Abstract
Human skeletal muscle precursor cells (myoblasts) have significant therapeutic potential and are a valuable research tool to study muscle cell biology. Oxygen is a critical factor in the successful culture of myoblasts with low (1–6%) oxygen culture conditions enhancing the proliferation, differentiation, and/or viability of mouse, rat, and bovine myoblasts. The specific effects of low oxygen depend on the myoblast source and oxygen concentration; however, variable oxygen conditions have not been tested in the culture of human myoblasts. In this study, muscle precursor cells were isolated from vastus lateralis muscle biopsies and myoblast cultures were established in 5% oxygen, before being divided into physiological (5%) or standard (20%) oxygen conditions for experimental analysis. Five percent oxygen increased proliferating myoblast numbers, and since low oxygen had no significant effect on myoblast viability, this increase in cell number was attributed to enhanced proliferation. The proportion of cells in the S (DNA synthesis) phase of the cell cycle was increased by 50%, and p21Cip1 gene and protein expression was decreased in 5 versus 20% oxygen. Unlike in rodent and bovine myoblasts, the increase in myoD, myogenin, creatine kinase, and myosin heavy chain IIa gene expression during differentiation was similar in 5 and 20% oxygen; as was myotube hypertrophy. These data indicate for the first time that low oxygen culture conditions stimulate proliferation, whilst maintaining (but not enhancing) the viability and the differentiation potential of human primary myoblasts and should be considered as optimum conditions for ex-vivo expansion of these cells.
Keywords: Human primary myoblasts, Oxygen, Proliferation, p21Cip1, Differentiation, Cell cycle, Species differences
Introduction
Human satellite cells isolated from skeletal muscle biopsies and proliferated as myoblasts are a useful research tool for studying various aspects of skeletal muscle cell biology, including regeneration, signal transduction and metabolism. Furthermore, these cells have potential for transplantation therapies to treat cardiovascular disease, muscular dystrophy, urinary incontinence, or severe skeletal muscle trauma (Péault et al. 2007). However, unlike bone marrow or cord blood stem cell transplants, it is impossible to isolate enough satellite cells from a muscle biopsy to have therapeutic value or to be used for in vitro research. Proliferation and expansion of myoblasts in culture are therefore essential to obtain adequate cell numbers. A limitation of standard cell culture techniques is that they do not mimic physiological conditions. Gases, oxygen (O2) in particular, are not accounted for in routine cell culture experiments (Csete 2005). This can affect cell behavior and confound the interpretation and physiological significance of experimental findings (Csete 2005).
Oxygen is essential for cell maintenance, but in vivo and in vitro oxygen levels have a significant impact on cellular redox state. Virtually all cellular processes are affected by the cellular redox state, including DNA synthesis, gene transcription, mRNA translation, enzyme kinetics, and signal transduction cascades (Hansen et al. 2007). However, if oxygen is in excess, it is a source of oxidative stress and is detrimental to cell health (Csete 2005). In adult skeletal muscles, normal tissue oxygen levels range from 1.8 to 10.5%, far less than standard cell culture oxygen concentrations of 20% (Csete et al. 2001). Indeed, recent research indicates that nearly all stem cells proliferate better at low, physiologically relevant oxygen concentrations as found in their in vivo niche (Fehrer et al. 2007). Cell culture oxygen levels can also regulate whether a proliferating stem cell maintains its “stemness potential” and its ability to respond appropriately to differentiation stimuli (Fehrer et al. 2007; Csete 2005). With regards to skeletal muscle stem cells, research using primary mouse, rat, or bovine myoblasts (Csete 2005; Chakravarthy et al. 2001; Kook et al. 2008) or C2C12 immortalized myoblasts (Hansen et al. 2007) has shown that low compared to standard tissue culture oxygen concentrations enhance variables such as myoblast viability, proliferation, and/or differentiation depending on the source of the cells (Li et al. 2007). For example, low oxygen culture conditions enhanced the proliferation rate of primary mouse myoblasts (Csete et al. 2001), but not of immortalized mouse C2C12 cells (Hansen et al. 2007). To date, it is not known how human myoblasts respond to estimated physiological (5%) oxygen cell culture conditions. To investigate this, we established human myoblast cultures from muscle biopsies obtained from elderly donors and tested the hypothesis that a low oxygen cell culture environment would enhance myoblast proliferation, viability and differentiation.
Methods
Human study subjects
Vastus lateralis biopsies (~250 mg) were taken by the orthopedic surgeon from healthy elderly patients during elective hip replacement surgery. Biopsies were placed in ice-cold DMEM cell culture medium and processed immediately for cell culture. Subjects (n = 6, 3 male and 3 female) were 69 ± 3 years of age, weighed 79 ± 6 kg, and were 168 ± 2 cm tall. None of these subjects had co-morbidities that compromise skeletal muscle health, such as type 2 diabetes, neuromuscular disorders, cardiovascular and renal disease. Prior to the surgery, informed written consent was obtained from all subjects. All experimental procedures were formally approved by the Deakin University and Barwon Health Human Research Ethics Committees.
Skeletal muscle growth medium and differentiation medium for myoblast proliferation and differentiation
Skeletal muscle growth medium (SkGM) containing low glucose (5.5 mM) DMEM plus Glutamax and supplemented with 2% fetal bovine serum (FBS; v/v), 0.5 mg/mL bovine serum albumin (BSA), 0.5 mg/mL fetuin, 20 ng/mL human epidermal growth factor, 0.39 μg/mL dexamethasone, 0.6% (v/v) penicillin/streptomycin and 0.6% (v/v) amphostat B was used for myoblast proliferation (Berggren et al. 2005). Growth medium supplemented with 2% FBS and EGF has been shown to maximize the fractional content of myoblasts in human primary skeletal muscle cell cultures (Gaster et al. 2001). Skeletal muscle differentiation medium (SkDM) containing low glucose (5.5 mM) DMEM plus Glutamax and supplemented with 2% horse serum (HS; v/v), 0.5 mg/mL bovine serum albumin (BSA), 0.5 mg/mL fetuin, 0.6% (v/v) penicillin/streptomycin and 0.6% (v/v) amphostat B was used to promote myoblast fusion and myotube growth (Berggren et al. 2005).
