Abstract
Chronic degenerative inflammatory diseases, such as chronic obstructive pulmonary disease and Alzheimer's dementia, afflict millions of people around the world, causing death and debilitation. Despite the global impact of these diseases, there have been few innovative breakthroughs into their cause, treatment or cure. As with many debilitating disorders, chronic degenerative inflammatory diseases may be associated with defective or dysfunctional responses to second messengers, such as cyclic adenosinemonophosphate (cAMP). The identification of the cAMP-activated guanine nucleotide exchange factors for Ras-like GTPases, Epac1 (also known as cAMP-GEF-I) and Epac2 (also known as cAMP-GEF-II), profoundly altered the prevailing assumptions concerning cAMP signalling, which until then had been solely associated with protein kinase A (PKA). Studies of the molecular mechanisms of Epac-related signalling have demonstrated that these novel cAMP sensors regulate many physiological processes either alone and/or in concert with PKA. These include calcium handling, cardiac and smooth muscle contraction, learning and memory, cell proliferation and differentiation, apoptosis, and inflammation. The diverse signalling properties of cAMP might be explained by spatio-temporal compartmentalization, as well as A-kinase anchoring proteins, which seem to coordinate Epac signalling networks. Future research should focus on the Epac-regulated dynamics of cAMP, and, hopefully, the development of compounds that specifically interfere with the Epac signalling system in order to determine the precise significance of Epac proteins in chronic degenerative inflammatory disorders.
Keywords: Epac, cAMP, AKAP, lung, neurons, inflammation
Introduction
The most common and versatile second messenger, cyclic adenosinemonophosphate (cAMP), controls a range of diverse physiological processes, including metabolic events, calcium handling, cardiac and smooth muscle contraction, secretion, ion channel conductance, learning and memory, cell growth and differentiation, apoptosis, and inflammation (Beavo and Brunton, 2002). G protein-coupled receptors tightly control the cellular content of cAMP via both adenylyl cyclases and cAMP phosphodiesterases (PDEs), and subcellular localization to lipid rafts and caveolae seems to serve as organizing centres for such signalling (Hanoune and Defer, 2001; Lugnier, 2006; Conti and Beavo, 2007; Patel et al., 2008a,b;). Interaction of cAMP effectors with A-kinase anchoring proteins (AKAPs) facilitates subcellular compartmentalization and spatio-temporal cAMP dynamics, and likely reveals the distinct signal transduction events that are driven by cAMP (Wong and Scott, 2004; Gold et al., 2006; Kinderman et al., 2006; Beene and Scott, 2008; Taylor et al., 2008; Baillie, 2009).
The novel cAMP mediators Epac1 (cAMP-GEF-I) and Epac2 (cAMP-GEF-II) are guanine nucleotide exchange factors for Ras-like small GTPases, directly activated by cAMP. The discovery of Epac altered prevailing assumptions concerning cAMP signalling, which had previously been associated with protein kinase A (PKA) (Cohen, 2002; Bos, 2003; Zambon et al., 2005; Bos, 2006; Schmidt et al., 2007a; Roscioni et al., 2008; Taylor et al., 2008). Epac proteins are now assumed to control a range of diverse effectors and to regulate several pivotal processes, including calcium handling and ion transport, cell proliferation and differentiation, cell survival and apoptosis, gene transcription and chromosomal integrity, vesicle trafficking and secretion, and barrier function and neuronal responses. As illustrated (Figures 1–4), cAMP-regulated Epac proteins seem to contribute to DNA repair (Huston et al., 2008), circadian pacemaker functioning (O'Neill et al., 2008), learning and memory (Gekel and Neher, 2008; Gelinas et al., 2008; Murray and Shewan, 2008; Ouyang et al., 2008), axon growth and regeneration (Murray and Shewan, 2008), hypertrophy (Morel et al., 2005; Oestreich et al., 2007; Ulucan et al., 2007; Métrich et al., 2008), fibrosis (Huang et al., 2007, 2008; Haag et al., 2008; Yokoyama et al., 2008c), and to inflammation (Aronoff et al., 2005; Aronoff et al. 2006a; Lorenowicz et al., 2006; Sands et al., 2006; Yarwood et al. 2008; Scheibner et al. 2008; Serezani et al. 2008; Borland et al. 2009a,b;).
Figure 1.
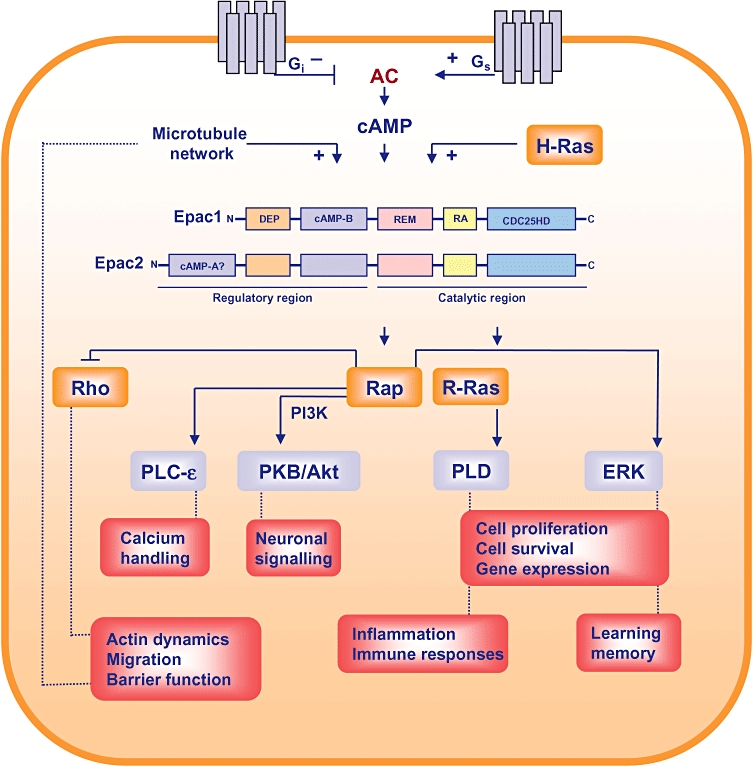
Epac: novel cyclic-adenosinemonophosphate (cAMP) mediators. Epac-driven signalling pathway. cAMP-modulating receptors are indicated in gray, small GTPases are presented in orange; other effectors are depicted in blue, and distinct cellular responses are shown in salmon. Epac2 is recruited by activated H-Ras to the plasma membrane, and Epac1 is regulated by the microtubule network. Inset, schematic representation of Epac1 and Epac1. AC, adenylyl cyclase; CDC25HD, guanine exchange factor domain; DEP, dishevelled, Egl-10, pleckstrin domain; ERK1/2, extracellular signal-regulated kinases 1 and 2; PKB/Akt, protein kinase B/Akt; PLC, phospholipase C; PLD, phospholipase D; RA, Ras-associating domain; REM, Ras-exchanger motif.
Figure 4.
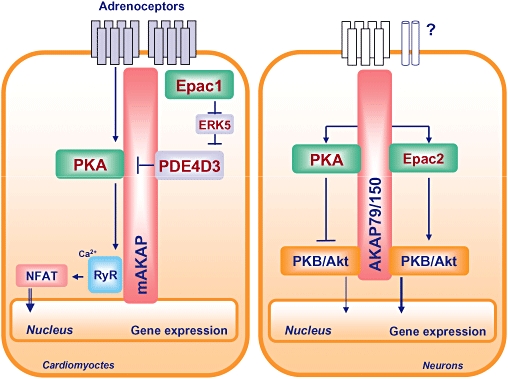
Coordination of protein kinase A (PKA)/Epac-driven cAMP dynamics by AKAPs. In neonatal rat cardiomyocytes, an AMP-responsive multiprotein complex is maintained by nuclear envelope-associated mAKAP, PKA, PDE4D3, ERK5 and Epac1, whereas in neuronal cells, such a functional cAMP-responsive multiprotein complex consists of plasma membrane-associated AKAP79/150, PKA, PKB/Akt and Epac2. Both cAMP organizing centers are most likely to facilitate distinct signal transduction events driven by cAMP. See text for further explanations. AKAP, A-kinase anchoring protein; ERK, extracellular signal-regulated kinase; NFAT, nuclear factor of activated T cells; PDE, phosphodiesterase.
It is interesting to note that Epac proteins exert their diverse biological functions either alone and/or in concert with PKA, and that distinct cAMP signalling complexes seem to be coordinated by AKAPs, which are known to interact with PKA and PDEs (Wong and Scott, 2004; Gold et al., 2006; Kinderman et al., 2006; Beene and Scott, 2008; Taylor et al., 2008). In 2005, a cAMP-responsive multiprotein complex, maintained by nuclear envelope-associated mAKAP, PKA, PDE4D3 and Epac1, was first identified in neonatal rat cardiomyocytes (Dodge-Kafka et al., 2005). Recent studies in our laboratory have reported a novel link between neuronal plasma membrane-associated AKAP79/150, PKA and Epac2 (Nijholt et al., 2008) (Figure 4). Such multiprotein complexes seem to alter the biological effects of cAMP (see later in the text).
Current insights into the molecular mechanisms of the regulation of multiple effectors and biological functions by Epac proteins can be found in several recent reviews on this topic (Bos, 2003; Holz et al., 2006; Holz et al., 2007; Roscioni et al., 2008; Borland et al., 2009b). Signalling of cAMP via cyclic nucleotide-gated channels, CNrasGEF and/or PSD-95/DlgA/ZO-1 (PDZ)-GEF has been outlined in prior reviews (Bos et al., 2007; Biel and Michalakis, 2009; Biel, 2009; Pannekoek et al., 2009; Raaijmakers and Bos, 2009). In this review, we will discuss our current understanding of Epac proteins as novel regulators of cAMP-dependent immune, lung and neuronal function. Some of this article was presented at EPHAR, Manchester 2008
Epac: novel cAMP mediators
Expression of Epac1 and Epac2
Epac1 (cAMP-GEF-1) and Epac2 (cAMP-GEF-II) are expressed in both mature and developing tissues. Recent studies have indicated that alterations in the cellular microenvironment are present in chronic degenerative inflammatory diseases and seem to affect the expression profile of Epac1 and Epac2. Initially, Epac1 mRNA expression was reported to be most abundant in the heart and kidney, although subsequently, it was found to be expressed in all human tissues being analysed (de Rooij et al., 1998). Additionally, expression of Epac1 has been found in monocytes, macrophages, B and T lymphocytes, eosinophils, neutrophils, platelets, and in CD34-positive haematopoietic cells (Tiwari et al., 2004; Bryn et al., 2006; Gerlo et al., 2006; Lorenowicz et al., 2006). Recently, developmental changes of Epac1 mRNA expression have been reported in the heart, vasculature, brain, kidneys and lungs (Ulucan et al., 2007; Yokoyama et al. 2008a,b; Murray and Shewan, 2008). Epac2 mRNA expression has been found to be prominent in the brain and adrenal glands (Kawasaki et al., 1998), whereas Epac2 was undetectable in all hematopoietic cell types being studied (Tiwari et al., 2004). It has also been reported that Epac2 mRNA expression in the heart, vasculature, brain, kidneys and lungs is subject to developmental changes (Ulucan et al., 2007; Murray and Shewan, 2008; Yokoyama et al., 2008a,b;). Due to the recent identification of a novel splice variant of Epac2, designated Epac2B, the previously identified Epac2 has been renamed Epac2A; it has been reported that Epac2A mRNA is expressed in pancreatic islets and cerebral cortex, whereas Epac2B mRNA expression is restricted to adrenal glands (Niimura et al., 2009).
There are distinct expression patterns of Epac1 and Epac2 proteins in rat brain, spinal cord and dorsal root ganglia at embryonic, neonatal and adult stages of development. Interestingly, it has been shown that the expression of Epac1 declines in adulthood, whereas Epac2 expression is dramatically up-regulated in adults. Such developmental regulation of Epac expression seems to promote axon growth and regeneration of the nervous system (Murray and Shewan, 2008). Expression of Epac1 mRNA, but not Epac2 mRNA, was found to be transiently increased in a mouse model of vascular injury, and up-regulation of Epac1 protein expression correlated with the progression of neointimal thickening (Yokoyama et al., 2008a). Although the expression of Epac1 and Epac2 mRNAs was up-regulated in rat ductus arteriosus during the perinatal period, Epac1 activity correlated with the intimal cushion formation (Yokoyama et al., 2008b). Studies on developmental changes of Epac1 and Epac2 mRNA expression have shown alterations of Epac1 and Epac2 in the heart, brain, kidneys and lungs of mice at different stages of development from foetus into adulthood. Interestingly, relative to Epac1, Epac2 became dominant in the adult brain and heart compared with fetal organs, whereas Epac1, relative to Epac2, became dominant in the adult kidney and lung compared with fetal organs (Ulucan et al., 2007), suggesting that Epac1 and Epac2 differentially contribute to fetal and adult organ function. In mycocardial hypertrophy induced by chronic catecholamine infusion, Epac1 and Epac2 mRNA were up-regulated, whereas only Epac1 mRNA was increased in pressure overload-induced hypertrophy (Ulucan et al., 2007). At present, it is not known whether Epac is a cause of the development of cardiac hypertrophy, or if its expression is a consequence of hypertrophy. Transforming growth factor β1 (TGF-β1) has been shown to reduce the expression of Epac1 mRNA in rat cardiac, lung and skin fibroblasts, and a decrease in Epac1 mRNA expression paralleled the increase in expression of TGF-β1 in a rat myocardial infarction model. Such a process may promote the synthesis of extracellular matrix at site of injury (Yokoyama et al., 2008c). The reduction of Epac1 mRNA expression in U937 monocytic cells by TGF-β1 might protect against aberrant transendothelial migration of leukocytes during inflammation (Basoni et al., 2005). Although the underlying molecular mechanisms have yet to be addressed, attenuation of Epac1 expression by (fibrogenic) agonists may represent a mode of adaptation of cAMP responses to pathophysiological alterations present in inflammatory diseases.
