Abstract
The antitumor activity of chemotherapeutic nitrogen mustards including chlorambucil, cyclophosphamide, and melphalan, is commonly attributed to their ability to induce DNA-DNA cross-links by consecutive alkylation of two nucleophilic sites within the DNA duplex. DNA-protein cross-linking by nitrogen mustards is not well characterized, probably because of its inherent complexity and the insufficient sensitivity of previous methodologies. If formed, DNA-protein conjugates are likely to contribute to both target and off-target cytotoxicity of nitrogen mustard drugs. Here we show that the DNA repair protein, O6-alkylguanine DNA alkyltransferase (AGT), can be readily cross-linked to DNA in the presence of nitrogen mustards. Both chlorambucil and mechlorethamine induced the formation of covalent conjugates between 32P-labeled double-stranded oligodeoxynucleotides and recombinant human AGT protein, which were detected by SDS-PAGE. Capillary HPLC-electrospray ionization mass spectrometry (ESI-MS) analysis of AGT that had been treated with guanine half mustards of chlorambucil or mechlorethamine revealed the ability of the protein to form either one or two cross-links to guanine. C145A AGT - a variant containing a single point mutation in the protein’s active site - was found capable of forming a single guanine conjugate, while cross-linking was virtually abolished upon treatment of the C145A/C150S AGT double mutant with the guanine half mustards. HPLC-ESI+-MS/MS sequencing of the tryptic peptides obtained from the wild type AGT protein that had been treated with nitrogen mustards in the presence of DNA confirmed that the cross-linking took place between the N7 position of guanine in DNA and two active site residues within the AGT protein (Cys145 and Cys150). The exact chemical structures of AGT-DNA cross-links induced by chlorambucil and mechlorethamine were identified as N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbuyric acid and N-(2-[Scysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)methylamine, respectively, based upon HPLC-MS/MS analysis of protein hydrolysates in parallel with the corresponding amino acid conjugates prepared synthetically. Mechlorethamine-induced AGT-DNA conjugates were isolated from protein extracts of AGT-expressing CHO cells, but not control cells, demonstrating that nitrogen mustards can cross-link the AGT protein to DNA in the presence of other nuclear proteins. Because AGT is overexpressed in many tumor types, further investigations of potential role of AGT-DNA cross-linking in the antitumor and mutagenic activity of antitumor nitrogen mustards is warranted.
Introduction
Nitrogen mustard drugs are broadly used in the clinic against a variety of neoplastic conditions, including lymphoma, leukemia, multiple myeloma, and ovarian carcinoma. These drugs can react with nucleophilic sites within DNA or proteins, giving rise to DNA-DNA and DNA-protein cross-links. DNA-DNA cross-linking by nitrogen mustards is well characterized and is thought to be largely responsible for their cytotoxic effects (1;2). In contrast, DNA-protein cross-linking by nitrogen mustards is not well studied, despite their potential role in cytotoxicity and/or mutagenicity. Many other bis-electrophiles including 1,2-dibromoethane (DBE), 1,2,3,4-diepoxybutane (DEB), platinum drugs, and dialhehydes, induce covalent cross-links between DNA and DNA binding proteins (histones, DNA repair proteins, and transcription factors) (3;4), raising the prospect that similar lesions may result from nitrogen mustard chemotherapy. If not repaired, DNA-protein cross-links can cause the blockage of the replication fork, accumulation of mutations, and the induction of pro-apoptotic processes (4).
One human protein that is readily cross-linked to DNA in the presence of bis-electrophiles is the DNA repair protein O6-alkylguanine DNA alkyltransferase (AGT) (5;6). The expression of human AGT in bacteria enhances the cytotoxic and mutagenic effects of DBE and DEB (5), presumably due to the formation of covalent AGT-DNA cross-links (5;6). The normal physiological function of AGT protein is to transfer the O6-alkyl group from promutagenic O6-alkylguanine lesions in DNA to an active site cysteine (Cys145 in the human protein), restoring normal guanine and preventing mutagenesis (7). Our present study employed a combination of mass spectrometry, site specific mutagenesis, and affinity capture techniques to demonstrate that antitumor nitrogen mustards can form covalent cross-links between the active site residues of AGT and DNA. We further show that this cross-linking reaction is not inhibited by free cysteine and takes place in the presence of other cellular proteins.
Materials and Methods
Chemicals and Reagents
Mechlorethamine hydrochloride and chlorambucil were purchased from Sigma-Aldrich (Milwaukee, WI). l-Cysteine and Boc-l-Cys-OH were obtained from Fluka (Buchs, Switzerland), and Boc-Lys-OMe was purchased from Bachem (King of Prussia, PA). 2'-Deoxyguanosine was obtained from TCI America (Portland, OR). Guanine half mustards of chlorambucil and mechlorethamine, N-(2-chloroethyl)-N-[2-(guan-7-yl)ethyl]-p-aminophenylbutyric acid (N7G-PBA-Cl) and N-(2-chloroethyl)-N-[2-(guan-7-yl)ethyl]methylamine (N7G-EMA-Cl), were prepared by reacting 2'-deoxyguanosine with the corresponding mustards as described in the Supporting Information (S-1). Amino acid-guanine conjugates of chlorambucil and mechlorethamine, N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbuyric acid (Cys-N7G-PBA), N-[2-[S-cysteinyl]ethyl]-N-[2-(guan-7-yl)ethyl]methylamine (Cys-N7G-EMA), and N-[2-[N-(lysyl)ethyl]-N-[2-(guan-7-yl)ethyl]methylamine (Lys-N7G-EMA), were prepared by reacting the corresponding protected amino acids with N7G-EMA-Cl or N7G-PBA-Cl, followed by deprotection and HPLC purification (Supporting Information S-1). [γ-32P]-ATP was from Perkin-Elmer (Boston, MA), and T4 polynucleotide kinase was obtained from New England Biolabs (Beverly, MA). Trypsin, carboxypeptidase Y, and proteinase K were purchased from Worthington Biochemical Corporation (Lakewood, NJ). Synthetic peptide GNPVPILIPCHR (which corresponds to positions 136–147 of AGT) and all synthetic DNA oligodeoxynucleotides were prepared at the University of Minnesota Microchemical Facility (Minneapolis, MN). Bovine histone H4 was purchased from Roche Applied Science (Indianapolis, IN). Human recombinant AGT proteins (C-terminal histidine-tagged wild type and N-terminal histidine-tagged C145A and C145A/C150S mutants) were prepared as described previously (8;9). Mouse monoclonal antibody specific for AGT (MGMT, clone MT3.1) was purchased from Millipore (Temecula, CA), and the alkaline phosphatase-conjugated anti-mouse IgG secondary antibody was obtained from Sigma (St. Louis, MO). Streptavidin sepharose high performance beads were purchased from GE Healthcare (Piscataway, NJ), and proteomics grade trypsin (Trypsin Gold) was obtained from Promega (Madison, WI).