Muscle satellite cell isolation and culture
Primary skeletal muscle cell cultures were established following the methods of Gaster et al., with modifications as described (Gaster et al. 2001). In brief, following collection the muscle biopsy was dissected free of blood, fat and connective tissue and minced finely in ice cold PBS. To release the satellite cells, the minced muscle was digested twice in fresh 0.1% (w/v) collagenase and 0.05% (w/v) dispase solution for ~20 min with agitation at 37 °C. The released satellite cells were re-suspended in SkGM and then pre-plated in an uncoated culture flask for 30 min in 5% CO2 and 5% O2 at 37 °C, to remove contaminating, adherent fibroblasts. The cell suspension was then transferred to an extra-cellular matrix (ECM)-coated flask (#E1270, Sigma–Aldrich; ECM diluted 1:75 in SkGM) to facilitate myoblast adherence and increase fractional myoblast content (Gaster et al. 2001) and cultured in 5% O2 and 5% CO2 at 37 °C. After 24 h of incubation, the SkGM was replaced with fresh medium. Thereafter, the SkGM was changed twice a week until cultures reached ~80% confluence. Myoblasts were expanded for 3 passages and experiments were carried out at passage 4. At each passage myoblasts were subcultured into new ECM-coated flasks at a density of 3,000 cells/cm2. Myoblasts were initially cultured in 5% oxygen which was defined as the control condition to best represent estimated physiological oxygen levels (Lees et al. 2008). The cells in culture were tested regularly for mycoplasma and no contamination was detected.
Differentiation of skeletal muscle myoblasts
For all differentiation experiments, myoblasts were cultured to ~90% confluence and induced to differentiate by changing the SkGM to SkDM (Berggren et al. 2005). Myoblasts were differentiated into multinucleated myotubes for up to 6 days.
Flow cytometry for determination of myoblast purity
To assess the myogenic purity of our primary human skeletal muscle cell cultures, desmin and NCAM expression was measured using flow cytometry in confluent myoblasts at passage 4 (Stewart et al. 2003). Direct immunofluorescence was performed on fresh cells to detect NCAM (CD56) with a FITC-conjugated mouse anti-CD56 antibody (#CBL510F, Cymbus Biotechnology; diluted 1:10 in PBS plus 1% BSA). To measure desmin positive myoblasts, cells were fixed in 2% paraformaldehyde at 37 °C for 10 min and then permeabilized in chilled absolute methanol (90% final concentration) on ice for 30 min. Indirect immunofluorescence was performed on these cells with a rabbit anti-desmin polyclonal antibody (#ab15200-1, Abcam; diluted 1:100 in PBS plus 1% BSA) and a swine anti-rabbit FITC-conjugate polyclonal secondary antibody (#F0205, Dako Corporation; diluted 1:50 in PBS plus 1% BSA). Cells were incubated with their respective primary and secondary antibodies for 30 min in the dark at room temperature. Stained cells were analyzed by flow cytometry using the FACS Calibur® system and CELL-Quest Software (Becton Dickson Biosciences). Approximately 2.5 × 105 cells were re-suspended in PBS and the percentage of either NCAM or desmin positive cells (FITC, FL-1 channel) was determined by flow cytometric quadrant analysis using CELLQuest software. Equivalent numbers of cells labeled with anti-IgG2a-FITC (Becton–Dickinson) or with a swine anti-rabbit FITC-conjugated polyclonal secondary antibody (#F0205, Dako Corporation) were used as negative controls for the CD56 and desmin analyses, respectively.
Desmin immunofluorescence staining for determination of myoblast purity
Myoblasts and myotubes grown in ECM-coated glass chamber slides (#254108, BD Falcon) were fixed in 2% paraformaldehyde and then permeabilized with 0.1% (v/v) Triton X-100 in PBS for 10 min. Slides were blocked with 10% normal goat serum in PBS, incubated overnight at 4 °C with a rabbit anti-desmin polyclonal antibody (#ab15200-1, Abcam; diluted 1:200 in PBS plus 1% BSA), and then exposed to a swine anti-rabbit FITC-conjugated polyclonal secondary antibody (#F0205, Dako Corporation). The cytoskeleton was counterstained with Alexa Fluoro 568 Phalloidin (#A12380, Molecular Probes—Invitrogen) and nuclei were stained with DAPI. Digital images were captured with a confocal microscope (Leica TCS SP5-AOBS confocal scanning laser with an inverted DMI 6000B microscope).
Manual cell counts to assess myoblast number and viability
To assess the effect of cell culture oxygen levels on proliferation and viability, myoblasts were seeded at 3,000 cells/cm2 in SkGM in 5% CO2, and either 5 or 20% O2, at 37 °C. At 24 h, 3 and 6 days post-seeding, myoblasts were harvested by trypsinization (0.1% trypsin (w/v)/5 mM EDTA), then stained with Trypan blue, a vital stain which is excluded by viable cells with an intact cell membrane. Manual cell counts of both stained and unstained cells were done in triplicate using a hemocytometer (n = 6 donors). Cell number was expressed as number of cells per cm2 and viability as the percentage of viable cells to total cell number. The myoblast viability values do not account for dead and detached cells, as these were discarded with the SkGM prior to trypsinization.
Cell cycle progression analysis using bromodeoxyuridine staining and flow cytometry
Bromodeoxyuridine (BrdU) incorporation was used to determine the proportion of myoblasts in each phase of the cell cycle. Myoblasts were seeded into 25 cm2 flasks in triplicate (n = 6 donors) and allowed to proliferate for 3 days in SkGM in 5% CO2, and either 5 or 20% O2, at 37 °C before being pulse-labeled with 10 μM BrdU for 5 h. Note, that the doubling time for myoblasts is 24–26 h (Pavlath and Gussoni 2005) and myoblast S phase is 6–8 h in duration (McGeachie and Grounds 1999), therefore not more than 14–20% of myoblasts would be expected to be in S phase of the cell cycle. Cells were then harvested by trypsinization and centrifuged for 5 min at 942g. The cells were washed in cold 1% (w/v) BSA and then fixed in 70% (v/v) ethanol overnight at 4 °C. Cells were incubated in 2 M HCl solution for 30 min at room temperature and washed with PBS. Any remaining acid was neutralized with 0.1 M di-sodium tetraborate (pH 8.5) and followed by a final wash in PBS plus 0.5% Tween®20 (v/v) and 1% BSA. Subsequently, the cells were incubated with FITC-conjugated anti-BrdU antibody (#347583, BD Biosciences; diluted 1:10 in 100 μl of PBS plus 0.5% Tween® 20 (v/v) and 1.0% BSA) for 2 h in the dark at room temperature. Cells were washed with PBS plus 0.5% Tween® 20 (v/v) to remove the antibody and counterstained with propidium iodide (50 μg/mL) for at least 1 h or overnight in the dark at 4 °C. Stained cells were analyzed by flow cytometry using the FACS Calibur® system and Cell Quest Software (Becton Dickson Biosciences). Approximately 2.5 × 105 cells were re-suspended in PBS and cells containing a single set of chromosomes (haploid) were discriminated from DNA doublets using FL-3-pulse area versus FL-3-pulse width. The BrdU positive cells (S-phase) were determined from the gated haploid population and the BrdU negative cells indicated either G1/G0 or G2/M phase according to the amount of DNA (FL-3 signal).