Localization of Epac1 and Epac2
Epac1 is localized in the plasma membrane, cytoplasm, perinuclear regions, nuclear membrane and mitochondria in various cell types, including HEK293, N1E-115, CHO, COS1(7), HeLa, PC12, PCCL3, primary hippocampal (cortical) neurons, peritoneal macrophages, rat cholangiocytes, primary rat neonatal ventriculocytes, and different tubular segmental cells of rat and human kidney (Qiao et al., 2002; DiPilato et al., 2004; Nikolaev et al., 2004; Ponsioen et al., 2004; Dodge-Kafka et al. 2005; Morel et al., 2005; Borland et al., 2006; Hochbaum et al., 2008; Li et al., 2008; Métrich et al., 2008; Di Benedetto et al. 2008; Banales et al., 2009). In particular, studies in HEK293 and COS1(7) cells demonstrated that subcellular (re)distribution of Epac1 is subject to cell cycle- and cytoskeleton-dependent dynamics (Qiao et al., 2002; Borland et al., 2006; Huston et al., 2008). Epac2 is localized to the (sub)plasma membrane, cytosolic fractions, actin cytoskeleton, meiotic midzone region and Golgi in several cells, such as MIN6, pancreatic islets, H1299, human microvascular endothelial cells, different tubular segmental cells of rat (human) kidney, rat cholangiocytes, adult rat ventricular myocytes and mouse spermatocytes (Ozaki et al., 2000; Hong et al., 2007; Shibasaki et al., 2007; Leroy et al., 2008; Li et al., 2008; Aivatiadou et al., 2009; Banales et al., 2009; Niimura et al., 2009). Clearly, Epac1 and Epac2 are localized to a number of subcellular regions, and their cell type-specific co-localization at the plasma membrane and cytoplasm indicates that Epacs might act in concert to modulate cellular responses.
Meanwhile, recent studies have reported on spatial and temporal regulation of cellular Epac signalling by using Epac-based fluorescence resonance energy transfer cAMP sensors or green fluorescent protein (GFP)/Flag-tagged Epacs (DiPilato et al., 2004; Nikolaev et al., 2004; Ponsioen et al., 2004, 2009; Di Benedetto et al., 2008; Leroy et al., 2008; Liu et al., 2008; Shafer et al., 2008). The Epac-based cAMP sensor studies were important for the concept that the concentration of cellular cAMP rises to a level sufficient to activate Epac1 and Epac2. Initially, Nikolaev and colleagues demonstrated, by using single-chain cAMP sensors based on the cAMP-binding domains of Epac and PKA, that β-adrenoceptor-induced cAMP signals are rapidly propagated throughout the entire cell body of primary hippocampal neurons and peritoneal macrophages (Nikolaev et al., 2004). Using Epac as an indicator of cAMP (either targeted to plasma membrane, mitchondria or nucleus), DiPilato and co-workers demonstrated differential dynamics of cAMP signaling in response to β-adrenoceptor or prostanoid receptor activation in HEK293, HeLa and in PC12 cells (DiPilato et al., 2004). In contrast, Ponsioen et al. showed, by using a CFP-Epac-YFP construct, that Epac activation was not limited to membranes, but rather occurs throughout the cell (A431, HEK293, N1E-115 and MCF-7 cells (Ponsioen et al., 2004). More recently, Ponsioen and colleagues reported, by using GFP-tagged Epac1, that activation of β- adrenoceptors induced a rapid translocation of Epac1 to the plasma membrane in A431, HEK293, OVCAR-3, ACHN, RCC10, MDCK, N1E-115, HeLa, Rat-1, GE11 and H1299 cells (Ponsioen et al., 2009). Similarly, Liu et al. demonstrated, by using Flag-tagged Epac2, that Epac2 activation requires compartmentalization of Epac2 to Ras-containing membranes, a process that operates in COS, HEK293 and PC12 cells (Liu et al., 2008). Although the concept of subcellular (re)distribution of Epac as a prerequisite for their activation remains controversial, Epac1 and Epac2 clearly represent novel cAMP sensors that contribute to the highly dynamic features of cAMP signalling.
Because the binding affinity of cAMP for PKA and for Epac has been found to be very similar (kd∼2.9 µM), it has been proposed that Epac and/or PKA are activated in response to moderate increases of cellular cAMP, and that such activation depends upon cellular compartmentalization of cAMP formation and effector protein availability (Dao et al., 2006). Indeed, studies in primary rat neonatal ventriculocytes have identified distinct intracellular cAMP signalling compartments composed of distinct PKA subtypes, Gs-coupled receptors and cAMP-hydrolyzing PDEs (Di Benedetto et al., 2008). Recently, Leroy and colleagues reported on spatio-temporal dynamics of β-adrenoceptor-induced cAMP signalling in adult rat ventricular myocytes, and speculated that PDE3 regulates a ‘constitutive’ cAMP pool linked to cardiac contractility, whereas PDE4 regulates the cAMP microdomains driven by β-adrenoceptor stimulation (Leroy et al., 2008). Intriguingly, very recent research suggests that different mechanisms contribute to the plasma membrane targeting of Epac1 and Epac2 (Liu et al., 2008; Niimura et al., 2009; Ponsioen et al., 2009). Thus, an increasing weight of experimental data infers that cellular compartmentalization of cAMP signalling affects the net outcome of biological functions. Spatio-temporal cAMP signalling is believed to involve members of the AKAP family (Wong and Scott, 2004), and indeed cAMP-responsive multiprotein complexes, including Epac1 and Epac2, have been identified in the heart and neurones (Dodge-Kafka et al., 2005; Nijholt et al., 2008) (Figure 4). In neonatal rat cardiomyocytes, Dodge-Kafka and colleagues identified a cAMP-responsive multiprotein complex composed of nuclear envelope-associated mAKAP, PKA, PDE4D3 and Epac1 (Dodge-Kafka et al., 2005). This cardiac-specific multiprotein complex was sensitive to differential cellular cAMP concentrations. Thus, at high cAMP concentrations, cardiac hypertrophy was attenuated upon Epac1-Rap1-dependent inhibition of extracellular signal-regulated kinase5 (ERK5) and subsequent activation of PDE4D3, whereas at low cAMP concentrations, cardiac hypertrophy was enhanced upon ERK5-mediated inactivation of PDE4D3 and subsequent increased PKA signalling (Dodge-Kafka et al., 2005) (Figure 4). Recent studies in our laboratory reported a novel link between neuronal plasma membrane-associated AKAP79/150, PKA, Epac2 and phosphatidylinositol 3-kinase (PI 3-kinase)-dependent protein kinase B (PKB)/Akt (Nijholt et al., 2008) (Figure 4). Direct activation of PKA or Epac2 complexed to AKAP79/150 exerted opposing effects on neuronal PKB/Akt: direct activation of PKA inhibited PKB/Akt phosphorylation, whereas direct activation of Epac2 enhanced PKB/Akt phosphorylation (Nijholt et al., 2008). At present, it is not known whether distinct PDEs are also tethered to the neuronal AKAP79/150 complex, and the input of distinct receptor-driven cAMP alterations has yet to be determined. Of particular interest, a recent study by Raymond and co-workers demonstrated the presence of distinct PKA- and Epac-based signalling complexes comprised of several PDEs and AKAPs (Raymond et al., 2007). Because co-localization of PKA and Epacs to distinct AKAPs correlates with opposing biological effects in cardiomyocytes and neurones (Figure 4), the capacity of AKAPs to interact with additional signalling components might be crucial (Wong and Scott, 2004; Beene and Scott, 2008). Clearly, further studies are required to analyze AKAP-dependent cellular cAMP compartmentalization and its effect on cAMP-dependent biological functions.
Structure of Epac1 and Epac2
Initial studies indicated that Epac1 and Epac2 consist of an auto-inhibitory N-terminal regulatory region that contains a DEP (dishevelled, Egl-10, pleckstrin) domain responsible for membrane association and a high-affinity cAMP-binding domain (cAMP-B), a C-terminal catalytic region bearing a CDC25 homology domain (CDC25HD) that exhibits GEF activity for Ras-like GTPases, a Ras-exchange motif (REM) domain believed to stabilize the GEF domain, and a Ras-associating (RA) domain present in several Ras-interacting proteins. Indeed, Epac2 has been shown to interact with GTP-bound Ras (Li et al., 2006) (Figure 1). In addition, Liu et al. demonstrated that the interaction of Epac2 with Ras via its RA domain is required to redirect Epac2 to Ras-containing membranes and to subsequently induce cAMP-dependent activation of Rap proteins (Liu et al., 2008). The authors proposed that coincident detection of both cAMP and Ras signals is required for Epac2 to activate Rap1 in a temporally and spatially controlled manner (Liu et al., 2008). Similarly, Ponsioen and colleagues recently characterized the molecular mechanisms underlying the direct spatial control of Epac1 by cAMP (Ponsioen et al., 2009). It has been reported that cAMP is required to release Epac1 from auto-inhibitory restraints, and that cAMP induces translocation of Epac1 to the plasma membrane, a process dependent on the DEP domain of Epac1 (Ponsioen et al., 2009). Until very recently, it was believed that the second low-affinity cAMP-A domain of Epac2 exerted an as-yet undetermined biological function. However, Niimura and co-workers identified a novel splice variant of Epac2, designated Epac2B, and the initially identified Epac2 has now been renamed as Epac2A (Niimura et al., 2009). Expression of Epac2A mRNA is restricted to pancreatic islets and cerebral cortex, and it has been shown that Epac2A-driven insulin secretion from pancreatic cells requires the cAMP-A domain, independent of its cAMP-binding capacity, to localize Epac2A near the plasma membrane (Niimura et al., 2009). Clearly, translocation of Epac1, Epac2A and Epac2B to the plasma membrane is driven by rather different mechanisms, and it will be of interest to analyse the impact of AKAP proteins in these processes. Recent X-ray crystallography of Epac2 and NMR spectroscopy of Epac1 have provided novel insights into the dynamic equilibrium of critical conformational switches encompassing a closed, auto-inhibited state in which the N-terminal regulatory region sterically blocks the C-terminal catalytic region to a completely different, open and catalytically active state (Rehmann et al., 2006, 2008; Mazhab-Jafari et al., 2007; Das et al., 2008; Harper et al., 2008). It is to be hoped that development of Epac-specific cAMP analogues will arise as a result of these types of studies.
Methods to validate cAMP signalling via PKA and Epac
Membrane-permeable cyclic nucleotide analogues have been synthesized, and these pharmacological tools seem to differentiate between PKA and Epac signalling (Table 1). For example, N6-benzyladenosine-3′,5′-cyclic monophosphate (6-Bnz-cAMP), 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-CPT-cAMPS), 8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-Me-cAMP), acetoxymethyl 8-pCPT-2′-O-Me-cAMP (8-pCPT-2′-O-Me-cAMP-AM), 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer (Sp-8-pCPT-2′-O-Me-cAMPS) and 8-(4-chlorophenylthio)-2′-O-methyl-cGMP (8-pCPT-2′-O-Me-cGMP) are now used to activate and/or inhibit PKA and Epac, and 8-pCPT-2′-O-Me-cGMP is used as a negative control for 8-pCPT-2″-O-Me-cAMP (Enserink et al., 2002; Christensen et al. 2003; Holz et al. 2006, 2007; Vliem et al. 2008; Haag et al. 2008) (Table 1). A 2′-O-methyl substitution on the ribose ring of cAMP of Epac-selective cAMP analogues confers specificity towards Epac, and such compounds are therefore used as pharmacological tools to analyse Epac-driven cAMP dynamics independent of PKA. However, currently available Epac activators do not differentiate between Epac1 and Epac2 (Table 1). As Epac1 and Epac2 are insensitive to inhibitors of PKA, such as Rp-8-CPT-cAMPS (Holz et al., 2007) (Table 1), PKA inhibitors are used to demonstrate that Epac-specific analogues act via Epac independently of PKA. Studies in Trypanosoma brucei indicated, however, that 8-pCPT-2′-O-Me-cAMP might act via its 5′-AMP derivative upon inhibition of PDEs (Laxman et al., 2006). In addition, recent studies in human platelets showed that various cyclic nucleotide analogues, including 6-Bnz-cAMP and 8-pCPT-2′-O-Me-cAMP might, in addition to their primary effects, also cause elevation of cAMP or cGMP upon inhibition of phosphodiesterases (Poppe et al., 2008). Altogether, these studies suggest caution when interpreting data obtained with cyclic nucleotide analogues of dubious selectivity, especially when such agents are used to dissect PKA-dependent and -independent versus Epac-dependent and -independent signalling properties. At present, highly specific pharmacological inhibitors of individual Epac isoforms, Epac1 and Epac2, are not available (Holz et al., 2007; Poppe et al. 2008). However, the successful suppression of the endogenous expression of Epac1 and Epac2 by specific siRNAs has been reported (López de Jesús et al., 2006; Haag et al. 2008, Huang et al. 2008; Yokoyama et al. 2008a,c;). Recently, Seino et al. developed Epac2-deficient mice and demonstrated that Epac2/Rap1 signalling is essential in the regulation of insulin granule dynamics (Shibasaki et al., 2007). Generation of additional Epac knockout mice and/or Epac reporter mice might help to gain further insights into Epac-related signalling.
