171, 301). Thus, EC-SOD contributes very little to serum SOD.
FIG. 13.
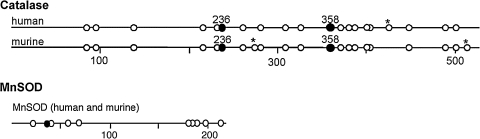
Tyrosine in catalase and MnSOD. Sequence location of 20 tyrosines in catalase and 10 tyrosine in MnSOD. (*) indicates sequence difference between murine and human. In catalase, Tyr 358 (filled circle) binds the proximal heme ligand and is critical for enzyme activity. Catalase contains a putative chlororination site (KXHY) at Tyr236. MnSOD Tyr34 (filled circle) is located in the active site of the enzyme and modification leads to inactivation of the enzyme. Figures are modified from Ghosh et al. (119).
Table 3.
Tyrosine Modifications in MnSOD and Catalase from Asthmatic Airway Epithelium
| mmol/mol | NiY/Y | BrY/Y | ClY/Y | mY/Phe | oY/Phe |
|---|---|---|---|---|---|
| MdSOD | 0.127 | 0.037 | 0.467 | 0.244 | 0.548 |
| Catalase | 0.379 | 0.061 | 7.976 | 0.006 | 0.000 |
Ranges in values of oxidative modifications observed in MnSOD and catalase from epithelial cell brushings from mild asthmatic subjects. The numbers are normalized to the content of the precursor amino acid (mmol oxidation product/mol precursor tyrosine or phenylalanine), which is monitored within the same injection. All data are representative of 4 asthmatic individuals. BrY, bromotyrosine; ClY, chlorotyrosine; DiY, dityrosine; mY, m-tyrosine; NO2Y, nitrotyrosine; oY, o-tyrosine; Phe, phenylalanine; Y, tyrosine. From Ghosh et al. (102) and Comhair et al. (56).
FIG. 14.
Redox abnormalities trigger apoptosis in airway epithelial cells. Exposure to ROS and/or RNS leads to extrusion of intracellular GSH and GSSG, and oxidative modification of MnSOD. Loss of SOD activity and/or extrusion of GSH activates BAX and caspases, and causes cytochrome c release from mitochondria, all of which trigger cell entry into programmed cell death pathways. This mechanism likely contributes to apoptosis and loss of airway epithelial cells, which is a hallmark of the remodeling in the asthmatic airway. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Recent studies also report a loss of circulating SOD activity in asthmatics. Similar to the correlation of airway SOD to lung function, serum SOD activity is related to asthma lung function, and this relationship appears to be unique to asthma since serum antioxidant capacity in chronic obstructive pulmonary diseases is unrelated to airflow limitation (62, 63, 263, 282). In vitro studies have shown that reactive oxygen and nitrogen species lead to oxidative and nitrative modification of tyrosine and inactivation of MnSOD and ECSOD, while Cu,ZnSOD can be inactivated by ROS and RNS through targeting of critical histidine residues and formation of histidinyl radicals (7, 213, 216).
Oxidative modification/inactivation of MnSOD is present in asthmatic airway epithelial cells (65). Altogether, the global loss of SOD activity reflects the increased oxidative and nitrative stress in asthmatic patients. This suggests that SOD may serve as a surrogate marker of oxidant stress and asthma severity (63, 65, 313).
Recent studies suggest that angiogenesis occurs in asthma (205, 295) and indicate a relation between the numbers of blood vessels in the bronchial wall and the severity of asthma (12, 260, 295, 338). VEGF-transgenic mice have an asthma-like phenotype with Th2-type inflammation, parenchymal and vascular remodeling, edema, mucus metaplasia, myocyte hyperplasia, and airway hyper-responsiveness (199). Reactive oxygen species such as superoxide and hydrogen peroxide enhance VEGF expression (192), while exogenous SOD prevents VEGF expression (192). These data suggest that the increased vascularization found in asthma may be due to the involvement of oxidative stress, perhaps via effects on hypoxia inducible factors.
b. Catalase inactivation
Red blood cells of asthmatic children were shown to have lower catalase activity than healthy children >15 years ago (251). Recently, catalase activity was found to be 50% lower in bronchoalveolar lavage of asthmatic lungs, as compared to healthy controls (120). The reduced catalase activity is not due to lower protein levels. Rather, catalase isolated from asthmatic airway epithelial cells has increased protein oxidation markers, including nitrotyrosine and chlorination and oxidation of sulfhydryls, linking oxidative modification to the reduced activity in vivo. Tyrosine oxidant modifications of catalase occur in asthma: chlorination of tyrosine by peroxidase-catalyzed halogenation, and oxidative cross-linking of tyrosine as monitored by dityrosine, a product of tyrosyl radical (120). The most extensive modification found in asthmatic lungs is tyrosine chlorination, which is 20-fold more extensive than tyrosine nitration (120). Unlike MnSOD, oxidation of phenylalanine to the nonphysiologic tyrosine isomers, m-Tyr and o-Tyr, is rare, indicating that exposure to hydroxyl radical-like oxidants through Fenton/Haber–Weiss reaction mechanisms is not prevalent in the oxidation of catalase.
Interestingly, catalase contains a recently identified putative chlorination site (KXHY) at Tyrosine 236, which may influence the susceptibility of the enzyme to peroxidase activity (26). On the other hand, tyrosine modification itself is not likely the complete cause of the loss of catalase activity. Other oxidative modifications, specifically oxidation of the cysteine 377 to cysteic acid, contribute to activity loss of the enzyme (120). Nevertheless, altogether the studies provide strong evidence that loss of antioxidant activities occur by multiple different oxidant mechanisms in asthmatic airways, which may be related to the enzyme structure and function and/or intracellular localization in different compartments of the cell.
c. Glutathione systems in asthma
In contrast to SODs and catalase, extracelluluar GPx (eGPx) is present at higher than normal levels in lungs of individuals with asthma (59, 60, 62, 64). The increase is due to induction of eGPx mRNA and protein expression by bronchial epithelial cells in response to increased intracellular or extracellular reactive oxygen species (59, 64). Not all oxidative stress will lead to increase of eGPx, for example, exposure to ozone decreases levels of eGPx protein and activity, whereas no change is detected with exposure to NO2 (14). It has been known for some time that alterations of GSH and GSSG levels and the ratio of GSSG/GSH are present in asthmatic airways (58, 59, 82, 312). Levels of glutathione in exhaled breath of children with asthma during acute asthma exacerbation are lower than control subjects (68), and the glutathione levels in exhaled breath of subjects with asthma increase after oral steroid treatment compared with pretreatment levels (68). Asthma and asthma exacerbations lead to rapid changes in intracellular as well as extracellular GSH and GSSG. Rapid changes in redox potential occur immediately after antigen challenge in epithelial lining fluid of asthmatics (58). Minutes after challenge, GSH levels drop and GSSG increases in the lung epithelial lining fluid, which verifies loss of reducing potential in asthmatic airways (58). GSH depletion in vivo and/or in vitro leads to inhibition of Th1-associated cytokine production and/or favors Th2-associated response (265). Thus, GSH facilitates a Th2 phenotype, and reduction in GSH levels supports the maintenance of Th2 response in asthma (265). Shifts of intracellular/extracellular pools of glutathione alter intracellular redox balance; efflux of GSH reproducibly activates BAX and cytochrome c release in cell lines (Hela cells and U937, monocyte cell line) and is one established mechanism for induction of apoptosis (117, 118, 167) (Fig. 14). Hence, alterations in the GSSG/GSH ratio and intracellular/extracellular distribution likely also contribute to the airway epithelial cell apoptosis in asthma.
Changes in the cellular redox status lead to formation of mixed disulfides between protein sulfhydrl groups and glutathione (S-glutathionylation) on multiple proteins. Glutathionylation of proteins is reversible, as those proteins can be reduced by glutaredoxins and thioredoxin (78, 79). Glutaredoxins are expressed in human alveolar macrophages and lung homogenates and to a lesser extent in bronchial epithelial cells (262, 279). A recent report by Reynaert et al. demonstrates that glutaredoxin 1 is upregulated in a mouse model of asthma (287). During asthma exacerbation in humans, the levels of serum TRX1 increase and are inversely correlated with airflow (350). This suggests that TRX may have a protective effect in asthma. In vitro studies have shown that exogenous TRX1 can prevent Th2 development by upregulating the expression of Th1-like cytokines, leading to a decrease in airway reactivity and airway inflammation (145). Because TRX1 reduces oxidization of proteins or the levels of hydrogen peroxide together with peroxiredoxin (247), the protective effects of TRX1 in asthma are thought to be partly dependent on its antioxidant effect (145). Through antioxidant effects, TRX1 regulates redox-sensitive signaling pathways (247), and may further affect pro-inflammatory pathways. One other target of TRX is the family of Prxs, which are reduced by TRX. Interestingly, a recent report by Avila et al, shows that Prx5 is increased in sputum of asthmatic and during viral-induced inflammation. Lehtonen et al. demonstrated that Prx1, 5 and 6 are upregulated in bronchial epithelial cells and alveolar macrophages of COPD (201).
3. Redox-dependent transcriptional regulation
Redox reactions have attracted attention as important chemical processes that regulate signal transduction. The response of a cell to a reactive oxygen- and nitrogen-rich environment often involves the activation of numerous intracellular signaling pathways, which can cause transcriptional changes and allow the cells to respond appropriately to the perceived oxidative stress. For example, at least two well-defined transcription factors, NF-κB and activation protein-1 (AP1), are regulated and influenced by the redox status and are implicated in the transcriptional regulation of a wide range of genes involved in oxidant stress and cellular response mechanisms (88, 221, 306). In addition to the activation of transcription factors, evidence suggest signaling pathways such as the family of mitogen-activated protein kinases (MAPKs) are directly or indirectly altered by redox changes (56, 209). In the nucleus, redox affects histone acetylation and deacetylation status, which at least partly regulates inflammatory gene expression by activation of the redox sensitive transcription factors (209).
a. Transcription factors NF-κB and AP1
Redox-sensitive molecular targets, such as transcription factors, usually contain highly conserved cysteine residues, and oxidation, nitrosylation, or the formation of disulfide links are crucial events in oxidant-redox signal.
Nuclear factor-κB (NF-κB) is activated in epithelial cells and inflammatory cells during oxidative stress, leading to the upregulation of a number of pro-inflammatory genes. NF-κB is a protein heterodimer made up of p65 and p50 subunits. There is evidence of activation of NF-κB in biopsies and sputum inflammatory cells such as macrophages and neutrophils of asthmatics (136). Many of the inflammatory genes responsible for the pathogenesis of asthma are regulated by NF-κB. Nitrosation of NF-κB subunits is an important mechanism for the redox sensing of NF-κB. In an elegant series of experiments, Reynaert et al. demonstrated that S-glutathionylation regulates activation of the NF-κB pathway (286, 287). Glutaredoxin-dependent reversal of S-glutathionylation of the inhibitory kappa-B kinase (IKKβ) modulates the activation of NF-κB in response to redox changes by protecting IKKβ from irreversible inactivation (286).
Activator protein-1 (AP-1) is a protein dimer, composed of a heterodimer of Fos and Jun proteins. The oxidant-sensitive cysteine in the DNA-binding site of c-Jun undergoes reversible S-glutathiolation during oxidative stress in the presence of physiologic levels of GSH (182). AP1 regulates many of the inflammatory and immune genes in oxidant-mediated diseases. Gene expression of γ-GCS, the rate-limiting enzyme for the GSH synthesis, is induced by the activation of AP1 (281). Asthmatic epithelial cells have increased expression of c-Fos. Cigarette smoke increases AP-1 DNA binding in human epithelial cells in vivo (281). High levels of NO and hydrogen peroxide cause increases in c-fos and c-jun mRNA of epithelial cells (281). The binding sites of the redox-regulated transcription factors NF-κB and AP-1 are located in the promoter regions of many antioxidant genes, such as NOSII and GPx, which are directly involved in lung diseases such as asthma (11, 221, 304, 348).
