Abstract
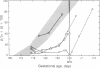
The switch from fetal to adult hemoglobin expression is regulated in many mammalian species by a developmental clock-like mechanism and determined by the gestational age of the fetus. Prolonging fetal globin gene expression is of considerable interest for therapeutic potential in diseases caused by abnormal beta-globin genes. Butyric acid, which is found in increased plasma concentrations in infants of diabetic mothers who have delayed globin gene switching, was infused into catheterized fetal lambs in utero during the time of the normal globin gene switch period. The globin gene switch was significantly delayed in three of four butyrate-treated fetuses compared with controls and was entirely prevented in one fetus in whom the infusion was begun before the globin switch was under way. These data provide a model for investigating and arresting the biologic clock of hemoglobin switching.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bard H., Prosmanne J. Relative rates of fetal hemoglobin and adult hemoglobin synthesis in cord blood of infants of insulin-dependent diabetic mothers. Pediatrics. 1985 Jun;75(6):1143–1147. [PubMed] [Google Scholar]
- Barker J. E., Pierce J. E., Nienhuis A. W. Hemoglobin switching in sheep: a comparison of the erythropoietin-induced switch to HbC and the fetal to adult hemoglobin switch. Blood. 1980 Sep;56(3):488–494. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Cockburn F., Blagden A., Michie E. A., Forfar J. O. The influence of pre-eclampsia and diabetes mellitus on plasma free amino acids in maternal, umbilical vein and infant blood. J Obstet Gynaecol Br Commonw. 1971 Mar;78(3):215–231. doi: 10.1111/j.1471-0528.1971.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Ginder G. D., Whitters M. J., Pohlman J. K. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3954–3958. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. J., Wood W. G. In vitro growth of sheep erythroid progenitors. Br J Haematol. 1986 Dec;64(4):757–765. doi: 10.1111/j.1365-2141.1986.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Dvir A., Ravid A., Shkolnik T., Stenzel K. H., Rubin A. L., Zaizov R. Effect of polar organic compounds on leukemic cells. Butyrate-induced partial remission of acute myelogenous leukemia in a child. Cancer. 1983 Jan 1;51(1):9–14. doi: 10.1002/1097-0142(19830101)51:1<9::aid-cncr2820510104>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Analysis of human hemoglobin switching in MEL x human fetal erythroid cell hybrids. Cell. 1986 Aug 1;46(3):469–476. doi: 10.1016/0092-8674(86)90667-7. [DOI] [PubMed] [Google Scholar]
- Partington G. A., Yarwood N. J., Rutherford T. R. Human globin gene transcription in injected Xenopus oocytes: enhancement by sodium butyrate. EMBO J. 1984 Dec 1;3(12):2787–2792. doi: 10.1002/j.1460-2075.1984.tb02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine S. P., Greene M. F., Faller D. V. Delay in the fetal globin switch in infants of diabetic mothers. N Engl J Med. 1985 Feb 7;312(6):334–338. doi: 10.1056/NEJM198502073120602. [DOI] [PubMed] [Google Scholar]
- Perrine S. P., Miller B. A., Greene M. F., Cohen R. A., Cook N., Shackleton C., Faller D. V. Butryic acid analogues augment gamma globin gene expression in neonatal erythroid progenitors. Biochem Biophys Res Commun. 1987 Oct 29;148(2):694–700. doi: 10.1016/0006-291x(87)90932-6. [DOI] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., Magarian C., Borun T. W. Resolution of hemoglobin subunits by electrophoresis in acid urea polyacrylamide gels containing Triton X-100. Anal Biochem. 1978 Apr;85(2):506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Bunch C., Kelly S., Gunn Y., Breckon G. Control of haemoglobin switching by a developmental clock? Nature. 1985 Jan 24;313(6000):320–323. doi: 10.1038/313320a0. [DOI] [PubMed] [Google Scholar]
- Zanjani E. D., Horger E. O., 3rd, Gordon A. S., Cantor L. N., Hutchinson D. L. Erythropoietin production in the fetal lamb. J Lab Clin Med. 1969 Nov;74(5):782–788. [PubMed] [Google Scholar]