Abstract
The worldwide yearly mortality from sepsis is substantial, greater than that of cancer of the lung and breast combined. Moreover, its incidence is increasing, and its response to therapy has not appreciably improved. In this condition, the secretion of procalcitonin (ProCT), the prohormone of calcitonin, is augmented greatly, attaining levels up to thousands of fold of normal. This hypersecretion emanates from multiple tissues throughout the body that are not traditionally viewed as being endocrine. The serum values of ProCT correlate with the severity of sepsis; they recede with its improvement and worsen with exacerbation. Accordingly, as highlighted in this review, serum ProCT has become useful as a biomarker to assist in the diagnosis of sepsis, as well as related infectious or inflammatory conditions. It is also a useful monitor of the clinical course and prognosis, and sensitive and specific assays have been developed for its measurement. Moreover, it has been demonstrated that the administration of ProCT to septic animals greatly increases mortality, and several toxic effects of ProCT have been elucidated by in vitro experimental studies. Antibodies have been developed that neutralize the harmful effects of ProCT, and their use markedly decreases the symptomatology and mortality of animals that harbour a highly virulent sepsis analogous to that occurring in humans. This therapy is facilitated by the long duration of serum ProCT elevation, which allows for a broad window of therapeutic opportunity. An experimental groundwork has been established that suggests a potential applicability of such therapy in septic humans.
Keywords: procalcitonin, calcitonin, sepsis, systemic inflammation, harmful marker, cytokine, therapeutic target
Introduction
Millions of patients in the world are increasingly exposed to sepsis each year, and the rate of sepsis mortality remains high (Angus et al., 2001; Harrison et al., 2006). Although sepsis is a dynamic and complex syndrome, certain serum markers have been implicated as playing a central, and potentially harmful, mediator role in this acute and devastating illness, for example, endotoxin, cytokines, chemokines, prostaglandins, oxygen free radicals, etc. Over several decades, various studies utilizing strategies, such as antibodies, soluble receptors or receptor antagonists, were initiated with much enthusiasm, only to be terminated with high cost and much chagrin due to lack of therapeutic impact. Indeed, based on these failures, it has became commonplace to conclude that such therapy is doomed to failure.
The present review demonstrates that many studies now indicate that the prohormone, procalcitonin (ProCT), is an excellent marker for sepsis and its related conditions, and that the immunoneutralization of this prohormone offers considerable therapeutic promise.
Sepsis and related conditions
The clinical term sepsis is characterized by a marked attack upon the host by pro-inflammatory cytokines that has been precipitated by an infection. Symptomatically, this illness often is manifested by two or more of the following: fever or hypothermia, tachypnea, tachycardia, leukocytosis or leukopenia. Not uncommonly, sepsis leads to one or more severe complications: for example, hypotension, cardiac failure, coma, renal failure, intravascular coagulation. This phenomenon is termed multiple organ dysfunction (Fry, 2000; Jean-Baptiste, 2007). Frequently, the disease leads to death. The mortality is greatest in infancy, the elderly, patients with other illnesses and the immunocompromised. When the offending microorganism is identified, bacteria are most often found to be the culprits. However, an identical condition is induced by the malarial parasite, and, rarely, by a fungal or viral infection.
Initially, a consensus meeting recommended that the term sepsis should be reserved for a patient with an infection (ACCP-SCCM Consensus Conference, 1992). However, it has been shown that microbiological cultures in patients strongly suspected of having sepsis are positive in only about 50% of cases, partially due to technical problems of the culture and the timing of specimen collection. Accordingly, it was concluded that the infection may only be strongly suspected, without being microbiologically confirmed (Levy et al., 2003). While some authors still insist on sepsis being characterized by proven infection, many others make the clinical diagnosis if infection is presumed or suspected even though not proven (Seam and Suffredini, 2007; Chen et al., 2009). Consequently, the terms culture-positive and culture-negative sepsis have been employed; the symptomatology and mortality of these two classifications have been reported to be similar (Rangel-Frausto, 1999). In sepsis, when cultures are positive, Gram-negative bacteria are moderately more common than Gram positive. However, the prevalence of Gram-positive bacterial pathogens is rapidly increasing (Opal and Cohen, 1999).
The systemic inflammatory response syndrome (SIRS) is the term given to patients who have two or more of the clinical symptoms noted in sepsis: fever or hypothermia, tachypnea, tachycardia and leukocytosis or leukopenia, although infection is not deemed to be present. As in sepsis, multiple organ dysfunction and death may also occur. Clearly, in view of the problems with bacterial culture mentioned earlier, the distinction between SIRS and sepsis is blurred. While in clinical practice, fever, hypothermia, tachypnea, tachycardia and/or leukocytosis or leukopenia can be non-specific findings, some authors believe that there is a hierarchical continuum towards sepsis (Rangel-Frausto, 1999; Brun-Buisson, 2000).
Conditions usually given the appellation SIRS include trauma, extensive surgery, heat stroke, pancreatitis, respiratory distress syndrome and burns. Importantly, as in sepsis, these conditions are frequently characterized by a hypersecretion of pro-inflammatory cytokines (Bone, 1996). It has been demonstrated that in these illnesses, there may be translocation across the gut wall, or across the respiratory or urogenital epithelial barriers of bacteria or of bacterial constituents, which are known to be potent stimuli to the secretion of pro-inflammatory cytokines (Berg, 1992; Ryan et al., 1992; Guyer et al., 2000; Li et al., 2001; Ammori et al., 2003a; Wang et al., 2003; Shibata et al., 2005; Duff et al., 2006; Pezzicoli et al., 2008). The same syndrome may also arise from non-bacterial sources, originating from the release of factors from dying cells, that is, damage-associated molecular patterns (Cinel and Opal, 2009).
Lymphocytes and their subsets (T cells, B cells and natural killer cells, as well as monocytes) are a basic part of the immune system, and these cells, along with neutrophils, play a major role in defending the host from infection when they are functioning appropriately (Majlessi et al., 2008; Zucchini et al., 2008; Ermert et al., 2009). These cells, as well as many others, secrete cytokines, which act as signalling peptides permitting different cells and tissues to intercommunicate and interact with one another. In sepsis, an expanding number of cytokines have been found to be involved in the pathophysiology of the disease (Bozza et al., 2007). For example, TNFα is an intrinsic part of the pro-inflammatory process; it influences immune cells, and can cause cell death. IL-1β, among other effects, induces fever, enables leukocytes to cross the capillary endothelium and increases sensitivity to pain; it is cytotoxic to various cell types and activates the caspase cascade of apoptosis. IL-6 is a multifunctional cytokine that is largely causative of the acute-phase response that follows injury and infection. Some actions of these and other pro-inflammatory cytokines are in part beneficial, but when they are over-expressed, they are counterproductive and lead to worsening of the illness or death (Slifka and Whitton, 2000; Bozza et al., 2007; Cinel and Opal, 2009).
ProCT
ProCT is the precursor for the hormone calcitonin (CT) (Roos et al., 1974; Jullienne et al., 1980). CT, which is found in the thyroid C cells and the pulmonary endocrine cells, has a metabolic role in calcium homeostasis (Hirsch et al., 1963; Zaidi et al., 1992). The amino acid sequence of ProCT is comprised of 116 amino acids (LeMoullec et al., 1984). It is composed of a centrally placed 33-amino acid immature CT that is not amidated, a 57-amino acid aminoterminus (NProCT) and a 21-amino acid CT carboxypeptide 1 (CCP1) at the carboxyl terminus (Figures 1 and 2). All of these component peptides, including ProCT, have been found to circulate at very low concentrations in normal serum, presumably produced by the neuroendocrine cells in the thyroid gland and in the lungs (Snider et al., 1997). However, all tissues throughout the body have the potential to elaborate ProCT (see below).
Figure 1.
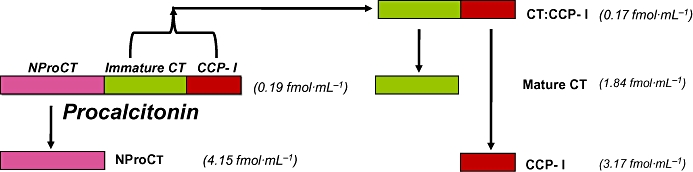
Procalcitonin and its constituent peptides in normal serum, all of which are found at the indicated low concentrations in the blood of normal humans (Snider et al., 1997).
Figure 2.