Recent evidence indicates that chromatin remodeling plays a critical role together with NF-κB and AP-1 in the activation of inflammatory genes such as NOS2, IL-8, and TNFα. Oxidative stress and other stimuli, such as cytokines, activate various signal transduction pathways leading to activation of transcription factors, such as NF-κB, and AP-1. Binding of transcription factors to DNA elements leads to recruitment of CREB-binding protein (CBP) and/or other co-activators to the transcriptional initiation complex on the promoter region of various genes. Activation of CBP leads to acetylation (Ac) of specific core histone lysine residues by an intrinsic histone acetyltransferase (HAT) activity (276). This results in the acetylation of core histones, opening up the chromatin structure to allow binding of RNA polymerase II, which initiates gene transcription. The process of acetylation and deacetylation of histone is also influenced by redox changes (274, 275). In biopsies and peripheral blood mononuclear cells from asthmatics, there is an increase in acetylation and a reduction in deacetylation activity, which upregulates some inflammatory gene expression and downregulates others (70). Redox changes also can activate members of the mitogen-activated protein kinase signaling (MAPK), such as extracellular signal-regulated kinase (ERK), c-jun N-terminal kinase (JNK), p38 kinase, and phosphoinositol-3 kinase, all of which may ultimately promote inflammation (47, 334).
b. Redox-dependent activation of JAK/STAT pathway
Binding of cytokines, including interleukin-4 and interferons, to their specific receptors leads to transphosphorylation of tyrosine residues on Janus kinases (JAK), which then recruit and phosphorylate the signal transducers and activators of transcription (STAT) family of transcription factors on tyrosine residues and result in gene expression of pro-inflammatory genes such as NOS2. Many of the inflammatory and immunomodulatory cytokines important in asthma pathogenesis signal through this canonical pathway. Multiple studies now support that STAT1 and STAT3 activation is redox regulated. Although STAT3 has not been evaluated in asthma, STAT1 is activated at high levels in asthmatic airway epithelium but not in healthy controls (129). Among the first reports, Simon et al. showed that members of the STAT family of transcription factors, including STAT1 and STAT3, are activated in response to H2O2 or GSH-depletion, and inhibited by antioxidants. STAT activation is H2O2 specific, not occurring in response to superoxide or NO (309), and effects are mediated via JAK2 and tyrosine kinase 2 (TYK2). Vanadium compounds in the particulate matter of air pollution, which are released from the industrial burning of fuel oil, activate signal transduction via the generation of H2O2; Wang et al. showed that vanadium leads to STAT-1 activation (339). Recently, the detailed redox mechanisms that regulate STAT activation by IL-4 have been identified (307). Upon ligand-receptor binding, ROS are rapidly generated by phosphatidylinositol 3-kinase-dependent activation of the NAD(P)H oxidase (NOX)1 and NOX5L. Consequently, cysteine oxidation by ROS inactivates protein tyrosine phosphatase 1B, which is then unable to inactivate/dephosphorylate the receptor. This amplifies the cytokine signal transduction and results in greater JAK/STAT activation and enhances the inflammatory response to cytokines (309). Thus, homeostatic control of cytokine-receptor activation and signal transduction occurs through ROS generation via activation of NOX enzymes; the ROS subsequently promote the receptor's own activation and the activation of other cytokine receptors in the cell, enabling cytokine signaling crosstalk through redox events.
F. Genetics of redox in asthma
Since SOD is decreased in asthma, and its activity is strongly related to asthma pathophysiology, it has been hypothesized that genetic variability of SODs may play a role in the development of asthma. Single nucleotide polymorphism Ala16Val of MnSOD is common and may change the secondary structure and mitochondrial targeting of the protein (180). A polymorphism (R213G) of EC-SOD causes more than ninefold higher levels of EC-SOD in plasma due loss of anchoring to heparin in the interstitium (301). The EC-SOD R213G polymorphism is associated with reduced exacerbations in COPD and lower rates of hospitalization (168). However, the functionally important genetic variants Ala16Val (MnSOD) and R213G (ECSOD) are not associated with genetic susceptibility to develop asthma (180). Genetic variation in the untranslated regions of transcripts, as well as intronic polymorphisms, can be functionally important, both of which have been described in the SOD gene loci. For example, two novel polymorphisms occur in the noncoding 5' untranslated region (Exon 1) and first intron (Intron 1) of the SOD3 gene (77). Both polymorphisms are situated in a conserved mammalian interspersed repetitive element, and may be functionally relevant to lung disease. Although not evaluated in asthma, a recent report by Dahl et al. found that EC-SOD homozygous for the Exon1/Intron1 polymorphism associates with reduced lung functions in individuals with chronic obstructive lung disease (77). This supports a role for EC-SOD in oxidant-mediated events influencing airway diseases and lung function (77).
The glutathione S-transferase superfamily includes a number of subclasses including glutathione S-transferase P1 and glutathione S-transferase M1 which are expressed in the lungs and have been implicated in asthma pathogenesis (317). The deletion allele of GSTM 1 (null-genotype) has been associated with increased risk of asthma and lower lung function (121, 325). A functional sequence variant in GSTP1 at codon 105 (Ile105Val- rs1695) (148, 323) has also been associated with asthma in some studies (249, 325). This variant has been reported to be both protective (249) or a risk factor (200, 325) for asthma. There are reports indicating a gene-pollution interact with asthma pathogenesis (210). A recent study by Islam et al. (155), reports that children with a Val105 mutation in GSTP1 variant allele may have a lower risk of asthma associated with exercise, especially with high ozone levels.
V. Clinical Implications
Inhaled glucocorticosteroids are the mainstay of therapies for most adult asthmatics (154). Other drugs such as cromolyn sodium, nedocromil, and leukotriene modifiers can also be used. Oral corticosteroids are used when inhaled therapies are inadequate. However, subsets of patients with severe asthma are refractory to treatment or develop significant side effects to the medications (154). Antioxidant therapy may be a safe and effective alternative. However, there is no evidence to support a benefit of antioxidants in severe asthma.
A. Clinical monitoring of redox in asthma
The redox abnormalities found in asthma result in distinct oxidant species. Protein bromination, lipid peroxidation, and NO production represent distinct biochemical pathways that have all been associated with the pathophysiology of asthma (217, 280, 347). Although the labile nature of oxidant species makes them difficult to quantify, the stable end-products of distinct oxidation pathways may be used as reliable indices of airway oxidative stress.
Elevated levels of 3-bromotyrosine and F2-IsoPs have been detected in both urine and exhaled breath condensates of asthmatics and are being evaluated as noninvasive biomarkers of asthma (9, 18, 95, 237, 240, 241, 259).
Numerous studies have demonstrated increased NO production in the airways of asthmatics, due at least in part to upregulation of inducible nitric oxide synthase (iNOS) in cells like bronchial epithelial cells (92, 93, 172–174). The technology for exhaled NO measurement has developed rapidly over the last 2 decades and uniform global standardization of techniques has enabled the use in clinical practice.
Exhaled NO is widely accepted as a measure of inflammation, in particular eosinophilic inflammation. NO monitoring is not likely helpful for smoking asthmatics, since smoking reduces exhaled NO levels. NO also reflects the airway redox in asthma; as the airway environment becomes more oxidizing and acidic; greater release from storage pools of GSNO also leads to high level of exhaled NO (113, 115). Exhaled breath condensate (EBC) pH is low in acute asthma exacerbations and increases with corticosteroid therapy. EBC-based pH assays may be of value in monitoring airway redox stress in the ambulatory environment. The rapid advances in the technologic processes for measure of stable oxidant endproducts provide confidence that many more biomarkers (or panels of biomarkers) with diagnostic and prognostic utility for evaluating the presence and activity of asthma will be available in the near future.
B. Antioxidant therapeutic strategies
1. Redox-sensitive transcription factors
NF-κB and AP1, are activated and contribute to the pathogenesis of asthma. Thus, several therapeutic strategies have been used to develop small antioxidant molecule inhibitors of these redox-regulated transcription factors. One small molecule inhibitor of AP1 transcription, PNRI-299, was discovered using a template that screened for molecules that bind to redox active substances. PNRI-299 selectively inhibits AP1 transcription but not NF-κB or thioredoxin (248); its intracellular molecular target is the oxidoreductase redox effector factor-1 (REF-1). In the murine model of asthma, PNRI-299 effectively reduces airway eosinophil infiltration, mucus hypersecretion, and IL-4 levels (248). Another small antioxidant molecule inhibitor of NF-κB and AP1 transcription, MOL 294, also successfully blunts airway inflammation and hyperreactivity in a mouse model of asthma. MOL 294 inhibits both NF-κB and AP1 via inhibition of thioredoxin. Intranasal administration of MOL 294 markedly reduces airway eosinophilia and mucus hypersecretion. Although not tested in humans, these studies suggest that therapies that target redox-regulated signal transduction pathways are feasible and can reduce allergic airway inflammation and reactivity (140). A limitation of this strategy is the relative lack of selectivity of transcription factor inhibition.
2. SOD therapies
Antioxidants have been proven beneficial in control of ischemia/reperfusion injury (76), shock and lung injury related to radiation therapy and chemotherapy (99), and in chronic inflammatory disorders such rheumatoid arthritis and osteoarthritis (298). Asthma is associated with decrease in antioxidant SOD, thus strategies aimed at increasing airway SOD levels may be a rational approach to more effective therapy for asthma. Further support for SOD therapy comes from murine models of asthma, which provide a link between antioxidants and airway hyper-responsiveness. For example, transgenic mice that overexpress SOD have decreased allergen-induced physiologic changes in the airway in comparison to controls (197). In other acute and chronic inflammation models, SOD mimetics reduced PARP immunofluorescence, providing evidence of a role for SOD in inhibition of apoptosis and inflammation (296, 297). Similarly, treatment with SOD mimetics reduces the magnitude of ovalbumin-induced airway hyper-responsiveness to methacholine in murine models of asthma (52). Exogenous EC-SOD given intratracheally to mice treated with asbestos, decreases neutrophil influx and oxidative matrix degradation (186). However, manipulation of endogenous SOD for therapeutic purposes has been problematic due to its short half-life and large molecular weight. A number of SOD mimetics based around organomanganese complexes have been developed, which retain their antioxidant properties in vivo. SOD mimetics include manganese (II) penta-azamacrocyclic complex (M40401, M40403, and M40419) (17, 231, 289, 298), manganese (III) (salen) (EUK134) (22, 23, 315) complexes, and manganese metaloporphyrin class 9 AEOL-10113, AEOL-10150 of SOD. Masini et al. demonstrated that SOD mimics given before antigen challenge of sensitized guinea pigs attenuate allergen-induced asthmatic bronchospasm (223). These animal studies collectively provide proof of the concept that SOD mimetic may be effective in treating asthmatic airway inflammation. However, clinical trials on the effects of SOD mimetics in patients with asthma have not been performed and thus efficacy of SOD mimetics in treatment of asthma is yet to be tested.
3. Glutathione system
Other potential untested strategies include the glutathione peroxidase mimetic, Ebselen, which is a nontoxic seleno-organic drug and an effective reductant of hydroperoxides (303). Ebselen inhibits airway inflammation by reducing neutrophil recruitment and chemokine expression in various animal models of inflammation (4, 225, 279). Resveratrol (3,5,4’-trhihydroxystilbene), a phytoalexin that is found in seeds of grapes, has been reported to have antioxidant, anti-inflammatory, and anticarcinogenic properties (188). Studies have shown that resveratrol effectively inhibits oxidative damage and scavenges free radicals such as lipid hydroperoxyl, hydroxyl (· OH), and superoxide (203). Resveratrol induces GSH synthesis and attenuates oxidative stress and depletion of GSH in lung epithelial cells (188). In primary lung epithelial cells, resveratrol (10 μM) attenuates cigarette smoke-mediated GSH depletion by inducing GSH synthesis which protects the epithelial cells (164) This compound may be benefical for patients with asthma (75).
4. Dietary antioxidants
Dietary antioxidants may improve asthma control or reduce its incidence. Epidemiological studies suggest associations between low dietary antioxidant intake, reduced lung function, and increased respiratory symptoms in asthmatics (34). The most recent Third National Health and Nutrition Examination Survey (NHANES III) findings indicate an association of some antioxidants with asthma in children who have environmental tobacco smoke exposure asthma (292). A large cross-sectional study in NHANES III shows that selenium and serum vitamin C are lower in young asthmatics as compared to nondiseased controls (292). Serum vitamin C and selenium were inversely associated with asthma, and the association was most evident in those exposed to the added oxidant stress of environmental cigarette smoke (292). The provocative findings suggest that dietary supplementation with selenium in those individuals with cigarette smoke exposure should be considered (292). Likewise, studies have revealed that asthmatics have lower than normal levels of coenzyme Q (CoQ) (116). Also known as ubiquinone, CoQ participates in electron transport in cellular respiration in the mitochondria. In its reduced form, ubiquinol (QH2) also serves as an antioxidant. One study in asthma and others in cardiovascular diseases has shown that CoQ increases SOD activity and thus therapy with CoQ may benefit in asthma (25, 130, 330). In a cross-over randomized study of 41 asthmatics who were all receiving corticosteroids, supplementation with CoQ [Q-Gel® (120 mg), 32 weeks] improved asthma control and enabled reduction of corticosteroid dose (130). These studies all support the concept that antioxidant supplementation and/or reduction in oxidant production or exposures will be beneficial in the treatment of asthma (Table 4).
Table 4.
