Abstract
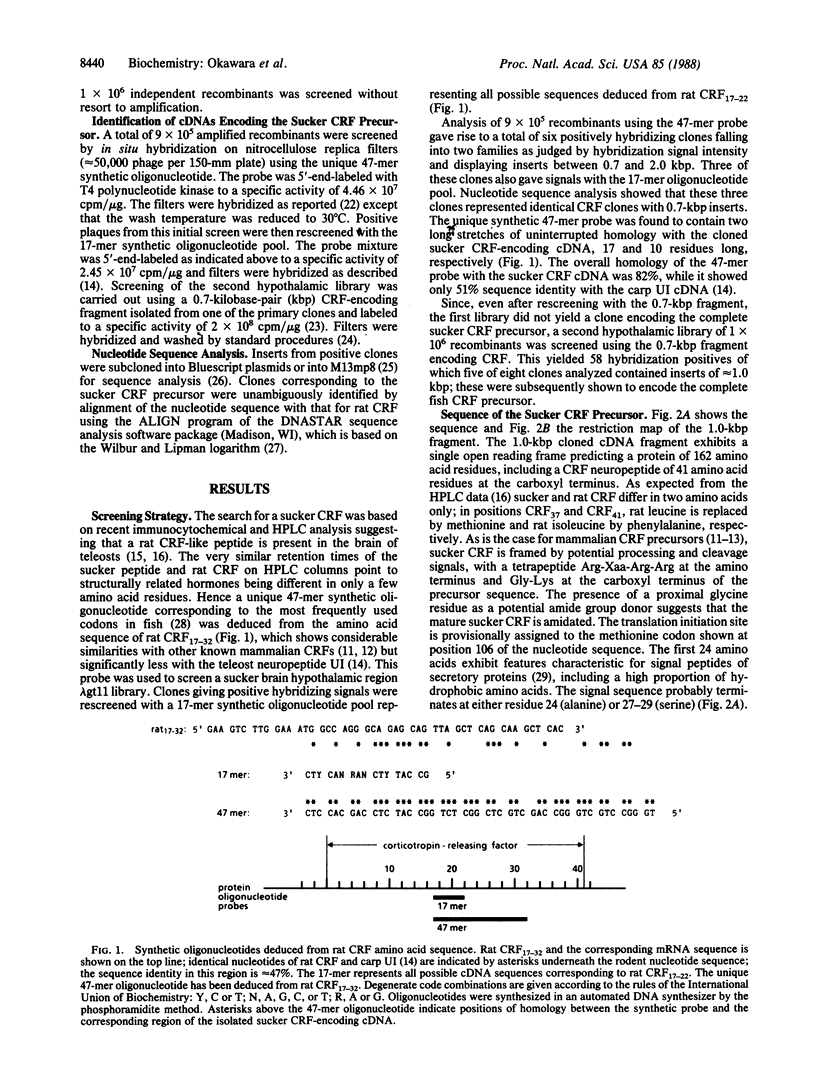
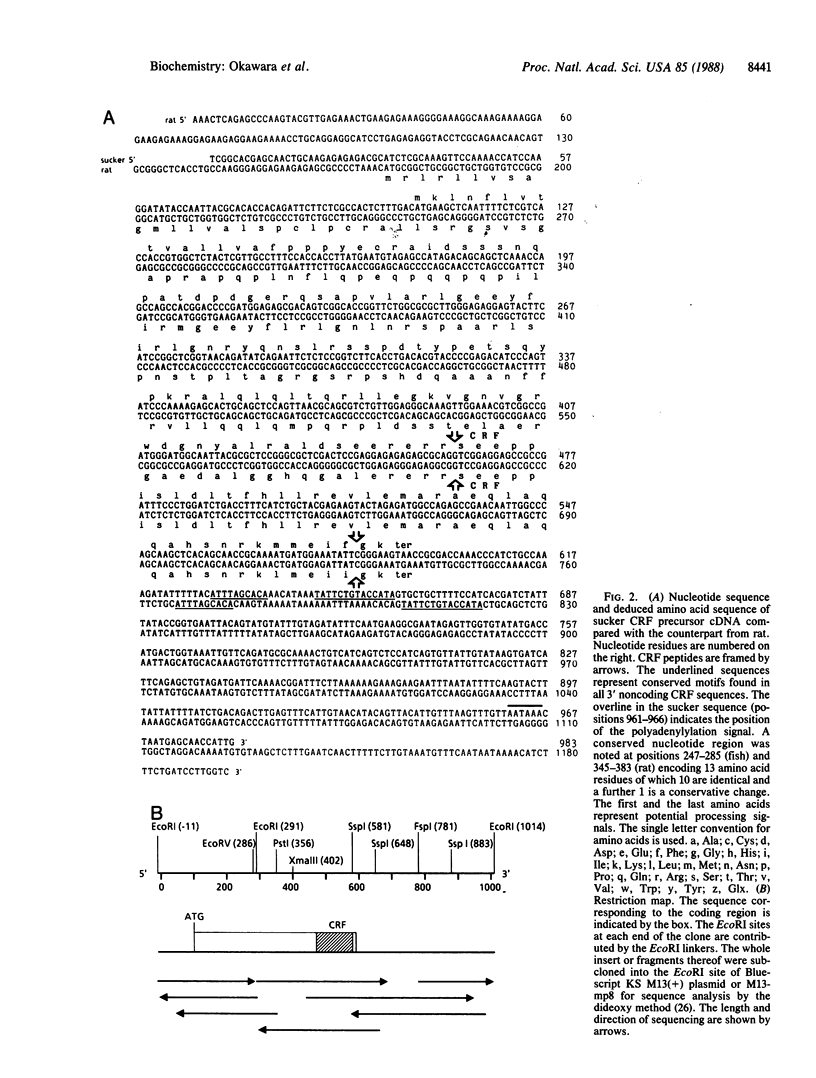
The sequence of a cDNA encoding the corticotropin-releasing factor precursor has been identified by screening lambda gt11 libraries constructed from poly(A)+ RNA of the hypothalamic region of the white sucker Catostomus commersoni brain with synthetic oligonucleotide probes deduced from the sequence of the rat corticotropin-releasing factor. The amino acid sequence of corticotropin-releasing factor of the sucker is strikingly conserved when compared to its counterpart from rat and differs only in two positions at the carboxyl terminus; in contrast, there is little similarity between their cryptic regions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern H. A., Pearson D., Larson B. A., Nishioka R. S. Neurohormones from fish tails: the caudal neurosecretory system. I. "Urophysiology" and the caudal neurosecretory system of fishes. Recent Prog Horm Res. 1985;41:533–552. doi: 10.1016/b978-0-12-571141-8.50016-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Esch F., Ling N., Bohlen P., Baird A., Benoit R., Guillemin R. Isolation and characterization of the bovine hypothalamic corticotropin-releasing factor. Biochem Biophys Res Commun. 1984 Aug 16;122(3):899–905. doi: 10.1016/0006-291x(84)91175-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Morimoto Y., Shibahara S., Noda M., Takahashi H., Hirose T., Asai M., Inayama S., Hayashida H., Miyata T. Cloning and sequence analysis of cDNA for ovine corticotropin-releasing factor precursor. Nature. 1983 Feb 10;301(5900):537–540. doi: 10.1038/301537a0. [DOI] [PubMed] [Google Scholar]
- GUILLEMIN R., ROSENBERG B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955 Nov;57(5):599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Ichikawa T., McMaster D., Lederis K., Kobayashi H. Isolation and amino acid sequence of urotensin I, a vasoactive and ACTH-releasing neuropeptide, from the carp (Cyprinus carpio) urophysis. Peptides. 1982 Sep-Oct;3(5):859–867. doi: 10.1016/0196-9781(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Ishida I., Ichikawa T., Deguchi T. Cloning and sequence analysis of cDNA encoding urotensin I precursor. Proc Natl Acad Sci U S A. 1986 Jan;83(2):308–312. doi: 10.1073/pnas.83.2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H., Mizuno N., Takahashi H., Shibahara S., Furutani Y., Imura H., Numa S. Cloning and sequence analysis of cDNA for rat corticotropin-releasing factor precursor. FEBS Lett. 1985 Oct 21;191(1):63–66. doi: 10.1016/0014-5793(85)80994-7. [DOI] [PubMed] [Google Scholar]