Table 1.
Cyclic nucleotide compounds
| Compound | Characteristics | References |
|---|---|---|
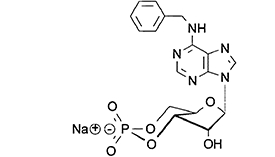 |
N6-benzyladenosine-3′,5′-cyclic monophosphate (6-Bnz-cAMP), selective and membrane-permeable protein kinase A (PKA) activator | Christensen, Selheim et al., 2003,Poppe et al., 2008 |
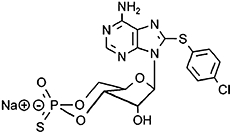 |
8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-8-CPT-cAMPS), competetive inhibitor of PKA | Christensen, Selheim et al., 2003,Poppe et al., 2008 |
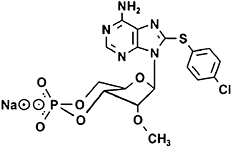 |
8-(4-chlorophenylthio)-2′-O-methyl-cAMP (8-pCPT-2′-O-Me-cAMP), selective and membrane-permeable activator of Epac1 and Epac2 | Enserink et al., 2002,Christensen, Selheim et al., 2003,Poppe et al., 2008 |
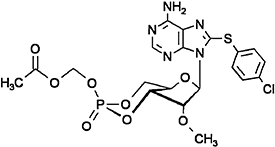 |
Acetoxymethyl 8-pCPT-2′-O-Me-cAMP (8-pCPT-2′-O-Me-cAMP-AM), see above | Vliem et al., 2008 |
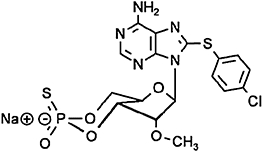 |
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer (Sp-8-pCPT-2′-O-Me-cAMPS), selective and membrane-permeable Epac activator, insensitive to phosphodiesterases | Laxman et al., 2006,Poppe et al., 2008 |
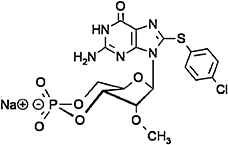 |
8-(4-chlorophenylthio)-2′-O-methyl-cGMP (8-pCPT-2′-O-Me-cGMP), negative control for 8-pCPT-2′-O-Me-cAMP | Haag et al., 2008 |
Epac effectors and biological functions
Epac1 and Epac2 were initially characterized as cAMP-activated GEFs for Rap1 and Rap2 (de Rooij et al., 1998; Kawasaki et al. 1998). Recent studies have indicated that Epac proteins also function as a molecular link between different Ras family members (Maillet et al., 2003; Krugmann et al., 2004; Morel et al., 2005; López de Jesús et al., 2006; Li et al. 2006; Métrich et al., 2008). Furthermore, our studies demonstrated that Epac1 binds to and activates R-Ras, a process that is likely to facilitate cytoskeleton dynamics and calcium handling driven by Epac (López de Jesús et al., 2006). Over the last 10 years, considerable progress has been made into the Epac-related cAMP dynamics associated with learning and memory (Gekel and Neher, 2008; Gelinas et al., 2008; Murray and Shewan, 2008; Ouyang et al., 2008), inflammation, fibrosis, and hypertrophy (Holz et al., 2006, 2007; Schmidt et al., 2007a; Roscioni et al., 2008; Schaafsma et al., 2008; Borland et al., 2009b). Epac1 and Epac2 seem to control these distinct cellular responses by signalling to a range of effectors. The number and diversity of these effectors is considerable. For example, phospholipase C-ε (Schmidt et al., 2001; Oestreich et al., 2007), phospholipase D (López de Jesús et al., 2006; Han et al., 2007) and ERK1/2 (Lin et al., 2003; Keiper et al., 2004; Kiermayer et al., 2005; Wang et al., 2006) have been implicated in the regulation of hypertrophic responses. ERKs (Ster et al., 2007, 2009; Gelinas et al., 2008; Ma et al., 2009) and PI 3-kinase-dependent PKB/Akt (Mei et al., 2002; Misra and Pizzo, 2005; Yano et al. 2007; Misra et al. 2008; Kwak et al., 2008; Nijholt et al. 2008) seem to modulate learning and memory. TGF-β1 receptor-regulated Smads are important for fibrogenic and inflammatory responses (Basoni et al., 2005; Conrotto et al., 2006; Yokoyama et al., 2008c). Finally, NF-κB (Fuld et al., 2005; Scheibner et al., 2008), the suppressor of cytokine signalling-3 (SOCS-3) (Sands et al., 2006; Yarwood et al., 2008; Borland et al., 2009a), the CCAAT/enhancer-binding protein C/EBP and glycogen synthase kinase-3 (GSK-3) (Jing et al., 2004; Xu et al., 2008) (Figure 1 and Figure 3), have been reported to regulate inflammation. Further details on the molecular mechanisms of the regulation of Epac-related effectors and their biological functions are outlined in recent excellent reviews on these topics (Cohen and Frame, 2001; Scheid and Woodgett, 2001; Kolch, 2005; Bunney and Katan, 2006; Oude Weernink et al., 2007; Perkins, 2007; Schmierer and Hill, 2007; Yoshimura et al., 2007; Borland et al., 2009b). Despite the novel insights into the molecular mechanisms linking Epac to a diversity of downstream effectors, it still remains to be determined whether co-localization of Epac to PKA-AKAP-based signalling complexes are of central importance to the net outcome of cAMP signalling.
Figure 3.
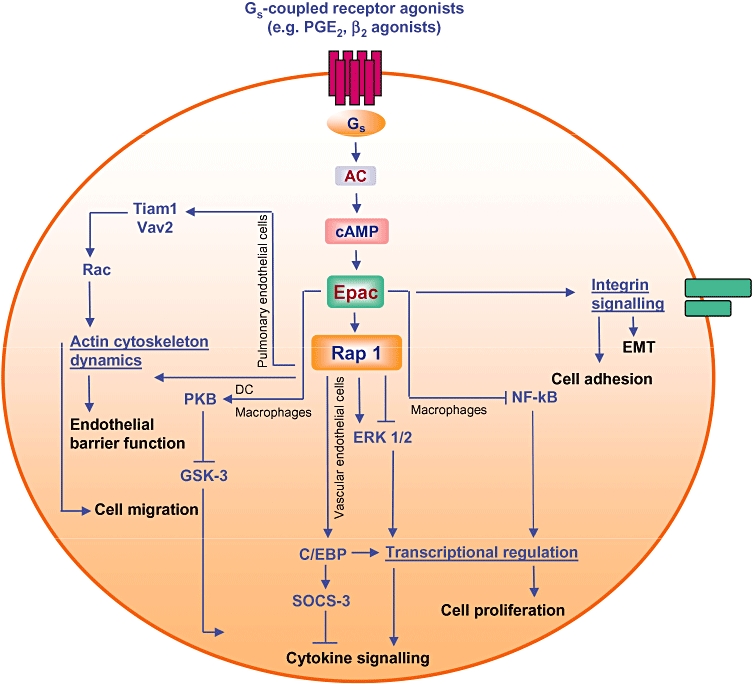
Epac and lung cell signalling. Activation of Epac proteins by Gs-coupled receptors profoundly alters actin dynamics, the microtubule network, gene expression and cytokine responsiveness and thereby modulates diverse processes, including barrier function, cell migration, cell division, cell adhesion and cell proliferation. See text for further details. AC, adenylyl cyclase; C/EBP, CCAAT/enhancer-binding protein; EMT, epithelial-mesenchymal transition; ERK, extracellular signal-regulated kinase; GSK-3, glycogen synthase kinase-3; PKB protein kinase B; PG, prostaglandin; SOCS-3, suppressor of cytokine signalling-3.
Epac and immune cells
The immune system consists of diverse cells derived from pluripotent haematopoietic stem cells. The myeloid cell lineages leukocytes and monocytes/macrophages are believed to mediate adaptive and innate immunity, whereas lymphocytes are believed to be responsible for adaptive immunity, and together they represent the key effector cells of host defense mechanisms (Vivier and Malissen, 2005; Medzhitov, 2007). cAMP drives several signalling cascades in immune cells, and thereby also plays a pivotal role in multiple immune cell responses, including growth and differentiation, growth arrest and apoptosis, and the production of chemokines and cytokines (Kammer, 1988). Until recently, modulation of immune response by cAMP had been assigned to PKA and PKA-mediated changes in protein expression and function (Cohen, 2002; Zambon et al., 2005). However, studies have indicated that Epac1 and Epac2 seem to act as novel cAMP mediators in the regulation of innate and adaptive immune cell functions (Serezani et al., 2008).
Epac in monocyctes and macrophages
Monocytes and macrophages are pivotal in host defense; after the entry of pathogens into the organism, they eliminate bacteria, viruses and parasites via phagocytosis. Significantly, most signalling pathways in monocytes have been described as cAMP dependent. In particular, cAMP has been reported to inhibit phagocytosis (Rossi et al., 1998; Aronoff et al., 2004) and the production of tumor necrosis factor-α (TNF-α) (Rowe et al., 1997). Traditionally, most inhibitory effects of cAMP were assigned to PKA; however, several earlier studies focused on mechanisms independent of PKA. In human monocytes, cAMP inhibits TNF-α secretion, phagocytosis and respiratory burst activity. Such actions of cAMP have been shown to be mediated via the classical PKA pathway (Bryn et al., 2006). Differentiation of monocytes to macrophages, however, was paralleled by increased expression of Epac1 and a contribution of Epac1 to macrophage responses (Bryn et al., 2006). Thus, the inhibitory effects of cAMP on macrophage activation and Fcγ receptor-mediated phagocytosis of pathogens have been reported to involve both PKA and Epac1 (Bryn et al., 2006). In contrast, cAMP-mediated inhibition of FcR-mediated phagocytosis in alveolar macrophages was independent of PKA via selective Epac1 activation, while synthesis of the inflammatory mediators leukotriene B4 and TNF-α in alveolar macrophages was inhibited by cAMP in a PKA-dependent manner independent of Epac (Aronoff et al., 2005). However, chemokine and cytokine production in bone marrow-derived dendritic cells involved both Epac1 and PKA (Aronoff et al., 2006a). In addition, Xu et al. recently showed in the murine macrophage-like cell line J774A.1 that the cAMP-induced suppressive effects on interferon-β production were mediated via Epac (Xu et al., 2008). Recent studies in our laboratory have indicated that activation of Epac (most likely Epac1) inhibited apoptosis in human leukocytic cells (Grandoch et al., 2009a). As illustrated in Figures 1–3, both cAMP mediators (either alone and/or in concert) are important in the regulation of distinct immune responses and are likely to depend on the differentiation status of the cells. Regarding the specific functions of Epac in the immune system, Epac1 regulates the differentiation of monocyctes to macrophages and subsequently controls cellular phagocytosis, as well as the production of inflammatory mediators, and apoptosis in human leukocytes. Future research will help to determine the molecular mechanism by which Epac proteins regulate the specific subset of immune functions.
Epac in integrin-mediated adhesion
Constant motion and recirculation of immune cells is pivotal for a proper function of the defense immune system. Immune cell trafficking is a highly regulated process that ensures appropriate cell migration and activation (Cahalan and Gutman, 2006). As illustrated in Figure 2, during inflammation, there is an excessive extravasation of leukocytes from the blood into the surrounding tissue, a process that requires the production of chemokines to induce leukocyte migration, integrin-mediated adhesion of circulating leukocytes, and finally transendothelial migration (Springer, 1994; Lorenowicz et al., 2007a, 2008). Again, cAMP plays a key role in these processes. However, the effect of cAMP on cell adhesion and chemotaxis of monocytes remains controversial (Zeidler et al. 2000; Fine et al., 2001; Lorenowicz et al. 2006; Pannekoek et al. 2009). Zeidler et al. (2000) reported that an elevation of cellular cAMP levels by prostaglandin E2 (PGE2) inhibited monocyte adhesion to endothelial cells. In contrast, Lorenowicz et al. (2006) reported on the opposing effects of cAMP on the same cellular response. The molecular mechanisms behind the cAMP effects on monocyte adhesion to endothelial cells have been the focus of recent studies. The Epac-Rap1 signalling pathway has been shown to be involved in integrin-mediated cell adhesion in non-myeloid cell lines (Rangarajan et al., 2003; Enserink et al., 2004). Moreover, in sickle (red blood) cells, the Epac-dependent Rap1 activation promotes cell adhesion to the extracellular matrix protein laminin (Murphy et al., 2005). Recent studies in monocytes reported that activation of Epac1 enhances monocyte adhesion to endothelial cells via fibronectin and promotes chemotaxis in the human promonocytic cell line U937 and primary monocytes (Lorenowicz et al., 2006). As illustrated in Figure 2, Epac1 induced polarization of leukocytes (including human monocytic U937 cells), which is a crucial process for the directed migration of the cells by chemokines (Lorenowicz et al., 2006, 2007a). Interestingly, Basoni et al. postulated that reduction of Epac1 mRNA expression by TGF-β1 and subsequent reduction of Rap1-driven leukocyte migration acts as an anti-inflammatory signal (Basoni et al., 2005). In contrast, Wittchen et al. reported that the Epac effector Rap1 inhibits endothelial transmigration of leukocytes upon enhancement of endothelial barrier functioning (Wittchen et al., 2005). In neutrophils, adhesion of the cells to endothelial surface has been shown to be inhibited by increased cAMP levels via inhibition of stimulus-induced L-selectin shedding and up-regulation of αMβ2 integrin (Berends et al., 1997; Derian et al. (1995). These effects are mediated via PKA (Derian et al., 1995), while the Epac-Rap pathway seems to promote leukocyte adhesion through activation of β1 integrins (Lorenowicz et al., 2007a) (see Figure 2). These results further underline the complexity of cAMP signalling and the various outcomes of elevated levels of cAMP, which are most likely to be immune cell-dependent and may be predicted by the relative contributions of PKA and Epac. Certainly, Epac1 modulates integrin function (most probably by barrier function) and leukocyte transmigration. However, the net outcome of leukocyte transmigration by Epacs remains controversial, and the molecular mechanisms have yet to be determined.