Possible Antioxidant Therapeutic Strategies for Asthma
| Strategy | Target |
|---|---|
| Small molecules | Redox-related transcription factors AP-1:PNRI-299 NF-κB and AP-1:MOL 295 |
| Antioxidant mimics | SOD mimetics: M40401, M40403, M40419, EUK 134, AEOL-10150 GPx mimetics: Ebselen GSH synthesis: Resveratrol |
| Dietary antioxidants | Vitamin C, selenium, and coenzyme Q |
VI. Conclusions and Future Directions
Asthma is a chronic inflammatory airway disease, and it is clear from multiple lines of evidence that the airway inflammation is defined by alterations of the airway redox. Redox-mediated post-transcriptional modifications lead to protein structure–function changes that are present even in mild asthmatics who are well-controlled. The abnormalities in redox are magnified in the asthmatic airway in response to exacerbating factors, including microbial infection, exposure to inhaled oxidizing pollutants, or allergen triggers in atopic individuals. During leukocyte activation, such as following allergen exposure, a respiratory burst occurs, generating O2•− and its dismutation product H2O2. Fenton/Haber–Weiss reactions affect endogenous proteins, such as MnSOD. Oxidative modifications of MnSOD amplify the oxidative milieu in the mitochondria, with potential adverse consequences on cellular respiration and cell survival. As eosinophils and/or neutrophils enter the inflamed airway, H2O2 is used in eosinophil peroxidase and/or myeloperoxidase-mediated reactions that oxidatively modify susceptible proteins in the airway environment. Among those proteins is catalase, the antioxidant enzyme that would otherwise act to control the amounts of H2O2 present in the airway. This enables more H2O2 to accumulate at the site of inflammation and further promotes peroxidase systems to produce high levels of nitrating, halogenating, and oxidizing injurious species. The greater toxic nitrogen oxides and airway acidity is accompanied by loss of beneficial nitrogen oxides, in particular nitrosothiols, which have adverse effects on smooth muscle relaxation and airway reactivity. In addition to injury of macromolecules, RNS and ROS amplify specific cytokine signal transduction by processes that include inhibition of deactivating signals. The loss of downregulatory signal transduction events further amplifies the inflammatory milieu and may contribute to Th2 lymphocyte polarization and development of the atopic environment typically seen in the asthmatic airway. Thus, alteration of redox participates in the pathophysiology of asthma. Finally, future therapy targeting redox will require the definition of the clinical pharmacology of antioxidant compounds. Furthermore, identification of noninvasive biomarkers of oxidative stress in patients with asthma will be critical for enabling assessment of treatment outcomes. Nevertheless, the cumulative data provide a compelling rationale to develop these types of therapeutic strategies for asthma that aim to correct the redox abnormalities.
Abbreviations Used
- CuZnSOD
copper-zinc superoxide dismutase
- EC-SOD
extracellular superoxide dismutase
- EPO
eosinophil peroxidase
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- GPx
glutathione peroxidase
- GRX
glutaredoxins
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- GSSG
oxidized glutathione
- HIF1
hypoxia-inducible factor-1
- H2O2
hydrogen peroxide
- HOCl
hypochlorous acid
- JAK
janus kinases
- MnSOD
manganese superoxide dismutase
- MPO
myeloperoxidase
- NO2−
nitrite
- NO3−
nitrate
- NOS
nitric oxide synthase
- NOS1/nNOS
neuronal NOS
- NOS2/iNOS
inducible NOS
- NOS3/eNOS
endothelial NOS
- O2•−
superoxide
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- Pr-SH
protein thiol groups
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SNO
S-nitrosothiols
- SOD
superoxide dismutases
- -S–S-
disulfide bridge
- STAT
signal transducers and activators of transcription
- TRX
thioredoxins
- VEGF
vascular endothelial growth factor
Footnotes
Reviewing Editors: Andrew Ghio, Vuokko Kinnula, Corrine Kliment, Paolo Montuschi, Sekhar Reddy, and Carl White
Acknowledgments
This study was supported by Grants HL081064, HL69170, AI70649, from the National Institutes of Health.
References
- 1.Abu-Soud HM. Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Soud HM. Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275:5425–5430. doi: 10.1074/jbc.275.8.5425. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Soud HM. Khassawneh MY. Sohn JT. Murray P. Haxhiu MA. Hazen SL. Peroxidases inhibit nitric oxide (NO) dependent bronchodilation: Development of a model describing NO-peroxidase interactions. Biochemistry. 2001;40:11866–11875. doi: 10.1021/bi011206v. [DOI] [PubMed] [Google Scholar]
- 4.Adcock IM. Brown CR. Kwon O. Barnes PJ. Oxidative stress induces NF kappa B DNA binding and inducible NOS mRNA in human epithelial cells. Biochem Biophys Res Commun. 1994;199:1518–1524. doi: 10.1006/bbrc.1994.1403. [DOI] [PubMed] [Google Scholar]
- 5.Agani FH. Pichiule P. Chavez JC. LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- 6.Alipour S. Deschamps F. Lesage FX. Effects of environmental tobacco smoke on respiratory symptoms and pulmonary function. Inhal Toxicol. 2006;18:569–573. doi: 10.1080/08958370600686267. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez B. Demicheli V. Duran R. Trujillo M. Cervenansky C. Freeman BA. Radi R. Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Andreadis AA. Hazen SL. Comhair SA. Erzurum SC. Oxidative and nitrosative events in asthma. Free Radic Biol Med. 2003;35:213–225. doi: 10.1016/s0891-5849(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 9.Antczak A. Montuschi P. Kharitonov S. Gorski P. Barnes PJ. Increased exhaled cysteinyl-leukotrienes and 8-isoprostane in aspirin-induced asthma. Am J Respir Crit Care Med. 2002;166:301–306. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- 10.Arcaroli JJ. Hokanson JE. Abraham E. Geraci M. Murphy JR. Bowler RP. Dinarello CA. Silveira L. Sankoff J. Heyland D. Wischmeyer P. Crapo JD. Extracellular superoxide dismutase haplotypes are associated with acute lung injury and mortality. Am J Resp Crit Care Med. 2009;179:105–112. doi: 10.1164/rccm.200710-1566OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambellan A. Leahy R. Xu W. Cruickshank PJ. Janocha A. Szabo K. Cannady SB. Comhair SA. Erzurum SC. Pivotal role of c-Fos in nitric oxide synthase 2 expression in airway epithelial cells. Nitric Oxide. 2009;20:143–149. doi: 10.1016/j.niox.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arner ES. Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 13.Aulak KS. Miyagi M. Yan L. West KA. Massillon D. Crabb JW. Stuehr DJ. Proteomic method identifies proteins nitrated in vivo during inflammatory challenge. Proc Natl Acad Sci USA. 2001;98:12056–12061. doi: 10.1073/pnas.221269198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avissar NE. Reed CK. Cox C. Frampton MW. Finkelstein JN. Ozone, but not nitrogen dioxide, exposure decreases glutathione peroxidases in epithelial lining fluid of human lung. Am J Respir Crit Care Med. 2000;162:1342–1347. doi: 10.1164/ajrccm.162.4.9912041. [DOI] [PubMed] [Google Scholar]
- 15.Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts) N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 16.Babior BM. Oxygen-dependent microbial killing by phagocytes (second of two parts) N Engl J Med. 1978;298:721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- 17.Baker K. Marcus CB. Huffman K. Kruk H. Malfroy B. Doctrow SR. Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: A key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 18.Baraldi E. Ghiro L. Piovan V. Carraro S. Ciabattoni G. Barnes PJ. Montuschi P. Increased exhaled 8-isoprostane in childhood asthma. Chest. 2003;124:25–31. doi: 10.1378/chest.124.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ. Nitric oxide and asthma. Res Immunol. 1995;146:698–702. doi: 10.1016/0923-2494(96)84921-2. [DOI] [PubMed] [Google Scholar]
- 20.Bartosz G. Superoxide dismutase and catalase. Handbook Environment Chem. 2005;2 part O:109–149. [Google Scholar]
- 21.Bast A. Haenen GR. Doelman CJ. Oxidants and antioxidants: Sstate of the art. Am J Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 22.Batinic-Haberle I. Manganese porphyrins and related compounds as mimics of superoxide dismutase. Methods Enzymol. 2002;349:223–233. doi: 10.1016/s0076-6879(02)49337-8. [DOI] [PubMed] [Google Scholar]
- 23.Batinic-Haberle I. Benov L. Spasojevic I. Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 24.Beckman JS. Ischiropoulos H. Zhu L. van der Woerd M. Smith C. Chen J. Harrison J. Martin JC. Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- 25.Belardinelli R. Mucaj A. Lacalaprice F. Solenghi M. Seddaiu G. Principi F. Tiano L. Littarru GP. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–2681. doi: 10.1093/eurheartj/ehl158. [DOI] [PubMed] [Google Scholar]
- 26.Bergt C. Fu X. Huq NP. Kao J. Heinecke JW. Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J Biol Chem. 2004;279:7856–7866. doi: 10.1074/jbc.M309046200. [DOI] [PubMed] [Google Scholar]
- 27.Biteau B. Labarre J. Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 28.Bolscher BG. Plat H. Wever R. Some properties of human eosinophil peroxidase, a comparison with other peroxidases. Biochim Biophys Acta. 1984;784:177–186. doi: 10.1016/0167-4838(84)90125-0. [DOI] [PubMed] [Google Scholar]
- 29.Brennan ML. Wu W. Fu X. Shen Z. Song W. Frost H. Vadseth C. Narine L. Lenkiewicz E. Borchers MT. Lusis AJ. Lee JJ. Lee NA. Abu-Soud HM. Ischiropoulos H. Hazen SL. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 30.Brigelius R. Muckel C. Akerboom TP. Sies H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol. 1983;32:2529–2534. doi: 10.1016/0006-2952(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 31.Bucchieri F. Puddicombe SM. Lordan JL. Richter A. Buchanan D. Wilson SJ. Ward J. Zummo G. Howarth PH. Djukanovic R. Holgate ST. Davies DE. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 32.Bucher TK M. Ege des Wasserstoffs in der Lebendigen Organisation. Angew Chem. 1958;70:552–570. [Google Scholar]
- 33.Buettner GR. The pecking order of free radicals and antioxidants: Lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 34.Burns JS. Dockery DW. Neas LM. Schwartz J. Coull BA. Raizenne M. Speizer FE. Low dietary nutrient intakes and respiratory health in adolescents. Chest. 2007;132:238–245. doi: 10.1378/chest.07-0038. [DOI] [PubMed] [Google Scholar]
- 35.Busse WW. Reed CE. Asthma, Definition and Pathogenesis. In: Middleton E Jr, editor; Reed CE, editor; Ellis EF, editor; Adkinson NF Jr, editor; Yunginger JW, editor; Busse WW, editor. Allergy, Principles and Practice. volume II. St Louis: Mosby-Year Book; 1992. p. 1173. [Google Scholar]
- 36.Busse W. Elias J. Sheppard D. Banks-Schlegel S. Airway remodeling and repair. Am J Respir Crit Care Med. 1999;160:1035–1042. doi: 10.1164/ajrccm.160.3.9902064. [DOI] [PubMed] [Google Scholar]
- 37.Calhoun WJ. Reed HE. Moest DR. Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–325. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 38.Candeias LP. Patel KB. Stratford MR. Wardman P. Free hydroxyl radicals are formed on reaction between the neutrophil-derived species superoxide anion and hypochlorous acid. FEBS Lett. 1993;333:151–153. doi: 10.1016/0014-5793(93)80394-a. [DOI] [PubMed] [Google Scholar]
- 39.Cantin AM. Begin R. Glutathione and inflammatory disorders of the lung. Lung. 1991;169:123–138. doi: 10.1007/BF02714149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cantin AM. Hubbard RC. Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 41.Cantin AM. North SL. Hubbard RC. Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 42.Cantin AM. Paquette Oxidant and antioxidants in lung injury. Lam and other Diseases characterized by smooth muscle proliferation. 1999:519–531. [Google Scholar]
- 43.Cantin A. Crystal RG. Oxidants, antioxidants and the pathogenesis of emphysema. Eur J Respir Dis. 1985;139:7–17. [PubMed] [Google Scholar]
- 44.Cao C. Leng Y. Kufe D. Catalase activity is regulated by c-Abl and Arg in the oxidative stress response. J Biol Chem. 2003;278:29667–29675. doi: 10.1074/jbc.M301292200. [DOI] [PubMed] [Google Scholar]
