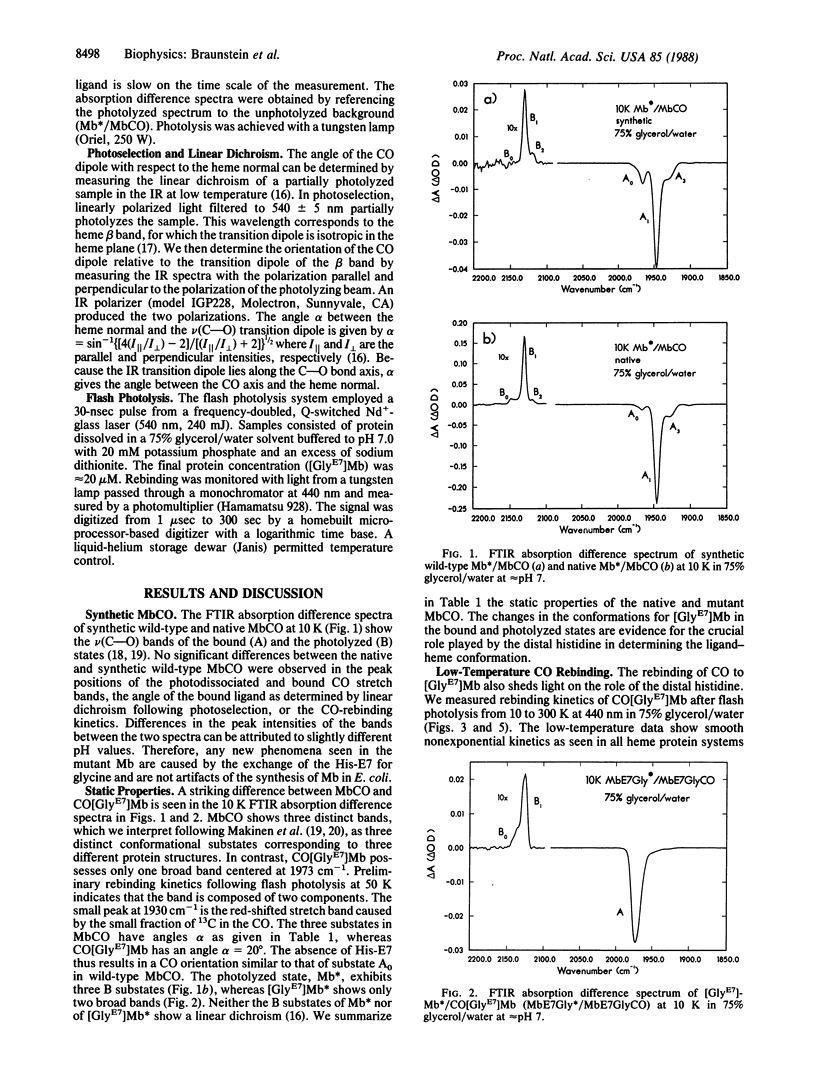
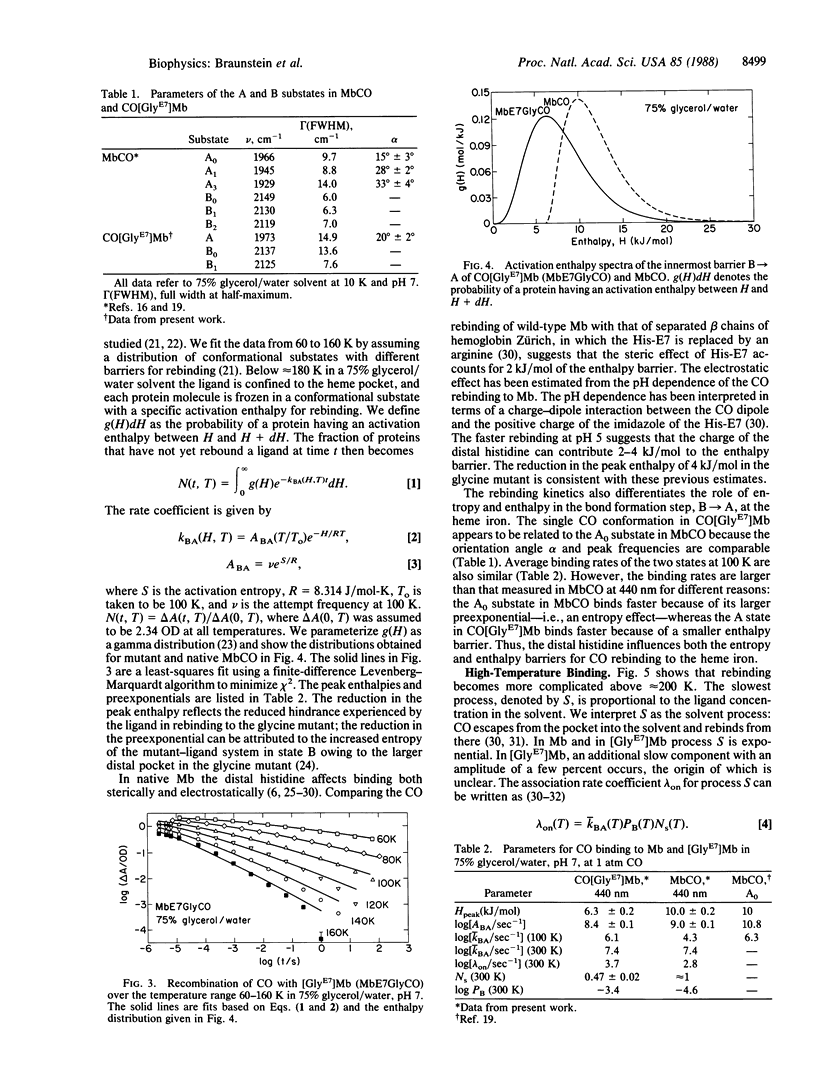
Abstract
Low-temperature flash photolysis with IR and visible spectroscopy was used to probe the influence of the distal histidine His-64(E7) of sperm-whale myoglobin (Mb) on the orientation of bound carbon monoxide (CO) and on the kinetics of CO rebinding. The synthesis and high-level expression of a sperm-whale myoglobin gene in Escherichia coli permits the efficient substitution of the distal histidine through site-directed mutagenesis. Substitution of His-E7 with glycine [GlyE7]Mb bound with CO (CO[GlyE7]Mb) results in one broad bound-CO IR stretch band, v(C-O), centered at 1973 cm-1 at 10 K, in contrast to three distinct bands for native and synthetic wild-type MbCO at 1966, 1945, and 1929 cm-1. After flash photolysis at 10 K, the unbound state of CO[GlyE7]Mb exhibits two CO stretch bands, whereas MbCO has three. Fourier transform IR spectroscopy measurements of the linear dichroism after photoselective flash photolysis of CO bound to [GlyE7]Mb at 10 K reveals the bound CO to be oriented at an angle of alpha = 20 degrees +/- 2 degrees with respect to the heme normal. Flash photolysis data from 10 to 300 K provide evidence for a larger distal pocket and a smaller enthalpy barrier (by approximately 4 kJ/mol) for [GlyE7]MbCO as compared with wild-type MbCO. These results reinforce the notion that the dominant control of the binding step at the heme iron comes from the proximal side through the protein structure.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Ansari A., DiIorio E. E., Dlott D. D., Frauenfelder H., Iben I. E., Langer P., Roder H., Sauke T. B., Shyamsunder E. Ligand binding to heme proteins: relevance of low-temperature data. Biochemistry. 1986 Jun 3;25(11):3139–3146. doi: 10.1021/bi00359a011. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The structure of human carbonmonoxy haemoglobin at 2.7 A resolution. J Mol Biol. 1980 Jan 15;136(2):103–128. doi: 10.1016/0022-2836(80)90308-3. [DOI] [PubMed] [Google Scholar]