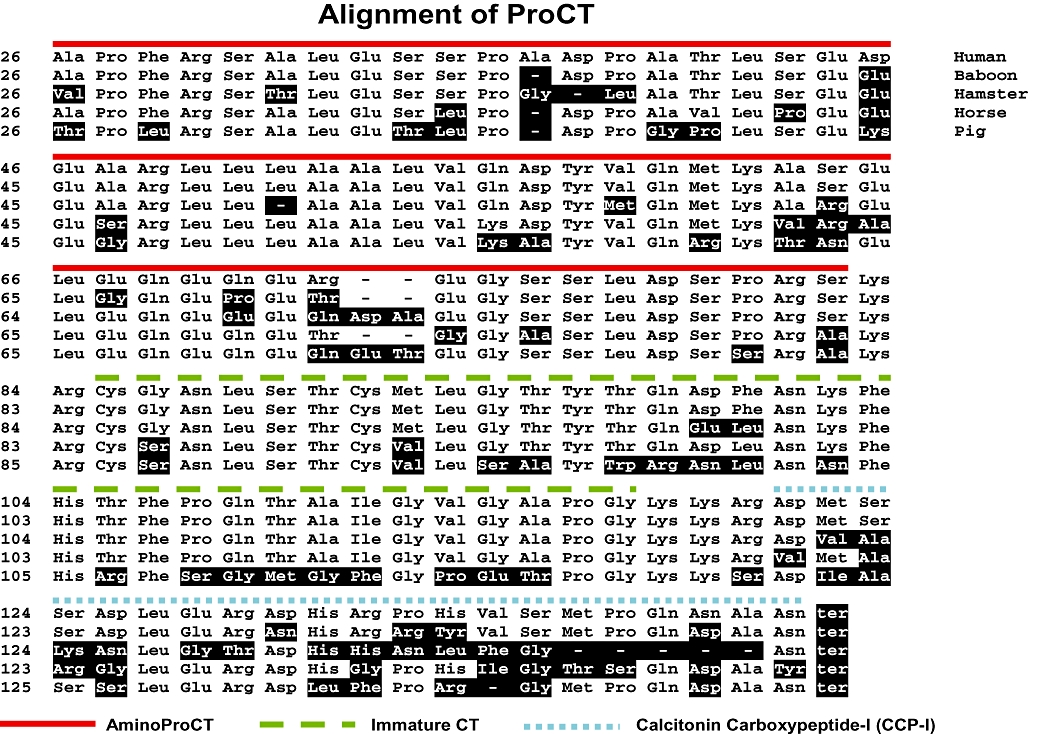
Comparative amino acid sequences of procalcitonin (ProCT) of different species. The human prohormone is derived from the CALC-I gene, via an alternative mRNA splicing that gives rise to the inclusion or exclusion of exons (Amara et al., 1982; Becker et al., 2002). The gene produces three different messenger RNAs. Two of these, after translation, eventuate into calcitonin precursor molecules. The first 25 amino acids that reside at the amino terminus of human pre-procalcitonin comprise the hydrophobic signal peptide that directs the prohormone through the endoplasmic reticulum. The following 57 amino acids comprise N-procalcitonin (NProCT), the next 33 amino acid residues is the immature calcitonin (the distal glycine residue is deleted if the calcitonin becomes amidated and is freed from the prohormone). The lysine–arginine at the terminus of NProCT and the lysine–lysine–arginine amino acids at the distal end of calcitonin are basic amino acid sites of cleavage. The 21 amino acid calcitonin carboxypeptide (CCP-I) is at the most distal end of the prohormone. (CCP-II, found in small concentrations in thyroid, pituitary and nervous tissue, differs by eight amino acids). All of the ProCT peptides are freed during the processing of the prohormone. The structure of human ProCT was reported by Dr Le Moullec et al., Paris, France (LeMoullec et al 1984); baboon ProCT was determined by Dr Andrew Russo et al., Iowa City, IA, USA; horse ProCT (Toribio et al., 2003); hamster and porcine ProCT determined by Dr Beat Muller et al., Aarau, Switzerland.
Hyperprocalcitonaemia in sepsis and related conditons
In sepsis, systemic infection and severe inflammation, the serum levels of ProCT usually increase markedly, attaining values of tens, to hundreds, to thousands-fold that of normal levels (Assicot et al., 1993; Whang et al., 1998; Müller et al., 2000). The same phenomenon has been found to occur in several species of animals, that is, hamster, rat, pig and baboon (Nylén et al., 1998; Redl et al., 2000; Wagner et al., 2002). Other than in some neuroendocrine tumours, clinical studies in humans reveal that the highest serum ProCT values occur in patients with sepsis. They also are increased in pneumonia (Nylén et al., 1996); acute inhalational injury (Nylén et al., 1992); and other severe infections and inflammations such as pancreatitis (Ammori et al., 2003b), appendicitis (Kafetzis et al., 2005), burns (Von Heimburg et al., 1998), heat stroke (Nylén et al., 1997), multitrauma (Maier et al., 2009) and extensive surgery (Meisner et al., 1998). While specific assay of serum ProCT in the healthy subject is less than 10 pg·mL−1 (Snider et al., 1997), it is not uncommon for levels to exceed 100 000 pg·mL−1 (we are unaware any other humoral substance that increases to such an extraordinary degree). In general, in humans and in experimental animals, these levels correlate with the severity of the condition, and remain elevated for the duration of the inflammatory process (Steinwald et al., 1999; Becker et al., 2007). Patients with SIRS may have very high levels of serum ProCT, and they overlap with sepsis. However, the highest levels tend to occur in sepsis. Notably, the levels that occur in systemic viral infections usually are considerably lower than bacterial infection (Nylén et al., 1996), but occasionally overlap (Thayvil et al., 2005).
Fluids other than blood can also manifest increased levels of ProCT. For example, salivary levels of this prohormone are increased in periodontitis (Bassim et al., 2008). Also, in persons with wartime extremity injuries, the ProCT in the wound exudate is significantly increased in those patients whose wounds dehisce when compared with wounds that subsequently heal (Forsberg et al., 2008). Serum ProCT levels below 500 pg·mL−1 are relatively uncommon in patients with classic sepsis symptomatology, but values below this level may indeed occur (Becker et al., 2007). Clinically, the daily determination of ProCT in sepsis is most useful (Becker et al., 2007; Castelli et al., 2009; Hochreiter et al., 2009). During the course of a septic process, there may be a marked increase in serum ProCT, often indicating an exacerbation of the illness. Moreover, a decreasing level often is a favourable sign (Figure 3) (Jensen et al., 2006). However, it should be emphasized that during the course of a septic process, complications may occur, such as hypotension, shock, heart failure, respiratory insufficiency or disseminated vascular coagulation. These conditions greatly influence the course and ultimate outcome of the disease without necessarily, in themselves, affecting ProCT levels. Moreover, the eventual outcome is influenced by the precipitating cause, as well as the clinical care. Thus, clinical severity-of-illness scores or prognostic scores, some of which involve parameters such as age or concomitant illness, for example, Acute Physiology and Chronic Health Evaluation score (Whang et al., 1998; Claeys et al., 2002), multiple organ failure (MOF) scores (Hensler et al., 2003), sequential organ assessment score (Castelli et al., 2004) or simplified acute physiology score II (Cheval et al., 2000) often correlate with serum ProCT levels, albeit only approximately.
Figure 3.
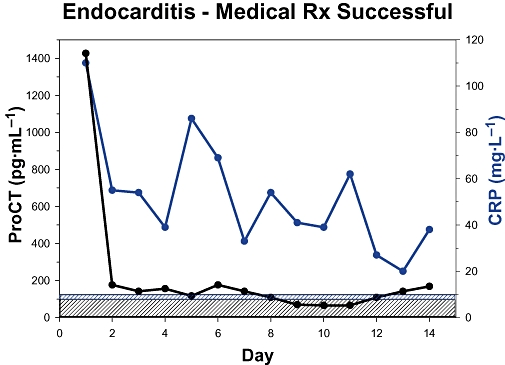
Serial mean serum procalcitonin (ProCT) levels from 19 patients with bacterial endocarditis (Duke classification) who were successfully treated with antibiotics. Within 1 day, ProCT levels had decreased markedly and these values remained close to normal. However, the CRP decline was much more gradual (unpublished data in collaboration with Dr Jonathan Sandoe, Leeds, Great Britain).
Initiators of hyperprocalcitonaemia
Studies of the initiation and the pattern of response of hyperprocalcitonaemia have been performed in experimental animals (Morgenthaler et al., 2003) and in humans (Dandona et al., 1994). In healthy human volunteers who were administered a single dose of endotoxin (lipopolysaccharide, LPS), serum levels of ProCT increased within 3 h, peaked within 24 h and then slowly declined (Preas et al., 2001). Moreover, high levels persisted, and remained above baseline for at least 7 days. In some instances, they did not normalize until 2 weeks. In contrast, in various laboratory and clinical settings, the classic cytokine markers have been found to be very evanescent (Thijs and Huck, 1995; Becker et al., 2004) and often exhibit marked inter-individual variations (Bone, 1996).
The primary pathophysiological trigger for the increase of serum ProCT is ‘infection’ (whether exogenous in origin or via endogenous translocation of bacterial toxins across the gut wall or other epithelial barriers). This often results in the appearance in the circulation of LPS, although it is likely that other constituents of microorganisms also are offenders. Soon thereafter, there is a secondary release of the putative principal pro-inflammatory and anti-inflammatory cytokine messengers. More study is required, and other messenger molecules may become identified, but current data indicate that mediators such as TNFα (as well as IL-1β and IL-6) comprise the specific proximate stimuli to hyperprocalcitonaemia (Redl et al., 2000, 2001; Whang et al., 2000; Domenech et al., 2001; Preas et al., 2001). Although there may be recurrent elevations of these cytokines during the course of sepsis, their short-lived duration and their erratic fluctuations differ markedly from the long-term persistence of hyperprocalcitonaemia, a ‘late’ and long-lasting marker and mediator.
When the secretion of ProCT becomes ubiquitous
As previously mentioned, secretion by the C cells of the thyroid, the pulmonary neuroendocrine cells of the lungs and perhaps other gastrointestinal neuroendocrine cells constitute the principal sources of serum ProCT in the healthy subject. Here, these cells produce CT from ProCT, the former being stored in dense-core secretion granules within the cytoplasm. These granules release their mature peptide contents when the appropriate stimulus appears at the cell surface, that is, a ‘regulated’ secretion (Burgess and Kelly, 1987).