- 45.Carlson MG. Peterson CG. Venge P. Human eosinophil peroxidase: Purification and characterization. J Immunol. 1985;134:1875–1879. [PubMed] [Google Scholar]
- 46.Carter EP. Garat C. Imamura M. Continual emerging roles of HO-1: protection against airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L24–25. doi: 10.1152/ajplung.00097.2004. [DOI] [PubMed] [Google Scholar]
- 47.Carvalho H. Evelson P. Sigaud S. Gonzalez–Flecha B. Mitogen-activated protein kinases modulate H(2)O(2)-induced apoptosis in primary rat alveolar epithelial cells. J Cell Biochem. 2004;92:502–513. doi: 10.1002/jcb.20070. [DOI] [PubMed] [Google Scholar]
- 48.Carver DJ. Gaston B. Deronde K. Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol. 2007;37:255–263. doi: 10.1165/rcmb.2006-0289SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai YC. Hoppe G. Sears J. Reversal of protein S-glutathiolation by glutaredoxin in the retinal pigment epithelium. Exp Eye Res. 2003;76:155–159. doi: 10.1016/s0014-4835(02)00309-3. [DOI] [PubMed] [Google Scholar]
- 50.Chance B. The properties of the enzyme-substrate compounds of horse-radish and lacto-peroxidase. Science. 1949;109:204–208. doi: 10.1126/science.109.2826.204-a. [DOI] [PubMed] [Google Scholar]
- 51.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 52.Chang LY. Crapo JD. Inhibition of airway inflammation and hyperreactivity by an antioxidant mimetic. Free Radic Biol Med. 2002;33:379–386. doi: 10.1016/s0891-5849(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 53.Chang LY. Kang BH. Slot JW. Vincent R. Crapo JD. Immunocytochemical localization of the sites of superoxide dismutase induction by hyperoxia in rat lungs. Lab Invest. 1995;73:29–39. [PubMed] [Google Scholar]
- 54.Cheeseman KH. Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993;49:481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 55.Choi AM. Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 56.Ciencewicki J. Trivedi S. Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. quiz 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clyde BL. Chang LY. Auten RL. Ho YS. Crapo JD. Distribution of manganese superoxide dismutase mRNA in normal and hyperoxic rat lung. Am J Respir Cell Mol Biol. 1993;8:530–537. doi: 10.1165/ajrcmb/8.5.530. [DOI] [PubMed] [Google Scholar]
- 58.Comhair SA. Bhathena PR. Dweik RA. Kavuru M. Erzurum SC. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet. 2000;355:624. doi: 10.1016/S0140-6736(99)04736-4. [DOI] [PubMed] [Google Scholar]
- 59.Comhair SA. Bhathena PR. Farver C. Thunnissen FB. Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: Evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- 60.Comhair SA. Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L246–255. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- 61.Comhair SA. Erzurum SC. The regulation and role of extracellular glutathione peroxidase. Antioxid Redox Signal. 2005;7:72–79. doi: 10.1089/ars.2005.7.72. [DOI] [PubMed] [Google Scholar]
- 62.Comhair SA. Lewis MJ. Bhathena PR. Hammel JP. Erzurum SC. Increased glutathione and glutathione peroxidase in lungs of individuals with chronic beryllium disease. Am J Respir Crit Care Med. 1999;159:1824–1829. doi: 10.1164/ajrccm.159.6.9810044. [DOI] [PubMed] [Google Scholar]
- 63.Comhair SA. Ricci KS. Arroliga M. Lara AR. Dweik RA. Song W. Hazen SL. Bleecker ER. Busse WW. Chung KF. Gaston B. Hastie A. Hew M. Jarjour N. Moore W. Peters S. Teague WG. Wenzel SE. Erzurum SC. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172:306–313. doi: 10.1164/rccm.200502-180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Comhair SA. Thomassen MJ. Erzurum SC. Differential induction of extracellular glutathione peroxidase and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O(2) or cigarette smoke. Am J Respir Cell Mol Biol. 2000;23:350–354. doi: 10.1165/ajrcmb.23.3.4076. [DOI] [PubMed] [Google Scholar]
- 65.Comhair SA. Xu W. Ghosh S. Thunnissen FB. Almasan A. Calhoun WJ. Janocha AJ. Zheng L. Hazen SL. Erzurum SC. Superoxide dismutase inactivation in pathophysiology of asthmatic airway remodeling and reactivity. Am J Pathol. 2005;166:663–674. doi: 10.1016/S0002-9440(10)62288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conner EM. Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 67.Cook DG. Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corradi M. Folesani G. Andreoli R. Manini P. Bodini A. Piacentini G. Carraro S. Zanconato S. Baraldi E. Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation. Am J Resp Crit Care Med. 2003;167:395–399. doi: 10.1164/rccm.200206-507OC. [DOI] [PubMed] [Google Scholar]
- 69.Corradi M. Montuschi P. Donnelly LE. Pesci A. Kharitonov SA. Barnes PJ. Increased nitrosothiols in exhaled breath condensate in inflammatory airway diseases. Am J Respir Crit Care Med. 2001;163:854–858. doi: 10.1164/ajrccm.163.4.2001108. [DOI] [PubMed] [Google Scholar]
- 70.Cosio BG. Mann B. Ito K. Jazrawi E. Barnes PJ. Chung KF. Adcock IM. Histone acetylase and deacetylase activity in alveolar macrophages and blood mononocytes in asthma. Am J Respir Crit Care Med. 2004;170:141–147. doi: 10.1164/rccm.200305-659OC. [DOI] [PubMed] [Google Scholar]
- 71.Cramer R. Soranzo MR. Patriarca P. Evidence that eosinophils catalyze the bromide-dependent decarboxylation of amino acids. Blood. 1981;58:1112–1118. [PubMed] [Google Scholar]
- 72.Crapo JD. Oury T. Rabouille C. Slot JW. Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci USA. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cross CE. Vasu VT. Lim Y. Gohil K. Combating oxidative stress at respiratory tract biosurfaces: Challenges yet to be resolved, a commentary on “Vitamin supplementation does not protect against symptoms in ozone-responsive subjects”. Free Radic Biol Med. 2006;40:1693–1697. doi: 10.1016/j.freeradbiomed.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 74.Crow JP. Sampson JB. Zhuang Y. Thompson JA. Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem. 1997;69:1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 75.Culpitt SV. Rogers DF. Fenwick PS. Shah P. De Matos C. Russell RE. Barnes PJ. Donnelly LE. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuzzocrea S. Riley DP. Caputi AP. Salvemini D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 77.Dahl M. Bowler RP. Juul K. Crapo JD. Levy S. Nordestgaard BG. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med. 2008;178:906–912. doi: 10.1164/rccm.200804-549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dalle-Donne I. Milzani A. Gagliano N. Colombo R. Giustarini D. Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 79.Dalle-Donne I. Rossi R. Giustarini D. Colombo R. Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Das KC. Lewis-Molock Y. White CW. Thiol modulation of TNF alpha and IL-1 induced MnSOD gene expression and activation of NF-kappa B. Mol Cell Biochem. 1995;148:45–57. doi: 10.1007/BF00929502. [DOI] [PubMed] [Google Scholar]
- 81.Davies KJ. Oxidative stress: The paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- 82.De Raeve HR. Thunnissen FB. Kaneko FT. Guo FH. Lewis M. Kavuru MS. Secic M. Thomassen MJ. Erzurum SC. Decreased Cu, Zn-SOD activity in asthmatic airway epithelium: Correction by inhaled corticosteroid in vivo. Am J Physiol. 1997;272:L148–154. doi: 10.1152/ajplung.1997.272.1.L148. [DOI] [PubMed] [Google Scholar]
- 83.Deisseroth A. Dounce AL. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970;50:319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- 84.Deneke SM. Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 85.Diaz M. Achkor H. Titarenko E. Martinez MC. The gene encoding glutathione-dependent formaldehyde dehydrogenase/GSNO reductase is responsive to wounding, jasmonic acid and salicylic acid. FEBS Lett. 2003;543:136–139. doi: 10.1016/s0014-5793(03)00426-5. [DOI] [PubMed] [Google Scholar]
- 86.Dominici S. Valentini M. Maellaro E. Del Bello B. Paolicchi A. Lorenzini E. Tongiani R. Comporti M. Pompella A. Redox modulation of cell surface protein thiols in U937 lymphoma cells: The role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623–635. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 87.Donzelli S. Switzer CH. Thomas DD. Ridnour LA. Espey MG. Isenberg JS. Tocchetti CG. King SB. Lazzarino G. Miranda KM. Roberts DD. Feelisch M. Wink DA. The activation of metabolites of nitric oxide synthase by metals is both redox and oxygen dependent: A new feature of nitrogen oxide signaling. Antioxid Redox Signal. 2006;8:1363–1371. doi: 10.1089/ars.2006.8.1363. [DOI] [PubMed] [Google Scholar]
- 88.Downey GP. Fialkow L. Forman HF, editor; Cadenas E, editor. Reactive oxygen intermediates as signaling molecules. Oxidative Stress and Signal Transduction. 1997:415–441. [Google Scholar]
- 89.Druilhe A. Wallaert B. Tsicopoulos A. Lapa e Silva JR. Tillie-Leblond I. Tonnel AB. Pretolani M. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–757. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- 90.Duan YJ. Komura S. Fiszer-Szafarz B. Szafarz D. Yagi K. Purification and characterization of a novel monomeric glutathione peroxidase from rat liver. J Biol Chem. 1988;263:19003–19008. [PubMed] [Google Scholar]
- 91.Dweik RA. The lung in the balance: Arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;292:L15–17. doi: 10.1152/ajplung.00322.2006. [DOI] [PubMed] [Google Scholar]
- 92.Dweik RA. Comhair SA. Gaston B. Thunnissen FB. Farver C. Thomassen MJ. Kavuru M. Hammel J. Abu–Soud HM. Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dweik RA. Laskowski D. Abu-Soud HM. Kaneko F. Hutte R. Stuehr DJ. Erzurum SC. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101:660–666. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dworski R. Murray JJ. Roberts LJ., 2nd Oates JA. Morrow JD. Fisher L. Sheller JR. Allergen-induced synthesis of F(2)-isoprostanes in atopic asthmatics. Evidence for oxidant stress. Am J Respir Crit Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- 95.Dworski R. Roberts LJ., 2nd Murray JJ. Morrow JD. Hartert TV. Sheller JR. Assessment of oxidant stress in allergic asthma by measurement of the major urinary metabolite of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2alpha) Clin Exp Allergy. 2001;31:387–390. doi: 10.1046/j.1365-2222.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- 96.Eaton P. Protein thiol oxidation in health and disease: Techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med. 2006;40:1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 97.Eisner MD. Klein J. Hammond SK. Koren G. Lactao G. Iribarren C. Directly measured second hand smoke exposure and asthma health outcomes. Thorax. 2005;60:814–821. doi: 10.1136/thx.2004.037283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erzurum SC. Danel C. Gillissen A. Chu CS. Trapnell BC. Crystal RG. In vivo antioxidant gene expression in human airway epithelium of normal individuals exposed to 100% O2. J Appl Physiol. 1993;75:1256–1262. doi: 10.1152/jappl.1993.75.3.1256. [DOI] [PubMed] [Google Scholar]
- 99.Escribano A. Garcia-Grande A. Montanes P. Miralles L. Garcia A. Aerosol orgotein (Ontosein) for the prevention of radiotherapy-induced adverse effects in head and neck cancer patients: A feasibility study. Neoplasma. 2002;49:201–208. [PubMed] [Google Scholar]
- 100.Fattman CL. Schaefer LM. Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 101.Fernandes AP. Holmgren A. Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 102.Filomeni G. Rotilio G. Ciriolo MR. Cell signalling and the glutathione redox system. Biochem Pharmacol. 2002;64:1057–1064. doi: 10.1016/s0006-2952(02)01176-0. [DOI] [PubMed] [Google Scholar]
- 103.Foote CS. Goyne TE. Lehrer RI. Assessment of chlorination by human neutrophils. Nature. 1983;301:715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- 104.Foresi A. Bertorelli G. Pesci A. Chetta A. Olivieri D. Inflammatory markers in bronchoalveolar lavage and in bronchial biopsy in asthma during remission. Chest. 1990;98:528–535. doi: 10.1378/chest.98.3.528. [DOI] [PubMed] [Google Scholar]