- Barlow C. H., Ohlsson P. I., Paul K. G. Infrared spectroscopic studies of carbonyl horseradish peroxidases. Biochemistry. 1976 May 18;15(10):2225–2229. doi: 10.1021/bi00655a031. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Ligand binding channels reflected in the resonance Raman spectra of cryogenically trapped species of myoglobin. J Biol Chem. 1987 Nov 5;262(31):14885–14890. [PubMed] [Google Scholar]
- Caughey W. S. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970 Oct 5;174(1):148–153. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Doxsee K. M. Carbon monoxide binding to iron porphyrins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6035–6039. doi: 10.1073/pnas.76.12.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughey W. S., Alben J. O., McCoy S., Boyer S. H., Charache S., Hathaway P. Differences in the infrared stretching frequency of carbon monoxide bound to abnormal hemoglobins. Biochemistry. 1969 Jan;8(1):59–62. doi: 10.1021/bi00829a009. [DOI] [PubMed] [Google Scholar]
- Dlott D. D., Frauenfelder H., Langer P., Roder H., DiIorio E. E. Nanosecond flash photolysis study of carbon monoxide binding to the beta chain of hemoglobin Zürich [beta 63(E7)His leads to Arg]. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6239–6243. doi: 10.1073/pnas.80.20.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doster W., Beece D., Bowne S. F., DiIorio E. E., Eisenstein L., Frauenfelder H., Reinisch L., Shyamsunder E., Winterhalter K. H., Yue K. T. Control and pH dependence of ligand binding to heme proteins. Biochemistry. 1982 Sep 28;21(20):4831–4839. doi: 10.1021/bi00263a001. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Wolynes P. G. Rate theories and puzzles of hemeprotein kinetics. Science. 1985 Jul 26;229(4711):337–345. doi: 10.1126/science.4012322. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Di Iorio E. E., Antonini E., Brunori M., Winterhalter K. H. Binding of carbon monoxide to hemoglobin Zürich. Proposal for a kinetic model. Eur J Biochem. 1977 May 2;75(1):267–273. doi: 10.1111/j.1432-1033.1977.tb11526.x. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Traylor T. G., Ascenzi P., Brunori M., Antonini E. Reactivity of ferrous myoglobin at low pH. J Biol Chem. 1977 Nov 10;252(21):7447–7448. [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Ikeda-Saito M., Iizuka T., Yamamoto H., Kayne F. J., Yonetani T. Studies on cobalt myoglobins and hemoglobins. Interaction of sperm whale myoglobin and Glycera hemoglobin with molecular oxygen. J Biol Chem. 1977 Jul 25;252(14):4882–4887. [PubMed] [Google Scholar]
