Abstract
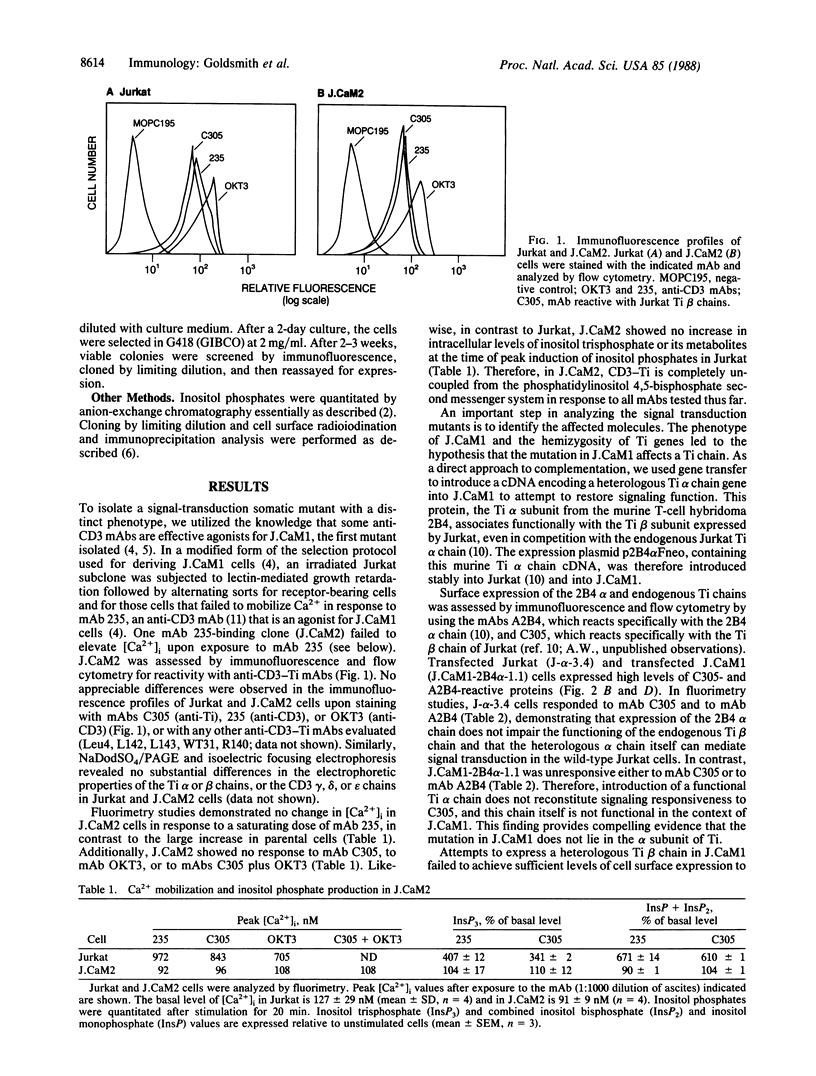
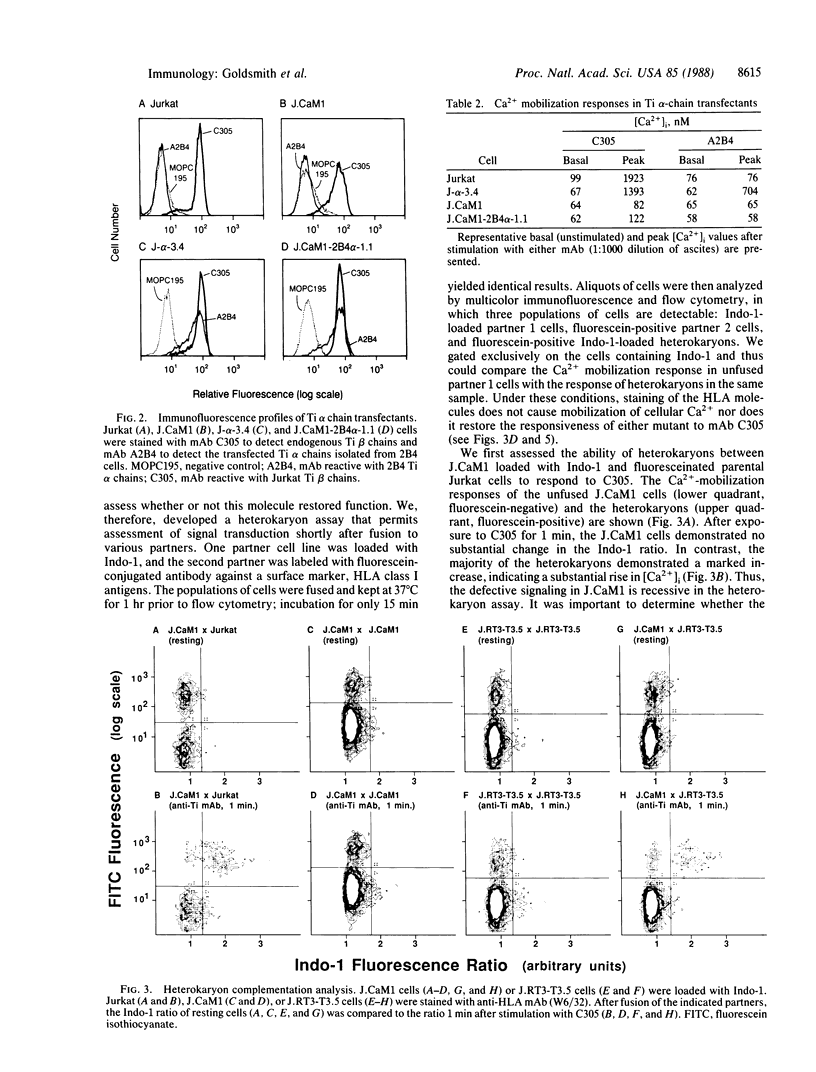
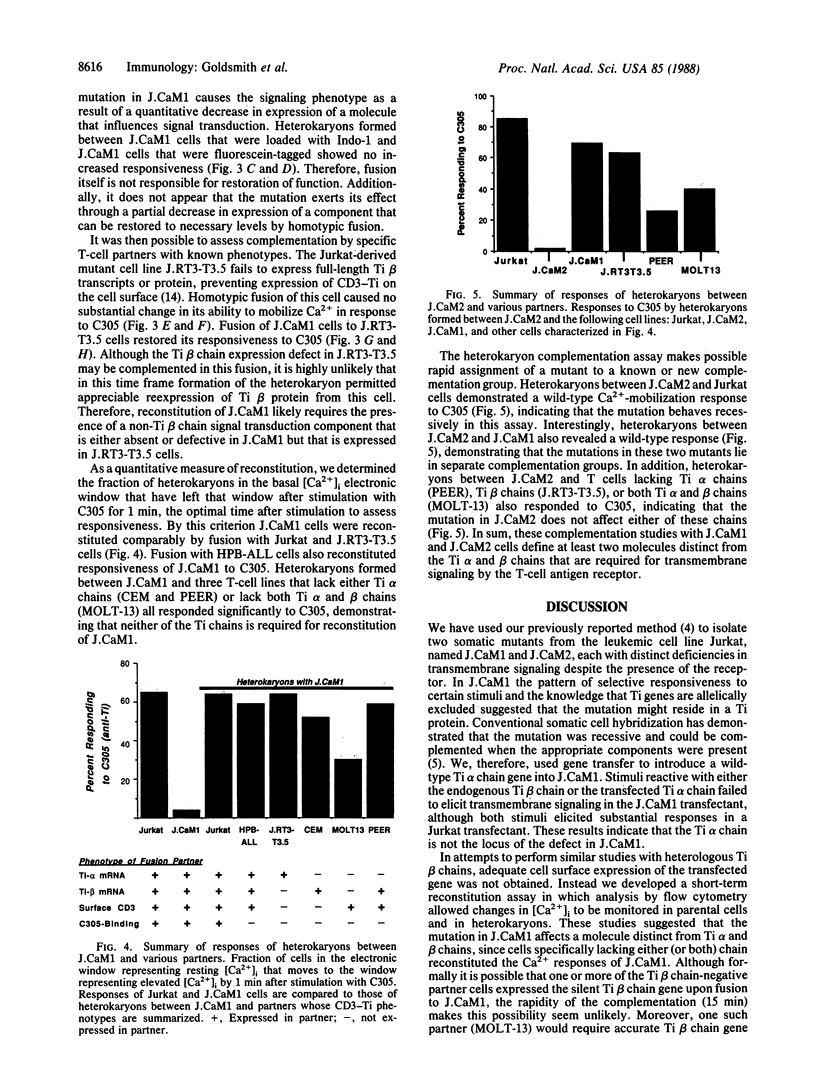
In the T-cell somatic mutant J.CaM1, the T-cell antigen receptor complex is poorly coupled to the inositolphospholipid second messenger system; some antibodies against the invariant CD3 subunit of the receptor retain their agonist function in J.CaM1. Here we show by a combination of complementation assays that the mutation in J.CaM1 affects a molecule other than the antigen-binding Ti subunit, suggesting that Ti is coupled indirectly to the signal transduction apparatus through a pathway involving the CD3 complex. We also describe another mutant, J.CaM2, in which the receptor complex is completely uncoupled from inositolphospholipid hydrolysis. J.CaM2 defines an additional complementation group, suggesting that signal transduction by the antigen receptor depends on at least two molecules distinct from Ti.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Borst J., Prendiville M. A., Terhorst C. Complexity of the human T lymphocyte-specific cell surface antigen T3. J Immunol. 1982 Apr;128(4):1560–1565. [PubMed] [Google Scholar]