- 105.Fradette C. Yamaguchi N. Du Souich P. 5-Hydroxytryptamine is biotransformed by CYP2C9, 2C19 and 2B6 to hydroxylamine, which is converted into nitric oxide. Br J Pharmacol. 2004;141:407–414. doi: 10.1038/sj.bjp.0705632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freedman JE. Frei B. Welch GN. Loscalzo J. Glutathione peroxidase potentiates the inhibition of platelet function by S-nitrosothiols. J Clin Invest. 1995;96:394–400. doi: 10.1172/JCI118047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 108.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 109.Fridovich I. Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- 110.Gao F. Kinnula VL. Myllarniemi M. Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal. 2008;10:343–354. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 112.Gardner PR. Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 113.Gaston B. Drazen JM. Loscalzo J. Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med. 1994;149:538–551. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 114.Gaston B. Sears S. Woods J. Hunt J. Ponaman M. McMahon T. Stamler JS. Bronchodilator S-nitrosothiol deficiency in asthmatic respiratory failure. Lancet. 1998;351:1317–1319. doi: 10.1016/S0140-6736(97)07485-0. [DOI] [PubMed] [Google Scholar]
- 115.Gaston B. Stamler JS. Nitrogen oxides and lung function. In: Crystal R, editor; West J, editor; Weibel E, editor; Barnes , editor. The Lung: Scientific Foundations. 2nd ed. Philadelphia: Lippincott Raven; 1997. pp. 239–253. [Google Scholar]
- 116.Gazdik F. Gvozdjakova A. Nadvornikova R. Repicka L. Jahnova E. Kucharska J. Pijak MR. Gazdikova K. Decreased levels of coenzyme Q(10) in patients with bronchial asthma. Allergy. 2002;57:811–814. doi: 10.1034/j.1398-9995.2002.23747.x. [DOI] [PubMed] [Google Scholar]
- 117.Ghibelli L. Coppola S. Fanelli C. Rotilio G. Civitareale P. Scovassi AI. Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- 118.Ghibelli L. Fanelli C. Rotilio G. Lafavia E. Coppola S. Colussi C. Civitareale P. Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh S. Janocha AJ. Aronica MA. Swaidani S. Comhair SA. Xu W. Zheng L. Kaveti S. Kinter M. Hazen SL. Erzurum SC. Nitrotyrosine proteome survey in asthma identifies oxidative mechanism of catalase inactivation. J Immunol. 2006;176:5587–5597. doi: 10.4049/jimmunol.176.9.5587. [DOI] [PubMed] [Google Scholar]
- 120.Ghosh S. Masri F. Comhair S. Andreadis A. Swaidani S. Aronica M. Aulak K. Erzurum S. Nitration of proteins in murine model of asthma. Am J Respir Crit Care Med. 2003;167:A889. [Google Scholar]
- 121.Gilliland FD. Gauderman WJ. Vora H. Rappaport E. Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med. 2002;166:710–716. doi: 10.1164/rccm.2112065. [DOI] [PubMed] [Google Scholar]
- 122.Giustarini D. Dalle-Donne I. Lorenzini S. Milzani A. Rossi R. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. J Gerontol. 2006;61:1030–1038. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 123.Gleich GJ. Ottesen EA. Leiferman KM. Ackerman SJ. Eosinophils and human disease. Int Arch Allergy Appl Immunol. 1989;88:59–62. doi: 10.1159/000234749. [DOI] [PubMed] [Google Scholar]
- 124.Goeptar AR. Scheerens H. Vermeulen NP. Oxygen and xenobiotic reductase activities of cytochrome P450. Crit Rev Toxicol. 1995;25:25–65. doi: 10.3109/10408449509089886. [DOI] [PubMed] [Google Scholar]
- 125.Goss SP. Singh RJ. Kalyanaraman B. Bicarbonate enhances the peroxidase activity of Cu,Zn-superoxide dismutase. Role of carbonate anion radical. J Biol Chem. 1999;274:28233–28239. doi: 10.1074/jbc.274.40.28233. [DOI] [PubMed] [Google Scholar]
- 126.Guo FH. Comhair SA. Zheng S. Dweik RA. Eissa NT. Thomassen MJ. Calhoun W. Erzurum SC. Molecular mechanisms of increased nitric oxide (NO) in asthma: Evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164:5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 127.Guo FH. De Raeve HR. Rice TW. Stuehr DJ. Thunnissen FB. Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo FH. Erzurum SC. Characterization of inducible nitric oxide synthase expression in human airway epithelium. Environ Health Perspect. 1998;106(Suppl 5):1119–1124. doi: 10.1289/ehp.98106s51119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo FH. Uetani K. Haque SJ. Williams BR. Dweik RA. Thunnissen FB. Calhoun W. Erzurum SC. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J Clin Invest. 1997;100:829–838. doi: 10.1172/JCI119598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gvozdjakova A. Kucharska J. Bartkovjakova M. Gazdikova K. Gazdik FE. Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. BioFactors (Oxford, England) 2005;25:235–240. doi: 10.1002/biof.5520250129. [DOI] [PubMed] [Google Scholar]
- 131.Haahtela T. Airway remodelling takes place in asthma—What are the clinical implications? Clin Exp Allergy. 1997;27:351–353. [PubMed] [Google Scholar]
- 132.Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 133.Halliwell B. Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 134.Hamann KJ. Barker RL. Ten RM. Gleich GJ. The molecular biology of eosinophil granule proteins. Int Arch Allergy Appl Immunol. 1991;94:202–209. doi: 10.1159/000235362. [DOI] [PubMed] [Google Scholar]
- 135.Harrison DG. Chapman MP. Christy JP. Marcus ML. Studies of functional site of origin of native coronary collaterals. Am J Physiol. 1986;251:H1217–1224. doi: 10.1152/ajpheart.1986.251.6.H1217. [DOI] [PubMed] [Google Scholar]
- 136.Hart LA. Krishnan VL. Adcock IM. Barnes PJ. Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Resp Critical Care Med. 1998;158:1585–1592. doi: 10.1164/ajrccm.158.5.9706116. [DOI] [PubMed] [Google Scholar]
- 137.Hazen SL. Hsu FF. Gaut JP. Crowley JR. Heinecke JW. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol. 1999;300:88–105. doi: 10.1016/s0076-6879(99)00117-2. [DOI] [PubMed] [Google Scholar]
- 138.Hazen SL. Zhang R. Shen Z. Wu W. Podrez EA. MacPherson JC. Schmitt D. Mitra SN. Mukhopadhyay C. Chen Y. Cohen PA. Hoff HF. Abu-Soud HM. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: Pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 139.Heffner JE. Repine JE. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989;140:531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- 140.Henderson WR., Jr. Chi EY. Teo JL. Nguyen C. Kahn M. A small molecule inhibitor of redox-regulated NF-kappa B and activator protein-1 transcription blocks allergic airway inflammation in a mouse asthma model. J Immunol. 2002;169:5294–5299. doi: 10.4049/jimmunol.169.9.5294. [DOI] [PubMed] [Google Scholar]
- 141.Higashi N. Mita H. Taniguchi M. Turikisawa N. Higashi A. Ozawa Y. Tohma S. Arimura K. Akiyama K. Urinary eicosanoid and tyrosine derivative concentrations in patients with vasculitides. J Allergy Clin Immunol. 2004;114:1353–1358. doi: 10.1016/j.jaci.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 142.Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989;264:13963–13966. [PubMed] [Google Scholar]
- 143.Horton MA. Larson KA. Lee JJ. Lee NA. Cloning of the murine eosinophil peroxidase gene (mEPO): Characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J Leukoc Biol. 1996;60:285–294. doi: 10.1002/jlb.60.2.285. [DOI] [PubMed] [Google Scholar]
- 144.Horwitz RJ. Busse WW. Inflammation and asthma. Clin Chest Med. 1995;16:583–602. [PubMed] [Google Scholar]
- 145.Hoshino T. Okamoto M. Takei S. Sakazaki Y. Iwanaga T. Aizawa H. Redox-regulated mechanisms in asthma. Antioxid Redox Signal. 2008;10:769–783. doi: 10.1089/ars.2007.1936. [DOI] [PubMed] [Google Scholar]
- 146.Hou Y. Guo Z. Li J. Wang PG. Seleno compounds and glutathione peroxidase catalyzed decomposition of S-nitrosothiols. Biochem Biophys Res Commun. 1996;228:88–93. doi: 10.1006/bbrc.1996.1620. [DOI] [PubMed] [Google Scholar]
- 147.Hu FB. Persky V. Flay BR. Richardson J. An epidemiological study of asthma prevalence and related factors among young adults. J Asthma. 1997;34:67–76. doi: 10.3109/02770909709071205. [DOI] [PubMed] [Google Scholar]
- 148.Hu X. Xia H. Srivastava SK. Herzog C. Awasthi YC. Ji X. Zimniak P. Singh SV. Activity of four allelic forms of glutathione S-transferase hGSTP1-1 for diol epoxides of polycyclic aromatic hydrocarbons. Biochem Biophys Res Commun. 1997;238:397–402. doi: 10.1006/bbrc.1997.7311. [DOI] [PubMed] [Google Scholar]
- 149.Hunt JF. Fang K. Malik R. Snyder A. Malhotra N. Platts–Mills TA. Gaston B. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 150.Hurd TR. Filipovska A. Costa NJ. Dahm CC. Murphy MP. Disulphide formation on mitochondrial protein thiols. Biochem Soc Trans. 2005;33:1390–1393. doi: 10.1042/BST0331390. [DOI] [PubMed] [Google Scholar]
- 151.Ignarro LJ. Buga GM. Wei LH. Bauer PM. Wu G. del Soldato P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Iijima H. Duguet A. Eum SY. Hamid Q. Eidelman DH. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am J Respir Crit Care Med. 2001;163:1233–1240. doi: 10.1164/ajrccm.163.5.2003145. [DOI] [PubMed] [Google Scholar]
- 153.Immenschuh S. Baumgart-Vogt E. Peroxiredoxins, oxidative stress, and cell proliferation. Antioxid Redox Signal. 2005;7:768–777. doi: 10.1089/ars.2005.7.768. [DOI] [PubMed] [Google Scholar]
- 154.Institute NNhlab. National asthma eduction and prevention program. Expert panel report 2:Guidelines for the diagnosis and management of asthma. 1997. p. 4051. p. Publication no. 97.
- 155.Islam T. Berhane K. McConnell R. Gauderman WJ. Avol E. Peters JM. Gilliland FD. Glutathione-S-transferase (GST) P1, GSTM1, exercise, ozone and asthma incidence in school children. Thorax. 2009;64:197–202. doi: 10.1136/thx.2008.099366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaakkola MS. Jaakkola JJ. Effects of environmental tobacco smoke on the respiratory health of adults. Scand J Work Environ Health. 2002;28(Suppl 2):52–70. [PubMed] [Google Scholar]
- 157.Jaakkola MS. Jaakkola JJ. Impact of smoke-free workplace legislation on exposures and health: Possibilities for prevention. Eur Respir J. 2006;28:397–408. doi: 10.1183/09031936.06.00001306. [DOI] [PubMed] [Google Scholar]
- 158.Janson C. Chinn S. Jarvis D. Zock JP. Toren K. Burney P. Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: A cross-sectional study. Lancet. 2001;358:2103–2109. doi: 10.1016/S0140-6736(01)07214-2. [DOI] [PubMed] [Google Scholar]
- 159.Jarjour NN. Busse WW. Calhoun WJ. Enhanced production of oxygen radicals in nocturnal asthma. Am Rev Respir Dis. 1992;146:905–911. doi: 10.1164/ajrccm/146.4.905. [DOI] [PubMed] [Google Scholar]
- 160.Jarjour NN. Calhoun WJ. Enhanced production of oxygen radicals in asthma. J Lab Clin Med. 1994;123:131–136. [PubMed] [Google Scholar]
- 161.Jatakanon A. Uasuf C. Maziak W. Lim S. Chung KF. Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 162.Jiang Q. Christen S. Shigenaga MK. Ames BN. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 163.Jindal SK. Gupta D. Singh A. Indices of morbidity and control of asthma in adult patients exposed to environmental tobacco smoke. Chest. 1994;106:746–749. doi: 10.1378/chest.106.3.746. [DOI] [PubMed] [Google Scholar]
- 164.Jindal SK. Jha LK. Gupta D. Bronchial hyper-responsiveness of women with asthma exposed to environmental tobacco smoke. Indian J Chest Dis Allied Sci. 1999;41:75–82. [PubMed] [Google Scholar]
- 165.Jorres R. Nowak D. Magnussen H. The effect of ozone exposure on allergen responsiveness in subjects with asthma or rhinitis. Am J Respir Crit Care Med. 1996;153:56–64. doi: 10.1164/ajrccm.153.1.8542163. [DOI] [PubMed] [Google Scholar]
- 166.Jourd'heuil D. Laroux FS. Miles AM. Wink DA. Grisham MB. Effect of superoxide dismutase on the stability of S-nitrosothiols. Arch Biochem Biophys. 1999;361:323–330. doi: 10.1006/abbi.1998.1010. [DOI] [PubMed] [Google Scholar]
- 167.Jungas T. Motta I. Duffieux F. Fanen P. Stoven V. Ojcius DM. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:27912–27918. doi: 10.1074/jbc.M110288200. [DOI] [PubMed] [Google Scholar]
- 168.Juul K. Tybjaerg-Hansen A. Marklund S. Lange P. Nordestgaard BG. Genetically increased antioxidative protection and decreased chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:858–864. doi: 10.1164/rccm.200509-1387OC. [DOI] [PubMed] [Google Scholar]
- 169.Kafoury RM. Pryor WA. Squadrito GL. Salgo MG. Zou X. Friedman M. Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am J Respir Crit Care Med. 1999;160:1934–1942. doi: 10.1164/ajrccm.160.6.9902025. [DOI] [PubMed] [Google Scholar]