Denaturing PAGE of Nitrogen Mustard-Induced DNA-Protein Cross-Links
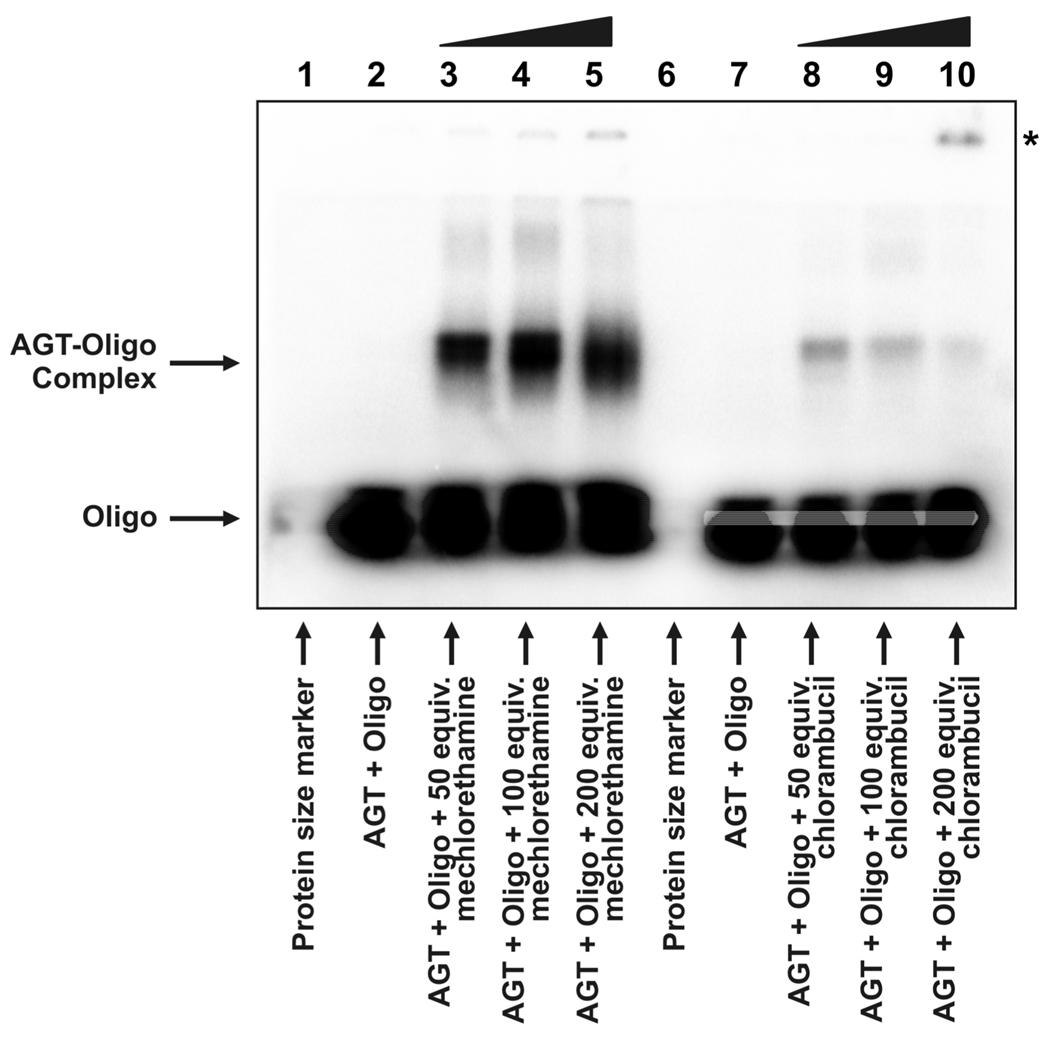
DNA 18-mer, 5'-GGAGCTGGTGGCGTAGGC-3' (200 pmol), was 5'-end labeled with 32P in the presence of [γ-32P]ATP and T4 polynucleotide kinase, purified by 12% denaturing PAGE, and desalted by SPE. The 32P-labeled duplex (0.93 nmol) was incubated with human recombinant AGT or Histone H4 (2.0 µg) in the presence of 50–200 molar equivalents of mechlorethamine or chlorambucil (4.7, 9.3, and 18.6 nmol respectively) for 3 h at 37°C. The reaction mixtures were separated by 12% SDS-PAGE, and the radiolabeled products were visualized using a Bio-Rad Molecular Imager FX. Nitrogen mustard-induced DNA-protein cross-linking was quantified by volume analysis employing Bio-Rad Quantity One Software, with cross-linking efficiency determined by the relative intensity of the reduced-mobility band versus the band corresponding to single-stranded DNA.
Reactions of Synthetic Peptide GNPVPILIPCHR with Guanine Half Mustards
Synthetic peptide GNPVPILIPCHR (76.1 nmol), representing residues 136–147 of WT AGT protein, was reacted with 5 equivalents of N7G-PBA-Cl or N7G-EMA-Cl (380.5 nmol) in 10 mM TRIS-HCl buffer, pH 7.2, at 37°C for 2 h, followed by HPLC-ESI+-MS/MS analysis as described below.
Reaction of Guanine Half Mustards with Recombinant AGT and Histone Proteins
Purified recombinant wild type AGT, its variants (C145A AGT and C145A/C150S AGT), or histone H4 (3.4 nmol) were incubated with N7G-PBA-Cl or N7G-EMA-Cl (85.8 nmol) in 10 mM TRIS-HCl buffer (pH 7.2) for 3 h at 37°C. In competition experiments, 100 and 500 molar equivalents l-cysteine (0.34 and 1.7 µmol respectively) was added. The alkylated proteins were subjected to HPLC-ESI+-MS analysis.
Tryptic digestion of alkylated proteins
Control or alkylated protein (~70 µg) was digested with trypsin (7.0 µg) in 100 mM ammonium bicarbonate buffer (pH 7.9) for 24 h at 37°C. Samples were dried, re-dissolved in 50 µL of 0.5% formic acid/0.01% TFA, and subjected to analysis by HPLC-ESI+-MS/MS.
Total digestion of alkylated proteins to amino acids
Tryptic peptides (from ~50 µg protein) were filtered through Microcon YM-10 membrane filters to remove trypsin. Carboxypeptidase Y (1.0 µg) and proteinase K (1.0 µg) were added to the filtrate, and proteolysis proceeded at room temperature for 24 h. Samples were dried and reconstituted in 15 mM ammonium acetate buffer (pH 5.0) prior to HPLC-ESI+-MS/MS analysis.
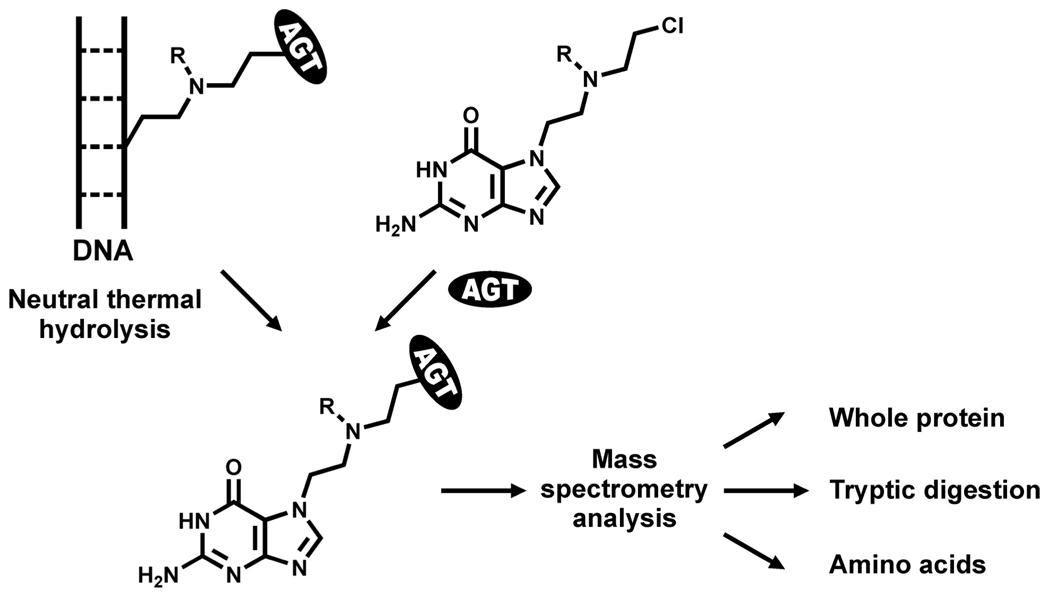
AGT Cross-Linking to Double-Stranded Oligodeoxynucleotides in the Presence of Chlorambucil and Mechlorethamine
Synthetic DNA 18-mer duplexes (5'-GGAGCTGGTGGCGTAGGC-3', + strand) were incubated with recombinant AGT (45 µg, 2.1 nmol) in the presence of mechlorethamine or chlorambucil (200 nmol) in 10 mM Tris-HCl buffer (pH 7.2) for 3 h at 37°C. Samples were heated at 70°C for 1 h to release N7-alkylated guanines, followed by tryptic digestion as described for half mustard-treated proteins and HPLC-ESI+-MS/MS analysis.
Mass Spectrometry
HPLC-ESI+-MS analysis of alkylated proteins was performed using an Agilent 1100 capillary HPLC-ion trap MS system operated in the ESI+ mode. Spectra were obtained by performing full scan MS within the m/z range of 100–1500. Separation was achieved with a Zorbax 300 SB-C3 column (150 mm × 0.5 mm, 5 um) eluted at a flow rate of 15 µL/min. The column was eluted with a gradient of 0.05% TFA in water (A) and 0.05% TFA in acetonitrile (B). Solvent composition was held at 30% B for the first 3 min, followed by a linear increase to 80% B in 20 min, and further to 90% B in 5 min.