Lactate dehydrogenase cytotoxicity assay
Lactate dehydrogenase (LDH) is released from dead or dying lysed cells and is an indicator of cell viability. A commercially available LDH cytotoxicity assay (# G1780, Promega) was used to assess the effect of cell culture oxygen levels on myoblast viability during proliferation. Unlike the manual viability cell counts using Trypan blue staining, myoblasts that have died and detached are accounted for by the LDH cytotoxicity assay. Myoblasts were seeded into 6-well plates in triplicate (n = 5 donors) in SkGM in 5% CO2, and either 5 or 20% O2, at 37 °C. Following 5 days of proliferation, the SkGM was replaced with 1 mL SkGM minus phenol red for 24 h. LDH activity was determined in medium and cell lysate samples following the manufacturer’s instructions. Viability was expressed as the ratio of absorbance of released LDH in the medium to total intracellular LDH from the cell lysate (mean ± SEM).
Total RNA isolation and reverse transcription
For gene expression analysis, myoblasts were seeded in triplicate into 25 cm2 flasks (proliferating cells) or 6-well plates (differentiating cells; n = 3–4 donors). A time-course experiment was conducted, whereby myoblasts were grown in 5% CO2, and either 5 or 20% O2, at 37 °C and samples were collected at 24 h and 4 days proliferation, at ~90% confluence and following 6, 24, 48 h and 6 days differentiation. Briefly, 1 mL TRIzol reagent (#15596-018, Invitrogen) was used per well or flask to lyse the cells and total RNA was extracted using the Illustra™ RNAspin Mini RNA Isolation Kit (#25-0500-71, GE Healthcare) as per manufacturer’s instructions. Complimentary DNA was synthesized by reverse transcription using AMV Reverse Transcriptase (#M9004, Promega) and random hexamer primers (#1181, Promega).
Real-time PCR
To assess cyclophilin, myogenin, myoD, myosin heavy chain (MyHC) IIa, creatine kinase (CK), cyclin A2, and CDK6 gene expression, specific SYBR primers were designed for all genes using Primer Express 2.0 software (Applied Biosystems, Foster City, CA) on sequences obtained from GenBank (see Table 1). To assess p21Cip1 and PPARγ gene expression, we used pre-designed Real-Time TaqMan® Gene Expression Assays (Applied Biosystems); these eliminate the need for primer design and PCR optimization (see Table 1).
Table 1.
Oligonucleotide sequences for real-time PCR
| Gene amplified | Oligonucleotide sequence 5′ → 3′ | Chemistry |
|---|---|---|
| Myogenin | For: GGTGCCCAGCGAATGC | SYBR |
| Rev: TGATGCTGTCCACGATCGA | ||
| MyoD | For: CCGCCTGAGCAAAGTAAATGA | SYBR |
| Rev: GCAACCGCTGGTTTGGATT | ||
| MyHC IIa | For: AAGGTCGGCAATGAGTATGTCA | SYBR |
| Rev: CAACCATCCACAGGAACATCTTC | ||
| Creatine Kinase | For: GGCATCTGGCACAATGAC | SYBR |
| Rev: GATGACCCGGAGGTGATC | ||
| Cyclin A2 | For: CGTGGACTGGTTAGTTGA | SYBR |
| Rev: ATGGCAAATACTTGAGGT | ||
| CDK6 | For: TGATGTGTGCACAGTGTCACGAAC | SYBR |
| Rev: CTGTATTCAGCTCCGAGGTGTTCT | ||
| p27Kip1 | For: GCGCAGGAGAGCCAGGAT | SYBR |
| Rev: TTGGGGAACCGTCTGAAACA | ||
| P21Cip1 | For: NA | TaqMan |
| Rev: NA | (Hs99999142) | |
| PPARγ | For: NA | TaqMan |
| Rev: NA | (Hs01115513) | |
| Cyclophilin | For: CATCTGCACTGCCAAGACTGA | SYBR |
| Rev: TTCATGCCTTCTTTCACTTTGC |
Real-time PCR was carried out using either SYBR Green PCR Master Mix (#4309155, Applied Biosystems) or TaqMan Universal PCR Master Mix (#4304437, Applied Biosystems) on an IQ5 Real-Time Detection System (Bio-Rad). The PCR conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, followed by 42 cycles of 95 °C for 15 s and 60 °C for 1 min. Gene expression levels were normalized to cyclophilin, a housekeeping gene suitable as an internal control for real-time PCR analysis in primary skeletal muscle cell cultures (McAinch et al. 2006). Data analysis was performed using the comparative method (ΔCT) and results are reported as arbitrary units or fold change. Each donor was done in triplicate and the real-time PCR reaction was run on all samples in duplicate (n = 3–4 donors).
p21Cip1 and CDK6 immunoblotting
As a preliminary assessment of whether oxygen modulates protein levels of cell cycle regulatory genes, myoblasts from three donors were chosen for analysis of p21Cip1 and CDK6 protein expression. Cells were seeded into 175 cm2 flasks in 5% CO2, and either 5 or 20% O2, at 37 °C. Following 4 days of proliferation, sub-confluent myoblasts were harvested by trypsinization and lysed in M-PER extraction buffer (#78501, Thermo Scientific) in the presence of protease inhibitors (Halt™ Protease Inhibitor Cocktail Kit, #78410, Thermo Scientific). Protein concentration was determined using the BCA method (#23225, Pierce). Nine μg of protein per sample was loaded into each well on 4–20% acrylamide gradient gels (#NG21-420, NuSep). Proteins were transferred onto PVDF membranes (#RPN303F, GE Healthcare), blocked with 5% non-fat milk in PBS, and incubated overnight with the respective primary antibody (#2946-p21Cip1 and #3136—CDK6; Cell Signaling) diluted 1:2000 with 5% non-fat milk in PBS with 0.05% (v/v) Tween-20 (PBST). The blots were then washed thoroughly with PBST and incubated with a secondary anti-mouse antibody (#7076; Cell Signaling) diluted 1:2000 in 5% non-fat milk in PBS. The reaction was developed using enhanced chemiluminescence (#RPN2132, GE Healthcare) and digital images of each blot were captured using a charge coupled device (CCD) camera (Nikon Digital Sight DSU2) attached to a ChemiDoc XRS (Bio-Rad) and Quantity One imaging software (Bio-Rad). For bands of interest, trace quantity (intensity x mm) was used to determine protein expression. Amount of protein loaded, antibody concentrations and exposure times were optimised to retain band intensity values within a linear and non-saturated range. Coomassie staining of the Western blot was used to confirm even loading and transfer. Protein levels of p21Cip1 and CDK6 were assessed for each donor in duplicate (n = 3 donors) and for each sample band intensities were normalised to the 5% oxygen sample.