- 170.Kafoury RM. Pryor WA. Squadrito GL. Salgo MG. Zou X. Friedman M. Lipid ozonation products activate phospholipases A2, C, and D. Toxicol App Pharmacol. 1998;150:338–349. doi: 10.1006/taap.1998.8418. [DOI] [PubMed] [Google Scholar]
- 171.Karlsson K. Marklund SL. Heparin-, dextran sulfate- and protamine-induced release of extracellular-superoxide dismutase to plasma in pigs. Biochim Biophys Acta. 1988;967:110–114. doi: 10.1016/0304-4165(88)90195-x. [DOI] [PubMed] [Google Scholar]
- 172.Kharitonov SA. Yates D. Robbins RA. Logan–Sinclair R. Shinebourne EA. Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/s0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- 173.Khatri SB. Hammel J. Kavuru MS. Erzurum SC. Dweik RA. Temporal association of nitric oxide levels and airflow in asthma after whole lung allergen challenge. J Appl Physiol. 2003;95:436–440. doi: 10.1152/japplphysiol.01127.2002. discussion 435. [DOI] [PubMed] [Google Scholar]
- 174.Khatri SB. Ozkan M. McCarthy K. Laskowski D. Hammel J. Dweik RA. Erzurum SC. Alterations in exhaled gas profile during allergen-induced asthmatic response. Am J Respir Crit Care Med. 2001;164:1844–1848. doi: 10.1164/ajrccm.164.10.2106119. [DOI] [PubMed] [Google Scholar]
- 175.Kierstein S. Krytska K. Sharma S. Amrani Y. Salmon M. Panettieri RA., Jr. Zangrilli J. Haczku A. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy. 2008;63:438–446. doi: 10.1111/j.1398-9995.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 176.Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax. 2005;60:693–700. doi: 10.1136/thx.2004.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kinnula VL. Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 178.Kinnula VL. Everitt JI. Whorton AR. Crapo JD. Hydrogen peroxide production by alveolar type II cells, alveolar macrophages, and endothelial cells. Am J Physiol. 1991;261:L84–91. doi: 10.1152/ajplung.1991.261.2.L84. [DOI] [PubMed] [Google Scholar]
- 179.Kinnula VL. Lehtonen S. Kaarteenaho-Wiik R. Lakari E. Paakko P. Kang SW. Rhee SG. Soini Y. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax. 2002;57:157–164. doi: 10.1136/thorax.57.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kinnula VL. Lehtonen S. Koistinen P. Kakko S. Savolainen M. Kere J. Ollikainen V. Laitinen T. Two functional variants of the superoxide dismutase genes in Finnish families with asthma. Thorax. 2004;59:116–119. doi: 10.1136/thorax.2003.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kirkman HN. Rolfo M. Ferraris AM. Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274:13908–13914. doi: 10.1074/jbc.274.20.13908. [DOI] [PubMed] [Google Scholar]
- 182.Klatt P. Molina EP. De Lacoba MG. Padilla CA. Martinez–Galesteo E. Barcena JA. Lamas S. Redox regulation of c-Jun DNA binding by reversible S-glutathiolation. FASEB J. 1999;13:1481–1490. doi: 10.1096/fasebj.13.12.1481. [DOI] [PubMed] [Google Scholar]
- 183.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93:480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 184.Klebanoff SJ. Hamon CB. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972;12:170–196. [PubMed] [Google Scholar]
- 185.Klebanoff SJCRA. The Neutrophil: Function and Clinical Disorders. Amsterdam: Elsevier/North Holland Biomedical Press; 1978. [Google Scholar]
- 186.Kliment CR. Englert JM. Gochuico BR. Yu G. Kaminski N. Rosas I. Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kobzik L. Bredt DS. Lowenstein CJ. Drazen J. Gaston B. Sugarbaker D. Stamler JS. Nitric oxide synthase in human and rat lung: Immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 188.Kode A. Rajendrasozhan S. Caito S. Yang SR. Megson IL. Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 189.Koh YY. Dupuis R. Pollice M. Albertine KH. Fish JE. Peters SP. Neutrophils recruited to the lungs of humans by segmental antigen challenge display a reduced chemotactic response to leukotriene B4. Am J Respir Cell Mol Biol. 1993;8:493–499. doi: 10.1165/ajrcmb/8.5.493. [DOI] [PubMed] [Google Scholar]
- 190.Koren HS. Bromberg PA. Respiratory responses of asthmatics to ozone. Intl Arch Allergy Immunol. 1995;107:236–238. doi: 10.1159/000236989. [DOI] [PubMed] [Google Scholar]
- 191.Kunzli N. Schwartz J. Stutz EZ. Ackermann-Liebrich U. Leuenberger P. Association of environmental tobacco smoke at work and forced expiratory lung function among never smoking asthmatics and non-asthmatics. The SAPALDIA-Team. Swiss Study on Air Pollution and Lung Disease in Adults. Soz Praventivmed. 2000;45:208–217. doi: 10.1007/BF01306015. [DOI] [PubMed] [Google Scholar]
- 192.Kuroki M. Voest EE. Amano S. Beerepoot LV. Takashima S. Tolentino M. Kim RY. Rohan RM. Colby KA. Yeo KT. Adamis AP. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J Clin Invest. 1996;98:1667–1675. doi: 10.1172/JCI118962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lakari E. Paakko P. Kinnula VL. Manganese superoxide dismutase, but not CuZn superoxide dismutase, is highly expressed in the granulomas of pulmonary sarcoidosis and extrinsic allergic alveolitis. Am J Respir Crit Care Med. 1998;158:589–596. doi: 10.1164/ajrccm.158.2.9711059. [DOI] [PubMed] [Google Scholar]
- 194.Lakari E. Paakko P. Pietarinen-Runtti P. Kinnula VL. Manganese superoxide dismutase and catalase are coordinately expressed in the alveolar region in chronic interstitial pneumonias and granulomatous diseases of the lung. Am J Resp Crit Care Med. 2000;161:615–621. doi: 10.1164/ajrccm.161.2.9904091. [DOI] [PubMed] [Google Scholar]
- 195.Lancaster JR., Jr. Gaston B. NO and nitrosothiols: Spatial confinement and free diffusion. Am J Physiol Lung Cell Mol Physiol. 2004;287:L465–466. doi: 10.1152/ajplung.00151.2004. [DOI] [PubMed] [Google Scholar]
- 196.Lane C. Knight D. Burgess S. Franklin P. Horak F. Legg J. Moeller A. Stick S. Epithelial inducible nitric oxide synthase activity is the major determinant of nitric oxide concentration in exhaled breath. Thorax. 2004;59:757–760. doi: 10.1136/thx.2003.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Larsen GL. White CW. Takeda K. Loader JE. Nguyen DD. Joetham A. Groner Y. Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol. 2000;279:L350–359. doi: 10.1152/ajplung.2000.279.2.L350. [DOI] [PubMed] [Google Scholar]
- 198.Larsson ML. Loit HM. Meren M. Polluste J. Magnusson A. Larsson K. Lundback B. Passive smoking and respiratory symptoms in the FinEsS Study. Eur Respir J. 2003;21:672–676. doi: 10.1183/09031936.03.00033702. [DOI] [PubMed] [Google Scholar]
- 199.Lee CG. Link H. Baluk P. Homer RJ. Chapoval S. Bhandari V. Kang MJ. Cohn L. Kim YK. McDonald DM. Elias JA. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee YL. Hsiue TR. Lee YC. Lin YC. Guo YL. The association between glutathione S-transferase P1, M1 polymorphisms and asthma in Taiwanese schoolchildren. Chest. 2005;128:1156–1162. doi: 10.1378/chest.128.3.1156. [DOI] [PubMed] [Google Scholar]
- 201.Lehtonen ST. Ohlmeier S. Kaarteenaho-Wiik R. Harju T. Paakko P. Soini Y. Kinnula VL. Does the oxidative stress in chronic obstructive pulmonary disease cause thioredoxin/peroxiredoxin oxidation? Antioxid Redox Signal. 2008;10:813–819. doi: 10.1089/ars.2007.1952. [DOI] [PubMed] [Google Scholar]
- 202.Leikauf GD. Zhao Q. Zhou S. Santrock J. Ozonolysis products of membrane fatty acids activate eicosanoid metabolism in human airway epithelial cells. Am J Respir Cell Mol Biol. 1993;9:594–602. doi: 10.1165/ajrcmb/9.6.594. [DOI] [PubMed] [Google Scholar]
- 203.Leonard SS. Xia C. Jiang BH. Stinefelt B. Klandorf H. Harris GK. Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 204.Leuenberger P. Schwartz J. Ackermann-Liebrich U. Blaser K. Bolognini G. Bongard JP. Brandli O. Braun P. Bron C. Brutsche M, et al. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am J Respir Crit Care Med. 1994;150:1222–1228. doi: 10.1164/ajrccm.150.5.7952544. [DOI] [PubMed] [Google Scholar]
- 205.Li X. Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 206.Li Y. Huang TT. Carlson EJ. Melov S. Ursell PC. Olson JL. Noble LJ. Yoshimura MP. Berger C. Chan PH, et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 207.Lillig CH. Berndt C. Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 208.Lind C. Gerdes R. Hamnell Y. Schuppe-Koistinen I. von Lowenhielm HB. Holmgren A. Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys. 2002;406:229–240. doi: 10.1016/s0003-9861(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 209.Liu H. Colavitti R. Rovira II. Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 210.London SJ. Gene-air pollution interactions in asthma. Proc Am Thor Soc. 2007;4:217–220. doi: 10.1513/pats.200701-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lundberg JO. Farkas-Szallasi T. Weitzberg E. Rinder J. Lidholm J. Anggaard A. Hokfelt T. Lundberg JM. Alving K. High nitric oxide production in human paranasal sinuses. Nat Med. 1995;1:370–373. doi: 10.1038/nm0495-370. [DOI] [PubMed] [Google Scholar]
- 212.Lundstrom J. Holmgren A. Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem. 1990;265:9114–9120. [PubMed] [Google Scholar]
- 213.MacMillan-Crow LA. Crow JP. Kerby JD. Beckman JS. Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.MacMillan-Crow LA. Crow JP. Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 215.Macmillan-Crow LA. Cruthirds DL. Invited review: Manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 216.MacMillan-Crow LA. Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 217.MacPherson JC. Comhair SA. Erzurum SC. Klein DF. Lipscomb MF. Kavuru MS. Samoszuk MK. Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: Characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 218.Maehly AC. Chance B. The assay of catalases and peroxidases. Meth Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- 219.Marklund SL. Holme E. Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126:41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- 220.Marklund SL. Karlsson K. Therapy and Preventive Medicine. New York: Plenum Press; 1990. Extracellular-superoxide dismutase, distribution in the body and therapeutic applications; pp. 1–4. edited by al. ELe. [DOI] [PubMed] [Google Scholar]
- 221.Marks–Konczalik J. Chu SC. Moss J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor kappaB-binding sites. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 222.Martin RJ. Cicutto LC. Smith HR. Ballard RD. Szefler SJ. Airways inflammation in nocturnal asthma. Am Rev Respir Dis. 1991;143:351–357. doi: 10.1164/ajrccm/143.2.351. [DOI] [PubMed] [Google Scholar]
- 223.Masini E. Bani D. Vannacci A. Pierpaoli S. Mannaioni PF. Comhair SA. Xu W. Muscoli C. Erzurum SC. Salvemini D. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med. 2005;39:520–531. doi: 10.1016/j.freeradbiomed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 224.Masri FA. Comhair SA. Koeck T. Xu W. Janocha A. Ghosh S. Dweik RA. Golish J. Kinter M. Stuehr DJ. Erzurum SC. Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005;172:597–605. doi: 10.1164/rccm.200411-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Matsue H. Edelbaum D. Shalhevet D. Mizumoto N. Yang C. Mummert ME. Oeda J. Masayasu H. Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 226.Mayeno AN. Curran AJ. Roberts RL. Foote CS. Eosinophils preferentially use bromide to generate halogenating agents. J Biol Chem. 1989;264:5660–5668. [PubMed] [Google Scholar]
- 227.McCay PB. Vitamin E: interactions with free radicals and ascorbate. Annu Rev Nutr. 1985;5:323–340. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- 228.McCord JM. Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 229.McCreanor J. Cullinan P. Nieuwenhuijsen MJ. Stewart–Evans J. Malliarou E. Jarup L. Harrington R. Svartengren M. Han IK. Ohman-Strickland P. Chung KF. Zhang J. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med. 2007;357:2348–2358. doi: 10.1056/NEJMoa071535. [DOI] [PubMed] [Google Scholar]
- 230.Meister A. Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 231.Melov S. Ravenscroft J. Malik S. Gill MS. Walker DW. Clayton PE. Wallace DC. Malfroy B. Doctrow SR. Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 232.Menzies D. Nair A. Williamson PA. Schembri S. Al-Khairalla MZ. Barnes M. Fardon TC. McFarlane L. Magee GJ. Lipworth BJ. Respiratory symptoms, pulmonary function, and markers of inflammation among bar workers before and after a legislative ban on smoking in public places. JAMA. 2006;296:1742–1748. doi: 10.1001/jama.296.14.1742. [DOI] [PubMed] [Google Scholar]