HPLC-ESI+-MS/MS analysis of tryptic peptides was performed using an Agilent 1100 capillary HPLCion trap MS system. Chromatographic separation was achieved using a Zorbax SB-C18 column (150 mm × 0.5 mm, 5 µm) eluted at a flow rate of 15 µL/min. The mobile phase consisted of 0.5% formic acid/0.01% TFA in water (A) and 0.5% formic acid/0.01% TFA in acetonitrile (B). The solvent composition was held at 3% B for the first 3 min, increased to 5% B over 7 min, held at 5% B for 10 min, increased to 35% B in 95 min, and further to 75% B in 10 min. The mass spectrometer was operated in ESI+ mode, and Auto MS2 was used to select and fragment the doubly-charged ions at m/z 658.4 (unmodified peptide G136NPVPILIPCHR147), m/z 849.6 (chlorambucil-induced guanine cross-link to G136NPVPILIPCHR147), m/z 775.4 (mechlorethamine-induced guanine cross-link to G136NPVPILIPCHR147), m/z 834.4 (unmodified peptide V148VCSSGAVGNYSGGLAVK165), m/z 1025.9 (chlorambucil-induced guanine cross-link to V148VCSSGAVGNYSGGLAVK165), and m/z 951.4 (mechlorethamine-induced guanine cross-link to V148VCSSGAVGNYSGGLAVK165).
HPLC-ESI+-MS/MS analysis of amino acid-guanine cross-links present in total digests of alkylated proteins was performed using an Agilent 1100 capillary HPLC-ion trap MS system operated in ESI+ mode. Auto MS2 was used to isolate and fragment the [M + H]+ ions of N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbutyric acid (Cys-N7G-PBA, m/z 504.2), N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)methylamine (Cys-N7G-EMA, m/z 356.2), and the corresponding conjugates to arginine, tyrosine, lysine and histidine. Chlorambucil-induced conjugates were separated using a Synergi C18 column (250 mm × 0.5 mm, 4 µm) eluted at a flow rate of 10 µL/min. The mobile phase consisted of 15 mM ammonium acetate, pH 5.0 in water (A) and acetonitrile (B), with a linear gradient of 2–65% B over the course of 30 min at 10°C. For separation of mechlorethamine-induced amino acid-guanine, solvent composition was held at 2% B for 15 min, followed by a linear increase to 30% B over the next 15 min.
Detection of Nitrogen Mustard-Induced AGT-DNA Cross-Links in Nuclear Protein Extracts
Chinese hamster ovary cells expressing human recombinant AGT (CHO-AGT) and their wild-type/empty vector equivalent (CHO-EV) (10) were maintained as exponentially growing monolayer cultures in α-MEM supplemented with 9% FBS and G418 (1 mg/mL) in a humidified incubator at 37°C with 5% CO2. Nuclear protein extracts from both cell lines were prepared as previously described (11) and dialyzed overnight at 4°C against 10 mM TRIS-HCl/10 mM KCl/10 mM MgCl2 (pH 7.4) using Slide-A-Lyzer dialysis cassettes (3.5 kDa molecular weight cut-off) from Pierce (Rockford, IL). Protein concentrations were determined via colorimetric assay (12).
5'-Biotinylated double-stranded oligodeoxynucleotides (5'-GGAGCTCGTGGCCTA-3' (+) strand, 3.12 nmol) were combined with the CHO-AGT nuclear extract (0.5 mg) in 10 mM TRIS-HCl – pH 7.4 in the absence and presence of mechlorethamine (100, 250, 500, 750, and 1000 µM). The reaction mixtures were incubated at 37°C for 3 h to induce cross-linking. Biotinylated DNA with any bound proteins was captured on streptavidin sepharose high performance beads. The beads were washed with 0.1% SDS, 4 M urea, and 1 M NaCl to remove any non-covalently attached proteins. Biotinylated DNA containing covalently bound proteins was released from the beads upon the addition of 4X SDS-PAGE loading buffer and heating to 90°C for 15 min. DNA-protein cross-links present in the elutes were separated by 10 or 12% SDS-PAGE, and the presence of AGT was detected by both Western blotting and tandem mass spectrometry.
Immunological experiments employed commercial anti-MGMT, clone MT3.1 (1:500 dilution) and alkaline phosphatase-conjugated anti-mouse IgG (1:10,000 dilution). The amount of AGT present was determined by direct comparison of the band intensity to that of known AGT standards analyzed in parallel. Percent cross-linking was determined by dividing the amount of AGT present in the biotin capture by the total amount of AGT in the reaction mixture.
For mass spectrometric identification of cross-linked proteins, SDS-PAGE gels were stained with SimplyBlue SafeStain (Invitrogen, Carlsbad, CA) and sample lanes were cut into 10 slices comprising the entire molecular weight range. Upon further dicing of the gel pieces into 1 mm pieces and washing with 100 mM ammonium bicarbonate, each fraction was subjected to reduction and alkylation with dithiothreitol and iodoacetaminde as previously described (13). Gel pieces were dehydrated with acetonitrile, dried under vacuum, and reconstituted in 25 mM ammonium bicarbonate containing mass spectrometry grade trypsin (~1 µg). The samples were digested overnight at 37°C. Tryptic peptides were extracted from the gel pieces using 0.1% aqueous formic acid/60% acetonitrile, evaporated to dryness, and resuspended in 0.1% aqueous formic acid. HPLC-ESI+-MS/MS analyses were performed on a Thermo LTQ linear ion trap MS system equipped with a Thermo Surveyor solvent delivery system and a microelectrospray source. Tryptic peptides were resolved on a 100 µm × 11 cm fused silica capillary column packed with 5 µm, 300 Å Jupiter C18 (Phenomenex, Torrence, CA) eluted at 0.6 µL/min with 0.1 formic acid in water (A) and 0.1% formic acid in acetonitrile (B). Solvent composition was kept at 2% B for the first 15 min, followed by a linear increase to 25% B over the next 35 min, and further to 90% B in the next 15 min, and held at 90% B for 10 min. MS/MS spectra of peptides were acquired using data-dependent scanning in which a single full MS spectrum (400–2000 m/z) was followed by four MS/MS spectra. Spectra were recorded using dynamic exclusion of previously analyzed precursors for 60 s. The MS/MS spectra were searched against human and Cricetulus griseus (Chinese hamster) database sequences using the SEQUEST algorithm (14). S-Carboxamidomethylation at cysteine (+57 Da), oxidation of methionine (+16 Da), and mechlorethamine-induced alkylation at cysteine (hydrolyzed monoadduct: +102 Da, guanine cross-link: +234 Da) were specified as dynamic modifications to identify spectra of adducted peptides.
Results
SDS PAGE Detection of Nitrogen Mustard-Induced AGT-DNA Cross-Links
To examine the ability of nitrogen mustards to cross-link AGT protein to DNA, recombinant human AGT and 5'-32P-end labeled DNA duplexes were incubated in the presence of increasing amounts of mechlorethamine or chlorambucil, followed by SDS-PAGE analysis. The results presented in Figure 1 reveal that a slow-migrating species corresponding to an AGT-DNA conjugate was formed when duplex DNA was exposed to nitrogen mustard drugs in the presence of AGT. No such product was formed in control experiments in which either AGT or oligonucleotide was omitted. Cross-linking of AGT to DNA by mechlorethamine displayed concentration-dependence, with increasing amounts of drug resulting in 15 to 25% of recombinant protein being cross-linked to DNA (lanes 3–5). Chlorambucil was less effective at cross-linking AGT to DNA (relative amount of AGT cross-linked by drug: 2–5%, lanes 8–10). Additional low-mobility bands were observed following treatment of AGT DNA mixtures with high concentrations of nitrogen mustards. These appear to represent higher-order complexes of AGT and DNA. In contrast, no conjugates were observed following similar treatment of Histone H4 (Supporting Information S-2).
Figure 1.
Detection of drug-induced AGT-DNA cross-links. Recombinant human AGT protein and radioactive duplex oligodeoxynucleotide (see Materials and Methods) were incubated in the presence of 50 (lanes 3 and 8), 100 (lanes 4 and 9) or 200 (lanes 5 and 10) equivalents of mechlorethamine (lanes 3–5) or chlorambucil (lanes 8–10) and subsequently resolved by 12% SDS-PAGE. Free duplex DNA (labeled 'Oligo') migrated to the bottom of the gel, whereas DNA cross-linked to AGT (labeled 'AGT-Oligo complex') displayed a substantially reduced mobility. The mobility of the higher-order complex of drug and AGT is indicated by an asterisk (see text).