Figure 2.
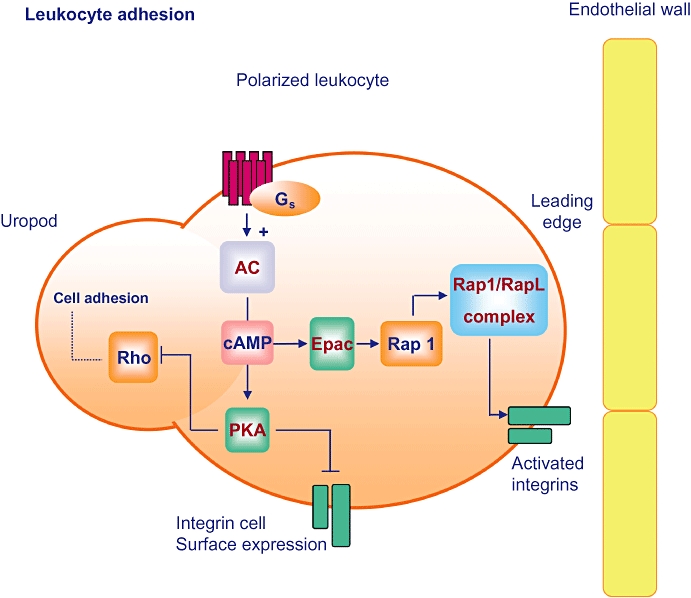
Epac: leukocyte migration and integrin-mediated adhesion. Gs-coupled receptors lead to adenylyl cyclase (AC)-dependent cellular cAMP elevations and subsequent activation of protein kinase A (PKA) and Epac. Whereas PKA inhibits expression of integrin receptors and Rho-dependent cell adhesion, Epac initiates Rap1 signalling to activate integrins via the Rap1 effector RapL and thereby promotes leukocyte polarization, migration and adhesion to endothelial cells.
Epac in B lymphocytes
Mature lymphocytes with their antigen-specific receptors are in permanent circulation from the blood stream to the lymphoid organs, and represent the main mediators of the adaptive immune response (Vivier and Malissen, 2005; Medzhitov, 2007). In B cells, signalling by the antigen-specific B cell antigen receptor (BCR) is a central step to maintain homeostasis and proper immune function, with cAMP functioning as an important physiological mediator (Kammer, 1988). Indeed, formation of cAMP has been detected in B cells after stimulation of the BCR, and has been implicated as a negative regulator of B cells (Wiener and Scarpa, 1989; Newell et al., 1993). By serving as a mediator of BCR-induced growth arrest and apoptosis, cAMP thereby adapts pro- and anti-apoptotic cell responses, and it is generally believed that these positive and negative regulatory signals are crucially important for a balanced immune response and immune homeostasis (Goodnow, 1996; Monroe, 2000; Niiro and Clark, 2002). Historically, the inhibitory cAMP effects on lymphocytes were primarily assigned to the classical PKA pathway (Cohen, 2002; Zambon et al., 2005), but recently, PKA-independent effectors have also been assumed to play a role. Indeed, expression of Epac1 (but not Epac2) has been reported in B cell chronic lymphotic leukaemia (Klein et al., 2001; Tiwari et al., 2004) (Table 2). It has been proposed that Epac acts as a mediator, and new cAMP target in BCR-induced growth arrest and apoptosis, although the underlying molecular mechanisms have not been addressed (Klein et al., 2001; Tiwari et al., 2004). Recently, we reported that the murine B lymphoma cell line WEHI-231 expresses both Epac1 and Epac2, and that Epac proteins signal to ERK1/2 and Akt upon activation of Rap1 and H-Ras, thus modulating BCR-induced growth arrest and apoptosis (Grandoch et al., 2009b). The relative contribution of PKA and Epac to BCR-induced growth arrest and apoptosis remains controversial; however, recent research suggests a role of Epac.
Table 2.
Epac and immune cell responses
| Cell system | Cellular responses | References |
|---|---|---|
| Sickle red blood cells | Adhesion to laminin via Epac-induced Rap1 activation | Murphy et al., 2005 |
| Monocyte-derived macrophages | FcR-mediated phagocytosis mediated via both protein kinase A (PKA) and Epac1-Rap1 | Bryn et al., 2006 |
| Human primary monocytes | Adhesion to endothelial cells via Epac1 activation | Lorenowicz et al., 2006 |
| Up-regulation of monocyte migration toward the chemokine CCL2 (MCP-1) via Epac1 activation | ||
| U937 (human monocytic cell line) | Epac activation promotes cell polarization | |
| Epac1 activation promotes adhesion to fibronectin by activation of β1-integrins | ||
| Epac1 activation up-regulates monocyte migration toward the chemokine CCL2 (MCP-1) | ||
| Human and rat (NR8383) alveolar macrophages | Activation of Epac1 suppresses FcR-mediated phagocytosis | Aronoff et al., 2005 |
| Stimulation of PKA or Epac1 inhibits bactericidal activity and H2O2 production | ||
| J774A.1 (murine macrophages) | Suppression of endotoxin-induced interferon-β production via cAMP-induced Epac activation | Xu et al., 2008 |
| Human T lymphocytes | PKA-dependent and -independent prolactin gene expression | Gerlo et al., 2006 |
| Jurkat T cells (human leukaemic T cell line) | Down-regulation of c-Jun activity via Epac activation | Fuld et al., 2005 |
| WEHI231 (murine B lymphoma cell line) | B cell antigen receptor-induced cAMP-dependent growth arrest and apoptosis involve Epac | Grandoch et al., 2009b |
| Human leukocytic cells (U937, HL-60, primary human mononuclear cells) | Epac activation inhibits apoptosis | Grandoch et al., 2009a |
CCL2, CCL identical to MCP-1; MCP-1, monocyte chemotactic protein-1.
Epac in T lymphocytes
In T lymphocytes, cAMP has been shown to inhibit cell proliferation and to reduce effector functions (Kammer, 1988; van Oirschot et al., 2001). Initially, the inhibitory effects of cAMP had been assigned to PKA (van Oirschot et al., 2001); however, upon identification of Epac1 and Epac2, the underlying molecular mechanisms have been re-evaluated (Table 2). Modulation of immune responses and inhibition of cellular growth by cAMP in human T lymphocytes has been reported to be PKA independent (Bryce et al., 1999; Staples et al., 2001). In addition, cAMP induces the expression of the immunomodulatory pituitary hormone prolactin in Jurkat T cells, as well as in human T lymphocytes. In Jurkat T cells, prolactin expression is mediated only by PKA, however, in human T lymphocytes, PKA-dependent and -independent mechanisms were observed, indicating a possible role of Epac (Gerlo et al., 2006). Moreover, in human leukemic Jurkat T cells, regulation of cell cycle and cAMP-dependent growth arrest has been shown to be mediated by PKA, thereby reducing T cell production and activation (Fuld et al., 2005). Intriguingly, Epac seems both to regulate and to suppress expression of a distinct subset of functional genes in the Jurkat T cells, including the NF-κB-related p53 phosphoprotein (Fuld et al., 2005). In addition, the Epac effector Rap1A positively regulates T cell signalling, and these effects were reported to be mediated via the activation of integrins (Sebzda et al., 2002; Bivona et al., 2004; Dustin et al., 2004). Indeed, PKA and the Epac-Rap pathway also seem to be important for the regulation of lymphocyte adhesion. While PKA-dependent signals promote T cell adhesion by different mechanisms, such as disassembly of the cytoskeleton (Rovere et al., 1996), Rap1 activation has been shown to be required for cell adhesion via αLβ2 and αLβ4 integrins in Jurkat T cells (de Bruyn et al., 2002). Again, it is reasonable to conclude that Epacs regulate integrin functioning, cell growth and gene expression in T lymphocytes. However, the precise regulation by Epacs of the molecular mechanisms in T lymphocyte functioning is still unclear.
Epac in immune cell responses
cAMP is a key mediator in the diverse cells of the immune system and acts as a pivotal modulator of different cell functions, such as migration, cell adhesion, gene transcription, cell proliferation and growth arrest (Table 2). For a long time, the diverse cAMP effects had been attributed solely to PKA; however, the identification of Epac proteins as novel cAMP mediators initiated studies that focused in more detail on the molecular mechanisms behind these diverse cAMP effects in order to discriminate between cellular responses driven by PKA and/or Epac. It is now known that distinct cellular responses require Epac, while others primarily require PKA. Thus, PKA and Epac seem to balance the diverse outcomes of cAMP effects in different cell systems in parallel pathways, and the net outcome depends on the respective distribution of both within the cell.
Epac and the lung
Chronic inflammatory lung diseases
Lung function is ensured by the cooperative action of diverse cells, and lung dysfunction may lead to lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) (Jeffery, 1998; Holgate, 2008), both of which are characterized by episodes of airway obstruction and structural changes in the bronchial wall, including increase in smooth muscle mass, epithelial cell damage and accumulation of the extracellular matrix (ECM) (Holgate, 2002). Unlike asthma, COPD is characterized by airway narrowing and progressive and irreversible decline in lung function (Holgate, 2002). Overall, these structural changes are known as airway remodelling, and they represent the hallmarks of both asthma and COPD (Jeffery, 2001). Structural alterations eventually impair lung function and sensitize the lung to react to external stimuli in ways that lead to cellular damage and inflammation (Bousquet et al., 2000; Cockcroft and Davis, 2006). Indeed, asthma and COPD are characterized by chronic inflammation and the infiltration into the airways of a variety of activated immune cells, including neutrophils, eosinophils and lymphocytes (Azzawi et al., 1990), which in turn produce inflammatory mediators and amplify the inflammatory response. Airway smooth muscle (ASM) cells are important because of their intrinsic capacity to migrate, to contract, to proliferate and to produce ECM components such as cytokines, growth factors and chemokines (Hirst 2000; Halayko and Amrani, 2003; Panettieri, 2003). Such observations have prompted researchers to assume that ASM cells play an important role in airway remodelling (Halayko et al., 1996; Hirst, 2003). Pulmonary fibroblasts produce the main ECM component collagen I and enzymes involved in collagen I degradation, and therefore play a role in the homeostasis of ECM (Racke et al., 2008). Although fibroblast recruitment, proliferation and collagen synthesis are important to regulate normal wound healing, excessive fibroblast activation can lead to pulmonary fibrosis, a common characteristic of many respiratory disorders (Huang et al., 2007). In addition to ASM and fibroblasts, epithelial cells are also of importance for normal lung function. Epithelial cells form cell layers characterized by gap, adherens and tight junctions and adhesion molecules, such as cadherins and certain integrins, and may convert into mesenchymal cells through a process known as epithelial-mesenchymal transition (EMT) (Thiery and Sleeman, 2006). Whereas EMT is important for physiological functioning in adult organisms, fibrogenic properties of mesenchymal cells have also been shown to contribute to pulmonary fibrosis (Radisky, 2005). In the lung, when the first contact with foreign external particles occurs, resident immune effector cells, such as dendritic cells and alveolar macrophages, are activated in order to process antigenic material. However, dendritic cells and alveolar macrophages are also a rich source of cytokines and chemokines, and they may also amplify inflammation (Suarez et al., 2008).