Myotube hypertrophy: protein content and MyHC immunohistochemistry
To assess the effects of oxygen on myotube hypertrophy, myoblasts were seeded into 24-well plates in 5% CO2, and either 5 or 20% O2, at 37 °C (n = 6 donors in quadruplicate). Following 6 days differentiation, cells were solubilized in 0.03% (w/v) SDS and total cellular protein was measured using a BCA™ Protein Assay Kit (#23225, Pierce). Myotubes 6 days post-differentiation, cultured in either 5 or 20% oxygen, were stained with an anti-myosin heavy chain (MyHC) antibody using immunohistochemistry. Cells grown in ECM-coated glass chamber slides (#254108, BD Falcon) were fixed in 2% paraformaldehyde, permeabilized with 0.1% (v/v) Triton X-100, blocked with 10% normal goat serum, incubated overnight at 4 °C with an anti-MyHC polyclonal antibody (#18-0075, Zymed—Invitrogen; 1:25 in PBS plus 1% BSA), and then were exposed to a mouse/rabbit dual link secondary antibody (#K4061, Dako Envision™+, Dako Corporation). Colour development was achieved with the AEC+ substrate chromogen (#3469, Dako Corporation), and nuclei were counterstained with hematoxylin.
Statistical analysis
Values are expressed as mean ± SEM. For the time course studies, results were analyzed using a general linear model ANOVA with the factors being time and oxygen, and a Tukey’s post hoc test was used to locate pair-wise significant differences, where appropriate. For the single time point data, results from individual subjects were compared using a paired t-test. A p value <0.05 was considered to be statistically significant.
Results
Purity of myogenic cultures
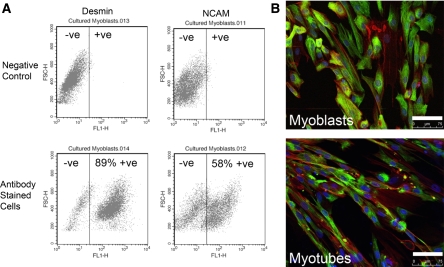
Myoblast desmin and NCAM expression was assessed at passage 4, because cells from this passage were used for all experimental analyses and data from cultured primary rat skeletal muscle cells indicate that myogenic purity (e.g. the percentage of desmin positive cells) can decrease with passage (Machida et al. 2004). Desmin is a very early marker of muscle precursor activation, expressed by undifferentiated muscle precursor cells in vivo and in vitro, and its expression precedes myoD and myogenin mRNA expression (Lawson-Smith and McGeachie 1998). NCAM expression has been associated with commitment to myoblast differentiation, with proliferating myoblasts being NCAM negative and differentiating myoblasts being NCAM positive (Capkovic et al. 2008). In the human primary myoblast cultures, the percentage of desmin positive cells was 84 ± 2% (range: 76–92%) and the percentage of NCAM positive cells was 45 ± 8% (range: 30–66%; Fig. 1a, b). The lower proportion of NCAM compared to desmin positive myoblasts may be attributed to desmin being an early myogenic marker, whereas NCAM is a late myogenic marker (Capkovic et al. 2008; Lawson-Smith and McGeachie 1998).
Fig. 1.
Assessment of myogenic purity of human primary skeletal muscle cell cultures using flow cytometry. a These representative graphs from myoblasts stained with anti-desmin or anti-NCAM antibodies, show that 89% of the cells were desmin-positive and 58% of the cells were NCAM positive. b To support the flow cytometry data are representative images of bona fide proliferating myoblasts and multinucleated myotubes where the desmin was stained green (FITC), nuclei were stained blue (DAPI) and the cytoskeleton was stained red (Alexa Fluoro 568 Phalloidin); white scale bar represents 75 μm. (please refer to online manuscript for colour images)
Low oxygen culture conditions enhanced the proliferation of human primary myoblasts
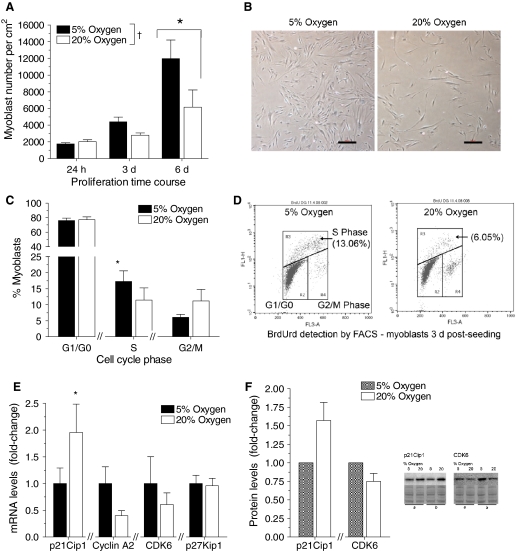
With time there was an increase in cell number in all proliferating myoblast cultures, reaching significance at 6 days post-seeding (p < 0.05; main effect time; Fig. 2a). Low oxygen culture conditions enhanced myoblast proliferation, such that cell numbers were greater in 5% compared to 20% oxygen (p < 0.05; main effect oxygen) and this was particularly evident at 3 and 6 days post-seeding (Fig. 2a, b). Although there was donor variability, low oxygen culture conditions consistently increased myoblast number in individual donors twofold to 5.5-fold (p < 0.05).
Fig. 2.