Mass Spectrometry Analysis of Half Mustard-Induced AGT-Guanine Cross-Links: Whole Protein Results
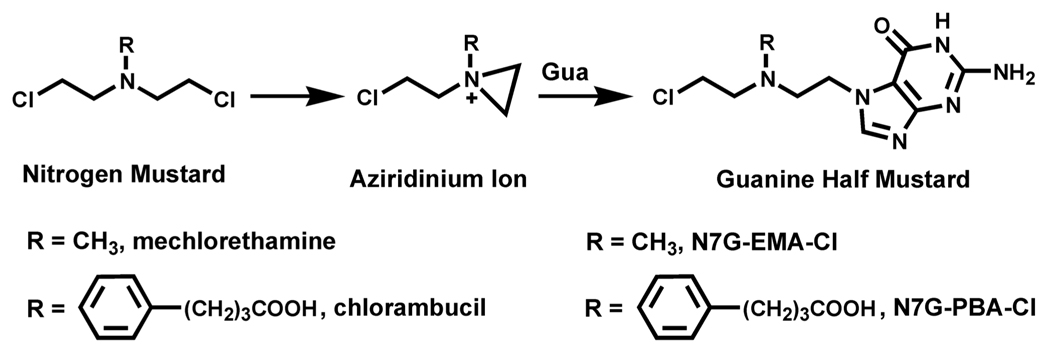
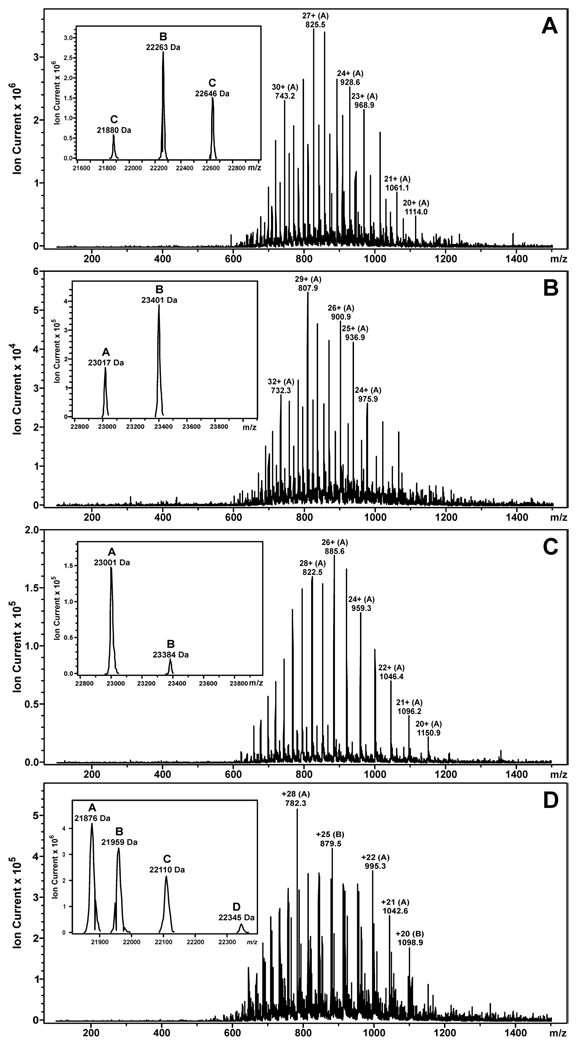
Initial DNA alkylation by mechlorethamine and chlorambucil leads to the formation of N7-guanine half mustards, which retain one of the chloroethyl groups (Scheme 1). These monoadducts can then react with a nucleophilic site within a neighboring protein, giving rise to DNA-protein cross-links. To gain insight into the nature of the nitrogen mustard-induced AGT-DNA linkages, recombinant AGT protein or its variants were incubated with synthetic guanine half mustards of mechlorethamine or chlorambucil as models of monoalkylated DNA, followed by capillary HPLC-ESI+-MS analysis of modified proteins (Scheme 2). When the experiment was performed with the wild type protein, deconvoluted mass spectra of the reaction mixture revealed three protein species: unreacted AGT (M = 21 880 Da), AGT containing a single chlorambucil crosslink to guanine (M = 22 263 Da), and a double chlorambucil cross-link to guanine (M = 22 646 Da) (Fig. 2A). The presence of a large molar excess of l-cysteine (100–500 equivalents) did not inhibit alkylation of AGT by N7G-PBA-Cl, suggesting that this low molecular weight thiol does not protect against the formation of DNA-protein cross-links upon exposure to nitrogen mustards (data not shown). These results were consistent with a previous study in which l-cysteine and glutathione did not prevent the formation of DEB-mediated AGT-DNA cross-links (6).
Scheme 1.
Chemical structures of nitrogen mustards and guanine half mustards employed in this work.
Scheme 2.
Mass Spectrometry-based approach used to characterize AGT-DNA cross-links of antitumor nitrogen mustards.
Figure 2.
ESI+-MS spectra and deconvoluted spectra (inset) of N7G-PBA-Cl and N7G-EMA-Cl treated AGT protein and its variants. (A) Wild type protein: A = unmodified AGT (calculated M = 21 876 Da, observed M = 21 880 Da), B = AGT containing a single chlorambucil cross-link to guanine (calculated M = 22 259 Da, observed M = 22 263 Da), C = AGT containing two chlorambucil cross-links to guanine (calculated M = 22 642 Da, observed M = 22 646 Da). (B) C145A AGT variant: A = unmodified C145A AGT (calculated M = 23 015 Da, observed M = 23 017 Da), B = C145A AGT containing a single chlorambucil cross-link to guanine (calculated M = 23 398 Da, observed M = 23 401 Da). (C) C145A/C150S variant: A = unmodified C145A/C150S AGT (calculated M = 22 996 Da, observed M = 23 001 Da), B = C145A/C150S AGT containing a single chlorambucil cross-link to guanine (calculated M = 23 379 Da, observed M = 23 384 Da). (D) Wild type protein: A = unmodified AGT (calculated M = 21 876 Da, observed M = 21 876 Da), B = AGT containing an intramolecular mechlorethamine cross-link (calculated M = 21 959 Da, observed M = 21 959 Da), C = AGT containing a single mechlorethamine cross-link to guanine (calculated M = 22 110 Da, observed M = 22 110 Da), D = AGT containing two mechlorethamine cross-links to guanine (calculated M = 22 344 Da, observed M = 22 345 Da).
When the same experiment was conducted with a variant form of AGT in which cysteine 145 was replaced by an alanine residue, a single half mustard-modified species was apparent (M = 23 401, mass increase of ~ 384 Da) (Figure 2B), suggesting that one of the cross-linking sites observed within the wild-type protein, namely Cys145, had been removed. Incubation of a third variant of AGT protein in which cysteine residues 145 and 150 had both been mutated (C145A/C150S) with chlorambucil half mustard yielded only trace amounts of cross-linked protein (Figure 2C). The significant differences between the observed molecular weights of the C145A and C145A/C150S AGT variants (M = 23 017 Da and M = 23 001 Da) and the wild type protein (M = 21 880) are due to the presence of an N-terminal histidine tag (MRGSHHHHHHGS).
Similar results were obtained following reaction of wild type AGT with the guanine half mustard of mechlorethamine (Figure 2D), with both single (M = 22 110 Da) and double (M = 22 345 Da) cross-links to guanine observed. A third species of alkylated AGT was also identified (M = 21 959 Da), corresponding to a mechlorethamine-induced intramolecular cross-link involving two amino acid residues within the protein. Taken together, these results support the interpretation that chlorambucil and mechlorethamine cross-link AGT to DNA via cysteine residues 145 and 150. In contrast, no alkylation of histone H4 was observed following exposure to N7G-PBA-Cl or N7G-EMA-Cl (Supporting Information S-3 and S4).