It has been reported that serum immunoreactive CT moeities persist in the serum of humans in spite of their having had a prior thyroidectomy (Silva et al., 1978). In humans, and also in thyroidectomized monkeys, small but measurable amounts of such immunoreactivity was extracted from many tissues (Becker et al., 1979; 1980;), and low levels of the mRNA of the CT gene, CALC 1, were later found (Russwurm et al., 2001). However, in sepsis, reverse transcriptase polymerase chain reaction studies revealed considerable amounts of CALC I mRNA expression in nearly all tissues examined (fat, liver, lung, muscle, stomach, kidney, brain, etc.) (Müller et al., 2001). Moreover, in situ hybridization studies demonstrated that multiple cell types within tissues participated in this up-regulation (Linscheid et al., 2003). Because of the huge mass of fat in the body, this phenomenon was studied ex vivo and in vitro with human fat cells. It was reported that the addition of LPS to these cells induced a large increase of both CALC I mRNA and hormonal secretion. Analogous increases were also produced by TNFα and IL-1β (Linscheid et al., 2003). This ubiquitous expression of ProCT in nearly all tissues appears to be a unique phenomenon. In essence, the body becomes an endocrine gland, secreting ProCT in an ongoing unregulated constitutive fashion (Burgess and Kelly, 1987). It has been postulated that this sepsis-related increase of CALC-I gene transcription is mediated via stimulus-specific response elements within the promotor of the gene. In this respect, the term ‘hormokine’ has been proposed; that is, the cytokine-like behaviour of a hormone (i.e. ProCT) during inflammation or infection (Müller et al., 2001; Nylén and Alarifi, 2001; Müller 2007). Because very little, if any, mature CT hormone is produced in sepsis, it can be hypothesized that non-neuroendocrine tissue lacks the enzymatic potential to adequately process and activate the immature peptide.
Immunoasssay of ProCT
In the medical literature, six assays for ProCT have been employed, three of which are available commercially. The authors have evaluated the performance of all of them, the characteristics of which are detailed in Table 1.
Table 1.
Characteristics of procalcitonin (ProCT) assays (as determined in the authors' laboratory)
| Assay | Source | Type of test | Status | Peptides identified |
Low assay standard |
Functional sensitivity |
Mean of healthy controlsa |
Assay time | Comments |
|---|---|---|---|---|---|---|---|---|---|
| (pg·mL−1) | (pg·mL−1) | (pg·mL−1) | |||||||
| NProCT | Becker et al. | elisa | Research | ProCT and NProCT | 10 | 20 | 33b | 16–18 h | Highly sensitive, quantitates most normals; equipment readily available Slow; requires plate reader |
| ProCa-S | BRAHMS | ILMA | Research | ProCT and CT : CCP-I | 5 | 20 | 31 | 3 h | The most sensitive ILMA assay, rapid assay Requires luminometer |
| PCT sensitive | BRAHMS | ILMA | Research | ProCT and CT : CCP-I | 5 | 50 | 13 | 3 h | Highly sensitive; rapid Requires luminometer |
| QPCT | BRAHMS | Solid-phase | Commercial | ProCT and CT : CCP-I | (500) | (500) | (500) | 5 min | Rapid bedside test needing no equipment Semiquantitative test; provides determinations only within very broad ranges |
| LUMItest | BRAHMS | ILMA | Commercial | ProCT and CT : CCP-I | 80 | 500 | 235 | 2 h 45 min | The most widely used test to date; rapid Relatively insensitive; cannot detect mild elevations or mild variations; expensive to do single determinations; requires luminometer |
| KRYPTOR | BRAHMS | TRACE | Commercial | ProCT and CT : CCP-I | 20 | 60 | 53 | Initial sample: 50 min Stat sample: 25–45 minc | Sufficiently sensitive for most purposes; readily detects daily changes, ideal for a large number of determinations; and hence inexpensive for this usage Most appropriate for large-volume usage as in hospitals, large infectious disease services, intensive care units or emergency rooms Requires Kryptor machine |
Note:As shown above, no currently available commercial assay measures exclusively procalcitonin. All assays detect at least one other constituent of this prohormone. High-performance liquid chromatography studies of calcitonin gene peptides extracted from concentrated normal human serum reveal very low levels of ProCT (2.5 pg·mL−1) plus its component peptides (NProCT, CT, CCP-I and CT : CCP-I) (Snider et al., 1997). In hyperprocalcitonemic states, procalcitonin and its constituent peptides all may be increased to varying extent from patient to patient. Other than the hyperprocalcitonaemia that is due to secretion from a neuroendocrine tumour, the amidated free CT in patients with sepsis, infection or systemic inflammation remains low. CT is not measurable in any of the above assays. For simplicity of expression, all these above assays are now referred to by investigators as ‘procalcitonin’ assays.
The discrepancy between functional sensitivity and healthy control values (i.e. LUMItest, PCT sensitive, KRYPTOR) implies considerable uncertainty of the values in this population. Because none of these cited assays are capable of measuring the actual values of ProCT in sera from healthy controls, the authors recommend that investigators not try to make comparisons between data points below the functional sensitivity of the assays because such comparisons are likely to be unreliable.
This level is 90% aminoprocalcitonin, based on chromatography studies. The actual mean level of ProCT in healthy controls is <4 pg·mL−1. ProCT + CT : CCP-I < 5 pg·mL−1 (Snider et al., 1997).
The initial sample time includes the time required for powering up the equipment; performing the daily maintenance; and running the calibrators, control samples and the first sample. During the week, the machine may be left on and the calibrators need to be run only once. The time required for daily maintenance is less than 20 min. Additional samples loaded into the Kryptor machine along with the first sample require 1–2 min per sample; thus, for example, five additional samples can be measured within 5–10 min. The stat sample time shown above is the time required to measure a single sample that is loaded by itself after starting the machine. This includes the time that may be required for the automatic dilution and recount of a sample which contains a concentration of ProCT > 50 ng·mL−1.
ProCT, procalcitonin; NProCT, aminoprocalcitonin; CT : CCP-I, the conjoined peptide consisting of calcitonin + calcitonincarboxypeptide I (see Snider et al., 1997); elisa, enzyme-linked immunosorbent assay; ILMA, immunoluminometric assay; TRACE, time-resolved amplified cryptate emission.
Currently, there is no available assay that detects ProCT exclusively. Initially, for the purpose of studying systemic infection, inflammation and sepsis, a very sensitive, single-antibody assay of NProCT was developed for research purposes (Nylén et al., 1995; Snider et al., 1997). This assay detects ProCT and the free NProCT peptide. All other assays detect ProCT and the free conjoined CT: CCP-I peptide, utilizing two antibodies in a sandwich assay.
When ProCT is high in patients with systemic infection or sepsis, with the exception of CT, all of the peptides shown in Figure 4 increase, albeit not to the same extent, and the pattern varies from patient to patient and at varying times during the illness. In spite of this phenomenon, on a practical level, the clinical utility of the assays is not compromised. For simplicity of expression, investigators utilizing these assays refer to the material being measured as ‘procalcitonin’.
Figure 4.
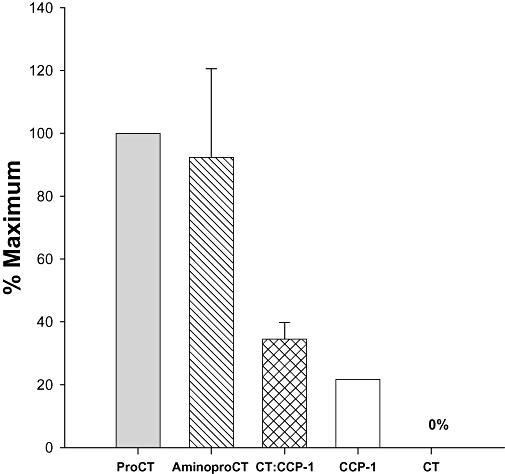
Mean peak concentrations of constituent peptides of procalcitonin (ProCT) of several patients with sepsis (G-75 Sephadex gel filtration and C18 reversed-phase high-performance liquid chromatography). The data are expressed in terms of the percentage of the mean peak level of the intact ProCT. In sepsis, some of the ProCT appears to have a two-amino acid truncation at the amino terminus (Weglöhner et al., 2001). The free, amidated, mature calcitonin remains absent or low, because the constitutive secretion of ProCT apparently lacks the enzymatic processing to cleave this peptide from the prohormone.
A very promising development in assays for ProCT is the recently available Kryptor procedure (Steinbach et al., 2004; Becker et al., 2007). Following preparation with calibration using control samples, the ProCT of a serum sample can be determined in 20 min, with excellent intra- and inter-assay precision. Although it should be recognized that the mean level for healthy controls is less than the functional sensitivity of the Kryptor assay (60 pg·mL−1), this instrument can still detect modest fluctuations from day to day, distinguish meaningful changes in ProCT (e.g. those changes associated with significant infection) and also perform multiple determinations at a cost that is comparatively low (see footnote to Table 1 for a more in-depth discussion of ProCT assays). It is now being utilized successfully to aid in the diagnosis of sepsis, and to follow the cause of this illness, as well as other severe infections such as pneumonia (Müller et al., 2007). Its reliability and sensitivity lend itself to the detection of the mild elevations that occur in a bacterial infection very early in the appearance of fever in infants (Dauber et al., 2008; Maniaci et al., 2008); it also offers useful diagnostic information in children with acute respiratory infection (Schützle et al., 2009). Other uses have been for identification of patients with paralytic ileus, the levels of whom are close to normal, as opposed to those with ileus of an obstructive or vascular causation (Maruna et al., 2008). Furthermore, this instrument should be able to detect the sudden increases of ProCT that herald the onset of systemic infection in patients with an intravascular catheter (Figure 5).