Expression of Epac in chronic inflammatory lung disease-associated cell types
Although chronic inflammatory lung disorders are a major cause of death and debilitation, there have been relatively few innovative breakthroughs into their cause, treatment or cure. Recently, research attention has been focused on new targets for therapeutic intervention (Barnes, 2007; Barnes, 2008; Chung and Adcock, 2008). Epacs represent a novel key effector in the lung due to their capacity to modulate airway inflammation and proliferation (Schaafsma et al., 2008), and as part of the signalling cascade triggered by β2-adrenoceptor agonists that target cAMP to alleviate the symptoms of asthma and COPD (Barnes and Hansel, 2004; Remington and DiGiovine, 2004; Balley and Tashkin, 2007). In the last few years, expression of Epac1 and Epac2 in several lung cell types has been successfully demonstrated (Figure 3 and Table 3). Epac1 and Epac2 mRNA were expressed in the lung epithelium and mesenchyme of mouse embryos at different developmental stages, and both cultured human ASM and bronchial epithelial cells express functional Epac1 and Epac2 (Schmidt et al., 2007b). In human pulmonary fibroblasts, mRNA and protein expression of Epac1 was also recently detected, while expression of Epac2 remained under detection limits (Haag et al., 2008; Huan et al. 2008). A few years ago, expression of Epac1 protein was identified in human and rat alveolar macrophages (Aronoff et al., 2005) and mouse dendritic cells (Aronoff et al., 2006b). Both cell types are believed to represent key effector cells in innate immunity. Studies on changes of Epac expression during development and under pathophysiological circumstances are still rather limited. Preliminary studies, however, reported on developmental changes of Epac1 and Epac2 in the heart, vasculature, brain, kidneys and lungs (Ulucan et al., 2007; Murray and Shewan, 2008; Yokoyama et al., 2008a,b;) (see above), up-regulation of Epac1 protein expression in rat inflamed neurons (Wang et al., 2007), up-regulation of Epac1 mRNA versus down-regulation Epac2 mRNA in Alzheimer's disease (McPhee et al., 2005), and up-regulation Epac1 and Epac2 mRNAs in myocardial hypertrophy (Ulucan et al., 2007) (see also above). Importantly, it has been recently discovered that the fibrogenic cytokine TGF-β1, a central mediator of inflammatory pulmonary diseases, induces conversion of resting fibroblasts to actively secrete myofibroblasts upon depletion of Epac1 (but not Epac2), which subsequently promotes the production of collagen and may increase pro-fibrotic signalling (Yokoyama et al., 2008c). Intriguingly, in epithelial cells Epac1 also directly interacts with TGF-β1 and inhibits Smad-dependent TGF-β signalling (Conrotto et al., 2006). Certainly, the expression of Epac (so far only reported for Epac1) is controlled by inflammatory mediators; however, the underlying molecular mechanisms have yet to be studied.
Table 3.
Epac and lung cell responses
| Cell system | Effect of Epac | References |
|---|---|---|
| Airway smooth muscle cells | Inhibition of cell proliferation | Kassel et al., 2008 |
| Pulmonary fibroblasts | Inhibition of cell proliferation | Haag et al., 2008;Huang et al., 2008 |
| Leukocytes | Adhesion and chemotaxis | Lorenowicz et al., 2006 |
| Human pulmonary artery endothelial cells | Endothelial barrier function | Birukova et al., 2007; Birukova et al., 2008 |
| Mouse alveolar macrophage-like line | Modulation of hyaluronan-dependent inflammatory gene expression | Scheibner et al., 2008 |
| Vascular endothelial cells | Regulation of IL-6 signalling | Sands et al., 2006 |
| Alveolar macrophages | Suppression of macrophage phagocytosis | Aronoff et al., 2005 |
| Bone marrow-derived mouse dendritic cells | Suppression of LPS-induced MIP-1α /1β production | Jing et al., 2004 |
| Mouse macrophage line | Suppression of LPS-induced IFN-β production | Xu et al., 2008 |
| Alveolar macrophages | No effect on cytokine production | Aronoff et al., 2006b |
| Mouse macrophage line | Production of IL-1-β and IL-6 | Tan et al., 2007a |
IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MIP, macrophage inflammatory protein.
Epac in cell proliferation
The distinct expression profile and localization of Epac1 and Epac2 provide mechanisms for a more integrated and precise control of cAMP signalling, but also increase the signalling complexity. Cell-type specific functions of Epac are most likely to be the result of different cellular environments, cell-to-cell contacts, and the relative abundance, distribution and function of its diverse effectors. Paradoxically, Epac exerts opposing effects even on the same cellular process. For example, the role of Epac in the regulation of cell proliferation is known to be of central importance for the pathophysiology of asthma, COPD and pulmonary fibrotic disorders. Epac has been shown to drive pro-proliferative processes in endothelial cells (Fang and Olah, 2007), macrophages (Misra and Pizzo, 2005), thyroid cells (Hochbaum et al., 2008) and osteoblasts (Fujita et al., 2002), a cellular response believed to involve activation of extracellular signal-regulated kinases via the Epac effector Rap. In contrast, recent studies, however, have reported on a novel anti-proliferative function of Epac in both ASM cells (Kassel et al., 2008) and pulmonary fibroblasts (Haag et al., 2008; Huang et al., 2008) (Figure 3 and Table 3). The findings open important new dimensions in cAMP-related signalling in the lung, particularly because in these studies, cell proliferation of the lung cells was not altered by the classical cAMP effector, PKA. Kassel et al. (2008) showed that β2-adrenoceptor agonists inhibit mitogen-stimulated cell proliferation of ASM cells, a process most likely driven by Epac via yet unknown molecular mechanisms. Haag et al. (2008) used specific Epac siRNA probes to diminish Epac expression in human pulmonary fibroblasts, and reported that Epac1 is responsible for anti-proliferative effects of PGE2. Inhibition of cell growth by the Epac effector Rap1 had previously been attributed to B-Raf and ERK1/2 (Dugan et al., 1999). Interestingly, inhibition of human fibroblast proliferation may not involve classical signalling routes, because PGE2, PKA or Epac1 did not inhibit mitogen-stimulated ERK1/2 (Huang et al., 2008). Direct interactions of Epac1/Rap1 with microtubules might be responsible for the inhibition of fibroblast proliferation (Huang et al., 2008), and indeed Epac-cytoskeleton interactions might be of importance for cell cycle-dependent dynamics (Qiao et al., 2002; Borland et al., 2006, 2009b; Lorenowicz et al., 2007b; Cheng et al., 2008; Huston et al., 2008) (Figure 3). Thus, Epac proteins (most likely Epac1) inhibit proliferation of human ASM and fibroblasts. However, future research should focus on unravelling the anti-proliferative signalling pathways driven by Epac.
Epac in cytoskeleton dynamics
The cAMP-elevating prostanoid PGE2 also inhibits a range of lung cell functions, including migration of fibroblasts (White et al., 2005) and differentiation of myofibroblasts (Kolodsick et al., 2003). The cAMP-dependency of these processes has led to speculation on the potential and specific roles of Epacs. Recent studies have indicated that Epacs exert important pro- and anti-inflammatory signalling properties in the lung (Roscioni et al., 2008; Schaafsma et al., 2008). The complex inflammatory processes in the lung are driven and maintained by the immunomodulatory capacities of lung cells. In fact, several lung cells produce and release inflammatory chemokines and cytokines, and express surface receptors that are important for cell adhesion and activation of leukocytes. Indeed, Epac/Rap signalling regulates cell migration and integrin-mediated cell adhesion in a wide variety of cell types (Bos et al., 2001, 2003; Lorenowicz et al., 2006; Pannekoek et al., 2009). Recently, Lyle et al. reported that cAMP-mediated Epac–Rap activation inhibits epithelial migration upon stabilization of focal adhesions and inhibition of membrane protrusions, possibly via regulation of cytoskeleton-integrin interactions (Lyle et al., 2008). Expression of Epac1 in primary leukocytes and leukemic cell lines was found to correlate with pro-inflammatory effects, including chemotaxis, β1-integrin dependent cell adhesion and Rap1-dependent cell polarization (Lorenowicz et al., 2006) (Figure 2). Indeed, chronic inflammatory responses are typically characterized by uncontrolled extravasation of leukocytes from the peripheral blood into tissues, a process mainly governed by the permeability of the endothelial cell monolayer, and in part determined by their cell contact strength (Vestweber, 2002; Pannekoek et al. 2009). Intriguingly, recent evidence suggests that both Epac and Rap regulate the formation of endothelial cell junctions upon redistribution of E-cadherin and recruitment of the cell junction molecule β-catenin to areas of cell contacts, processes believed to enhance endothelial barrier function (Wittchen et al., 2005). Recent studies on human pulmonary artery endothelial cells have indicated that enhanced endothelial barrier function involves Epac/Rap-induced membrane localization of the Rac-specific guanine nucleotide exchange factors Tiam1 and Vav2 and subsequent activation of Rac, and that such endothelial barrier-protecting mechanisms were shared by a variety of cAMP-elevating stimuli, including PGE2, prostacyclin and atrial natriuretic peptides (Birukova et al., 2007, 2008) (Figure 3 and Table 3). Certainly, inhibition of (inappropriate) leukocyte transmigration and enhancement of barrier function by Epac could serve to reduce inflammation.
Epac in inflammatory mediator production
Epac and Rap also regulate cell contact with the ECM proteins laminin (Enserink et al., 2004) and fibronectin (Rangarajan et al., 2003) in several cell lines, a process involving integrin-driven cytoskeleton dynamics and modulation of cellular adhesion and migration (reviewed in (Pannekoek et al., 2009). Epac/Rap signalling to laminin-5 is of particular interest, because laminin-5 functions as a signal adaptor for growth factor-induced invasive growth in lung cancer (Kodama et al., 2005). ECM might be envisioned not only as a target of inflammation, but also as a key effector in the activation of inflammatory cells. Indeed, fragments of the ECM component hyaluronan have been shown to modulate negatively mice lung inflammation and fibrosis (Jiang et al., 2005). It has also been reported that during inflammation, hyaluronan is degraded to lower molecular weight fragments that subsequently stimulate macrophages and airway epithelial cells to produce mediators of tissue injury and repair, including TNF-α, interleukin (IL)-12, macrophage inflammatory protein (MIP)-1α, MIP-2, IL-8, tissue inhibitor of metalloproteinase-1 and inducible nitric oxide synthase (McKee et al., 1996; Scheibner et al., 2006). Scheibner et al. (2008) showed that adenosine, known to be up-regulated and released into extracellular space during inflammation, exerts anti-inflammatory effects by acting on the Gs-coupled A2A adenosine receptor expressed in macrophages, dendritic cells, T and B lymphocytes, and epithelial cells. Importantly, it has been reported that the anti-inflammatory signalling properties of adenosine seem to involve Epac, which in turn inhibit NF-kB and modulated induction of inflammatory gene expression by lower molecular weight fragments of hyaluronan in in vitro and in vivo models (Scheibner et al., 2008). Negative regulation of cytokine signalling by Epac seems not to be limited to these cells. In vascular endothelial cells, Epac/Rap signalling regulates expression of SOCS-3 and subsequently inhibits the IL-6 receptor trans-signalling complex (Sands et al., 2006) (Figure 3). Recent studies in human umbilical vein endothelial cells indicate that Epac1 induces the C/EBP family of transcription factors, a process that seems to involve SOCS-3 induction by PKCα and ERK1/2 (Yarwood et al., 2008; Borland et al. 2009a). C/EBP proteins regulate cell differentiation and inflammation (Ramji and Foka, 2002), processes partly depending on cAMP (Pelletier et al., 1998). The lack of PKA phosphorylation sites on cAMP-responsive C/EBP domains (Wilson and Roesler, 2002) further points to PKA-independent but Epac-dependent transcriptional events. Finally, Epacs exert functions in anti-inflammatory and immune responses mediated by alveolar macrophages and dendritic cells (Aronoff et al., 2006b) (Table 3). Activation of Epac suppressed dose-dependent phagocytosis in alveolar macrophages, a process likely mediated by the Epac effector Rap (Aronoff et al., 2006b). In bone marrow-derived mouse dendritic cells, Epac inhibited the release of the inflammatory chemokines, MIP-1α and MIP-1β, induced by lipopolysaccaride upon activation of PKB/Akt and the subsequent phosphorylation/inhibition of GSK-3, which promoted DNA binding of the transcriptional repressor CCAT displacement protein (Jing et al., 2004). In the mouse macrophage cell line J774A.1, Epac also reduced the lipopolysaccaride-induced production of IFN-β via signalling to PKB/Akt and GSK-3 (Xu et al., 2008). Epac did not alter the production of MIP-1α and MIP-1β in alveolar macrophages (Aronoff et al., 2006b), whereas in the RAW264.7, mouse macrophage cell line activation of Epac increased the production of the pro-inflammatory mediators IL-1β and IL-6 (Tan et al., 2007b). Together, these studies indicate that Epacs reduced and/or increased the production of inflammatory mediators in different phagocytotic cells. Clearly, the underlying molecular mechanisms of the regulation of inflammation by Epacs remain to be studied and might be strictly cell type-dependent.
Epac in lung cell responses
The subcellular localization of Epac and/or its effectors might differ in dendritic cells, alveolar macrophages and in other lung-related cells. Such mechanisms might also contribute to the distinct cellular responses and provide an explanation of opposing Epac-related responses (Figure 3 and Table 3). Epac-driven signals through activation of Rap might be also envisaged, but these have not yet been studied in the airways. In epithelial cells, down-regulation of EMT-related E-cadherin has been shown to signal to integrins via Rap1, the latter being activated upon E-cadherin endocytosis (Balzac et al., 2005), suggesting that Epac/Rap signals might contribute to the complex network of EMT signalling. Rap1 clearly represents the main Epac effector in several systems (Roscioni et al., 2008; Yarwood et al., 2008; Pannekoek et al., 2009). However, upon signalling to different additional key effectors, Epac might fulfil its complex and partly opposing functions in cells of lung origin. Future studies on Epac-related signalling in the lung may help to develop novel therapeutic strategies against inflammatory pulmonary diseases.