Capillary HPLC-ESI+-MS/MS Analysis of Half Mustard-Induced AGT-Guanine Cross-Links: Peptide Mapping
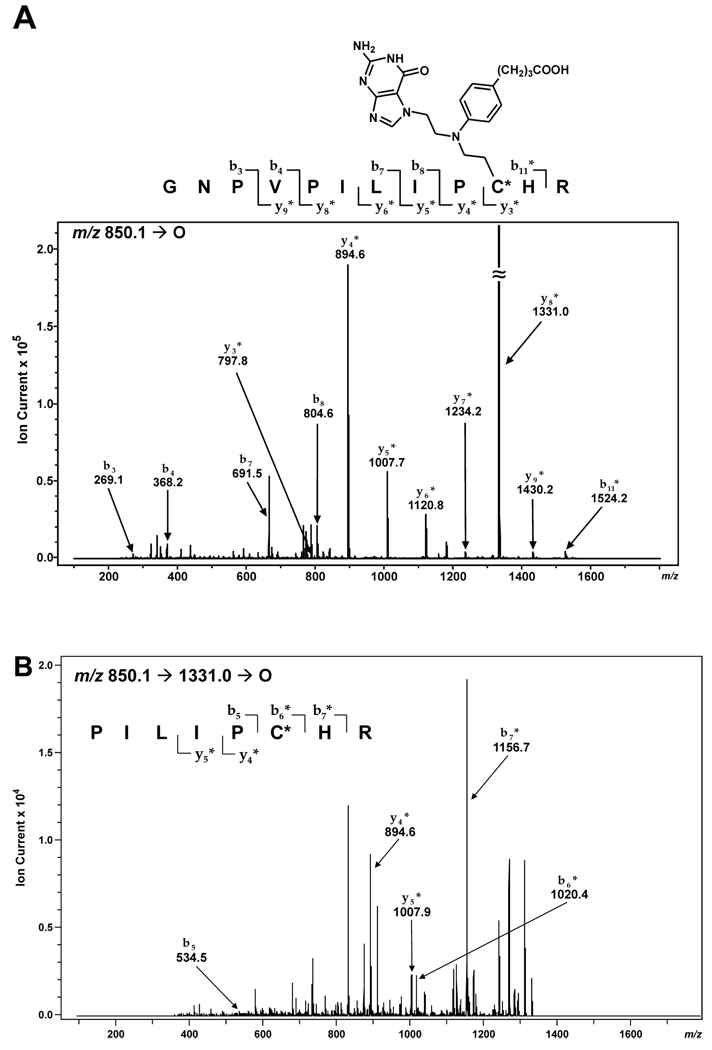
Further evidence for nitrogen mustard-mediated cross-linking of AGT to DNA via the two active site cysteines within the protein was obtained through MS/MS sequencing of peptides resulting from proteolytic digestion of alkylated AGT. HPLC-ESI+-MS/MS analysis of a tryptic digest of N7G-PBA-Cl-treated AGT detected a prominent doubly-charged ion at 850.1 m/z corresponding to a drug-induced conjugate involving peptide G136NPVPILIPCHR147 and a guanine base (calculated M = 1697.8 Da). The MS/MS spectrum (Figure 3A) was consistent with the presence of a chlorambucil-guanine adduct located at either Cys145 or His146. While the masses of product ions b3–b8 were in agreement with the theoretical values for the unmodified peptide, the mass of b11 was increased by 383 Da (observed M = 1523.2 Da versus calculated M = 1140.5 Da for the unmodified peptide). The y4–y9 ions also contained the chlorambucil-guanine cross-link as indicated by the 383 Da mass shift. MS3 analysis of the y8* fragment ion (P140ILIPCHR147, m/z 1331.0 [M + H]+) yielded product ions corresponding to b6, b7, y5, and y4 + 383 Da, mapping the site of modification to Cys145 (Figure 3B). Further proof of modification at Cys145 was obtained upon analysis of synthetic peptide GNPVPILIPCHR containing a chlorambucil cross-link to guanine (m/z 849.7 [M + 2H]2+) which displayed similar HPLC retention time and MS/MS fragmentation as the AGT-derived peptide-guanine cross-link (Supporting Information S-5). The second cross-linking site was similarly identified at Cys150 by MS2 sequencing of the peptide V148VCSSGGAVGNYSGGLAVK165 (calculated M = 2049.8 Da, with C corresponding to the chlorambucil-guanine conjugate of Cys (Supporting Information S-6). Analogous results were obtained upon analysis of tryptic fragments resulting from proteolytic digestion of N7G-EMA-Cl-treated AGT (Supporting Information S-7 and S-8). In neither series of experiments were other peptides found to contain guanine half mustard-induced lesions, suggesting that nitrogen mustard-mediated cross-linking of AGT to DNA occurs only via cysteines 145 and 150 of the protein.
Figure 3.
MS/MS (A) and MS3 spectrum (B) of the AGT tryptic peptide G136NPVPILIPCHR147 containing chlorambucil-guanine cross-link at C (Cys145).
To confirm this interpretation, additional experiments were performed in which recombinant AGT protein was incubated with nitrogen mustard drugs in the presence of double-stranded DNA (Scheme 2). Analysis of tryptic digests detected alkylation of the same two cysteine residues - Cys145 and Cys150 - suggesting that they are inherently reactive towards nitrogen mustard-derived aziridinium ions (Supporting Information S-9 and S-10). In addition, treatment of AGT with mechlorethamine in the presence of DNA produced an intramolecular AGT cross-link involving cysteines 145 and 150 according to MS/MS sequencing of tryptic peptides (Supporting Information S-11).
Capillary HPLC-ESI+-MS/MS Analysis of AGT Total Digests
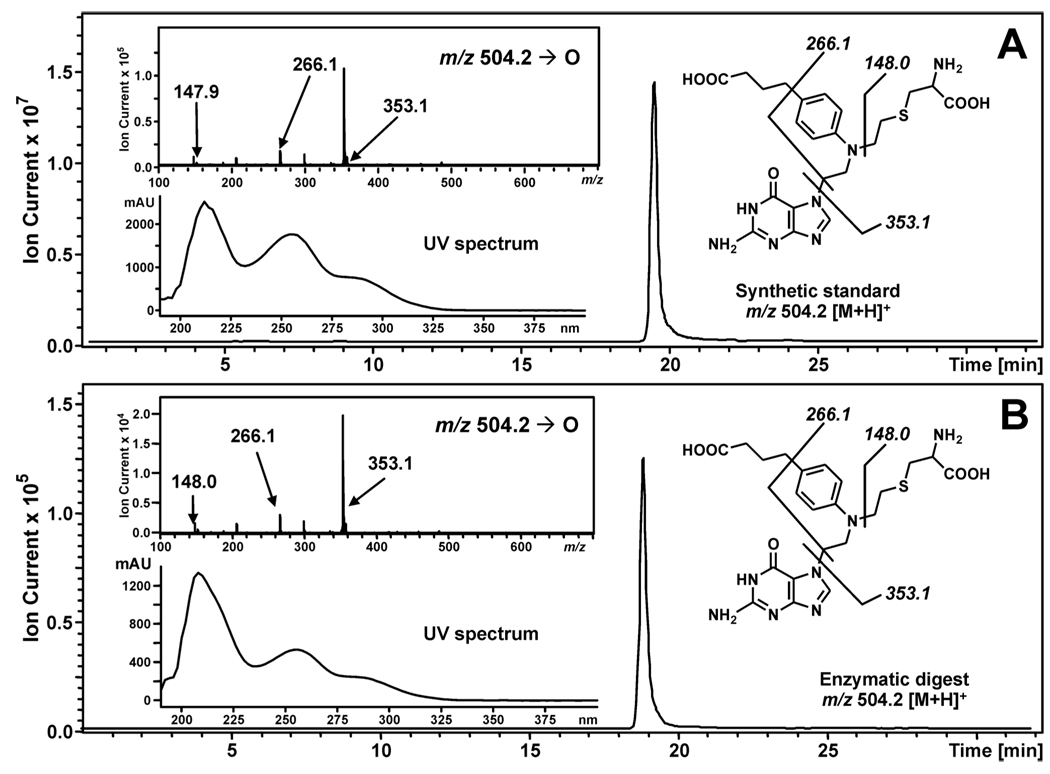
To establish the exact molecular structures of AGT-DNA conjugates, N7G-PBA-Cl and N7G-EMA-Cl treated AGT proteins were subjected to complete hydrolysis, followed by capillary HPLC-ESI+-MS/MS analysis of the resulting amino acids (Figure 4). Synthetic cysteine-guanine conjugates, N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbuyric acid (Cys-N7G-PBA) and N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)methylamine (Cys-N7G-EMA), were used as authentic standards. HPLC-ESI+-MS/MS analysis of AGT digests following exposure to N7G-PBA-Cl detected a prominent peak at m/z 504.2 which had the same retention time, MS/MS fragmentation pattern, and UV spectrum as Cys-N7G-PBA (Figure 4). In contrast, no HPLC-ESI+-MS/MS signals were observed in the ion channels corresponding to chlorambucil-induced guanine conjugates to other nucleophilic residues (Lys, Arg, His, Tyr). Similar results were observed for N7G-EMA-Cl (Supporting Information S-12). These data establish the covalent structures of amino acid-nucleobase conjugates induced by chlorambucil and mechlorethamine as Cys-N7G-PBA and Cys-N7G-EMA, respectively.