Figure 5.
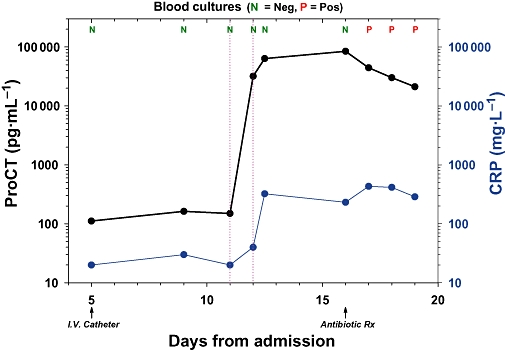
Study of a trauma patient admitted to the intensive care unit who had an intravascular catheter inserted at day 5 following hospitalization with a consequent risk of ensuing infection (see Maki et al., 2006). Antibiotics were started at day 16 because of the onset of fever, and the catheter was removed a day later. Retrospective studies revealed that on the morning of day 11, procalcitonin (ProCT) suddenly increased from a value that was close to normal (120 pg·mL−1) to 25 000 pg·mL−1, and by that evening had increased further to 60 000 pg·mL−1. However, on the same morning, the CRP had only slightly increased, and then subsequently increased further. Blood cultures remained negative (N) until day 17, when they became positive (P). Thus, the initial alarming increase of serum ProCT had occurred 5 days prior to the clinical diagnosis of catheter-related sepsis.
Perhaps the most exciting benefit from ProCT measurements obtained by Kryptor technology is its use as a marker to reduce overutilization of antimicrobial therapy. Throughout the world, the needless or excess administration of antibiotics has led to a marked increase of drug-related morbidity, immunological sensitization and expense, and, above all, has resulted in the emergence of drug-resistant bacteria on a massive scale (Patterson, 2001; Cetinkaya and Cag, 2004; Cohen, 2007; Cameron and Maling, 2008). In this respect, a series of publications have shown the value of ProCT-guided parameters in decreasing unnecessary antibiotic therapy or its duration of administration (Christ-Crain et al., 2004; 2006; Stolz et al., 2007; Briel et al., 2008; Nobre et al., 2008; Schroeder et al., 2009).
Because most acute respiratory infections are viral, the routine antibiotic therapy for this condition, that is, so commonly encountered in primary care settings, offers very little benefit (Briel et al., 2008). Nevertheless, it is likely that viral infections compose the greatest use of antibiotics (i.e. employed in over half of such cases). Recently, in a large study, 53 primary care physicians recruited 458 adults with acute respiratory infections who were thought to be in need of antibiotics (Briel et al., 2008). They were then randomly assigned to ProCT-guided therapy or standard therapy. The guidelines were: ProCT serum level < 100 pg·mL−1– antibiotics strongly discouraged; ProCT 100–250 pg·mL−1– antibiotics discouraged; ProCT > 250 pg·mL−1– antibiotics recommended. Follow-up examinations were weekly for 1 month. There was no difference in number of days missed from work, and no difference in ongoing infection or relapse. The ProCT-guided group had a prescription rate that was significantly lower than the standard therapy group.
Utilizing a ProCT guidance strategy, similar impressive diminutions of antibiotic treatment have been demonstrated in hospitalized patients with community-acquired pneumonia (Christ-Crain et al., 2006) and in exacerbations of chronic obstructive pulmonary disease (Stolz et al., 2007), and may have shortened the duration of treatment in patients with sepsis (Nobre et al., 2008; Hochreiter et al., 2009). Thus, in addition to its role in assisting in the diagnosis and follow-up of respiratory diseases (Müller et al., 2007), the measurement of ProCT shows much hope as a weapon against antibiotic overusage.
The toxicity of ProCT in sepsis
Toxicity of ProCT in vivo
To date, all of the physio-pharmacological studies that have been performed to evaluate the effects of ProCT have indicated potentially harmful effects. Based on multiple prior studies revealing that ProCT metabolism in hamsters was very similar to that of humans, a model for virulent septic peritonitis was developed, utilizing the intraperitoneal placement of agar pellets containing premeasured quantities of Escherichia coli (Nylén et al., 1998; Steinwald et al., 1999). The 72 h mortality of the hamsters, as well as serum levels of ProCT, correlated well with the number of bacteria administered. The bacterial dose was then adjusted to achieve a mortality of approximately 50%. Subsequently, ProCT that had been shown previously to be non-toxic in normal animals, was injected intraperitoneally into septic hamsters. In repeatedexperiments, this resulted in mortalities close to 100% (Nylén et al., 1998).
ProCT induces proinflammatory-like effects on leukocytes
In a manner similar to the inflammatory action of LPS, ProCT increased the expression of surface markers on human neutrophils and lymphocytes (CD16 and CD14, respectively) (Wei et al., 2008). This is thought to reflect the motion of intracellular secretory vesicles towards the cell surface. In this study, ProCT also increased the concentration of intracellular calcium ions, similar to the action of the proinflammatory cytokine, IL-8. In another study, ProCT decreased both phagocytic and candidacidal activity of neutrophils in a dose-dependent manner (Pincikova et al., 2005). In agar plate studies, ProCT also inhibited the microbicidal effect of serum and blood upon cultured E. coli. This prohormone also suppressed the blastic transformation of activated T lymphocytes of normal human blood, but increased the activity of unstimulated autologous lymphocytes (Bucova et al., 2006).
ProCT increases leukocyte-derived cytokines
In recent experiments, human recombinant human ProCT was added to whole human blood, as well as to human peripheral blood lymphocytes that had been treated by gradient centrifugation and incubated (Liappis et al., 2007a). In whole blood, there was a marked dose-dependent increase of pro-inflammatory cytokines above control levels. Among the isolated lymphocytes, TNFα was found to be the highest responder. Thus, TNFα, a potent stimulant of ProCT production (Whang et al., 2000; Redl et al., 2001) may, along with other cytokines, further reinforce the already high ProCT levels of sepsis in a self-perpetuating cascade fashion (Figure 6).
Figure 6.
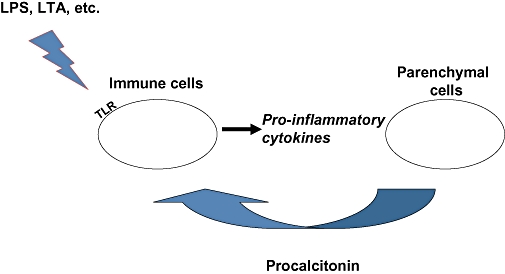
Illustrative sequence of events by which endotoxin (lipopolysaccharide) from Gram-negative bacteria or other bacterial products, such as lipotechoic acid (LTA) from Gram-positive bacteria (Ryu et al., 2009), interact with immune cells via toll-like receptors (TLRs) to initiate a pro-inflammatory cytokine response that induces the systemic hypersecretion of procalcitonin (ProCT) from parenchymal cells. In turn, in a feedback manner, the blood cell production of these same cytokines may further augment the local levels of ProCT.
ProCT effects on leukocyte migration
Polymorphonucleocytes mediate much of the complex cytokine and inflammatory responses that occur in infection and sepsis (Wagner and Roth, 1999; Martins et al., 2006). In a study, these cells were separated from the heparinized blood of normal human volunteers and were incubated at 37°C with recombinant human ProCT at several dilutions (Liappis et al., 2007b). The chemotactic index was calculated by comparing migration towards the chemoattractant, formyl methionyl leucylphenylalanine peptide. A dose-dependent decrease in the chemotactic index was repeatedly found. These findings suggest that the marked malfunction of neutrophils that is known to occur during sepsis (Egger et al., 2004; Alves-Filho et al., 2008) is in part due to ProCT. Interestingly, a similar inhibition of migration towards a chemoattractant has been noted for mononuclear lymphocytes (Wiedermann et al., 2002). This diminution of chemoattractant stimulation may conceivably impact upon macrophage activation, as well as on phagocytosis. The effect on these cells appears to involve an interaction of ProCT with a cyclic AMP-dependent protein kinase A.
ProCT augments nitric oxide
Increased levels of the vasodilator, nitric oxide (NO), occur in sepsis, and these levels correlate with severity (Brealey et al., 2002; Mitaka et al., 2003). Particularly high values are found in patients with complicating hypotension (i.e. severe sepsis, septic shock). Studies with cultured rat aortic vascular smoth muscle cells have documented that ProCT alone does not raise inducible NO synthase (iNOS) gene expression. However, if these are previously primed with LPS plus TNFα and interferon-γ in order to institute a pro-inflammatory stimulus, the later addition of ProCT strikingly augments iNOS and NO levels (Hoffmann et al., 2002). Thus, as is the case for in vivo studies, ProCT itself is not primarily an initial pro-inflammatory stimulus. Instead, it is a potent amplifier of the inflammatory cascade.