Increased myoblast proliferation rate in 5% compared to 20% oxygen culture conditions. For all graphs, 5% oxygen culture conditions are represented by black bars and 20% oxygen culture conditions are represented by white bars. a *With time there was an increase in cell number in proliferating myoblast cultures, reaching significance at 6 days post-seeding (p < 0.05; main effect time). †Low oxygen culture conditions enhanced myoblast proliferation, such that cell numbers were greater in 5% compared to 20% oxygen (p < 0.05; main effect oxygen). b Representative images showing greater myoblast proliferation in 5% than 20% oxygen at 4 days post-seeding; red scale bar represents 100 μm. c Percentage of myoblasts in the G1/G0, S (DNA synthesis) and G2/M phase of the cell cycle as measured by BrdU incorporation and FACS analysis. *At 3 days post-seeding, there were more cells in S phase in 5% than 20% oxygen (p < 0.05; paired t-test) and this was associated with a non-significant reduction in the proportion of cells in the G2/M phase. Oxygen had no effect on the proportion of myoblasts in the G1/G0 phase. d Representative FACS graphs, obtained 3 days post-seeding, showing a greater percentage of cells gated in the S phase of the cell cycle at 5% than 20% oxygen. e *At 4 days post-seeding, mRNA levels of the negative cell cycle regulator p21Cip1 were higher in 20% compared to 5% oxygen (p < 0.05; paired t-test). Cyclin A2, CDK6 and p27Kip1 gene expression was not significantly affected by cell culture oxygen levels. f In concordance with the gene expression results, qualitative Western blot data indicate that p21Cip1 protein levels were increased ~1.5-fold in proliferating myoblasts cultured in 20 versus 5% oxygen; whereas CDK6 protein levels were unaffected by oxygen. To support the graphed group data, note the representative lanes from the p21Cip1 and CDK6 western blots from myoblasts cultured in 5 and 20% oxygen from two different subjects. (please refer to online manuscript for colour images)
BrdU incorporation and flow cytometry were used to investigate whether increased myoblast number in the 5% oxygen culture conditions could be attributed to increased DNA replication or changes in cell cycle. At 3 days post-seeding, myoblasts cultured in 5% oxygen had on average ~ 50% more cells in the S (DNA synthesis) phase of the cell cycle compared to cells cultured in 20% oxygen (p < 0.05; Fig. 2c). Furthermore, in the 5% oxygen cultures there was a trend for a lower proportion of myoblasts in the G2/M phase (6%) compared to the 20% oxygen cultures (11%). Oxygen had no effect on the proportion of cells in G1/G0 phase.
Gene and protein expression of cell cycle regulators in low oxygen culture
Real-time PCR was performed 4 days post-seeding to determine whether low oxygen culture conditions were associated with reduced expression of negative cell cycle regulators (p21Cip1 and p27Kip1) and increased expression of positive cell cycle regulators (cyclin A2 and CDK6) in proliferating myoblasts. mRNA levels of p21Cip1, but not p27Kip1, were significantly down-regulated in 5% compared to 20% oxygen (p < 0.05; Fig. 2e). The gene expression of the positive cell cycle regulators cyclin A2 and CDK6 was not significantly affected by oxygen, (Fig. 2e). In concordance with the gene expression results, qualitative Western blot data indicate that p21Cip1 protein levels were increased ~1.5-fold in proliferating myoblasts cultured in 20 versus 5% oxygen, while CDK6 protein levels were not altered (Fig. 2f).
Low oxygen culture conditions do not improve myoblast viability during proliferation
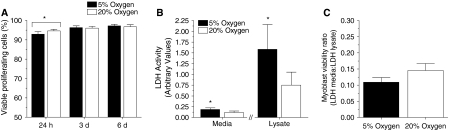
Low oxygen culture conditions can improve cell viability (Csete 2005), hence Trypan blue staining and a LDH cytotoxicity assay were used to investigate whether improved myoblast viability contributed to the increase in cell number observed in 5% oxygen. The viability of proliferating myoblasts, as assessed by Trypan blue staining, was the same in 5 and 20% oxygen culture conditions at 24 h, 3, and 6 days post-seeding (Fig. 3a).
Fig. 3.
No effect of cell culture oxygen levels on the viability of proliferating myoblasts. For all graphs, 5% oxygen culture conditions are represented by black bars and 20% oxygen culture conditions are represented by white bars. a Cell viability, as determined by Trypan blue staining, was similar in proliferating myoblasts cultured in 5 or 20% oxygen at 24 h, 3 and 6 days post-seeding. b Indicative of the increase in myoblast numbers in low oxygen culture conditions, LDH activity was greater in the cell lysate and medium from 5% oxygen compared to 20% oxygen cultures (p < 0.05; paired t-test). c In concordance with the Trypan blue data, when medium and cell lysate LDH activity were expressed as a viability ratio, oxygen concentration had no significant effect on the viability of proliferating myoblasts
The findings from the LDH cytotoxicity assay also indicated that oxygen had minimal effect on human myoblast viability during proliferation. Due to increased cell numbers, the medium (dead cells) LDH activity and the cell lysate (viable cells) LDH activity was higher in myoblasts proliferated in 5% compared with 20% oxygen culture conditions (p < 0.05; Fig. 3b). However, when expressed as a viability ratio (medium to cell lysate LDH activity), there was no significant difference between myoblasts proliferated in 5 or 20% oxygen (Fig. 3c).
In proliferating myoblasts, myoD and myogenin mRNA levels are similar in both 5 and 20% oxygen culture conditions
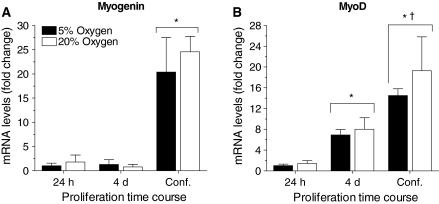
With increasing cell density, myoD and myogenin mRNA levels were up-regulated between 24 h post-seeding and 90% confluence (p < 0.05; main effect time; Fig. 4a, b). The increase in myoD gene expression preceded the increase in myogenin gene expression which is indicative of spontaneous myoblast differentiation. Compared to 24 h proliferation, myoD gene expression was increased 7.5-fold at 4 days post-seeding and 15- to 20-fold at 90% confluence. Myogenin mRNA levels were similar at 24 h and 4 days post-seeding, but at 90% confluence gene expression was increased 20-fold. The increase in myoD and myogenin mRNA transcripts was independent of cell culture oxygen concentration (Fig. 3a, b).
Fig. 4.
Oxygen does not modulate myoregulatory factor gene expression in proliferating myoblasts. For all graphs, 5% oxygen culture conditions are represented by black bars and 20% oxygen culture conditions are represented by white bars. a, b *MyoD and myogenin gene expression was upregulated in proliferating myoblasts with time (p < 0.05; main effect time), indicating spontaneous differentiation with increasing cell density. MyoD and myogenin mRNA levels were similar in 5 and 20% oxygen at all time points measured
Low oxygen culture conditions have no effect on myoblast differentiation and myotube hypertrophy
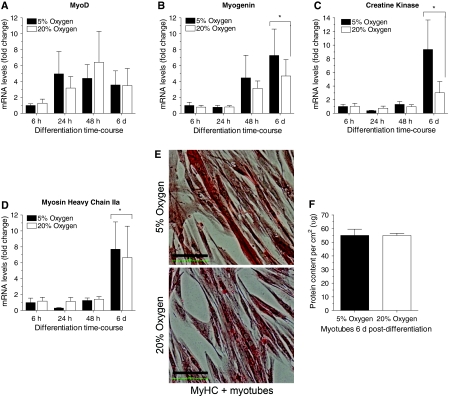
Myoblast differentiation is characterized by an early increase in myoD and myogenin gene expression, followed by a later increase in creatine kinase and MyHC gene expression (Chargé and Rudnicki 2004). MyoD mRNA transcripts were increased three to six-fold between 24 h and 6 days post-differentiation versus 6 h (p = N.S.). Gene expression of the later markers of differentiation, specifically myogenin, creatine kinase, and MyHC IIa, was increased significantly at 6 days post-differentiation in both the 5 and 20% oxygen culture conditions compared to 6, 24 and 48 h post-differentiation (p < 0.05; main effect time). MyoD, myogenin, creatine kinase and MyHC IIa gene expression were not significantly different between the 5 and 20% oxygen culture conditions at any post-differentiation time point examined (Fig. 5a–d).