Epac and neurones
Epac in neuronal functions
Our current understanding of the molecular mechanisms underpinning the neuronal basis of learning and memory has progressed substantially. cAMP-dependent PKA is thought to be the major, if not the sole neuronal cAMP mediator, and its importance in memory consolidation is generally accepted (Abel and Nguyen, 2008). In the neuronal system, however, recent studies have reported that expression of Epac is subject to alteration during development and under pathophysiological conditions (McPhee et al., 2005; Ulucan et al., 2007; Murray and Shewan, 2008). It has been postulated that the developmental regulation of Epac expression promotes axon growth and regeneration in the nervous system (Murray and Shewan, 2008), and McPhee and co-workers reported up-regulation of Epac1 mRNA versus down-regulation of Epac2 mRNA in those regions of the human brain associated with Alzheimer's disease (McPhee et al., 2005) (Figure 1, see above).
Studies on the functional role of Epac in the neuronal system have shown that Epac enhances neurotransmitter release in glutamatergic synapses (Sakaba and Neher, 2003; Zhong and Zucker, 2005; Gekel and Neher, 2008), and that Epac1 and Epac2 modulate neuronal excitability (Ster et al., 2007). In dorsal root ganglions, Epac mediates the translocation and activation of protein kinase C (PKC), leading to the establishment of inflammatory pain (Hucho et al., 2005), and promotes neurite outgrowth (Murray and Shewan, 2008). In spinal cord tissue, Epac advances neurite regeneration (Murray and Shewan, 2008). In suprachiasmatic nuclei of the hypothalamus, it has been very recently reported that Epac1 and Epac2 represent a novel core component of the circadian pacemaker (O'Neill et al., 2008). These data suggest that Epacs may be key modulators in the regulation of neuronal functions as diverse as neurotransmitter release and neuronal excitability.
Epac and neuronal effectors
Epac seems to activate distinct molecular mechanisms in the neuronal system. In cultures from mouse hippocampus, Epac-induced elevation of neurotransmitter release seems to require functional PKC (Gekel and Neher, 2008). In cultured mouse cerebellar neurons, however, Epac-regulated neuronal excitability required Rap and ERKs (Ster et al., 2007, 2009). Thus far, evidence for a role of Epac in the process of learning and memory is limited. Gelinas et al. (2008) reported that Epac activation enhances the maintenance of long-lasting synaptic potentiation in area CA1 of mouse hippocampal slices, a process that involves a transient increase in ERK1/2 immunoreactivity. Co-application of a selective PKA and a selective Epac activator has been shown to rescue the memory retrieval impairment observed in dopamine β-hydroxylase deficient mice, and the Epac effector Rap seemed to be important for these neuronal responses (Ouyang et al., 2008). Although recent research efforts indicate that Epac regulates neuronal cAMP signalling, it remains controversial as to whether Epac requires input of PKA, localization to specific brain areas, and signalling to distinct effectors in a time- and space-limited manner.
Subcellular compartmentalization of Epac signalling
Increase in cellular cAMP can simultaneously induce activation of the two cAMP mediators PKA and Epac, and thus specificity and coordination of cAMP signalling requires tight regulation. Subcellular compartmentalization and spatio-temporal cAMP dynamics are believed to involve members of the AKAP family (Wong and Scott, 2004; Beene and Scott, 2008). As outlined above, a functional cAMP-responsive multiprotein complex, maintained by nuclear envelope-associated mAKAP, PKA, PDE4D3 and Epac1, has been identified in neonatal rat cardiomyocytes (Dodge-Kafka et al., 2005). Additionally, it has been reported that the AKAP-based signalling complex was of importance in sensing subtle alterations in cardiac cAMP levels and in regulating hypertrophic functions (Dodge-Kafka et al., 2005). It is worth noting that both PKA anchored to neuronal AKAP79/150 and the Epac effector Rap have been reported to regulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking during synaptic plasticity (Zhu et al., 2002, 2005; Snyder et al. 2005). It is therefore reasonable to assume that AKAP79/150 might integrate cAMP signals in order to coordinate distinct signalling properties of PKA and Epac in neuronal cells. Indeed, research in our laboratory has recently identified a novel neuronal plasma membrane-associated AKAP79/150-based signalling complex comprised of PKA, Epac2 and PKB/Akt (Nijholt et al., 2008) (Figure 4).
Epac in neuronal dysfunction
The dysfunction of signalling events driven by cAMP and the Epac effector PKB/Akt seems to be a key feature of inflammatory disease, including Alzheimer's dementia (Martinez et al., 1999; Halliday et al., 2000; Lin et al., 2001; Griffin et al., 2005; Rogers, 2008). To date, however, the exact nature of the connection of cAMP-dependent signalling with downstream PKB/Akt phosphorylation (activation) remains unknown. However, recent research in our laboratory suggests that this connection exists. In murine primary cortical neurons and HT-4 cells, activation of cAMP-dependent PKA reduced PKB/Akt phosphorylation, whereas activation of Epac enhanced PKB/Akt phosphorylation in a Rap-dependent manner. Studies with PKA-binding deficient neuronal AKAP79/150 and peptides-disrupting PKA anchoring to AKAPs have indicated that AKAP79/150 acts as a key regulator in the two cAMP pathways to control PKB/Akt phosphorylation (Nijholt et al., 2008) (Figure 4). The novel link between neuronal AKAP79/150, PKA, PKB/Akt and Epac2 provides a molecular mechanism to exert a reciprocal effect on neuronal PKB/Akt phosphorylation. Importantly, the neuronal cAMP-responsive multiprotein complex exerted opposing effects on PKB/Akt signals (Nijholt et al., 2008). Clearly, Epac2 (most likely complexed to or associated with AKAP79/150) enhances PKB/Akt phosphorylation, and most likely subsequent PKB/Akt signaling as well. Because Epac2 expression is reduced in those regions of the human brain associated with Alzheimer's disease (McPhee et al., 2005), it is reasonable to assume that Epac2-induced PKB/Akt phosphorylation is reduced in patients suffering from such pathophysiological conditions. Such a mechanism might explain the dysfunction of PKB/Akt signals during inflammatory disorders, such as Alzheimer's dementia. Clearly, further research is required to unravel the molecular mechanisms driven by Epacs.
Future perspectives
Activation of GSK-3 by PKB/Akt might contribute to the regulation of many early and late neuronal functions, ranging from cell differentiation, proliferation, and survival to learning and memory (Peineau et al., 2008), and the interaction of PKA and Epac with AKAPs might indicate distinct cAMP signalling properties. It has been reported that members of the AKAP family interact with muscarinic receptors and β2-adrenoceptors (Hoshi et al., 2003, 2005; Giembycz and Newton, 2005). Both receptor subtypes are coupled to cAMP driven signalling, and are important for proper lung function. Indeed, dysfunction of cholinergic transmission contributes to the development and progression of chronic inflammatory lung disorders (Belmonte, 2005; Barnes, 2008; Chung and Adcock, 2008; Schaafsma et al., 2008). Recent research has reported that the existence of a GSK-3/β-catenin signalling axis in ASM cells is of key importance to mitogenic signalling (Gosens et al., 2008; Nunes et al., 2008). Interaction of AKAPs with muscarinic receptors and β2-adrenoceptors might also coordinate compartmentalization and spatio-temporal cAMP dynamics in the lung, and subsequent signalling to PKB/Akt-regulated targets. Dysfunction of such mechanisms might contribute to the development and perpetuation of inflammatory disorders of the pulmonary and neuronal system.
Concluding remarks
Studies in diverse cell types, animal models and in genetically modified mice indicate that the novel cAMP mediators, Epac1 and Epac2, are important for several processes under physiological and pathophysiological circumstances. Further insights into the spatio-temporal dynamics of Epac-driven cAMP signalling will unravel the molecular mechanisms underlying the development and the progression of chronic degenerative inflammatory diseases, such as Alzheimer's dementia and COPD. The development of novel Epac-specific compounds for innovative therapeutic intervention for the causes, treatment or cure of these diseases definitely represents the primary challenge of the future.
Acknowledgments
As Epac-related research is very dynamic, we apologize that were not able to include all recent work. We thank Dr. Oude Weernink for critical reading of the manuscript and helpful comments, and Dr. Frank Schwede for helpful discussion (Biolog Life Science, http://www.biolog.de). Sara S. Roscioni is recipient of an Ubbo Emmius Fellowship from the School of Behavioral and Cognitive Neurosciences (BCN), University of Groningen, and Martina Schmidt is a Rosalind Franklin Fellow at the University of Groningen.
Glossary
Abbreviations:
- 8-pCPT-2′-O-Me-cAMP
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate
- 8-pCPT-2′-O-Me-cAMP-AM
8-(4-chlorophenylthio)-2′-O- methyladenosine-3′,5′-cyclic monophosphate acetoxymethyl
- AKAP
A-kinase anchoring protein
- ASM
airway smooth muscle
- BCR
B cell antigen receptor
- COPD
chronic obstructive pulmonary disease
- C/EBP
CCAAT/enhancer-binding protein
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- Epac
guanine nucleotide exchange factors for Ras-like small GTPases
- ERK
extracellular signal-regulated kinase
- GSK-3
glycogen synthase kinase-3
- PDE
phosphodiesterase
- PI 3-kinase
phosphatidylinositol 3-kinase
- PKA, PKB, PKC
protein kinase A, B and C
- PG
prostaglandin
- PLC
phospholipase C
- SOCS-3
suppressor of cytokine signalling-3
- TGF-β1
transforming growth factor β1
- TNF-α
tumor necrosis factor-α
Conflicts of interest
None provided.