- Lederis K., Fryer J., Rivier J., MacCannell K. L., Kobayashi Y., Woo N., Wong K. L. Neurohormones from fish tails. II: Actions of urotensin I in mammals and fishes. Recent Prog Horm Res. 1985;41:553–576. [PubMed] [Google Scholar]
- Lederis K., Letter A., McMaster D., Moore G., Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science. 1982 Oct 8;218(4568):162–165. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- Ling N., Esch F., Böhlen P., Baird A., Guillemin R. Isolation and characterization of caprine corticotropin-releasing factor. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1218–1224. doi: 10.1016/0006-291x(84)91222-1. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Gojobori T., Aota S., Ikemura T. Codon usage tabulated from the GenBank genetic sequence data. Nucleic Acids Res. 1986;14 (Suppl):r151–r197. doi: 10.1093/nar/14.suppl.r151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Montecucchi P. C., Anastasi A., de Castiglione R., Erspamer V. Isolation and amino acid composition of sauvagine. An active polypeptide from methanol extracts of the skin of the South American frog Phyllomedusa sauvagei. Int J Pept Protein Res. 1980 Sep;16(3):191–199. [PubMed] [Google Scholar]
- SAFFRAN M., SCHALLY A. V. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955 May;33(3):408–415. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Morimoto Y., Furutani Y., Notake M., Takahashi H., Shimizu S., Horikawa S., Numa S. Isolation and sequence analysis of the human corticotropin-releasing factor precursor gene. EMBO J. 1983;2(5):775–779. doi: 10.1002/j.1460-2075.1983.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Rivier C., Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulis C. R., Lederis K. The distribution of 'extraurophyseal' urotensin I-immunoreactivity in the central nervous system of Catostomus commersoni after urophysectomy. Neurosci Lett. 1986 Sep 25;70(1):75–80. doi: 10.1016/0304-3940(86)90440-4. [DOI] [PubMed] [Google Scholar]
- Yulis C. R., Lederis K., Wong K. L., Fisher A. W. Localization of urotensin I- and corticotropin-releasing factor-like immunoreactivity in the central nervous system of Catostomus commersoni. Peptides. 1986 Jan-Feb;7(1):79–86. doi: 10.1016/0196-9781(86)90065-3. [DOI] [PubMed] [Google Scholar]