Impact on energy homeostasis
As discussed, ProCT contains an aminoterminal peptide, NProCT. Following processing, this segment also is found free in the blood, having been cleaved from the prohormone by a convertase enzyme. It was reported that a single intracerebroventricular administration of NProCT in rats induces a significant decrease of food intake and weight over a period of over 48 h. It also increases body temperature and locomotor activity, and appears to disrupt the integration of hormonal signals that may affect hypothalamic–pituitary energy homeostasis (Tavares et al., 2007). Curiously, ProCT has been alleged to be located within the normal hypothalamus by immunostaining, although it is not otherwise known to be stored in cells (Ojeda et al., 2006). Moreover, the administration of LPS in the rat was noted to induce CT gene expression in the neighbouring pituitary (Kiriyama et al., 2002). Whether these phenomena play a role in the clinical manifestations of sepsis remains to be determined.
ProCT is a blocker of CT-gene-related peptide (CGRP) activity
ProCT derives from one of a family of genes. The gene in question possesses an alternative splice variant, CT-gene-related mRNA, which gives rise to CGRP, a hormone possessing a slight homology with CT (Amara et al., 1982; Becker et al., 2002). CGRP normally functions as a peptidergic agent and is involved in various aspects of neurotransmission. In the healthy, non-infected state, there is a preferential synthesis of either CT mRNA or CGRP mRNA, according to ambient conditions and perhaps neuroendocrine cell phenotype. Similar to the case for CT mRNA in sepsis or other varieties of severe infection and systemic inflammation, there is also a tissue-wide constitutive expression of CGRP mRNA (Domenech et al., 2001). As a result, CGRP is increased in sepsis (although not to the same extent as is ProCT) (Linscheid et al., 2005).
CGRP exerts effects that are anti-inflammatory and that would be of potential benefit in sepsis (i.e. increased phagocytosis, down-regulation of TNFα, dilatation of coronary arteries, positive cardiac inotropic and chronotropic effects, antibacterial actions, etc.) (Dong et al., 1993; Ichinose and Sawada, 1996; Sheykhzade and Berg Nyborg, 2001; Okajima and Harada, 2006; El Karim et al., 2008; Li et al., 2008). Both CT and CGRP act via a group of receptors that are formed by complexes between the receptors (‘calcitonin receptor’ and ‘calcitonin receptor-like receptor’) and one of three receptor activity-modifying proteins (RAMPs) (McLatchie et al., 1998; Poyner et al., 2002). It was hypothesized that in sepsis, ProCT might blunt the actions of CGRP at its receptor site. Accordingly, the biological activity of ProCT upon this group of human receptors was explored. These receptors were transiently expressed into COS-7 cells, alone or together with individual RAMPs, so as to generate receptors for CGRP (Sexton et al., 2008). Subsequently, ProCT was examined for its ability to influence the action of exogenous CGRP on its receptor, as assessed by intracellular cyclic adenosine monophosphate (cAMP) accumulation. In repeated experiments, using concentrations comparable to those observed during sepsis, there was a marked inhibition of the CGRP response at its specific receptor. Interestingly, a study in which septic animals were treated with CGRP reported a marked increase of survival when CGRP was administered before endotoxemic shock was produced (Gomes et al., 2005). Importantly, this same therapy was unsuccessful if administered with a CGRP receptor antagonist. These studies strongly suggest that the hyperprocalcitonaemia occuring in sepsis blocks the effects of CGRP and prevents its action. The demonstration of the strong attenuation of the action of CGRP at its receptor provides an insight into one of the several mechanisms underlying the ProCT toxicity in sepsis and its related conditions (Christ-Crain and Müller, 2008; Sexton et al., 2008).
Immunoneutralization of ProCT in sepsis
Hamsters
Based on clinical and in vivo animal studies, as well as many in vitro findings, it is apparent that ProCT per se is a toxic factor in sepsis that adversely influences survival. Accordingly, a goat antiserum was raised to the midportion of ProCT, that is, a segment of the immature non-amidated CT that is cross-reactive with the prohormone (Figure 2). The administration of this antiserum markedly increased survival of hamsters. Importantly, this beneficial effect occurred both when the immunoneutralization was performed at the initiation of sepsis, as well as after its onset (Nylén et al., 1998).
Pigs
To further investigate in detail the physiological and metabolic consequences of sepsis, to evaluate how ProCT might affect these parameters and to determine whether survival would be increased, a large animal model of a rapidly fatal, porcine polymicrobial sepsis was developed during a series of experiments (Martinez et al., 2001; Wagner et al., 2002; Becker et al., 2003). It was also desirable to evaluate whether such immunoneutralization of the harmful effects of sepsis could be achieved by an antiserum raised to another portion of the ProCT molecule. For this purpose, an antiserum was produced in rabbits that was specific to the aminoterminus portion of this prohormone. A highly virulent sepsis was induced in adult male Yorkshire pigs by the intraperitoneal instillation of a suspension of cecal content (1 g·kg−1 bodyweight) that was accompanied by the further addition of a measured amount of E. coli. Within 4 h, all control animals were premorbid and manifested a symptomatology similar to the syndrome of MOF that occurs in humans with preterminal sepsis, that is, acidosis, renal failure, cardiac failure and shock. (Becker et al., 2003).
In these experiments, highly purified ProCT-reactive rabbit IgG was administered intravenously, and the control animals received non-ProCT-reactive IgG. All animals had physiological data determined (e.g. urine output, core body temperature, arterial pressure, heart rate, cardiac index, stroke index), as well as metabolic data (e.g. blood urea nitrogen, serum creatinine, arterial lactate and pH). These were obtained and recorded hourly until death, or until the predetermined time of sacrifice, that is, 15 h after induction of sepsis. (At this latter time, all non-treated animals were dead.)
In these studies, which were repeated several times, the immunoneutralization of ProCT resulted in amelioration or stabilization of several of the physiological and metabolic data. Importantly, both early immunoneutralization (i.e. concomitant with induction of sepsis) or late immunoneutralization (i.e. when the animals were deemed to be premorbid), greatly reduced short-term mortality. In early immunoneutralization, none of the control animals survived, while the survival of the treated animals was 85% (Wagner et al., 2002). For late immunoneutralization, there were no survivors for the controls, while 80% of the treated pigs survived (Martinez et al., 2001).
Thus, in studies involving two species of animals, immunoneutralization of ProCT improved both the symptomatology and the survival of highly virulent sepsis. In pigs, this occurred not only early in the course of the illness, but also after the advent of a very advanced septic state. Hence, immunoneutralization of ProCT may be useful in treating well-established sepsis and, perhaps, also in preventing the possible occurrence of this complication in non-septic patients with severe infections.
Comments and conclusions
Several characteristics of a marker–mediator of sepsis that would be most applicable to a successful therapy by immunoneutralization in the human are shown in Table 2. Few sepsis markers–mediators have met all or even half of these features (e.g. endotoxin, TNFα, IL-1, enterobacterial common antigen) (Seam and Suffredini, 2007). However, ProCT meets all of them. Therefore, therapeutic immunoneutralization of this prohormone merits serious consideration.
Table 2.
Characteristics of a marker–mediator of sepsis that would be most applicable to a succesful therapy by immunoneutralization
| 1. toxic marker that is elevated in most patients with sepsis |
| 2. A correlation between the titre of the serum marker and the severity and outcome of sepsis |
| 3. Demonstration that the marker is a toxic mediator |
| 4. Persistence of the marker for a sufficiently long duration to allow for a suitable window for appropriate therapy |
| 5. A rationale for clinical trials that is based upon results in animals having a bacterial-induced sepsis that is analogous to that occurring in humans |
| 6. Study of more than species of experimental animal |
| 7. A marker that is measurable with sensitivity, reproducibility and specificity. Accordingly, a presumptive diagnosis of sepsis that was based only on clinical criteria could be confirmed prior to a therapeutic trial. |
| 8. As a corollary, the selection and/or triage of the candidate patients would be based upon a rapid determination of the blood level of the marker. Moreover, such a marker-based diagnosis and a marker-driven selection might greatly reduce the number of patients needed to be enrolled for a clinical trial. |
Multiple studies have demonstrated that serum levels of ProCT are markedly increased in humans with sepsis, severe infection and severe inflammation. The high levels last as long as the inflammatory process persists, and tend to correlate with the outcome of the illness. ProCT was found to be toxic to septic animals, and in vitro studies of this prohormone also documented several noxious effects. Therapeutic immunoneutralization of animals with severe sepsis has been proven successful in two species. Such findings strongly indicate that ProCT immunoneutralization in humans with these conditions offers considerable promise. Moreover, the rapid onset of increased serum ProCT with the advent of the illness and the very long-lasting duration of this elevation provide a broad clinical window for therapeutic intervention. Furthermore, the ease and rapidity of ProCT measurement allow for a swift documentation of the presence of the illness, and permit the selection and stratification of the cases to be treated. Conceivably, not only sepsis, but also SIRS might be amenable to such therapy. These multiple studies provide a groundwork for clinical pharmacotherapy trials with engineered humanized monoclonal antibodies.