Fig. 5.
Similar myoblast differentiation efficacy and myotube growth in 5 and 20% oxygen culture conditions. For all graphs, 5% oxygen culture conditions are represented by black bars and 20% oxygen culture conditions are represented by white bars. a Compared to the 6 h time point MyoD gene expression tended to increase three to six-fold between 24 h and 6 days post-differentiation. b–d In differentiating myotubes, myogenin, creatine kinase, and MyHC IIa mRNA levels were significantly increased at 6 days post-differentiation in both the 5 and 20% oxygen culture conditions (p < 0.05; main effect time). e Representative light microscopy image of multi-nucleated myotubes at 6 post-differentiation cultured in 5 or 20% oxygen and stained with an antibody reactive against all adult myosin heavy chain isoforms. Myosin heavy chain protein stained red; black scale bar represents 100 μm. f As a marker of hypertrophy, the total protein content of 0.25% SDS-solubilized 6 days old myotubes was determined and it was not different in the 5 and 20% oxygen culture conditions. (please refer to online manuscript for colour images)
In concordance with the gene expression data, at 6 days post-differentiation total protein content of myotubes cultured in 5 and 20% oxygen was similar (Fig. 5f). No indication of myotube hypertrophy was observed when myotubes cultured in 5 or 20% oxygen were examined under a microscope. Figure 5e is a representative image of myotubes differentiated in 5 or 20% oxygen for 6 days and then probed with an anti-MyHC antibody; demonstrating the similarity in fiber size and MyHC protein expression. Thus, cell culture oxygen levels neither enhanced nor impaired myoblast differentiation efficacy or hypertrophy.
In contrast to previous reports in primary mouse skeletal muscle cultures (Csete et al. 2001), oxygen had no effect on adipogenesis in proliferating or differentiating human myoblasts. In proliferating myoblasts PPARγ gene expression, the key transcription factor regulating adipocyte differentiation, was similar in 5 and 20% oxygen cultures (data not shown). Furthermore, Oil Red O staining for lipids was carried out on myotubes differentiated in 5 or 20% oxygen and no evidence of staining was observed (data not shown).
Discussion
Low, estimated physiological oxygen culture conditions (3–6%) have been shown to enhance the proliferation, differentiation, and viability of primary mouse and rat myoblasts (Lees et al. 2008; Chakravarthy et al. 2001; Csete et al. 2001). Here we report for the first time, that the proliferative capacity of human myoblasts was also enhanced by low oxygen culture conditions, but viability and differentiation of human myoblasts were similar in low and standard oxygen culture conditions. There was a significant increase in cell number when proliferating myoblasts, obtained from the vastus lateralis muscle of elderly donors, were cultured in 5% compared to 20% oxygen. Although enhanced proliferation of rodent myoblasts in low oxygen culture conditions has been well described in the literature (Lees et al. 2008; Csete et al. 2001), because of species differences it was not known whether human myoblasts would respond similarly. Proliferation is far more responsive to environmental regulation, such as low oxygen culture conditions, in rodent compared to human cells (Smogorzewska and de Lange 2002; Mouly et al. 2006).
Low oxygen culture conditions increased myoblasts numbers ~ 1.5-fold at 3 days post-seeding and ~two-fold at 6 days post-seeding. Although increased myoblast proliferation in low oxygen has been reported in primary mouse (Csete et al. 2001), rat (Lees et al. 2008; Chakravarthy et al. 2001), and bovine (Kook et al. 2008) myoblasts, none of these studies report a proliferation time course making it difficult to exactly compare the responsiveness seen in this study. The increase in myoblast number observed in low compared to standard oxygen culture conditions may be attributed to a faster progression through the cell cycle (Lees et al. 2008; Kook et al. 2008) and/or to improved myoblast viability (Csete et al. 2001).
The effect of oxygen on myoblast cell cycle progression was assessed using pulse BrdU labeling and flow cytometric analysis. At 3 days post-seeding, the 5% oxygen cultures had more myoblasts in S phase and a trend towards fewer myoblasts in the G2/M phase compared to the 20% oxygen cultures; the proportion of cells in G1/G0 phase was similar. Together with the increase in myoblast number, the flow cytometry data suggest that low oxygen culture conditions may stimulate a faster G1 to S phase transition and attenuate the proportion of cells arrested in the G2/M phase (with the net effect being a similar proportion of cells in G1/G0). Using immunohistochemistry rather than flow cytometry, a significant increase in BrdU positive nuclei was observed in primary rat (Lees et al. 2008) and mouse (Csete et al. 2001) myoblasts cultured in low compared to standard oxygen. However, the phase of the cell cycle affected by low oxygen culture conditions cannot accurately be determined from these immunohistochemistry studies. In contrast to our cell cycle data, Kook et al. (2008) reported that the increase in proliferation observed in bovine myoblasts cultured in 1 versus 20% oxygen was due to a decrease in the proportion of cells in S phase and a corresponding increase in the proportion of cells in the G2/M phase. This discrepancy may be mediated by differences in oxygen concentration, as 1% compared to 5% oxygen is more representative of hypoxia rather than physiological normoxia. Species differences might also be involved.
The mechanism(s) by which low oxygen enhanced myoblast proliferation are not well understood, but are likely mediated by the differential expression of cell cycle regulators. Up-regulation of positive cell cycle regulators and down-regulation of negative cell cycle regulators have been reported in primary myoblasts cultured in low compared to standard oxygen levels, although the specific regulators affected depend on cell culture oxygen concentration and myoblast source (Chakravarthy et al. 2001; Lees et al. 2008; Kook et al. 2008). In proliferating human primary myoblasts at 4 days post-seeding, the gene and protein expression of p21Cip1 was found to be lower in 5% than 20% oxygen, whereas p27Kip1 mRNA levels were not affected by oxygen. Whilst p27Kip1 expression and activity are regulated by both transcriptional and translational pathways, proteolytic degradation is a particularly important regulatory mechanism (Besson et al. 2006) and as such the information provided by gene expression data can be limited. In primary myoblast cultures established from adult rat skeletal muscles, 5% oxygen also reduced mRNA levels of p21Cip1, but not p27Kip1. This reduction in p21Cip1 gene expression was associated with reduced protein expression and it was attributed to decreased mRNA stability, as promoter activity was not affected by oxygen concentration (Lees et al. 2008). In a follow-up mechanistic study, they showed that cell culture oxygen levels modulate Sirt1 expression and that adenovirus mediated over-expression of Sirt1 increased myoblast proliferation and suppressed p21Cip1 expression. Paradoxically, p27Kip1 expression was increased (Rathbone et al. 2009). Hence, we believe that p21Cip1 may be more significant than p27Kip1 in regulating primary myoblast proliferation in low oxygen culture conditions. In human primary myoblasts oxygen had no significant effect on the expression of the positive cell cycle regulators, specifically cyclin A2 and CDK6. However, the regulation of cell cycle progression is complex and other positive cell cycle regulators responsive to oxygen in human primary myoblasts could include cyclin D1, CDK4, CDK2, and PCNA (Lees et al. 2008).