- 233.Mezzetti A. Lapenna D. Pierdomenico SD. Calafiore AM. Costantini F. Riario-Sforza G. Imbastaro T. Neri M. Cuccurullo F. Vitamins E, C and lipid peroxidation in plasma and arterial tissue of smokers and non-smokers. Atherosclerosis. 1995;112:91–99. doi: 10.1016/0021-9150(94)05403-6. [DOI] [PubMed] [Google Scholar]
- 234.Migler R. DeChatelet LR. Human eosinophilic peroxidase: Biochemical characterization. Biochem Med. 1978;19:16–26. doi: 10.1016/0006-2944(78)90003-0. [DOI] [PubMed] [Google Scholar]
- 235.Mikerov AN. Umstead TM. Gan X. Huang W. Guo X. Wang G. Phelps DS. Floros J. Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am J Physiol Lung Cell Mol Physiol. 2008;294:L121–130. doi: 10.1152/ajplung.00288.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Mita H. Higashi N. Taniguchi M. Higashi A. Akiyama K. Increase in urinary leukotriene B4 glucuronide concentration in patients with aspirin-intolerant asthma after intravenous aspirin challenge. Clin Exp Allergy. 2004;34:1262–1269. doi: 10.1111/j.1365-2222.2004.02034.x. [DOI] [PubMed] [Google Scholar]
- 237.Mondino C. Ciabattoni G. Koch P. Pistelli R. Trove A. Barnes PJ. Montuschi P. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol. 2004;114:761–767. doi: 10.1016/j.jaci.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 238.Montefort S. Gratziou C. Goulding D. Polosa R. Haskard DO. Howarth PH. Holgate ST. Carroll MP. Bronchial biopsy evidence for leukocyte infiltration and upregulation of leukocyte-endothelial cell adhesion molecules 6 hours after local allergen challenge of sensitized asthmatic airways. J Clin Invest. 1994;93:1411–1421. doi: 10.1172/JCI117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Montuschi P. Barnes PJ. Isoprostanes and asthma. Drug Discov Today: Therap Strat. 2006;3:287–292. [Google Scholar]
- 240.Montuschi P. Corradi M. Ciabattoni G. Nightingale J. Kharitonov SA. Barnes PJ. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am J Respir Crit Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- 241.Montuschi P. Mondino C. Koch P. Barnes PJ. Ciabattoni G. Effects of a leukotriene receptor antagonist on exhaled leukotriene E4 and prostanoids in children with asthma. J Allergy Clin Immunol. 2006;118:347–353. doi: 10.1016/j.jaci.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 242.Montuschi P. Nightingale JA. Kharitonov SA. Barnes PJ. Ozone-induced increase in exhaled 8-isoprostane in healthy subjects is resistant to inhaled budesonide. Free Radic Biol Med. 2002;33:1403–1408. doi: 10.1016/s0891-5849(02)01084-5. [DOI] [PubMed] [Google Scholar]
- 243.Moore WC. Bleecker ER. Curran-Everett D. Erzurum SC. Ameredes BT. Bacharier L. Calhoun WJ. Castro M. Chung KF. Clark MP. Dweik RA. Fitzpatrick AM. Gaston B. Hew M. Hussain I. Jarjour NN. Israel E. Levy BD. Murphy JR. Peters SP. Teague WG. Meyers DA. Busse WW. Wenzel SE. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mudway IS. Kelly FJ. Ozone and the lung: A sensitive issue. Mol Aspects Med. 2000;21:1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 245.Nakamura H. De Rosa SC. Yodoi J. Holmgren A. Ghezzi P. Herzenberg LA. Herzenberg LA. Chronic elevation of plasma thioredoxin: Inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc Natl Acad Sci USA. 2001;98:2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nakamura H. Nakamura K. Yodoi J. Redox regulation of cellular activation. Ann Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 247.Nakamura T. Nakamura H. Hoshino T. Ueda S. Wada H. Yodoi J. Redox regulation of lung inflammation by thioredoxin. Antioxid Redox Signal. 2005;7:60–71. doi: 10.1089/ars.2005.7.60. [DOI] [PubMed] [Google Scholar]
- 248.Nguyen C. Teo JL. Matsuda A. Eguchi M. Chi EY. Henderson WR., Jr. Kahn M. Chemogenomic identification of Ref-1/AP-1 as a therapeutic target for asthma. Proc Natl Acad Sci USA. 2003;100:1169–1173. doi: 10.1073/pnas.0437889100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nickel R. Haider A. Sengler C. Lau S. Niggemann B. Deichmann KA. Wahn U. Heinzmann A. Association study of glutathione S-transferase P1 (GSTP1) with asthma and bronchial hyper-responsiveness in two German pediatric populations. Pediatr Allergy Immunol. 2005;16:539–541. doi: 10.1111/j.1399-3038.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 250.Nordberg J. Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 251.Novak Z. Nemeth I. Gyurkovits K. Varga SI. Matkovics B. Examination of the role of oxygen free radicals in bronchial asthma in childhood. Clin Chim Acta. 1991;201:247–251. doi: 10.1016/0009-8981(91)90375-m. [DOI] [PubMed] [Google Scholar]
- 252.O'Connor GT. Weiss ST. Tager IB. Speizer FE. The effect of passive smoking on pulmonary function and nonspecific bronchial responsiveness in a population-based sample of children and young adults. Am Rev Respir Dis. 1987;135:800–804. doi: 10.1164/arrd.1987.135.4.800. [DOI] [PubMed] [Google Scholar]
- 253.O'Sullivan MP. Tyner JW. Holtzman MJ. Apoptosis in the airways: Another balancing act in the epithelial program. Am J Respir Cell Mol Biol. 2003;29:3–7. doi: 10.1165/rcmb.F273. [DOI] [PubMed] [Google Scholar]
- 254.Olsen RL. Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983;209:781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Olsson I. Persson AM. Stromberg K. Winqvist I. Tai PC. Spry CJ. Purification of eosinophil peroxidase and studies of biosynthesis and processing in human marrow cells. Blood. 1985;66:1143–1148. [PubMed] [Google Scholar]
- 256.Oury TD. Chang LY. Marklund SL. Day BJ. Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 257.Oury TD. Day BJ. Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Rad Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 258.Oury TD. Schaefer LM. Fattman CL. Choi A. Weck KE. Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- 259.Paredi P. Kharitonov SA. Barnes PJ. Analysis of expired air for oxidation products. Am J Respir Crit Care Med. 2002;166:S31–37. doi: 10.1164/rccm.2206012. [DOI] [PubMed] [Google Scholar]
- 260.Pascual RM. Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: An overview. J Allergy Clin Immunol. 2005;116:477–486. doi: 10.1016/j.jaci.2005.07.011. quiz 487. [DOI] [PubMed] [Google Scholar]
- 261.Peden DB. Boehlecke B. Horstman D. Devlin R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J Allergy Clin Immunol. 1997;100:802–808. doi: 10.1016/s0091-6749(97)70277-x. [DOI] [PubMed] [Google Scholar]
- 262.Peltoniemi M. Kaarteenaho-Wiik R. Saily M. Sormunen R. Paakko P. Holmgren A. Soini Y. Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Human Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 263.Perrin-Nadif R. Auburtin G. Dusch M. Porcher JM. Mur JM. Blood antioxidant enzymes as markers of exposure or effect in coal miners. Occup Environ Med. 1996;53:41–45. doi: 10.1136/oem.53.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Persson MG. Zetterstrom O. Agrenius V. Ihre E. Gustafsson LE. Single-breath nitric oxide measurements in asthmatic patients and smokers. Lancet. 1994;343:146–147. doi: 10.1016/s0140-6736(94)90935-0. [DOI] [PubMed] [Google Scholar]
- 265.Peterson JD. Herzenberg LA. Vasquez K. Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pietarinen-Runtti P. Raivio KO. Saksela M. Asikainen TM. Kinnula VL. Antioxidant enzyme regulation and resistance to oxidants of human bronchial epithelial cells cultured under hyperoxic conditions. Am J Respir Cell Mol Biol. 1998;19:286–292. doi: 10.1165/ajrcmb.19.2.2836. [DOI] [PubMed] [Google Scholar]
- 267.Podrez EA. Schmitt D. Hoff HF. Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Poss KD. Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Postma DS. Renkema TE. Noordhoek JA. Faber H. Sluiter HJ. Kauffman H. Association between nonspecific bronchial hyperreactivity and superoxide anion production by polymorphonuclear leukocytes in chronic air-flow obstruction. Am Rev Respir Dis. 1988;137:57–61. doi: 10.1164/ajrccm/137.1.57. [DOI] [PubMed] [Google Scholar]
- 270.Pou S. Keaton L. Surichamorn W. Rosen GM. Mechanism of superoxide generation by neuronal nitric-oxide synthase. J Biol Chem. 1999;274:9573–9580. doi: 10.1074/jbc.274.14.9573. [DOI] [PubMed] [Google Scholar]
- 271.Pou S. Pou WS. Bredt DS. Snyder SH. Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 272.Powis G. Metabolism and reactions of quinoid anticancer agents. Pharmacol Ther. 1987;35:57–162. doi: 10.1016/0163-7258(87)90105-7. [DOI] [PubMed] [Google Scholar]
- 273.Que LG. Liu L. Yan Y. Whitehead GS. Gavett SH. Schwartz DA. Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rahman I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: Antioxidant therapeutic targets. Current Drug Targets. 2002;1:291–315. doi: 10.2174/1568010023344607. [DOI] [PubMed] [Google Scholar]
- 275.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36:95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- 276.Rahman I. Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 277.Rahman I. Antonicelli F. MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. J Biol Chem. 1999;274:5088–5096. doi: 10.1074/jbc.274.8.5088. [DOI] [PubMed] [Google Scholar]
- 278.Rahman I. Bel A. Mulier B. Donaldson K. MacNee W. Differential regulation of glutathione by oxidants and dexamethasone in alveolar epithelial cells. Am J Physiol. 1998;275:L80–86. doi: 10.1152/ajplung.1998.275.1.L80. [DOI] [PubMed] [Google Scholar]
- 279.Rahman I. Biswas SK. Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 280.Rahman I. MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- 281.Rahman I. Smith CA. Lawson MF. Harrison DJ. MacNee W. Induction of gamma-glutamylcysteine synthetase by cigarette smoke is associated with AP-1 in human alveolar epithelial cells. FEBS Lett. 1996;396:21–25. doi: 10.1016/0014-5793(96)01027-7. [DOI] [PubMed] [Google Scholar]
- 282.Rahman I. Swarska E. Henry M. Stolk J. MacNee W. Is there any relationship between plasma antioxidant capacity and lung function in smokers and in patients with chronic obstructive pulmonary disease? Thorax. 2000;55:189–193. doi: 10.1136/thorax.55.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Reaume AG. Elliott JL. Hoffman EK. Kowall NW. Ferrante RJ. Siwek DF. Wilcox HM. Flood DG. Beal MF. Brown RH., Jr. Scott RW. Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 284.Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993;260:1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- 285.Reid TJ., 3rd Murthy MR. Sicignano A. Tanaka N. Musick WD. Rossmann MG. Structure and heme environment of beef liver catalase at 2.5 A resolution. Proc Natl Acad Sci USA. 1981;78:4767–4771. doi: 10.1073/pnas.78.8.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Reynaert NL. Ckless K. Guala AS. Wouters EF. van der Vliet A. Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta. 2006;1760:380–387. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 287.Reynaert NL. Wouters EF. Janssen-Heininger YM. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Rhee SG. Kim KH. Chae HZ. Yim MB. Uchida K. Netto LE. Stadtman ER. Antioxidant defense mechanisms: A new thiol-specific antioxidant enzyme. Ann NY Acad Sci. 1994;738:86–92. doi: 10.1111/j.1749-6632.1994.tb21793.x. [DOI] [PubMed] [Google Scholar]
- 289.Roberts MC. Pang Y. Riley DE. Hillier SL. Berger RC. Krieger JN. Detection of Tet M and Tet O tetracycline resistance genes by polymerase chain reaction. Mol Cell Probes. 1993;7:387–393. doi: 10.1006/mcpr.1993.1057. [DOI] [PubMed] [Google Scholar]
- 290.Roum JH. Buhl R. McElvaney NG. Borok Z. Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 291.Rowley DA. Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide and iron salts by superoxide- and ascorbate-dependent mechanisms: relevance to the pathology of rheumatoid disease. Clin Sci. 1983;64:649–653. doi: 10.1042/cs0640649. [DOI] [PubMed] [Google Scholar]
- 292.Rubin RN. Navon L. Cassano PA. Relationship of serum antioxidants to asthma prevalence in youth. Am J Respir Crit Care Med. 2004;169:393–398. doi: 10.1164/rccm.200301-055OC. [DOI] [PubMed] [Google Scholar]
- 293.Sakamaki K. Tomonaga M. Tsukui K. Nagata S. Molecular cloning and characterization of a chromosomal gene for human eosinophil peroxidase. J Biol Chem. 1989;264:16828–16836. [PubMed] [Google Scholar]