References
- Abel T, Nguyen PV. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog Brainres. 2008;169:97–115. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aivatiadou E, Ripolone M, Brunetti F, Berruti G. cAMP-Epac2-mediated activation of Rap1 in developing male germ cells: RA-RhoGAP as a possible direct down-stream effector. Mol Reprod Develop. 2009;76:407–416. doi: 10.1002/mrd.20963. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E-2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006a;26:827–833. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006b;26:827–833. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- Baillie GS. Compartmenalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009;276:1790–1799. doi: 10.1111/j.1742-4658.2009.06926.x. [DOI] [PubMed] [Google Scholar]
- Balley WC, Tashkin DP. Pharmacological therapy. Novel approaches for chronic obstructive pulmonary disease. Proc Am Thor Soc. 2007;4:543–548. doi: 10.1513/pats.200701-017FM. [DOI] [PubMed] [Google Scholar]
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–4783. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase A (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49:160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Chronic opbstructive pulmonary disease: a growing but neglected global epidemic. PlosMed. 2007;4:e112–e113. doi: 10.1371/journal.pmed.0040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Hansel TT. Prospects for new drugs for chronic obstructive pulmonary disease. Lancet. 2004;364:985–996. doi: 10.1016/S0140-6736(04)17025-6. [DOI] [PubMed] [Google Scholar]
- Basoni C, Nobles M, Grimshaw A, Desgranges C, Davies D, Perretti M, et al. Inhibitory control of TGF-β1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J. 2005;19:822–824. doi: 10.1096/fj.04-3085fje. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research – still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol. 2008;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte KE. Cholinergic pathways in the lung and anticholinergic therapy for chronic obstructive pulmonary diesease. Proc Am Thor Soc. 2005;2:297–304. doi: 10.1513/pats.200504-043SR. [DOI] [PubMed] [Google Scholar]
- Berends C, Dijkhuizen B, deMonchy JGR, Dubois AEJ, Gerritsen J, Kauffman HF. Inhibition of PAF-induced expression of CD11b and shedding of L-selectin on human neutrophils and eosinophils by the type IV selective PDE inhibitor, rolipram. Eur Respir J. 1997;10:1000–1007. doi: 10.1183/09031936.97.10051000. [DOI] [PubMed] [Google Scholar]
- Biel M. Cyclic nucleotide-regulated cation channels. J Biol Chem. 2009;284:9017–9021. doi: 10.1074/jbc.R800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Michalakis S. Cyclic nucleotide-gated channels. Handb Exp Pharmacol. 2009;191:111–136. doi: 10.1007/978-3-540-68964-5_7. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, et al. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivona TG, Wiener HH, Ahearn IM, Silletti J, Chiu VK, Philips MR. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;3:461–470. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Gupta M, Magiera MM, Rundell CJ, Fuld S, Yarwood SJ. Microtubule-associated protein 1B-light chain 1 enhances activation of Rap1 by exchange protein activated by cyclic AMP but not intracellular targeting. Mol Pharmacol. 2006;69:374–384. doi: 10.1124/mol.105.016337. [DOI] [PubMed] [Google Scholar]
- Borland G, Bird RJ, Palmer TM, Yarwood SJ. Activation of protein kinase C α by EPAC1 is required for the ERK- and C/EBPβ-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J Biol Chem. 2009a;284:17391–17403. doi: 10.1074/jbc.M109.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009b;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bos JL, de RJ, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2:369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- Bos JL, de BK, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of samll G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- de Bruyn KMT, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn2+- and antibody-induced LFA-1-and VIA-4-mediated cell adhesion. J Biol Chem. 2002;277:29468–29476. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- Bryce PJ, Dascombe MJ, Hutchinson IV. Immunomodulatory effects of pharmacological elevation of cyclic AMP in T lymphocytes via a protein kinase A independent mechanisms. Immunpharmacol. 1999;41:139–146. doi: 10.1016/s0162-3109(98)00060-5. [DOI] [PubMed] [Google Scholar]
- Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–7370. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. Phospholipase C epsilon: linking second messengers and small GTPases. Trends Cell Biol. 2006;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Gutman GA. The sense of place in the immune system. Nat Immunol. 2006;7:329–332. doi: 10.1038/ni0406-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. ActaBiochim Biophys Sin. 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooji J, Dremier S, Schwede F, Dao KK, et al. cAMP analog mapping of Epac 1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118:551–559. doi: 10.1016/j.jaci.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Cohen P. Protein kinases – the major drug targets of the twenty-first century. Nat Rev Drug Discovery. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- Cohen P, Frame S. The renaissance of GSK-3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- Conrotto P, Yakymoych I, Yakymoych M, Souchelnytskyi S. Interactome of transforming growth factor-β type I receptor (TβRI): inhibition of TGFβ signaling by Epac1. J Prot Res. 2006;6:287–297. doi: 10.1021/pr060427q. [DOI] [PubMed] [Google Scholar]
- Conti M, Beavo JA. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, et al. Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem. 2006;281:21500–21511. doi: 10.1074/jbc.M603116200. [DOI] [PubMed] [Google Scholar]
- Das R, Mazhab-Jafari MT, Chowdhury S, SilDas S, Selvaratnam R, Melacini G. Entropy-driven cAMP-dependent allosteric control of inhibitory interactions in exchange proteins directly activated by cAMP. J Biol Chem. 2008;283:19691–19703. doi: 10.1074/jbc.M802164200. [DOI] [PubMed] [Google Scholar]
- Derian CK, Santulli RJ, Rao PE, Solomon HF, Barrett JA. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by camp modulators. J Immunol. 1995;154:308–317. [PubMed] [Google Scholar]
- Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, et al. Protein kinase A type I and II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge-Kafka KLSJ, Pare GC, Michel JJC, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Kim JS, Zhang Y, Bart RD, Sun Y, Holtzman DM, et al. Differential effects of cAMP in neurons and astrocytes. Role of B-raf. J Biol Chem. 1999;274:25842–25848. doi: 10.1074/jbc.274.36.25842. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Bivona TG, Philips MR. Membranes as messengers in T cell adhesion signaling. Nat Immunol. 2004;5:363–372. doi: 10.1038/ni1057. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de RJ, van TM, Schwede F, Genieser HG, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, et al. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem. 2004;279:44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- Fang Y, Olah ME. Cyclic AMP-dependent, protein kinase A-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: role of exchange protein activated by cAMP 1 (Epac1) J Pharmacol Exp Ther. 2007;322:1189–1200. doi: 10.1124/jpet.107.119933. [DOI] [PubMed] [Google Scholar]
- Fine JS, Byrnes HD, Zavodny PJ, Hipkin RW. Evaluation of signal transduction pathways in chemoattractant-induced human monocyte chemotaxis. Inflammation. 2001;25:61–67. doi: 10.1023/a:1007152903135. [DOI] [PubMed] [Google Scholar]
- Fujita T, Meguro T, Fukuyama R, Nakamuta H, Koida M. New signaling pathway for parathyroid hormone and cyclic AMP action on extracellular-regulated kinase and cell proliferation in bone cells. Checkpoint of modulation by cyclic AMP. J Biol Chem. 2002;277:22191–22200. doi: 10.1074/jbc.M110364200. [DOI] [PubMed] [Google Scholar]
- Fuld S, Borland G, Yarwood SJ. Elevation of cyclic AMP in Jurkat T-cells provokes distinct transcriptional responses through the protein kinase A (PKA) and exchange protein activated by cyclic AMP (EPAC) pathways. Exp Cell Res. 2005;309:161–173. doi: 10.1016/j.yexcr.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ, Nguyen PV. Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem. 2008;15:403–411. doi: 10.1101/lm.830008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlo S, Verdood P, Hooghe-Peters E, Kooijman R. Multiple cAMP-induced signaling cascades regulate prolactin expression in T cells. Cell Mol Life Sci. 2006;63:92–99. doi: 10.1007/s00018-005-5433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giembycz MA, Newton R. Beyond the dogma: novel β2-adrenoceptor signaling in the airways. Eur Respir J. 2005;27:1286–1306. doi: 10.1183/09031936.06.00112605. [DOI] [PubMed] [Google Scholar]
- Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Taskén K, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocytes repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R, Meurs H, Schmidt M. The GSK-3/β-catenin-signalling axis in smooth muscle and its relationship with remodelling. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:185–191. doi: 10.1007/s00210-008-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandoch M, Bujok V, Fleckenstein D, Schmidt M, Fischer JW, Weber A-A. Epac inhibits apoptosis of human leukocytes. J Leukoc Biol. 2009a;86:847–849. doi: 10.1189/jlb.0109048. [DOI] [PubMed] [Google Scholar]
- Grandoch M, López de Jesús M, Oude Weernink PA, Weber A-A, Jakobs KH, Schmidt M. B cell receptor-induced grwoth arrest and apoptosis in WEH-231 immature B lymphoma cells involve cyclic AMP and Epac proteins. Cell Signal. 2009b;21:609–621. doi: 10.1016/j.cellsig.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Haag S, Warnken M, Juergens UR, Racke K. Role of Epac1 in mediating anti-proliferative effects of prostanoid EP(2) receptors and cAMP in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:617–630. doi: 10.1007/s00210-008-0334-3. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol. 2003;137:209–222. doi: 10.1016/s1569-9048(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol. 1996;270:L1040–L1051. doi: 10.1152/ajplung.1996.270.6.L1040. [DOI] [PubMed] [Google Scholar]
- Halliday G, Robinson SR, Shepherd C, Kril J. Alzheimer's disease and inflammation: a review of cellular and therapeutic mechanisms. Clin Experiment Pharmacol Physiol. 2000;27:1–8. doi: 10.1046/j.1440-1681.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- Han L, Stope MB, López de Jesús M, Oude Weernink PA, Urban M, Wieland T, et al. Direct stimulation of receptor-controlled phospholipase D1 by phospho-cofilin. EMBO J. 2007;26:4189–4202. doi: 10.1038/sj.emboj.7601852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- Harper SM, Wienk H, Wechselberger RW, Bos JL, Boelens R, Rehmann H. Structural dynamics in the activation of Epac. J Biol Chem. 2008;283:6501–6508. doi: 10.1074/jbc.M707849200. [DOI] [PubMed] [Google Scholar]
- Hirst SJ. Airway smooth muscle as a target in asthma. Clin Exp Allergy. 2000;30(Suppl 1):54–59. [PubMed] [Google Scholar]
- Hirst SJ. Regulation of airway smooth muscle cell immunomodulatory function: role in asthma. Respir Physiol Neurobiol. 2003;137:309–326. doi: 10.1016/s1569-9048(03)00155-1. [DOI] [PubMed] [Google Scholar]
- Hochbaum D, Hong K, Barila G, Ribeiro-Neto F, Altschuler DL. Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis. J Biol Chem. 2008;283:4464–4468. doi: 10.1074/jbc.C700171200. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Airway inflammation and remodeling in asthma: current concepts. Mol Biotechnol. 2002;22:179–189. doi: 10.1385/MB:22:2:179. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. Epac-selelctive cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2007;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Doebele RC, Lingen MW, Quilliam LA, Tang W-J, Rosner MR. Anthrax edema toxin inhibits endothelial cell chemotaxis via Epac and Rap1. J Biol Chem. 2007;282:19781–19787. doi: 10.1074/jbc.M700128200. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Zhang J-S, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, et al. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi N, Langeberg LK, Scott JD. Distinct enyzme combinations in AKAP signalling complexes permit functional diversity. Nat CellBiol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–L413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:482–489. doi: 10.1165/rcmb.2008-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston E, Lynch MJ, Mohamed A, Collins DM, Hill EV, MacLeod R, et al. Epac and PKA allow cAMP dual control over DNA-PK nuclear translocation. Proc Natl Acad Sci USA. 2008;105:12791–12796. doi: 10.1073/pnas.0805167105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53:129–136. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jing H, Yen JH, Ganea D. A novel signaling pathway mediates the inhibition of CCL3/4 expression by prostaglandin E2. J Biol Chem. 2004;279:55176–55186. doi: 10.1074/jbc.M409816200. [DOI] [PubMed] [Google Scholar]
- Kammer GM. The adenylate cyclase-camp-protein kinase a pathway and regulation of the immune-response. Immunol Today. 1988;9:222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kassel KM, Wyatt TA, Panettieri RA, Jr, Toews ML. Inhibition of human airway smooth muscle cell proliferation by beta 2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol. 2008;294:L131, L138. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Keiper M, Stope MB, Szatkowski D, Bohm A, Tysack K, Vom DF, et al. Epac- and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S, et al. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell. 2005;16:5639–5648. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinderman FS, Kim C, van Daake S, Ma Y, Pham BQ, Spraggon G, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Stolovitzky GA, Mattioli M, Cattoretti G, Husson H, Freedman A, et al. Gene expression profiling of B cell chronic lymphotic leukemia reveals a homogeneous phenotype related to memory B-cells. J Exp Med. 2001;194:1625–1638. doi: 10.1084/jem.194.11.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K, Ishii G, Miyamoto S, Goya M, Zhang SC, Sangai T, et al. Laminin 5 expression protects against anoikis at aerogenous spread and lepidic growth of human lung adenocarcinoma. Int J Cancer. 2005;116:876–884. doi: 10.1002/ijc.21136. [DOI] [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Williams R, Stephens L, Hawkins PT. ARAP3 is a PI3K- and Rap-regulated GAP for RhoA. Curr Biol. 2004;14:1380–1384. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Kwak H-J, Park K-M, Choi H-E, Chung K-S, Lim H-J, Park H-Y. PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal. 2008;20:803–814. doi: 10.1016/j.cellsig.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Laxman S, Riechers A, Sadilek M, Schwede F, Beavo JA. Hydrolysis products of cAMP analogs cause transformation of Trypanosoma brucei from slender to stumpy-like forms. Proc Natl Acad Sci USA. 2006;103:19194–19199. doi: 10.1073/pnas.0608971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechêne P, Mazet J-L, et al. Spatiotemporal dymanics of β-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes. Circ Res. 2008;102:1091–1100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- Li Y, Asuri S, Rebhun JF, Castro AF, Paranavitana NC, Quilliam LA. The RAP1 guanine nucleotide exchange factor Epac2 couples cyclic AMP and Ras signals at the plasma membrane. J Biol Chem. 2006;281:2506–2514. doi: 10.1074/jbc.M508165200. [DOI] [PubMed] [Google Scholar]