References
- ACCP-SCCM Consensus Conference. Definition of sepsis and multiple organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- Alves-Filho JC, de Freitas A, Spiller F, Souto Fo, Cunha FQ. The role of neutrophils in severe sepsis. Shock. 2008;30(Suppl. 1):3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA procession in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, et al. Calcitonin precursors: early markers of gut barrier dysfunction in patients with acute pancreatitis. Pancreas. 2003a;27:239–243. doi: 10.1097/00006676-200310000-00008. [DOI] [PubMed] [Google Scholar]
- Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, et al. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. Br J Surg. 2003b;90:197–204. doi: 10.1002/bjs.4036. [DOI] [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States) analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentration in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassim CW, Redman RS, DeNucci DJ, Becker KL, Nylén ES. Salivary procalcitonin and periodontitis in diabetes. J Dent Res. 2008;87:630–634. doi: 10.1177/154405910808700707. [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Moore CF, Monaghan KG, Silva OL. Calcitonin in extrathyroidal tissues of man. Acta Endocrinol. 1979;92:746–751. doi: 10.1530/acta.0.0920746. [DOI] [PubMed] [Google Scholar]
- Becker KL, Geelhoed G, O'Neill W, Monaghan KG, Snider RH, Moore CF, et al. Calcitonin in tissues of thyroidectomized monkey. Experientia. 1980;36:609–610. doi: 10.1007/BF01965831. [DOI] [PubMed] [Google Scholar]
- Becker KL, Müller B, Nylén ES, Cohen R, White JG, Snider RH., Jr . Calcitonin gene family of peptides. Structure, molecular biology, and effects. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2nd. San Diego, CA: Academic Press; 2002. pp. 619–639. [Google Scholar]
- Becker KL, Nylén ES, Snider RH, Müller B, White JC. Immunoneutralization of procalcitonin as therapy of sepsis. J Endotoxin Res. 2003;9:367–374. doi: 10.1179/096805103225003295. [DOI] [PubMed] [Google Scholar]
- Becker KL, Nylén ES, White JC, Müller B, Snider RH., Jr Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–1525. doi: 10.1210/jc.2002-021444. [DOI] [PubMed] [Google Scholar]
- Becker KL, Snider RH, Nylén ES. Procalcitonin assay in systemic inflammation infection, and sepsis: clinical utility and limitations. Crit Care Med. 2007;36:941–952. doi: 10.1097/CCM.0B013E318165BABB. [DOI] [PubMed] [Google Scholar]
- Berg RD. Bacterial translocation from the gastrointestinal tract. J Med. 1992;23:217–244. [PubMed] [Google Scholar]
- Bone RC. Toward a theory regarding the pathogenesis of the systemic inflammatory response syndrome: what we do and do not know about cytokine regulation. Crit Care Med. 1996;24:163–172. doi: 10.1097/00003246-199601000-00026. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, et al. Cytokine profile as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49. doi: 10.1186/cc5783. Available at http://ccforum.com/content/11/2/R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- Briel M, Schuetz P, Müller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections. Arch Int Med. 2008;168:2000–2007. doi: 10.1001/archinte.168.18.2000. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26(Suppl 1):S64–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucova M, Zahorec R, Buc M. Immunomodulatory effect of recombinant human procalcitonin on mitogenic activity of lymphocytes. Cent Eur J Immunol. 2006;31:87–93. [Google Scholar]
- Burgess TC, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–294. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Cameron C, Maling T. Fatal allergic reactions to antibiotics. NZ Med J. 2008;121:132–133. [PubMed] [Google Scholar]
- Castelli GP, Pognani C, Meisner M, Stuani A, Bellomi D, Sgarbi L. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis, and organ failure. Crit Care. 2004;8:R234–R242. doi: 10.1186/cc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli GP, Pognani C, Cita M, Paladini R. Procalcitonin as a prognostic and diagnostic tool for septic complications after major trauma. Crit Care Med. 2009;37:1845–1849. doi: 10.1097/CCM.0b013e31819ffd5b. [DOI] [PubMed] [Google Scholar]
- Cetinkaya F, Cag Y. Penicillin sensitivity among children without a positive history for penicillin allergy. Pediatr Allergy Immunol. 2004;15:278–280. doi: 10.1111/j.1399-3038.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Jenq C-C, Tian Y-C, Chang MY, Lin CY, Chang CC, et al. Rifle classification for predicting in-hospital mortality in critically ill sepsis patients. Shock. 2009;31:139–145. doi: 10.1097/SHK.0b013e31817d419e. [DOI] [PubMed] [Google Scholar]
- Cheval C, Timsit JF, Garrouste-Orgeas M, Assicot M, De Jonghe B, Misset B, et al. Procalcitonin (PCT) is useful in predicting the bacterial origin of an acute circulatory failure in critically ill patients. Intensive Care Med. 2000;26:S153–S158. doi: 10.1007/BF02900729. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Müller B. Calcitonin peptides – the mediators in sepsis or just another fairy tale? Crit Care Med. 2008;36:1684–1687. doi: 10.1097/CCM.0b013e3181726819. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomized, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M, Jaccard-Stolz D, Bingisser R, Mueller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- Claeys R, Vinken S, Spapen H, ver Elst K, Decochez K, Huyghens L, et al. Plasma procalcitonin and C-reactive protein in acute septic shock: clinical and biological correlates. Crit Care Med. 2002;30:757–762. doi: 10.1097/00003246-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infections: a review of epidemiology, clinical features, management, and prevention. Int J Dermatol. 2007;46:1–11. doi: 10.1111/j.1365-4632.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- Dandona P, Nix D, Wilson MR, Aljada A, Love J, Assicot M, et al. Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab. 1994;79:1605–1608. doi: 10.1210/jcem.79.6.7989463. [DOI] [PubMed] [Google Scholar]
- Dauber A, Weiss S, Maniaci V, Nylén E, Becker KL, Bachur R. Procalcitonin levels in febrile infants after recent immunization. Pediatrics. 2008;122:1119–1122. doi: 10.1542/peds.2008-1884. See Editorial, 2008, 122: 1117–1118. [DOI] [PubMed] [Google Scholar]
- Domenech VS, Nylén ES, White JC, Snider RH, Becker KL, Landmann R, et al. Calcitonin gene-related peptide expression in sepsis: postulation of microbial infection-specific response elements within the calcitonin 1 gene promoter. J Investig Med. 2001;49:514–521. doi: 10.2310/6650.2001.33628. [DOI] [PubMed] [Google Scholar]
- Dong LW, Tong LJ, Zhang L, Su JY, Tang CS. Changes of calcium transport capacity of myocardium and myocardial mitochondria during sepsis. Sheng Li Xue Bao. 1993;45:158–163. [PubMed] [Google Scholar]
- Duff C, Murphy PG, Callaghan M, McClean S. Differences in invasion and translocation of Burkholderia cepacia complex species in polarized lung epithelial cells in vitro. Microb Pathog. 2006;41:183–192. doi: 10.1016/j.micpath.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Egger G, Aigner R, Glasner P, Hofer HP, Mitterhammer H, Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infection in severe trauma: comparison with various inflammation markers. Intensive Care Med. 2004;30:331–334. doi: 10.1007/s00134-003-2111-6. [DOI] [PubMed] [Google Scholar]
- El Karim IA, Linden GJ, Orr DF, Lundy FT. Antimicrobial activity of neuropeptides against a range of microorganisms from skin, oral, respiratory and gastrointestinal tract sites. J Neuroimmunol. 2008;200:11–16. doi: 10.1016/j.jneuroim.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Ermert D, Zychlinsky A, Urban C. Fungal and bacterial killing by neutrophils. Methods Mol Biol. 2009;470:293–312. doi: 10.1007/978-1-59745-204-5_21. [DOI] [PubMed] [Google Scholar]
- Forsberg JA, Elster EA, Anderson RC, Nylen E, Brown TS, Rose MW, et al. Correlation of procalcitonin and cytokine expression with dihiscence of wartime extremity wounds. J Bone Joint Surg Am. 2008;90:580–588. doi: 10.2106/JBJS.G.00265. [DOI] [PubMed] [Google Scholar]
- Fry DE. Multiple organ dysfunction syndrome: past, present and future. Surg Infect (Larchmt) 2000;1:155–161. doi: 10.1089/109629600750018088. [DOI] [PubMed] [Google Scholar]
- Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, et al. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endoxemia. Shock. 2005;24:590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- Guyer DM, Henderson IR, Nataro JP, Mobley HL. Identification of sat, an autotransporter toxin produced by uropathogenic. Escherichia coli. Mol Microbiol. 2000;38:53–66. doi: 10.1046/j.1365-2958.2000.02110.x. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Welch CA, Edleston JM. The epidemiology of severe sepsis in England, Wales, and Northern Ireland, 1996 to 2004. Crit Care. 2006;10:R42. doi: 10.1186/cc4854. Available at http://ccforum.com/content/10/2/R42 (accessed 00 Xxxx 0000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler T, Sauerland S, Lefering R, Nagelschmidt M, Bouillon B, Andermahr J, et al. The clinical value of procalcitonin and neopterin in predicting sepsis and organ failure after major trauma. Shock. 2003;20:420–426. doi: 10.1097/01.shk.0000093541.78705.38. [DOI] [PubMed] [Google Scholar]
- Hirsch PF, Gauthier GG, Munson PL. Thyroid hypocalcemic principle and recurrent laryngeal injury as factors affecting the response to parathyroidectomy in rats. Endocrinology. 1963;73:244–251. doi: 10.1210/endo-73-2-244. [DOI] [PubMed] [Google Scholar]
- Hochreiter M, Kohler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83. doi: 10.1186/cc7903. Available at http://ccforum.com/content/13/3/R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G, Czechowski M, Schloesser M, Schobersberger W. Procalcitonin amplifies inducible nitric oxide synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit Care Med. 2002;30:2091–2095. doi: 10.1097/00003246-200209000-00023. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Sawada M. Enhancement of phagocytosis by calcitonin-gene related peptide (CGRP) in cultured mouse peritoneal macrophages. Peptides. 1996;17:1405–1414. doi: 10.1016/s0196-9781(96)00198-2. [DOI] [PubMed] [Google Scholar]
- Jean-Baptiste E. Cellular mechanisms in sepsis. Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- Jensen JU, Heslet L, Hartvig T, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006;34:2596–2602. doi: 10.1097/01.CCM.0000239116.01855.61. [DOI] [PubMed] [Google Scholar]
- Jullienne A, Segond N, Calmettes C, Moukhtar MS, Milhaud G. Biosynthesis of human calcitonin. Evidence for a prohormone. Biochem Biophys Res Commun. 1980;95:932–937. doi: 10.1016/0006-291x(80)91562-4. [DOI] [PubMed] [Google Scholar]
- Kafetzis DA, Velissariou IM, Nikolaides P, Sklavos M, Maktabi M, Spyridis G, et al. Procalcitonin as a predictor of severe appendicitis in children. Eur J Clin Microbiol Infect Dis. 2005;24:484–487. doi: 10.1007/s10096-005-1360-4. [DOI] [PubMed] [Google Scholar]
- Kiriyama Y, Nomura Y, Tokumitsu Y. Calcitonin gene expression induced by lipopolysaccharide in the rat pituitary. Am J Physiol Endocrinol Metab. 2002;282:E1380–E1384. doi: 10.1152/ajpendo.00453.2001. [DOI] [PubMed] [Google Scholar]
- LeMoullec JM, Jullienne A, Chenais J, Lasmoles F, Guliana JM, Milhaud G, et al. The complete sequence of human preprocalcitonin. FEBS Lett. 1984;167:93–97. doi: 10.1016/0014-5793(84)80839-x. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Li D, Li NS, Chen QQ, Guo R, Xu PS, Deng HW, et al. Calcitonin gene-related peptide-mediated cardioprotection of postconditioning in isolated rat hearts. Regul Pept. 2008;147:4–8. doi: 10.1016/j.regpep.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Li Z, Yang X, Lu L, Yu Y, Yao Y. Gut barrier function damage following multiple firearm injuries in a porcine model. Clin Med Sci J. 2001;16:209–213. [PubMed] [Google Scholar]
- Liappis A, Snider RH, Nylén ES, Becker KL. Human leukocyte and whole blood cytokine response to exogenous procalcitonin. 2007a. Endocrine Society, P1-367, p. 250, June 2–5, Toronto, Canada.
- Liappis A, Gibbs KW, Yoon B, Snider RH, Nylen ES, Gao B, et al. Exogenous procalcitonin alters neutrophil chemotaxis 47th Interscience Conference on Antimicrobial Agents and Chemotherapy. Chicago, IL: 2007b. September 17–20. [Google Scholar]
- Linscheid P, Seboek D, Nylén ES, Langer I, Schlatter M, Becker KL, et al. In vitro and in vivo calcitonin-1 gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144:5578–5584. doi: 10.1210/en.2003-0854. [DOI] [PubMed] [Google Scholar]
- Linscheid P, Seboek D, Zulewski H, Keller U, Müller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005;146:2699–2708. doi: 10.1210/en.2004-1424. [DOI] [PubMed] [Google Scholar]
- Maier M, Wutzler S, Lehnert M, Szermutzky M, Wyen H, Bingold T, et al. Procalcitonin levels in patients with multiple injuries including visceral trauma. J Trauma. 2009;66:243–249. doi: 10.1097/TA.0b013e31817c966f. [DOI] [PubMed] [Google Scholar]
- Majlessi L, Lo-Man R, Leclerc C. Regulatory B and T cells in infections. Microbes Infect. 2008;10:1030–1035. doi: 10.1016/j.micinf.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- Maniaci V, Dauber A, Weiss S, Nylén ES, Becker KL, Bachur R. Procalcitonin in young febrile infants for the detection of serious bacterial infections. Pediatrics. 2008;122:701–710. doi: 10.1542/peds.2007-3503. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Wagner KE, Snider RH, Nylen ES, Müller B, Sarani B, et al. Late immunoneutralization of procalcitonin arrests the progression of lethal porcine sepsis. Surg Infect (Larchmt) 2001;2:193–201. doi: 10.1089/109629601317202678. discussion 202–203. [DOI] [PubMed] [Google Scholar]
- Martins PS, Brunialti MK, da Luz Fernandez M, Martos LS, Gomes NE, Rigato, et al. Bacterial recognition and induced cell activation in sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:183–191. doi: 10.2174/187153006777442350. [DOI] [PubMed] [Google Scholar]
- Maruna P, Frasko R, Gürlich R. Plasma procalcitonin in patients with ileus. Relations to other inflammatory parameters. Physiol Res. 2008;57:481–486. doi: 10.33549/physiolres.931249. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentration of procalcitonin after different types of surgery. Intensive Care Med. 1998;24:680–684. doi: 10.1007/s001340050644. [DOI] [PubMed] [Google Scholar]
- Mitaka C, Hirata Y, Yokoyama K, Wakimoto H, Hirokawa M, Nosaka T, et al. Relationships of circulating nitrite/nitrate levels to severity and multiple organ dysfunction syndrome in systemic inflammatory response syndrome. Shock. 2003;19:305–309. doi: 10.1097/00024382-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Weglöhner W, Weglöhner W, Agay D, Bohuon C, et al. Production of procalcitonin (PCT) in nonthyroidal tissue after LPS injection. Horm Metab Res. 2003;35:290–295. doi: 10.1055/s-2003-41304. [DOI] [PubMed] [Google Scholar]
- Müller B. Endocrine aspects of critical illness. Ann Endocrinol (Paris) 2007;68:290–298. doi: 10.1016/j.ando.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000;28:977–983. doi: 10.1097/00003246-200004000-00011. [DOI] [PubMed] [Google Scholar]
- Müller B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- Müller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, et al. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre V, Harbarth S, Graf J-D, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- Nylén ES, Alarifi A. Humoral markers of severity and prognosis in critical illness. Best Pract Res Clin Endocrinol Metab. 2001;15:553–573. doi: 10.1053/beem.2001.0169. [DOI] [PubMed] [Google Scholar]
- Nylén ES, O'Neill W, Jordan MH, Snider RH, Moore CF, Lewis M, et al. Serum procalcitonin as an index of inhalation injury in burns. Horm Metab Res. 1992;24:439–442. doi: 10.1055/s-2007-1003354. [DOI] [PubMed] [Google Scholar]
- Nylén ES, Jeng J, Jordan MH, Snider RH, Thompson KA, Lewis MS, et al. Late pulmonary sequela following burns: persistence of hyperprocalcitonemia using a 1–57 amino acid N-terminal flanking peptide assay. Respir Med. 1995;89:41–46. doi: 10.1016/0954-6111(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Nylén ES, Snider RH, Thompson BS, Rohatgi P, Becker KL. Pneumonitis-associated hyperprocalcitonemia. Am J Med Sci. 1996;312:12–18. doi: 10.1097/00000441-199607000-00003. [DOI] [PubMed] [Google Scholar]
- Nylén ES, Alarifi AA, Becker KL, Alzeer A. Effect of classical heatstroke on serum procalcitonin. Crit Care Med. 1997;25:1362–1365. doi: 10.1097/00003246-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Nylén ES, Whang KL, Steinwald PM, Snider RH, White JC, Becker KL. Procalcitonin increases mortality and procalcitonin-recognizing antiserum improves mortality in an experimental model of sepsis. Crit Care Med. 1998;26:1001–1006. doi: 10.1097/00003246-199806000-00015. See Editorial 26: 977–978. [DOI] [PubMed] [Google Scholar]
- Ojeda ML, Ambrosiani J, Tavares E, Maldonado R, Minano FJ. Identification and localization of procalcitonin-like immunoreactivity in the rat. Neurosci Lett. 2006;408:40–45. doi: 10.1016/j.neulet.2006.07.076. [DOI] [PubMed] [Google Scholar]
- Okajima K, Harada N. Regulation of inflammatory responses by sensory neurons: molecular mechanism(s) and possible therapeutic applications. Curr Med Chem. 2006;13:2241–2251. doi: 10.2174/092986706777935131. [DOI] [PubMed] [Google Scholar]