Figure 4.
HPLC-ESI+-MS/MS analysis of amino acid-guanine conjugates of chlorambucil. (A) Extracted ion chromatogram of synthetic Cys-N7G-PBA (m/z 356.2 [M + H]+); Inset: MS/MS fragmentation and UV spectrum. (B) Extracted ion chromatogram of AGT-derived Cys-N7G-PBA (m/z 356.2 [M + H]+; Inset: MS/MS fragmentation and UV spectrum.
AGT-DNA Cross-Linking in the Presence of Other Cellular Proteins
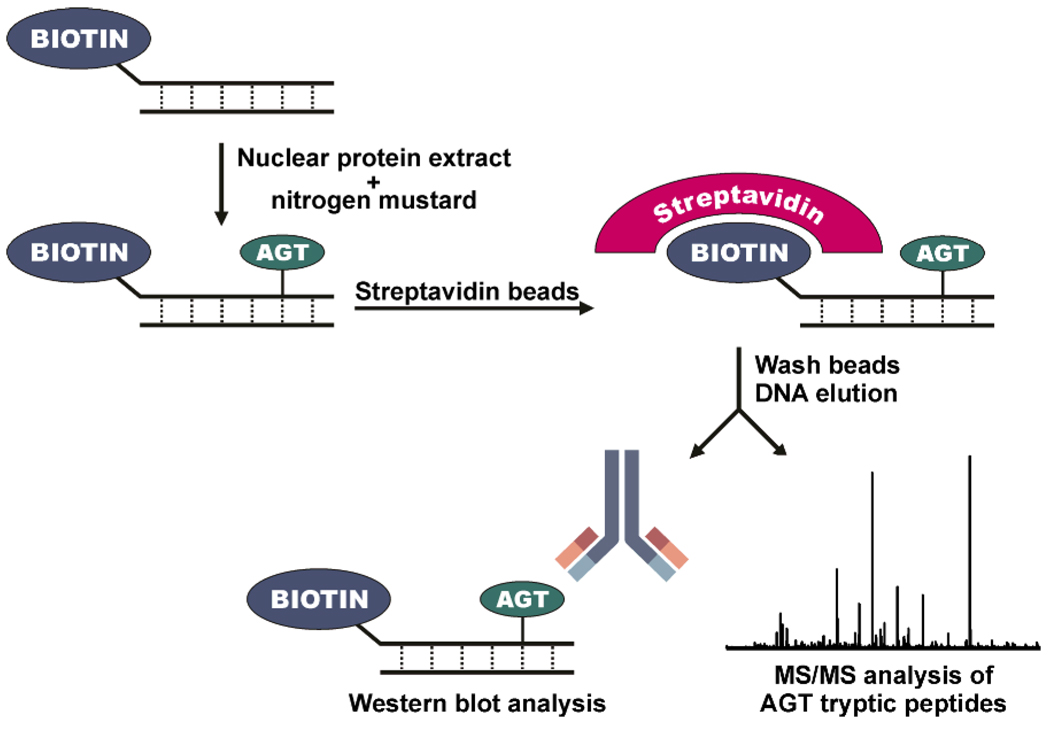
To determine whether AGT-DNA cross-linking can occur in the presence of other cellular proteins, an affinity based approach was developed (Scheme 3). Nuclear protein extracts were prepared from transgenic Chinese hamster ovary (CHO) cells that express recombinant human AGT (10) and from Chinese hamster ovary cells transfected with an empty vector which does not express human AGT. These extracts were incubated with synthetic DNA duplexes containing a 5' biotin tag (5'-GGAGCTGGTGGCGTAGGC-3', (+) strand) in the presence and absence of mechlorethamine. Streptavidin beads were then employed to capture any proteins chemically cross-linked to the biotinylated DNA. Following stringent washing procedures to remove any non-covalently bound proteins, biotinylated DNA along with any covalently attached proteins was eluted with SDS-PAGE loading buffer, and the resulting proteins were analyzed by Western blotting and HPLC-ESI+-MS/MS of tryptic digests.
Scheme 3.
Biotin capture assay for AGT-DNA cross-links.
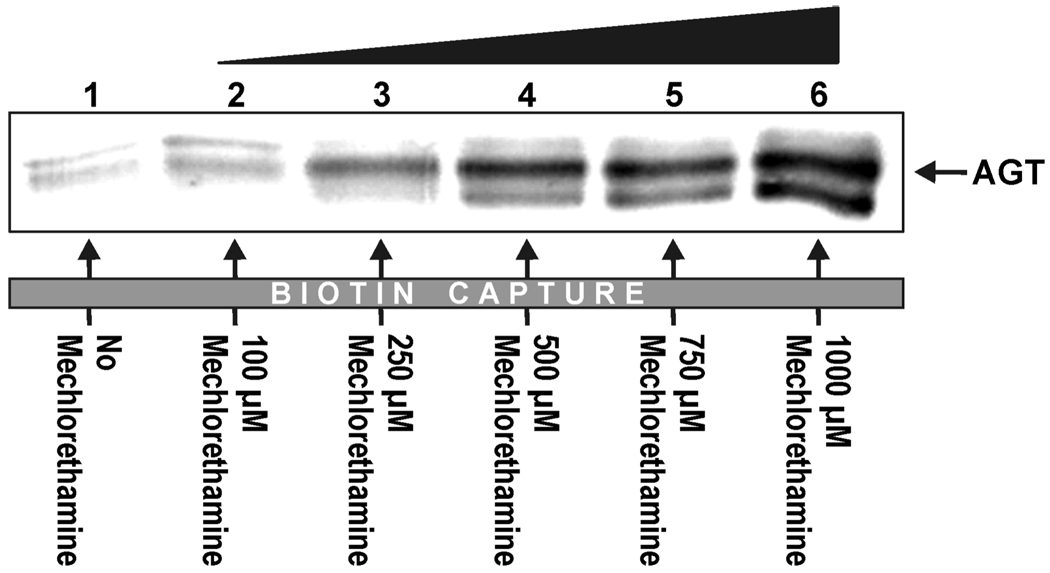
Immunological detection with anti-MGMT antibody revealed the concentration-dependent formation of drug-induced AGT-DNA cross-links in nuclear protein extracts from the AGT-expressing CHO cell line (Figure 5, lanes 2–6). Cross-linking of AGT to DNA increased from ~ 2.0% to 10.0% as the mechlorethamine concentration increased from 100 to 1000 µM based upon signal comparison to known AGT standards. Very little AGT was detected in the biotin capture of reactions lacking mechlorethamine (Figure 5, lane 1), DNA, or AGT (data not shown).
Figure 5.
Mechlorethamine induces AGT-DNA cross-links in nuclear protein extracts as indicated by Biotin capture assay. Biotinylated double-stranded oligodeoxynucleotides (5'-GGAGCTCGTGGCCTA-3' (+) strand, 3.12 nmol) were incubated with nuclear protein extracts from Chinese hamster ovary cells (CHO) expressing human AGT in the absence (lane 1) or presence of increasing amounts of mechlorethamine (lanes 2–6). Botinylated DNA and AGT-DNA cross-links were captured using streptavidin sepharose high performance beads, washed with buffers containing SDS, urea, and NaCl to remove non-covalently bound proteins, and eluted via heating in SDS-PAGE loading buffer. The presence of AGT in the biotin capture fractions following DNA elution was detected by Western blot analysis using a monoclonal antibody against AGT.
Identification of AGT protein from biotin capture experiments was further confirmed by mass spectrometry of tryptic digests. Protein mixtures were separated by SDS-PAGE, and the proteins were subjected to in-gel tryptic digestion prior to HPLC-ESI+-MS/MS. Upon analysis of peptides present in the biotin capture of mechlorethamine-treated samples, three distinct AGT tryptic peptides were identified: F108GEVISYQQLAALAGNPK125, V148VCSSGAVGNYSGGLAVK165, and L176GKPGLGGSSGLAGAWLK193 (see example in Figure 6). In contrast, no AGT peptides were detected in the biotin capture from the control reaction lacking mechlorethamine. Taken together, our results support the formation of AGT-DNA cross-links in the presence of other cellular proteins.
Figure 6.
HPLC-ESI+-MS/MS identification of AGT tryptic peptide F108GEVISYQQLAALAGNPK125 resulting from biotin capture of AGT-DNA cross-links.