- Li Y, Konings IBM, Zhao J, Price LS, de Heer E, Deen PMT. Renal expression of exchange protein directly activated by cAMP (Epac)1 and 2. Am J Physiol Renal Physiol. 2008;295:F525, F533. doi: 10.1152/ajprenal.00448.2007. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, et al. A role of PI-3-kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin SL, Johnson-Farley NN, Lubinsky DR, Cowen DS. Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J Neurochem. 2003;87:1076–1085. doi: 10.1046/j.1471-4159.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Takahashi M, Li Y, Song S, Dillon TJ, Shinde U, et al. Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol. 2008;28:7109–7125. doi: 10.1128/MCB.01060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Jesús M, Stope MB, Oude Weernink PA, Mahlke Y, Börgermann C, Ananaba VN, et al. Cyclic AMP-dependent and Epac-mediated activation of R-Ras by G protein-coupled receptors leads to phospholipase D stimulation. J Biol Chem. 2006;281:21837–21847. doi: 10.1074/jbc.M604156200. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol. 2006;80:1542–1552. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, Hordijk PL. cAMP signaling in leukocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2007a;27:1014–1022. doi: 10.1161/ATVBAHA.106.132282. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, van Stalborch A-MD, van Sterkenburg MAJA, Hiemstra PS, Hordijk PL. Microtubule dynamics and Rac-1 signaling independently regulate barrier function in lung epithelial cells. Am J Respir Cell Mol Biol. 2007b;293:L1321, L1331. doi: 10.1152/ajplung.00443.2006. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, Kooistra MRH, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration though parallel and independent pathways. Eur J CellBiol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Therap. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lyle KS, Raaijmakers JH, Bruinsma W, Bos JL, de RJ. cAMP-induced Epac-Rap activation inhibits epithelial cell migration by modulating focal adhesion and leading edge dynamics. Cell Signal. 2008;20:1104–1116. doi: 10.1016/j.cellsig.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Ma N, Abel T, Hernadez PJ. Exchange protein activated by cAMP enhances long-term memory formation independent of protein kinase A. Learn Mem. 2009;23:367–370. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- Martinez M, Fernandez E, Frank A, Guaza C, de la Feunte M, Hernanz A. Increased cerebrospinal fluid cAMP levels in Alzheimer's disease. BrainRes. 1999;846:265–267. doi: 10.1016/s0006-8993(99)01981-2. [DOI] [PubMed] [Google Scholar]
- Mazhab-Jafari MT, Das R, Fotheringham SA, SilDas S, Chowdhury S, Melacini G. Understanding cAMP-dependent allostery by NMR spectroscopy: comparative analysis of the Epac1 cAMP-binding domain in its apo and cAMP-bound state. J Am Chem Soc. 2007;129:14482–14492. doi: 10.1021/ja0753703. [DOI] [PubMed] [Google Scholar]
- McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, et al. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer's disease. Biochem Soc Trans. 2005;33:1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recogniyion of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- Métrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, et al. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- Misra UK, Kaczowka S, Pizzo SV. The cAMP-activated GTP exchange factor, Epac1 upregulates plasma membrane and nuclear Akt kinase activities in 8-pCPT-2-O-Me-cAMP-stimulated macrophages: gene silencing of the cAMP-activated GTP exchange Epac1 prevents 8-pCPT-2-O-cAMP activation of Akt activity in macrophages. Cell Signal. 2008;20:1459–1470. doi: 10.1016/j.cellsig.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Coordinate regulation of forskolin-induced cellular proliferation in macrophages by protein kinase A/cAMP-response element-binding protein (CREB) and Epac1-Rap1 signaling: effects of silencing CREB gene expression on Akt activation. J Biol Chem. 2005;280:38276–38289. doi: 10.1074/jbc.M507332200. [DOI] [PubMed] [Google Scholar]
- Monroe JG. Balancing signals for negative selection and activation of developing B lymphocytes. Clin Immunol. 2000;95:S8, S13. doi: 10.1006/clim.1999.4817. [DOI] [PubMed] [Google Scholar]
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier ALAM, et al. cAMP-binding protein Epac induces cardiomyocytes hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Zayed MA, Evans A, Parker CE, Ataga KI, Telen MJ, et al. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood. 2005;105:3322–3329. doi: 10.1182/blood-2004-07-2881. [DOI] [PubMed] [Google Scholar]
- Murray AJ, Shewan DA. Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol Cell Neurosci. 2008;38:578–588. doi: 10.1016/j.mcn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Newell MK, VanderWall J, Beard KS, Freed JH. Ligation of major histocompatibility complex class II molecules mediates apoptotic cell death in resting B lymphoctes. Proc Natl Acad Sci USA. 1993;90:10459–10463. doi: 10.1073/pnas.90.22.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol. 2009;219:652–658. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- Nijholt IM, Dolga AM, Ostroveanu A, Luiten PGM, Schmidt M, Eisel ULM. Neuronal AKAP150 coordinates PKA and Epac mediated PKB/Akt phosphorylation. Cell Signal. 2008;20:1715–1724. doi: 10.1016/j.cellsig.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünnemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- Nunes RO, Schmidt M, Dueck G, Baarsma H, Halayko AJ, Kerstjens HAM, et al. GSK-3/β-catenin signaling axis in ariway smooth muscle: role in mitogenic signaling. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1110, L1118. doi: 10.1152/ajplung.00500.2007. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hasings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, et al. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- Oude Weernink PA, López de Jesús M, Schmidt M. Phospholipase D signaling: orchestration of PIP2 and small GTPases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:399–411. doi: 10.1007/s00210-007-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang M, Zhang L, Zhu J, Schwede F, Thomas SA. Epac signaling is required for hippocampal dependent memory retrieval. Proc Natl Acad Sci USA. 2008;105:11993–11997. doi: 10.1073/pnas.0804172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, et al. cAMP-GEFII is a direct target of cAMP in regulated excocytosis. Nat CellBiol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Panettieri RA., Jr Airway smooth muscle: immunomodulatory cells that modulate airway remodeling? Respir Physiol Neurobiol. 2003;137:277–293. doi: 10.1016/s1569-9048(03)00153-8. [DOI] [PubMed] [Google Scholar]
- Pannekoek W-J, Kooistra MRH, Zwartkruis FJT, Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction mol. Annu Rev Pharmacol Toxicol. 2008a;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane rafts and caveolae microdomains. Handb Exp Pharmacol. 2008b;186:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, et al. The role of GSK-3 in synaptic plasticity. Brit J Pharmacol. 2008;153:S428, S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier N, Boudreau F, Yu SJ, Zannoni S, Boulanger V, Asselin C. Activation of haptoglobin gene expression by cAMP involves CCAAT/enhancer-binding protein isoforms in intestinal epithelia cells. FEBS Lett. 1998;439:275–280. doi: 10.1016/s0014-5793(98)01388-x. [DOI] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, et al. Detecting cAMP-induced Epac activation by fluorescent resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponsioen B, Gloereich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol. 2009;29:2521–2531. doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang X-B, Christensen AE, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods. 2008;5:277–278. doi: 10.1038/nmeth0408-277. [DOI] [PubMed] [Google Scholar]
- Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- Raaijmakers JH, Bos JL. Specificity in Ras and Rap signaling. J Biol Chem. 2009;284:10995–10999. doi: 10.1074/jbc.R800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke K, Haag S, Bahulayan A, Warnken M. Pulmonary fibroblasts, an emerging target for anti-obstructive drugs. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:193–201. doi: 10.1007/s00210-008-0264-0. [DOI] [PubMed] [Google Scholar]
- Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de RJ, Price LS, Schwede F, et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond DR, Wilson LS, Carter RL, Maurice DH. Numerous PKA-, or EPAC-based, signalling complexes allow selectvie phosphodiesterase 3 and phosphodiesterase 4 coordination of cell adhesion. Cell Signal. 2007;19:2507–2518. doi: 10.1016/j.cellsig.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL. Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature. 2008;455:124–127. doi: 10.1038/nature07187. [DOI] [PubMed] [Google Scholar]
- Remington TL, DiGiovine B. Long-acting β-agonists: anti-inflammatory properties and synergy with corticosteroids in asthma. Curr Opin Pulm Med. 2004;11:74–78. doi: 10.1097/01.mcp.0000146784.56834.ff. [DOI] [PubMed] [Google Scholar]
- Rogers J. The inflammatory response in Alzheimer's disease. J Periodontol. 2008;79:1535–1543. doi: 10.1902/jop.2008.080171. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–357. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- Rossi AG, McCutcheon JC, Roy N, Chilvers ER, Haslett C, Dransfield I. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. J Immunol. 1998;160:3562–3568. [PubMed] [Google Scholar]
- Rovere P, Inverardi L, Bender JR, Pardi R. Feedback modulation of ligand-engaged alpha L/beta(2), leukocyte integrin (LFA-1) by cyclic AMP-dependent protein kinase. J Immunol. 1996;156:2273–2279. [PubMed] [Google Scholar]
- Rowe J, FinlayJones JJ, Nicholas TE, Bowden J, Morton S, Hart PH. Inability of histamine to regulate TNF-alpha production by human alveolar macrophages. Am J Respir Cell Mol Biol. 1997;17:218–226. doi: 10.1165/ajrcmb.17.2.2722. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Sands WA, Woolson HD, Milne GR, Rutherford C, Palmer TM. Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells. Mol Cell Biol. 2006;26:6333–6346. doi: 10.1128/MCB.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma D, Roscioni SS, Meurs H, Schmidt M. Monomeric G-proteins as signal transducers in airway physiology and pathophysiology. Cell Signal. 2008;20:1705–1714. doi: 10.1016/j.cellsig.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- Scheibner KA, Boodoo S, Collins S, Black KE, Chan-Li Y, Zarek P, et al. The adenosine A2a receptor inhibits matrix induced inflammation in a novel fashion. Am J Respir Cell Mol Biol. 2008;40:251–259. doi: 10.1165/rcmb.2008-0168OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Woodgett JR. PKB/Akt: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–766. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Oude Weernink PA, vom Dorp F, Rehmann H, Lomasney JW, et al. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nature Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sand C, Jakobs KH, Michel MC, Oude Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol. 2007a;7:193–200. doi: 10.1016/j.coph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Elzinga CRS, Bos IST, Beemsterboer JJ, Warnders FJ, van den Hoff MJB, et al. A key role for Epac in airway development and biology. Am Respir Crit Care Medi. 2007b;175:A524. c. [Google Scholar]
- Schmierer B, Hill CS. TGF-β-Smad signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–982. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- Sebzda E, Bracke M, Tugal T, Hoog N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP. Master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. Role for A-kinase anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration – the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Staples KJ, Bergmann M, Tomita K, Houslay MD, McPhee I, Barnes PJ, et al. Adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent inhibition of IL-5 from human T lymphocytes is not mediated by the cAMP-dependent protein kinase A. J Immunol. 2001;167:2074–2080. doi: 10.4049/jimmunol.167.4.2074. [DOI] [PubMed] [Google Scholar]
- Ster J, De BF, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, et al. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proc Natl Acad Sci USA. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ster J, de Bock F, Bertaso F, Abitbol K, Daniel H, Bockaert J, et al. Epac mediates PACAP-dependetn long-term depression in the hippocampus. J Physiol. 2009;587:101–113. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez CJ, Parker NJ, Finn PW. Innate immune mechanism in allergic asthma. Curr Allergy Asthma Rep. 2008;8:451–459. doi: 10.1007/s11882-008-0085-8. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007a;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. β2 adrenergic receptor activation stimulates pro-inflammtory cytokine production in macrophages via PKA- and NF-κb-independent mechanisms. Cell Signal. 2007b;19:251–260. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Cheng CY, Brown SHJ, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta. 2008;1784:16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Felekkis K, Moon E-Y, Flies A, Sherr DH, Lerner A. Among circulating hematopoietic cells, B-CLL uniquely expresses functional EPAC1, but EPAC1-mediated Rap1 activation does not account for PDE4 inhibitor-induced apotosis. Blood. 2004;103:2661–2667. doi: 10.1182/blood-2003-06-2154. [DOI] [PubMed] [Google Scholar]
- Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662, H1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- van Oirschot BA, Stahl M, Lens SMA, Medema RH. Protein kinase a regulates expression of p27(kip1) and cyclin D3 to suppress proliferation of leukemic T cell lines. J Biol Chem. 2001;276:33854–33860. doi: 10.1074/jbc.M104395200. [DOI] [PubMed] [Google Scholar]
- Vestweber D. Regulation of endothelial cell contacts during leukocyte extravasation. Curr Opin Cell Biol. 2002;14:587–593. doi: 10.1016/s0955-0674(02)00372-1. [DOI] [PubMed] [Google Scholar]
- Vivier E, Malissen B. Innate and adaptive immunity: specificities and signaling hierachies revisited. Nat Immunol. 2005;6:17–21. doi: 10.1038/ni1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliem MJ, Ponsioen B, Schwede F, Pannekoek W-J, Riedl J, Kooistra MRH, et al. 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chem Bio Chem. 2008;9:2052–2054. doi: 10.1002/cbic.200800216. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, et al. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26:2130–2145. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Gu Y, Li GW, Huang LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. J Physiol. 2007;584:191–203. doi: 10.1113/jphysiol.2007.135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, et al. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32:135–141. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener E, Scarpa A. Activation of protein kinase-C modulates the adenylate-cyclase effector system of lymphocytes-B. J Biol Chem. 1989;264:4324–4328. [PubMed] [Google Scholar]
- Wilson HL, Roesler WJ. CCAAT/enhancer binding proteins: do they possess intrinsic cAMP-inducible activity? Mol Cell Endocrinol. 2002;188:15–20. doi: 10.1016/s0303-7207(01)00754-7. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL, Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano N, Suzuki D, Endoh M, Zhao TC, Padbury JF, Tseng Y-T. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J Biol Chem. 2007;282:18819–18830. doi: 10.1074/jbc.M610537200. [DOI] [PubMed] [Google Scholar]
- Yarwood SJ, Borland G, Sands WA, Palmer TM. Identification of CCAAT/enhancer-binding proteins as exchange protein activated by cAMP-activated transcription factors that mediate the induction of the SOCS-3 gene. J Biol Chem. 2008;283:6843–6853. doi: 10.1074/jbc.M710342200. [DOI] [PubMed] [Google Scholar]
- Yokoyama U, Minamisawa S, Quan H, Akaike T, Jin M, Otsu K, et al. Epac1 is upregulated during neointima formation and promotes vascular smooth muscle migration. Am J Physiol Heart Circ Physiol. 2008a;295:H1547, H1555. doi: 10.1152/ajpheart.01317.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama U, Minamisawa S, Quan H, Akaike T, Suzuki S, Jin M, et al. PGE2-activate EPAC promotes neointimal cushion formation of the rat ductus arteriosus by a process distinct from that of PKA. J Biol Chem. 2008b;283:28702–28709. doi: 10.1074/jbc.M804223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA. 2008c;105:6386–6391. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, et al. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci USA. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler R, Csanady M, Gires O, Lang S, Schmitt B, Wollenberg B. Tumor cell-derived prostaglandin E2 inhibits monocyte function by interfering with CCR5 and Mac-1. Faseb J. 2000;14:661–668. doi: 10.1096/fasebj.14.5.661. [DOI] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. cAMP acts on exchange protein activated by cAMP/cAMP-regulated guanine nucleotide exchange protein to regulate transmitter release at the crayfish neruomuscular junction. J Neurosci. 2005;25:208–214. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Zhao M, van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]