Low oxygen culture conditions have been previously shown to improve stem cell viability (Csete 2005), and during proliferation this can lead to increased myoblast numbers. Unpublished data from Csete et al. indicate that mouse muscle satellite cells undergo less apoptosis when cultured in 6% rather than 20% oxygen (Csete 2005). The survival of isolated single mouse muscle fibers after 48 h in 6% oxygen was 74% compared to 22% for fibers cultured in 20% oxygen (Csete et al. 2001). However, in this current study there was no effect of oxygen on the viability of proliferating human myoblasts as assessed by vital Trypan blue staining and the LDH cytotoxicity assay. Thus, the increase in myoblast number in low oxygen culture conditions was attributed solely to greater proliferation.
A close and functional cross-talk relationship exists between cell cycle regulators and the myoregulatory transcription factors. They act in parallel but independently to regulate terminal cell cycle withdrawal and differentiation (Kitzmann and Fernandez 2001). In proliferating myoblasts p27Kip1 acts upstream of myoD to influence its half-life (Messina et al. 2005), whereas p21Cip1 is a suggested downstream target of myoD and it can help regulate myoblast differentiation at the myogenin step (Zhang et al. 2008). Given that low oxygen decreased p21Cip1 expression in proliferating human myoblasts and reports that low oxygen culture conditions can help maintain proliferating adult stem cells in an undifferentiated state (Fehrer et al. 2007), myoD and myogenin mRNA transcripts were measured to assess whether oxygen affects myoblast “stem cell potential”. During proliferation with increasing myoblast density, myoD gene expression increased followed by a delayed increase in myogenin gene expression, indicative of spontaneous differentiation and a loss of myoblast “stem cell potential”. Despite lower p21Cip1 expression in 5% oxygen, the increase in myoD and myogenin mRNA transcripts during myoblast proliferation was not affected by oxygen concentration. This was not unexpected, since with increasing myoblast density there is greater cell-to-cell contact which can stimulate differentiation signalling pathways (Messina et al. 2005). Our gene expression data suggest that in human cells, oxygen neither helped maintain myoblast stem cell potential nor enhanced spontaneous myoblast differentiation. Future experiments would need to investigate the effects of oxygen on the protein level and transcriptional activity of myoregulatory transcription factors to draw definitive conclusions about the effects of low oxygen culture conditions on human myoblast “stem cell potential”. In contrast to human myoblasts, low oxygen increased myoD and myogenin gene expression after 24 h in culture in freshly isolated proliferating mouse primary myoblasts (Csete et al. 2001), suggesting that in these cells low oxygen culture conditions may promote a more differentiated phenotype. This is in concordance with the stimulatory effect of oxygen on myotube formation in rodent cells (Chakravarthy et al. 2001; Hansen et al. 2007; Csete et al. 2001).
Low oxygen culture conditions are thought to enhance differentiation, myotube formation and hypertrophy in rodent or bovine myoblasts by providing a reducing environment which may have favourable effects on cellular redox state and downstream redox-sensitive signalling pathways (Hansen et al. 2007; Chakravarthy et al. 2001; Kook et al. 2008). Protein levels of the differentiation markers myoD, myogenin, and MyHC were greater in primary bovine myoblasts differentiated in 1% oxygen compared to 20% oxygen (Kook et al. 2008). Primary rat myotubes differentiated in 3% oxygen were larger than those differentiated in 20% oxygen (Chakravarthy et al. 2001). The fusion index was higher for primary bovine (Kook et al. 2008) and C2C12 myoblasts (Hansen et al. 2007) differentiated in low compared to standard oxygen culture conditions. During differentiation of human myoblasts, we observed the expected increase in myoD, myogenin, creatine kinase, and MyHC IIa and IIx gene expression. But in contrast to bovine or rodent myoblasts (Kook et al. 2008; Hansen et al. 2007), this increase was similar in 5 and 20% oxygen. Human myotube hypertrophy was also independent of oxygen concentration, as after 6 days of differentiation the protein content of myotubes grown in 5 or 20% oxygen was similar.
In primary mouse myoblasts low oxygen culture conditions not only stimulated myogenesis, but also inhibited adipogenesis through the down regulation of the key transcriptional regulator PPARγ (Csete et al. 2001). This was not relevant in human primary skeletal muscle cell cultures, as PPARγ gene expression in myoblasts was unaffected by oxygen concentration and, using Oil Red O staining, we observed no evidence of lipid accumulation in differentiated myotubes.
To minimize the invasiveness of the study, muscle biopsies were obtained from healthy elderly donors during elective hip replacement surgery. Given the literature, we believe that the response of human primary myoblasts, proliferated for a low number of passages, to 5% oxygen culture conditions would be independent of donor age. Whilst there is a dramatic decrease in the replicative potential of cultured human myoblasts between birth and ~20 years, this is followed by a period of relative stability even into old age (Decary et al. 1997). The impairment in muscle regeneration observed with aging in vivo, is likely mediated by the external host environment, rather than an irreversible loss of the regenerative potential of satellite cells (Conboy and Rando 2005). Furthermore, if myoblasts are to be used in autologous myoblast transplantation therapies for cardiovascular disease, for example, then primary muscle cell cultures would need to be established from elderly donors.