Discussion
Historically, DNA-protein cross-links have received less attention than other DNA lesions. As a result, the biological consequences and cellular mechanisms for the repair of these lesions are not fully understood. As bulky, helix-distorting lesions, DNA-protein cross-links can block the binding and progression of protein complexes, potentially interfering with crucial cellular processes including DNA replication, transcription, DNA repair, recombination, and chromatin remodeling (4). The formation of DNA-protein cross-links can occur upon exposure to a variety of cytotoxic, mutagenic, and carcinogenic agents, including ionizing radiation (15), metals (4), endogenous and exogenous aldehydes (16), and chemotherapeutic drugs (17;18). Because certain types of DNA-protein cross-links have been shown to persist through several cycles of DNA replication due to lack of repair (4), DNA-protein cross-linking could play an important role in the cytotoxic activity of many antitumor drugs traditionally known for cross-linking DNA, including the nitrogen mustards.
We present the first evidence for the cross-linking of a specific DNA repair protein, O6-alkylguanine DNA alkyltransferese, to DNA in the presence of antitumor nitrogen mustards. Denaturing gel electrophoresis detected a concentration-dependent formation of AGT-DNA conjugates following incubation of human recombinant AGT protein with 32P-labeled oligonucleotides in the presence of nitrogen mustards (Figure 1). Mass spectrometry analysis of recombinant human AGT that had been incubated with guanine half mustards as models for monoalkylated DNA confirmed these findings and revealed that cross-linking can take place at two different sites within the protein (Figures 2A and 2D). Mutagenesis studies indicated that the two observed cross-linking sites reside at the active site cysteine residues, Cys145 and Cys150, since their replacement with Ala and Ser, respectively, blocked cross-linking (Figures 2B and 2C). These results were fully supported by HPLCESI+-MS/MS sequencing of tryptic peptides obtained from AGT treated with guanine half mustards (Figure 3and Supporting Information S-6 through S-8) or mustards in the presence of double stranded DNA (Supporting Information S-9 and S-10). The exact structures of the cross-links were established as N-[2-[S-cysteinyl]ethyl]- N-[2-(guan-7-yl)ethyl]methylamine and N-(2-[S-cysteinyl]ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbuyric acid for mechlorethamine and chlorambucil, respectively, based on HPLC-ESI+-MS/MS analysis of amino acidnucleobase conjugates in total protein digests in comparison with the corresponding synthetic standards (Figure 4 and Supporting Information S-12).
In comparing the overall cross-linking ability of the two nitrogen mustards used in this study, several interesting observations were made. For reactions in which cross-linking was initiated between double-stranded oligodeoxynucleotides and AGT via the addition of free drug, mechlorethamine displayed a greater cross-linking efficiency (Figure 1). This is not surprising since nitrogen mustard drugs, including chlorambucil, were specifically designed to afford compounds with reduced reactivity/toxicity for chemotherapeutic applications (19). However, reactions in which cross-linking was achieved upon incubation of AGT with synthetic guanine half mustards, chlorambucil appeared to be a more efficient cross-linker (Figure 2). This may be explained by a higher affinity of N7G-PBA-Cl with its aromatic substituent for the primarily hydrophobic active site pocket of AGT (20).
Using a novel biotin/streptavidin enrichment strategy in combination with immunodetection and tandem mass spectrometry, AGT-DNA cross-linking in the presence of other cellular proteins was established. Upon incubation of 5'-biotinylated oligonucleotides with nuclear proteins from AGT-expressing CHO cells (in which AGT comprises ~ 0.5% of total protein), AGT-DNA cross-linking displayed concentration dependence, with cross-link amounts increasing from 2 to 10% as the mechlorethamine concentration increased from 100–1000 µM (Figure 5). Similar results were obtained in HeLa nuclear extracts, a cell line with endogenous expression of AGT (Loeber and Tretyakova, unpublished results). Although these studies employed a large excess of mechlorethamine to afford levels of cross-linking detectable by Western blotting/HPLC-ESI+-MS/MS, these results may still hold biological relevance as DNA-protein cross-links would likely cause significant disruption to normal cellular processes, including DNA replication and transcription.
Our results support nitrogen mustard-induced AGT-DNA cross-linking involving two active site cysteine residues (Cys145 and Cys150), despite the presence of three other cysteine residues within the protein. These same two sites were also modified when the recombinant protein was treated with chlorambucil or mechlorethamine in the absence of DNA (Supporting Information S-13 through S-18), suggesting selective reactivity of these residues towards nitrogen mustards. These results are consistent with our previous results for AGT-DNA cross-linking by diepoxybutane (6).
To gain further insight into the specificity of AGT in forming DNA-protein cross-links, similar experiments were conducted with another DNA-binding protein, histone H4. Upon SDS-PAGE analysis of reaction mixtures in which histone H4 had been incubated with both nitrogen mustards in the presence of 32P-end labeled oligodeoxynucleotides, no histone-DNA cross-links were observed (Supporting Information S-2). Likewise, ESI+-MS analysis of histone H4 treated with N7G-PBA-Cl or N7G-EMA-Cl yielded the same deconvoluted MS spectra (Supporting Information S-3 and S-4), suggesting that no cross-linking had taken place. Upon total digestion of half mustard-treated histone H4 to amino acids, no amino acid-guanine conjugates were detected (data not shown). These negative results were consistent with a previous study that showed that no DNA-histone cross-linking had occurred following treatment of nuclear protein extracts with mechlorethamine (21). Unlike AGT, histone H4 contains no cysteines. Instead, it contains numerous nucleophillic lysine and arginine residues. The apparent lack of nitrogen mustard-induced histone-DNA cross-linking may be attributed to the fact that the side chains of lysine and arginine are protonated at physiological pH, thus preventing attack by the positively charged aziridinium ions (Scheme 1).
As nitrogen mustard-mediated DNA-protein cross-linking displays selectivity for AGT, questions are raised regarding the role of this important repair protein in the cellular response to bifunctional alkylating agents. Within the cell, AGT is responsible for the irreversible transfer of promutagenic O6-alkylguanine lesions in DNA to an active site cysteine residue, Cys145, restoring normal guanine and preventing mutagenesis. Levels of AGT expression are highly variable between different tissues and tumor types, opening the possibility that it may mediate the biological effects of DNA alkylating drugs. Previous studies have yielded conflicting reports on the possible role of AGT in mediating cytotoxicity of nitrogen mustards, specifically cyclophosphamide and chlorambucil. Several papers provide evidence that AGT partially protects against the toxicity and mutagenicity of cyclophosphamide metabolites and imparts resistance to treatment of tumor xenografts (22). In CHO cells, expression of human AGT was found to protect against the toxic and mutagenic effects of cyclophosphamide, and that the metabolite acrolein was responsible for generating the lesions in which AGT afforded protection (23). Although the idea that AGT may be acting as a molecular scavenger was proposed, a more recent study has shown that DNA binding is required for AGT-mediated protection against acrolein toxicity, suggesting that the active site thiol is not simply sequestering this reactive metabolite (24). However, in other studies in cultured cells, survival was not affected by AGT activity (25), and a comparison of the therapeutic response of 23 tumor xenografts showed no correlation with AGT levels (26). The presence of AGT renders E. coli strains more sensitive to killing by chlorambucil (27) but no effect of AGT status on drug sensitivity was seen in a studies of HeLa or CHO cells (25). Clinical studies have also yielded conflicting results on the role of AGT levels in the outcome of therapy with cyclophosphamide [reviewed (24)]. Recently, it was shown that MGMT does not protect against mutations induced by cyclophosphamide in mice (28).
The present results showing that AGT can be cross-linked to DNA by nitrogen mustards adds another factor that must be considered in the overall effect of AGT levels to the response to treatment. This cross-linking could increase the toxicity, and the extent to which this is balanced or exceeded by the ability to repair other adducts may determine the overall biological response.
Supplementary Material
Acknowledgements
We thank Kris Murphy, Danae Quirk Dorr, Vallabha Tantry, Melissa Goggin, and Zhou Xin (University of Minnesota Cancer Center) for synthesizing guanine half mustards and guanine-amino acid conjugates of nitrogen mustards; Brock Matter (University of Minnesota Cancer Center), Simona Codreanu and Daniel Liebler (Mass Spectrometry Research Center, Vanderbilt University) for help with mass spectrometry experiments, and Bob Carlson (University of Minnesota Cancer Center) for preparing figures for this manuscript. Funding for this research was from Leukemia Research Foundation and the National Cancer Institute grants to N.T. (R01-CA-100670) and A.P. (R01-CA-018137). R.L. was partially supported by the NIH Chemistry-Biology Interface Training Grant (T32-GM08700) and the University of Minnesota Cancer Center.