- Opal SM, Cohen J. Clinical Gram-positive sepsis: does it fundamentally differ from Gram-negative sepsis? Crit Care Med. 1999;27:1682–1683. doi: 10.1097/00003246-199908000-00039. [DOI] [PubMed] [Google Scholar]
- Patterson JE. Antibiotic utilization: is there an effect on antimicrobial resistance? Chest. 2001;19(2) Suppl.:426S–430S. doi: 10.1378/chest.119.2_suppl.426s. [DOI] [PubMed] [Google Scholar]
- Pezzicoli A, Santi I, Lauer P, Rosini R, Rinaudo D, Grandi G, et al. Pilus backbone contributes to group B Streptococcus paracellular translocation through epithelial cells. J Infect Dis. 2008;198:890–898. doi: 10.1086/591182. [DOI] [PubMed] [Google Scholar]
- Pincikova T, Bucova M, Slobodnikova L. Influence of recombinant human procalcitonin on phagocytic and candidacidal ability of polymorphonuclear leukocytes and on killing mechanisms of serum and blood against bacteria, Staphylococcus aureus and Escherichia coli. Vnitr Lek. 2005;51:1365–1370. [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Preas HL, Nylén ES, Snider RH, Becker KL, White JC, Agosti JM, et al. Effects of anti-inflammatory agents on serum levels of calcitonin precursors during human experimental endotoxemia. J Infect Dis. 2001;184:373–376. doi: 10.1086/322031. [DOI] [PubMed] [Google Scholar]
- Rangel-Frausto MS. The epidemiology of bacterial sepsis. Infect Dis Clin North Am. 1999;13:299–312. doi: 10.1016/s0891-5520(05)70076-3. [DOI] [PubMed] [Google Scholar]
- Redl H, Schlag G, Tögel E, Assicot M, Bohuon C. Procalcitonin release patterns in a baboon model of trauma and sepsis: relationship to cytokines and neopterin. Crit Care Med. 2000;28:3569–3663. doi: 10.1097/00003246-200011000-00021. [DOI] [PubMed] [Google Scholar]
- Redl H, Schiesser A, Tögel E, Assicot M, Bohuon C. Possible role of TNF on procalcitonin release in a baboon model of sepsis. Shock. 2001;16:25–27. doi: 10.1097/00024382-200116010-00005. [DOI] [PubMed] [Google Scholar]
- Roos BA, Okano K, Deftos LJ. Evidence for a procalcitonin. Biochem Biophys Res Commun. 1974;60:134–140. doi: 10.1016/0006-291x(74)90430-6. [DOI] [PubMed] [Google Scholar]
- Russwurm S, Stonans I, Stonane E, Wiederhold M, Luber A, Zipfel PF, et al. Procalcitonin and CGRP-1 mRNA expression in various tissues. Shock. 2001;16:109–112. doi: 10.1097/00024382-200116020-00004. [DOI] [PubMed] [Google Scholar]
- Ryan CM, Yarmush ML, Burke JF, Tompkins RG. Increased gut permeability early after burn injury. Crit Care Med. 1992;20:1508–1512. doi: 10.1097/00003246-199211000-00005. [DOI] [PubMed] [Google Scholar]
- Ryu YH, Baik JE, Yang JS, Kang SS, Im J, Yun CH, et al. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acid. Int Immunopharmacol. 2009;9:127–133. doi: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Schroeder S, Hochreiter M, Koehler T, Schweiger A-M, Bein B, Keck FS, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intesive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394:221–226. doi: 10.1007/s00423-008-0432-1. [DOI] [PubMed] [Google Scholar]
- Schützle H, Forster J, Superti-Furga A, Berner R. Is serum procalcitonin a reliable diagnostic marker in children with acute respiratory tract infections? A retrospective analysis. Eur J Pediatr. 2009;168:1117–1124. doi: 10.1007/s00431-008-0899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seam N, Suffredini AF. Mechanisms of sepsis and insights into clinical trials. Drug Discov Today Dis Mech. 2007;4:83–93. doi: 10.1016/j.ddmec.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton PM, Christopoulos G, Christopoulos A, Nylén ES, Snider RH, Becker KL. Procalcitonin has bioactivity at calcitonin receptor family complexes: potential mediator implications in sepsis. Crit Care Med. 2008;36:1684–1687. doi: 10.1097/CCM.0b013e318170a554. [DOI] [PubMed] [Google Scholar]
- Sheykhzade M, Berg Nyborg NC. Mechanism of CGRP-induced relaxation in rat intramural coronary arteries. Br J Pharmacol. 2001;132:1235–1246. doi: 10.1038/sj.bjp.0703936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Uno T, Riedel W, Nishimaki M, Watanabe K. Transiently enhanced LPS-induced fever following hyper thermic stress in rabbits. Int J Biometeorol. 2005;50:67–74. doi: 10.1007/s00484-005-0272-4. [DOI] [PubMed] [Google Scholar]
- Silva OL, Wisneski LA, Cyrus J, Snider RH, Moore CF, Becker KL. Calcitonin in thyroidectomized patients. Am J Med Sci. 1978;275:159–164. doi: 10.1097/00000441-197803000-00005. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. J Mol Med. 2000;78:74–80. doi: 10.1007/s001090000086. [DOI] [PubMed] [Google Scholar]
- Snider RH, Nylén ES, Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Invest Med. 1997;45:552–560. [PubMed] [Google Scholar]
- Steinbach G, Rau B, Debard AL, Javourez JF, Bienvenu J, Ponzio A, et al. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor system. Clin Chem Lab Med. 2004;42:440–449. doi: 10.1515/CCLM.2004.077. [DOI] [PubMed] [Google Scholar]
- Steinwald PM, Whang KT, Becker KL, Snider RH, Jr, Nylén ES, White JC. Elevated calcitonin precursor levels are related to mortality in an animal model of sepsis. Crit Care. 1999;3:11–16. doi: 10.1186/cc300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz D, Christ-Crain M, Bingisser R, Leuppi J, Miedinger D, Müller C, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- Tavares E, Maldonado R, Minano FJ. N-procalcitonin: central effects on feeding and energy homeostasis in rats. Endocrinology. 2007;148:1891–1901. doi: 10.1210/en.2006-0792. [DOI] [PubMed] [Google Scholar]
- Thayvil S, Shenoy M, Hamaluba M, Gupta A, Frater J, Verber IG, et al. Is procalcitonin useful in early diagnosis of serious bacterial infections in childen? Acta Pediatr. 2005;94:155–158. doi: 10.1111/j.1651-2227.2005.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Thijs LG, Huck CE. Time course of cytokine levels in sepsis. Intensive Care Med. 1995;21:S258–S263. doi: 10.1007/BF01740764. [DOI] [PubMed] [Google Scholar]
- Toribio RE, Kohn CW, Leone GW, Capen CG, Rosol TJ. Molecular cloning of equine calcitonin, calcitonin gene-related peptide-1, calcitonin gene-related peptide-II. Mol Cell Endocrinol. 2003;199:119–128. doi: 10.1016/s0303-7207(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Von Heimburg D, Stieghorst W, Khorram-Sefat R, Pallua N. Procalcitonin – a sepsis parameter in severe burn injuries. Burns. 1998;24:745–750. doi: 10.1016/s0305-4179(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J Leukocyte Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- Wagner KE, Martinez JM, Vath SD, Snider RH, Nylén ES, Becker KL, et al. Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med. 2002;30:2313–2321. doi: 10.1097/00003246-200210000-00021. [DOI] [PubMed] [Google Scholar]
- Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipotechoic acid in Gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock. 2003;20:402–414. doi: 10.1097/01.shk.0000092268.01859.0d. [DOI] [PubMed] [Google Scholar]
- Weglöhner W, Struck J, Fischer-Schulz C, Morgenthaler NG, Otto A, Bohuon C, et al. Isolation and characterization of serum procalcitonin from patients with sepsis. Peptides. 2001;12:2099–2103. doi: 10.1016/s0196-9781(01)00541-1. [DOI] [PubMed] [Google Scholar]
- Wei JX, Verity A, Garle M, Mahajan R, Wilson V. Examination of the effect of procalcitonin on human leukocytes and the porcine isolated coronary artery. BJA. 2008;100:612–621. doi: 10.1093/bja/aen073. [DOI] [PubMed] [Google Scholar]
- Wiedermann FJ, Kaneider N, Egger P, Tiefenthaler W, Wiedermann CJ, Lindner KH, et al. Migration of human monocytes in response to procalcitonin. Crit Care Med. 2002;30:1112–1117. doi: 10.1097/00003246-200205000-00025. [DOI] [PubMed] [Google Scholar]
- Whang K, Steinwald PM, White JC, Nylén ES, Snider RH, Simon GL, et al. Serum calcitonin precursors in sepsis and systemic infection. J Clin Endocrinol Metab. 1998;83:3296–3301. doi: 10.1210/jcem.83.9.5129. [DOI] [PubMed] [Google Scholar]
- Whang KT, Vath SD, Becker KL, Snider RH, Nylen ES, Muller B, et al. Procalcitonin and pro-inflammatory cytokine interactions in sepsis. Shock. 2000;14:73–78. doi: 10.1097/00024382-200014010-00013. [DOI] [PubMed] [Google Scholar]
- Zaidi M, Alam ASMT, Shakar VS, Bax BE, Moonga BS, Bevis PJ, et al. Quantitative description of components of in vitro morphometric change in the rat osteoclast model. Relationships with cellular function. Eur Biophys J. 1992;21:349–355. doi: 10.1007/BF00188348. [DOI] [PubMed] [Google Scholar]
- Zucchini N, Crozat K, Baranek T, Robbins SH, Altfeld M, Dalod M. Natural killer cells in immunodefense against infective agents. Expert Rev Anti Infect Ther. 2008;6:867–885. doi: 10.1586/14787210.6.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]