- Imai K., Ikeda-Saito M., Yonetani T. Studies on cobalt myoglobins and hemoglobins, XIII. A consequence of the occurrence of glutamine at the E7 (58) site of alpha subunits in opossum hemoglobin. J Mol Biol. 1980 Dec 25;144(4):551–565. doi: 10.1016/0022-2836(80)90336-8. [DOI] [PubMed] [Google Scholar]
- Kerr E. A., Yu N. T., Bartnicki D. E., Mizukami H. Resonance Raman studies of CO and O2 binding to elephant myoglobin (distal His(E7)----Gln). J Biol Chem. 1985 Jul 15;260(14):8360–8365. [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Luisi B., Shih D., Miyazaki G., Imai K., Poyart C., De Young A., Kwiatkowsky L., Noble R. W., Lin S. H. Distal residues in the oxygen binding site of haemoglobin studied by protein engineering. 1987 Oct 29-Nov 4Nature. 329(6142):858–860. doi: 10.1038/329858a0. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Ormos P., Braunstein D., Frauenfelder H., Hong M. K., Lin S. L., Sauke T. B., Young R. D. Orientation of carbon monoxide and structure-function relationship in carbonmonoxymyoglobin. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8492–8496. doi: 10.1073/pnas.85.22.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRYER L., KENDREW J. C., WATSON H. C. THE MODE OF ATTACHMENT OF THE AZIDE ION TO SPERM WHALE METMYOGLOBIN. J Mol Biol. 1964 Jan;8:96–104. doi: 10.1016/s0022-2836(64)80152-2. [DOI] [PubMed] [Google Scholar]
- Satterlee J. D., Teintze M., Richards J. H. Spectroscopic studies of the nature of ligand bonding in carbonmonoxyhemoglobins: evidence of a specific function for histidine-E7 from infrared and nuclear magnetic resonance intensities. Biochemistry. 1978 Apr 18;17(8):1456–1462. doi: 10.1021/bi00601a015. [DOI] [PubMed] [Google Scholar]
- Springer B. A., Sligar S. G. High-level expression of sperm whale myoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8961–8965. doi: 10.1073/pnas.84.24.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]