Abbreviations
- Cys-N7G-EMA
N-[2-[S-cysteinyl]ethyl]-N-[2-(guan-7-yl)ethyl]methylamine
- Cys-N7G-PBA
N-(2-[Scysteinyl] ethyl)-N-(2-[guan-7-yl]ethyl)-p-aminophenylbuyric acid
- N7G-EMA-Cl
N-(2-chloroethyl)-N-[2-(guan-7-yl)ethyl]methylamine
- N7G-PBA-Cl
N-(2-chloroethyl)-N-[2-(guan-7-yl)ethyl]-p-aminophenylbutyric acid
- O6-alkylguanine DNA alkyltransferase
AGT
Footnotes
Supporting Information Available: Synthetic procedures for the preparation of the guanine half mustards and amino acid-guanine conjugates of chlorambucil and mechlorethamine, SDS-PAGE analysis of nitrogen mustard-induced histone H4-DNA cross-links, MS spectra of histone H4 following exposure to guanine half mustards, MS/MS spectra of AGT tryptic peptides containing nitrogen mustard-induced lesions, and amino acid sequences of human AGT variants and bovine histone H4 can be found in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference List
- 1.Lawley PD, Brookes P. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature. 1965;206(983):480–483. doi: 10.1038/206480a0. [DOI] [PubMed] [Google Scholar]
- 2.Osborne MR, Lawley PD. Alkylation of DNA by melphalan with special reference to adenine derivatives and adenine-guanine cross-linking. Chem. Biol. Interact. 1993;89(1):49–60. doi: 10.1016/0009-2797(93)03197-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Hachey DL, Valadez G, Williams KM, Guengerich FP, Loktionova NA, Kanugula S, Pegg AE. Characterization of a mutagenic DNA adduct formed from 1,2-dibromoethane by O6-alkylguanine-DNA alkyltransferase. J. Biol. Chem. 2004;279(6):4250–4259. doi: 10.1074/jbc.M311105200. [DOI] [PubMed] [Google Scholar]
- 4.Barker S, Weinfeld M, Murray D. DNA-protein crosslinks: their induction, repair, and biological consequences. Mutat. Res. 2005;589(2):111–135. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Valadez JG, Liu L, Loktionova NA, Pegg AE, Guengerich FP. Activation of bis-electrophiles to mutagenic conjugates by human O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 2004;17(7):972–982. doi: 10.1021/tx049897u. [DOI] [PubMed] [Google Scholar]
- 6.Loeber R, Rajesh M, Fang Q, Pegg AE, Tretyakova N. Cross-linking of the human DNA repair protein O6-alkylguanine DNA alkyltransferase to DNA in the presence of 1,2,3,4-diepoxybutane. Chem. Res. Toxicol. 2006;19(5):645–654. doi: 10.1021/tx0600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pegg AE. Repair of O6-alkylguanine by alkyltransferases. Mutat. Res. 2000;462(2–3):83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu L, Xu-Welliver M, Kanugula S, Pegg AE. Inactivation and degradation of O6-alkylguanine-DNA alkyltransferase after reaction with nitric oxide. Cancer Res. 2002;62(11):3037–3043. [PubMed] [Google Scholar]
- 9.Edara S, Kanugula S, Goodtzova K, Pegg AE. Resistance of the human O6-alkylguanine-DNA alkyltransferase containing arginine at codon 160 to inactivation by O6-benzylguanine. Cancer Res. 1996;56(24):5571–5575. [PubMed] [Google Scholar]
- 10.Cai Y, Wu MH, Xu-Welliver M, Pegg AE, Ludeman SM, Dolan ME. Effect of O6-benzylguanine on alkylating agent-induced toxicity and mutagenicity in Chinese hamster ovary cells expressing wild-type and mutant O6-alkylguanine-DNA alkyltransferases. Cancer Res. 2000;60(19):5464–5469. [PubMed] [Google Scholar]
- 11.Jessberger R, Berg P. Repair of deletions and double-strand gaps by homologous recombination in a mammalian in vitro system. Mol. Cell Biol. 1991;11(1):445–457. doi: 10.1128/mcb.11.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–257. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 13.Shin NY, Liu Q, Stamer SL, Liebler DC. Protein targets of reactive electrophiles in human liver microsomes. Chem. Res. Toxicol. 2007;20(6):859–867. doi: 10.1021/tx700031r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5(11):976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 15.Barker S, Weinfeld M, Zheng J, Li L, Murray D. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J. Biol. Chem. 2005;280(40):33826–33838. doi: 10.1074/jbc.M502477200. [DOI] [PubMed] [Google Scholar]
- 16.Murata-Kamiya N, Kamiya H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001;29(16):3433–3438. doi: 10.1093/nar/29.16.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ewig RA, Kohn KW. DNA damage and repair in mouse leukemia L1210 cells treated with nitrogen mustard, 1,3-bis(2-chloroethyl)-1-nitrosourea, and other nitrosoureas. Cancer Res. 1977;37(7 Pt 1):2114–2122. [PubMed] [Google Scholar]
- 18.Kloster M, Kostrhunova H, Zaludova R, Malina J, Kasparkova J, Brabec V, Farrell N. Trifunctional dinuclear platinum complexes as DNA-protein cross-linking agent. Biochemistry. 2004;43(24):7776–7786. doi: 10.1021/bi030243e. [DOI] [PubMed] [Google Scholar]
- 19.Mutschler E, Derendorf H, editors. Chemotherapy of Malignant Tumors. Drug Actions: Basic Principles and Therapeutic Aspects. Stuttgart: Medpharm Scientific Publishers; 1995. pp. 600–601. [Google Scholar]
- 20.Daniels DS, Woo TT, Luu KX, Noll DM, Clarke ND, Pegg AE, Tainer JA. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nat. Struct. Mol. Biol. 2004;11(8):714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 21.Berger SL. Histone modifications in transcriptional regulation. Curr. Opin. Genet. Dev. 2002;12(2):142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 22.Friedman HS, Pegg AE, Johnson SP, Loktionova NA, Dolan ME, Modrich P, Moschel RC, Struck R, Brent TP, Ludeman S, Bullock N, Kilborn C, Keir S, Dong Q, Bigner DD, Colvin OM. Modulation of cyclophosphamide activity by O6-alkylguanine-DNA alkyltransferase. Cancer Chemother. Pharmacol. 1999;43(1):80–85. doi: 10.1007/s002800050866. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Wu MH, Ludeman SM, Grdina DJ, Dolan ME. Role of O6-alkylguanine-DNA alkyltransferase in protecting against cyclophosphamide-induced toxicity and mutagenicity 1. Cancer Res. 1999;59(13):3059–3063. [PubMed] [Google Scholar]
- 24.Hansen RJ, Ludeman SM, Pegg AE, Dolan ME. Role of MGMT in protecting against cyclophosphamide-Induced toxicity in cells and animals. DNA Repair. 2007;6:1145–1154. doi: 10.1016/j.dnarep.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preuss I, Thust R, Kaina B. Protective effect of O6-methylguanine-DNA methyltransferase (MGMT) on the cytotoxic and recombinogenic activity of different antineoplastic drugs. Int. J. Cancer. 1996;65(4):506–512. doi: 10.1002/(SICI)1097-0215(19960208)65:4<506::AID-IJC19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.D'Incalci M, Bonfanti M, Pifferi A, Mascellani E, Tagliabue G, Berger D, Fiebig HH EORTC SPG and PAMM Groups. The antitumour activity of alkylating agents is not correlated with the levels of glutathione, glutathione transferase and O6-alkylguanine-DNA-alkyltransferase of human tumour xenografts. Eur. J. Cancer. 1998;34(11):1749–1755. doi: 10.1016/s0959-8049(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 27.Salmelin C, Hovinen J, Vilpo J. Polymyxin permeabilization as a tool to investigate cytotoxicity of therapeutic aromatic alkylators in DNA repair-deficient Escherichia coli strains. Mutat. Res. 2000;467(2):129–138. doi: 10.1016/s1383-5718(00)00026-7. [DOI] [PubMed] [Google Scholar]
- 28.Hansen RJ, Nagasubramanian R, Delaney SM, Samson LD, Dolan ME. Role of O6-methylguanine-DNA methyltransferase in protecting from alkylating agent-induced toxicity and mutations in mice. Carcinogenesis. 2007;28(5):1111–1116. doi: 10.1093/carcin/bgl218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.