In conclusion, we assessed the effects of oxygen on myoblast proliferation and differentiation in human primary skeletal muscle cell cultures established from elderly donors. Our data indicate for the first time in cultured human skeletal muscle cells, that low estimated physiological oxygen concentrations enhanced myoblast proliferation by potentially stimulating a faster G1 to S phase transition and perhaps reducing G2/M phase lag. Our findings also showed that low oxygen culture conditions neither enhanced nor compromised human myoblast differentiation. This is in contrast to rat, mouse, or bovine skeletal muscle cell culture models, where low oxygen (1–6%) enhanced both myoblast proliferation and differentiation (Kook et al. 2008; Csete et al. 2001; Lees et al. 2008; Chakravarthy et al. 2001). The activity and behavior of muscle precursor cells is regulated by various local signals from the environment whether in vitro or in vivo. Cell culture oxygen concentration is one such important regulator (Cornelison 2008). Our data, in context with the relevant literature, indicate that how oxygen regulates muscle precursor cell behavior is highly dependent upon species of origin (e.g. mouse, rat, bovine or human) and whether the myoblasts are primary or immortalised. Furthermore, our data also demonstrate that 5% oxygen culture conditions are a viable strategy to obtain increased myoblast numbers for in vitro skeletal muscle stem cell research.
Acknowledgments
We thank Assoc Prof David Cameron-Smith for his expertise with primer design; Prof Geoff C Nicholson for his assistance with the human ethics applications; the Orthopedic Surgeons—Drs David Bainbridge, Rick Angliss, and Rob Wood—for collection of the muscle biopsy samples; and Lisa Coleman, the Orthopedic Liaison Nurse, for her participation in subject recruitment. We gratefully acknowledge all of their contributions towards the completion of this project. The work presented in this report was supported by a NHMRC Postdoctoral Fellowship to NS and by the Deakin University Central Research Grants Scheme.
Abbreviations
- BrdU
Bromodeoxyuridine
- CK
Creatine kinase
- LDH
Lactate dehydrogenase
- MyHC
Myosin heavy chain
- SkGM
Skeletal muscle growth medium
- SkDM
Skeletal muscle differentiation medium
References
- Berggren JR, Tanner CJ, Koves TR, Muoio DM, Houmard JA. Glucose uptake in muscle cell cultures from endurance-trained men. Med Sci Sports Exerc. 2005;37:579–584. doi: 10.1249/01.MSS.0000158180.11224.E8. [DOI] [PubMed] [Google Scholar]
- Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that control p27Kip1 stability, subcellular localization, and tumour suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capkovic KL, Stevenson S, Johnson MC, Thelen JJ, Cornelison DDW. Neural cell adhesion molecule (NCAM) marks adult myogenic cells committed to differentiation. Exp Cell Res. 2008;314:1553–1565. doi: 10.1016/j.yexcr.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscle. Cell Mol Life Sci. 2001;58:1150–1158. doi: 10.1007/PL00000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé SBP, Rudnicki MA. Cellular and molecular regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Cornelison DDW. Context matters: in vivo and in vitro influences on muscle satellite cell activity. J Cell Biochem. 2008;105:663–669. doi: 10.1002/jcb.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Barbara W. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- Decary S, Mouly V, Hamida BC, Barbet JP, Butler-Browne GS. Replicative potential of telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Brunauer R, Laschober G, Unterluggauer H, Reitinger S, Kloss F, Gülly C, Gassner R, Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- Gaster M, Beck-Nielsen H, Schrøder HD. Proliferation conditions for human satellite cells: the fractional content of satellite cells. APMIS. 2001;109:726–734. doi: 10.1034/j.1600-0463.2001.d01-139.x. [DOI] [PubMed] [Google Scholar]
- Hansen JM, Klass M, Harris C, Csete M. A reducing redox environment promotes C2C12 myogenesis: implications for regeneration in aged muscle. Cell Biol Int. 2007;31:546–553. doi: 10.1016/j.cellbi.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann M, Fernandez A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol Life Sci. 2001;58:571–579. doi: 10.1007/PL00000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S-H, Son Y-O, Lee K-Y, Lee H-J, Chung W-T, Choi K-C, Lee J-C. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of myoD. Cell Biol Int. 2008;32:871–878. doi: 10.1016/j.cellbi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Lawson-Smith MJ, McGeachie JK. The identification of myogenic cells in skeletal muscle, with emphasis on the use of tritiated thymidine autoradiography and desmin antibodies. J Anat. 1998;192:161–171. doi: 10.1046/j.1469-7580.1998.19220161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees SJ, Childs TE, Booth FW. p21Cip1 expression is increased in ambient oxygen, compared to estimated physiological (5%) levels in rat muscle precursor cells. Cell Prolif. 2008;41:193–207. doi: 10.1111/j.1365-2184.2008.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu L, Chen X, Fan M. Effects of hypoxia on proliferation and differentiation of myoblasts. Med Hypotheses. 2007;69:629–636. doi: 10.1016/j.mehy.2006.12.050. [DOI] [PubMed] [Google Scholar]
- McAinch AJ, Steinberg GR, Mollica J, O’Brien PE, Dixon JB, Macaulay SL, Kemp BE, Cameron-Smith D. Differential regulation of adiponectin receptor gene expression by adiponectin and leptin in myotubes derived from obese and diabetic individuals. Obesity. 2006;14:1898–1904. doi: 10.1038/oby.2006.221. [DOI] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD. The timing between skeletal muscle myoblast replication and fusion into myotubes, and the stability of regenerated dustrophic myofibres: an autoradiographic study in mdx mice. J Anat. 1999;194:287–295. doi: 10.1046/j.1469-7580.1999.19420287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G, Blasi C, La Rocca SA, Pompili M, Calconi A, Grossi M. p27Kip1 acts downstream of N-cadherin-mediated cell adhesion to promote myogenesis beyond cell cycle regulation. Mol Biol Cell. 2005;16:1469–1480. doi: 10.1091/mbc.E04-07-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, Gidaro T, Jacquemin V, Mamchaoui K, Negroni E, Périé S, Renault V, Silva-Barbosa SD, Butler-Browne GS. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2006;184:3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Gussoni E. Human myoblasts and muscle derived SP cells. Methods Mol Med. 2005;107:97–110. doi: 10.1385/1-59259-861-7:097. [DOI] [PubMed] [Google Scholar]
- Péault B, Rudnicki MA, Torrente Y, Cossu G, Tremblay JP, Partridge T, Gussoni E, Kunkel LM, Huard J. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Therapy. 2007;15:867–872. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Rathbone CR, Booth FW, Lees SJ. Sirt1 increases skeletal muscle precursor cell proliferation. Eur J Cell Biol. 2009;88:35–44. doi: 10.1016/j.ejcb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, Lange T. Different telomere damage signalling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JD, Masi TL, Cumming AE, Molnar GM, Wentworth BM, Sampath K, McPherson JM, Yaeger PC. Characterization of proliferating human skeletal muscle-dervived cells in vitro: differential modulation of myoblast markers by TGF-b2. J Cell Physiol. 2003;196:70–78. doi: 10.1002/jcp.10322. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, Liu D, Finegold M, Harper WJ, Elledge SJ. p21CIP1 and p57KIP2 control muscle differentiation at the myogenin step. Genes Dev. 2008;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]