- 294.Saleh D. Ernst P. Lim S. Barnes PJ. Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: Effect of inhaled glucocorticoid. Faseb J. 1998;12:929–937. [PubMed] [Google Scholar]
- 295.Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–906. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Salvemini D. Mazzon E. Dugo L. Riley DP. Serraino I. Caputi AP. Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Salvemini D. Mazzon E. Dugo L. Serraino I. De Sarro A. Caputi AP. Cuzzocrea S. Amelioration of joint disease in a rat model of collagen-induced arthritis by M40403, a superoxide dismutase mimetic. Arthritis Rheum. 2001;44:2909–2921. doi: 10.1002/1529-0131(200112)44:12<2909::aid-art479>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 298.Salvemini D. Wang ZQ. Zweier JL. Samouilov A. Macarthur H. Misko TP. Currie MG. Cuzzocrea S. Sikorski JA. Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 299.Sanders SP. Nitric oxide in asthma. Pathogenic, therapeutic, or diagnostic? Am J Respir Cell Mol Biol. 1999;21:147–149. doi: 10.1165/ajrcmb.21.2.f158. [DOI] [PubMed] [Google Scholar]
- 300.Sanders SP. Zweier JL. Harrison SJ. Trush MA. Rembish SJ. Liu MC. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med. 1995;151:1725–1733. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 301.Sandstrom J. Nilsson P. Karlsson K. Marklund SL. 10-fold increase in human plasma extracellular superoxide dismutase content caused by a mutation in heparin-binding domain. J Biol Chem. 1994;269:19163–19166. [PubMed] [Google Scholar]
- 302.Schafer FQ. Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 303.Schewe T. Molecular actions of ebselen—An antiinflammatory antioxidant. Gen Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 304.Schulze-Osthoff K. Los M. Baeuerle PA. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes. Biochem Pharmacol. 1995;50:735–741. doi: 10.1016/0006-2952(95)02011-z. [DOI] [PubMed] [Google Scholar]
- 305.Sedgwick JB. Calhoun WJ. Vrtis RF. Bates ME. McAllister PK. Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 306.Sen CK. Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 307.Sharma P. Chakraborty R. Wang L. Min B. Tremblay ML. Kawahara T. Lambeth JD. Haque SJ. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Silkoff PE. Caramori M. Tremblay L. McClean P. Chaparro C. Kesten S. Hutcheon M. Slutsky AS. Zamel N. Keshavjee S. Exhaled nitric oxide in human lung transplantation. A noninvasive marker of acute rejection. Am J Respir Crit Care Med. 1998;157:1822–1828. doi: 10.1164/ajrccm.157.6.9707159. [DOI] [PubMed] [Google Scholar]
- 309.Simon AR. Rai U. Fanburg BL. Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 310.Singh RJ. Goss SP. Joseph J. Kalyanaraman B. Nitration of gamma-tocopherol and oxidation of alpha-tocopherol by copper-zinc superoxide dismutase/H2O2/NO2-: Role of nitrogen dioxide free radical. Proc Natl Acad Sci USA. 1998;95:12912–12917. doi: 10.1073/pnas.95.22.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Singh S. Evans TW. Nitric oxide, the biological mediator of the decade: Fact or fiction? Eur Respir J. 1997;10:699–707. [PubMed] [Google Scholar]
- 312.Smith LJ. Houston M. Anderson J. Increased levels of glutathione in bronchoalveolar lavage fluid from patients with asthma. Am Rev Respir Dis. 1993;147:1461–1464. doi: 10.1164/ajrccm/147.6_Pt_1.1461. [DOI] [PubMed] [Google Scholar]
- 313.Smith LJ. Shamsuddin M. Sporn PH. Denenberg M. Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–1307. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- 314.Soini Y. Kahlos K. Napankangas U. Kaarteenaho-Wiik R. Saily M. Koistinen P. Paaakko P. Holmgren A. Kinnula VL. Widespread expression of thioredoxin and thioredoxin reductase in non-small cell lung carcinoma. Clin Cancer Res. 2001;7:1750–1757. [PubMed] [Google Scholar]
- 315.Spasojevic I. Batinic–Haberle I. Stevens RD. Hambright P. Thorpe AN. Grodkowski J. Neta P. Fridovich I. Manganese(III) biliverdin IX dimethyl ester: a powerful catalytic scavenger of superoxide employing the Mn(III)/Mn(IV) redox couple. Inorg Chem. 2001;40:726–739. doi: 10.1021/ic0004986. [DOI] [PubMed] [Google Scholar]
- 316.Starke DW. Chock PB. Mieyal JJ. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem. 2003;278:14607–14613. doi: 10.1074/jbc.M210434200. [DOI] [PubMed] [Google Scholar]
- 317.Strange RC. Spiteri MA. Ramachandran S. Fryer AA. Glutathione-S-transferase family of enzymes. Mutation Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 318.Stuehr D. Pou S. Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–14536. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 319.Stuehr DJ. Enzymes of the L-arginine to nitric oxide pathway. J Nutr. 2004;134:2748S–2751S. doi: 10.1093/jn/134.10.2748S. discussion 2765S–2767S. [DOI] [PubMed] [Google Scholar]
- 320.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 321.Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Ann Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- 322.Stuehr DJ. Griffith OW. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- 323.Sundberg K. Johansson AS. Stenberg G. Widersten M. Seidel A. Mannervik B. Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 324.Sutton A. Khoury H. Prip-Buus C. Cepanec C. Pessayre D. Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- 325.Tamer L. Calikoglu M. Ates NA. Yildirim H. Ercan B. Saritas E. Unlu A. Atik U. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004;9:493–498. doi: 10.1111/j.1440-1843.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 326.Tanizaki Y. Kitani H. Mifune T. Mitsunobu F. Kajimoto K. Sugimoto K. Effects of glucocorticoids on humoral and cellular immunity and on airway inflammation in patients with steroid-dependent intractable asthma. J Asthma. 1993;30:485–492. doi: 10.3109/02770909309056758. [DOI] [PubMed] [Google Scholar]
- 327.Ten RM. Pease LR. McKean DJ. Bell MP. Gleich GJ. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Thomas JA. Poland B. Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- 329.Thorn J. Brisman J. Toren K. Adult-onset asthma is associated with self-reported mold or environmental tobacco smoke exposures in the home. Allergy. 2001;56:287–292. doi: 10.1034/j.1398-9995.2001.00805.x. [DOI] [PubMed] [Google Scholar]
- 330.Tiano L. Belardinelli R. Carnevali P. Principi F. Seddaiu G. Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur Heart J. 2007;28:2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 331.Trautmann A. Akdis M. Schmid-Grendelmeier P. Disch R. Brocker EB. Blaser K. Akdis CA. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001;108:839–846. doi: 10.1067/mai.2001.118796. [DOI] [PubMed] [Google Scholar]
- 332.Trautmann A. Schmid-Grendelmeier P. Kruger K. Crameri R. Akdis M. Akkaya A. Brocker EB. Blaser K. Akdis CA. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J Allergy Clin Immunol. 2002;109:329–337. doi: 10.1067/mai.2002.121460. [DOI] [PubMed] [Google Scholar]
- 333.Tredaniel J. Boffetta P. Saracci R. Hirsch A. Exposure to environmental tobacco smoke and adult non-neoplastic respiratory diseases. Eur Respir J. 1994;7:173–185. doi: 10.1183/09031936.94.07010173. [DOI] [PubMed] [Google Scholar]
- 334.Usatyuk PV. Vepa S. Watkins T. He D. Parinandi NL. Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 335.USEPA. US Environmental Protection Agency; Washington, DC: 2006. Air Quality Criteria for Ozone and Related Photochemical Oxidants (Final) EPA/600/R–05/004aF-cF. [Google Scholar]
- 336.van Acker SA. Koymans LM. Bast A. Molecular pharmacology of vitamin E: Structural aspects of antioxidant activity. Free Radic Biol Med. 1993;15:311–328. doi: 10.1016/0891-5849(93)90078-9. [DOI] [PubMed] [Google Scholar]
- 337.Venarske DL. Busse WW. Griffin MR. Gebretsadik T. Shintani AK. Minton PA. Peebles RS. Hamilton R. Weisshaar E. Vrtis R. Higgins SB. Hartert TV. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Vrugt B. Wilson S. Bron A. Holgate ST. Djukanovic R. Aalbers R. Bronchial angiogenesis in severe glucocorticoid-dependent asthma. Eur Respir J. 2000;15:1014–1021. doi: 10.1034/j.1399-3003.2000.01507.x. [DOI] [PubMed] [Google Scholar]
- 339.Wang YZ. Ingram JL. Walters DM. Rice AB. Santos JH. Van Houten B. Bonner JC. Vanadium-induced STAT-1 activation in lung myofibroblasts requires H2O2 and P38 MAP kinase. Free Radic Biol Med. 2003;35:845–855. doi: 10.1016/s0891-5849(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 340.Wardlaw AJ. Eosinophils in the 1990s: New perspectives on their role in health and disease. Postgrad Med J. 1994;70:536–552. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Weiss SJ. Test ST. Eckmann CM. Roos D. Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986;234:200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]
- 342.Wink DA. Hanbauer I. Laval F. Cook JA. Krishna MC. Mitchell JB. Nitric oxide protects against the cytotoxic effects of reactive oxygen species. Ann NY Acad Sci. 1994;738:265–278. doi: 10.1111/j.1749-6632.1994.tb21812.x. [DOI] [PubMed] [Google Scholar]
- 343.Wink DA. Nims RW. Darbyshire JF. Christodoulou D. Hanbauer I. Cox GW. Laval F. Laval J. Cook JA. Krishna MC, et al. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 344.Wispe JR. Clark JC. Burhans MS. Kropp KE. Korfhagen TR. Whitsett JA. Synthesis and processing of the precursor for human mangano-superoxide dismutase. Biochim Biophys Acta. 1989;994:30–36. doi: 10.1016/0167-4838(89)90058-7. [DOI] [PubMed] [Google Scholar]
- 345.Wong GH. Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: Possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 346.Wood LG. Garg ML. Simpson JL. Mori TA. Croft KD. Wark PA. Gibson PG. Induced sputum 8-isoprostane concentrations in inflammatory airway diseases. Am J Respir Crit Care Med. 2005;171:426–430. doi: 10.1164/rccm.200408-1010OC. [DOI] [PubMed] [Google Scholar]
- 347.Wu W. Samoszuk MK. Comhair SA. Thomassen MJ. Farver CF. Dweik RA. Kavuru MS. Erzurum SC. Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Xu W. Comhair SA. Zheng S. Chu SC. Marks-Konczalik J. Moss J. Haque SJ. Erzurum SC. STAT-1 and c-Fos interaction in nitric oxide synthase-2 gene activation. Am J Physiol Lung Cell Mol Physiol. 2003;285:L137–1–48. doi: 10.1152/ajplung.00441.2002. [DOI] [PubMed] [Google Scholar]
- 349.Xu W. Kaneko FT. Zheng S. Comhair SA. Janocha AJ. Goggans T. Thunnissen FB. Farver C. Hazen SL. Jennings C. Dweik RA. Arroliga AC. Erzurum SC. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 350.Yamada Y. Nakamura H. Adachi T. Sannohe S. Oyamada H. Kayaba H. Yodoi J. Chihara J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol Lett. 2003;86:199–205. doi: 10.1016/s0165-2478(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 351.Yamakura F. Taka H. Fujimura T. Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- 352.Yeh CT. Ching LC. Yen GC. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem. 2009;20:163–171. doi: 10.1016/j.jnutbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 353.Yoo JH. Erzurum SC. Hay JG. Lemarchand P. Crystal RG. Vulnerability of the human airway epithelium to hyperoxia. Constitutive expression of the catalase gene in human bronchial epithelial cells despite oxidant stress. J Clin Invest. 1994;93:297–302. doi: 10.1172/JCI116959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Yoshimura S. Suemizu H. Taniguchi Y. Arimori K. Kawabe N. Moriuchi T. The human plasma glutathione peroxidase-encoding gene: organization, sequence and localization to chromosome 5q32. Gene. 1994;145:293–297. doi: 10.1016/0378-1119(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 355.Zhang H. Joseph J. Felix C. Kalyanaraman B. Bicarbonate enhances the hydroxylation, nitration, and peroxidation reactions catalyzed by copper, zinc superoxide dismutase. Intermediacy of carbonate anion radical. J Biol Chem. 2000;275:14038–14045. doi: 10.1074/jbc.275.19.14038. [DOI] [PubMed] [Google Scholar]
- 356.Zhang Y. Marcillat O. Giulivi C. Ernster L. Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]
- 357.Zheng L. Nukuna B. Brennan ML. Sun M. Goormastic M. Settle M. Schmitt D. Fu X. Thomson L. Fox PL. Ischiropoulos H. Smith JD. Kinter M. Hazen SL. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]