- Goldsmith M. A., Weiss A. Isolation and characterization of a T-lymphocyte somatic mutant with altered signal transduction by the antigen receptor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6879–6883. doi: 10.1073/pnas.84.19.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987 Apr 1;165(4):1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Ruitenberg J. J., Allison J. P., Weiss A. Distinct epitopes on the T cell antigen receptor of HPB-ALL tumor cells identified by monoclonal antibodies. J Immunol. 1986 Oct 1;137(7):2286–2292. [PubMed] [Google Scholar]
- Loh E. Y., Lanier L. L., Turck C. W., Littman D. R., Davis M. M., Chien Y. H., Weiss A. Identification and sequence of a fourth human T cell antigen receptor chain. Nature. 1987 Dec 10;330(6148):569–572. doi: 10.1038/330569a0. [DOI] [PubMed] [Google Scholar]
- Minami Y., Weissman A. M., Samelson L. E., Klausner R. D. Building a multichain receptor: synthesis, degradation, and assembly of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1987 May;84(9):2688–2692. doi: 10.1073/pnas.84.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Patel M. D., Samelson L. E., Klausner R. D. Multiple kinases and signal transduction. Phosphorylation of the T cell antigen receptor complex. J Biol Chem. 1987 Apr 25;262(12):5831–5838. [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Smith A. V., Trowbridge I. S. Changes in gene expression induced by a phorbol diester: expression of IL 2 receptor, T3, and T cell antigen receptor. J Immunol. 1987 Jan 15;138(2):613–619. [PubMed] [Google Scholar]
- Sussman J. J., Bonifacino J. S., Lippincott-Schwartz J., Weissman A. M., Saito T., Klausner R. D., Ashwell J. D. Failure to synthesize the T cell CD3-zeta chain: structure and function of a partial T cell receptor complex. Cell. 1988 Jan 15;52(1):85–95. doi: 10.1016/0092-8674(88)90533-8. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]


