Abstract
Cellular mechanisms that maintain redox homeostasis are crucial, providing buffering against oxidative stress. Glutathione, the most abundant low molecular weight thiol, is considered the major cellular redox buffer in most cells. To better understand how cells maintain glutathione redox homeostasis, cells of Saccharomyces cerevisiae were treated with extracellular oxidized glutathione (GSSG), and the effect on intracellular reduced glutathione (GSH) and GSSG were monitored over time. Intriguingly cells lacking GLR1 encoding the GSSG reductase in S. cerevisiae accumulated increased levels of GSH via a mechanism independent of the GSH biosynthetic pathway. Furthermore, residual NADPH-dependent GSSG reductase activity was found in lysate derived from glr1 cell. The cytosolic thioredoxin-thioredoxin reductase system and not the glutaredoxins (Grx1p, Grx2p, Grx6p, and Grx7p) contributes to the reduction of GSSG. Overexpression of the thioredoxins TRX1 or TRX2 in glr1 cells reduced GSSG accumulation, increased GSH levels, and reduced cellular glutathione Eh′. Conversely, deletion of TRX1 or TRX2 in the glr1 strain led to increased accumulation of GSSG, reduced GSH levels, and increased cellular Eh′. Furthermore, it was found that purified thioredoxins can reduce GSSG to GSH in the presence of thioredoxin reductase and NADPH in a reconstituted in vitro system. Collectively, these data indicate that the thioredoxin-thioredoxin reductase system can function as an alternative system to reduce GSSG in S. cerevisiae in vivo.
Keywords: Glutathione, Organisms/Yeast, Sulfhydryls/Thiol, Redox, Glutaredoxin, Glutathione Reductase, Thioredoxin, Thioredoxin Reductase
Introduction
Aerobic organisms maintain a reducing intracellular environment to facilitate appropriate functioning of numerous processes, including the proper folding of proteins and maintenance of their activity. Cellular mechanisms that maintain redox homeostasis are crucial, because they provide a buffer against conditions that may perturb the redox environment of cells and/or induce oxidative stress (1, 2). Changes to redox state such as exposure to reactive oxygen species (ROS) can lead to detrimental effects including oxidation of sulfhydryl groups in proteins that can result in loss of activity (3). Given the importance of redox homeostasis, it is vital to understand the mechanisms involved in maintaining normal redox homeostasis. The high abundance of glutathione (1–10 mm) in cells and its low redox potential (−240 mV) make the glutathione system a major intracellular redox buffer in most cells (1, 4, 5). Previous studies have indicated that cells lacking Cu,Zn-superoxide dismutase, genes involved in glutathione homeostasis or those involved in the pentose phosphate pathway can alter intracellular the glutathione redox balance (6–9), demonstrating the complexity of redox systems. Therefore, understanding the factors that affect cellular glutathione homeostasis contributes importantly to our overall understanding of how cells maintain intracellular redox homeostasis.
In Saccharomyces cerevisiae, reduced glutathione (GSH)3 biosynthesis occurs in the cytosol via two ATP-dependent steps. The first is catalyzed by γ-glutamylcysteine synthetase encoded by GSH1 (10), which catalyzes condensation of glutamate and cysteine. Glutathione synthetase encoded by GSH2 (11) catalyzes the addition of glycine to γ-glutamylcysteine to form GSH (12). GSH is an important antioxidant, because yeast strains altered in their GSH redox state are sensitive to oxidant-induced stress (13–16).
Several cellular processes use the reducing power of GSH including glutathione peroxidases, which mediates the reduction of hydrogen peroxide to water, generating stoichiometric quantities of oxidized glutathione (GSSG) in the process. GSSG can be recycled to GSH by glutathione reductase using reducing equivalents supplied by NADPH (2). GLR1 encodes the only known GSSG reductase in S. cerevisiae. Mutants deleted for GLR1 are viable, overaccumulate GSSG, and are hypersensitive to oxidants (17, 18). Besides glutathione, cells have other redox-active molecules such as the thioredoxins and glutaredoxins that participate in oxidative stress defense.
Thioredoxins are low molecular weight thiol-disulfide oxidoreductases with a conserved CGPC active site and are involved in the reduction of enzymes that are oxidized to form a disulfide (19). These enzymes include 3′-phosphoadenosine 5′-phosphosulfate (PAPS) reductase required for sulfur assimilation and ribonucleotide reductase (RNR) required for maintaining dNTPs for DNA synthesis (3). S. cerevisiae contains two cytoplasmic thioredoxins encoded by TRX1 and TRX2. Mutants deleted for both TRX1 and TRX2 are defective in sulfate assimilation, consistent with the role of the two thioredoxins as hydrogen donors for PAPS reductase (20). Trx2p is involved in oxidative stress defense because the trx2 mutant is sensitive to H2O2 (21, 22). Thioredoxin reductase encoded by TRR1 reduces oxidized thioredoxins directly. Mutants deleted for TRR1 are sensitive to hydrogen peroxide and are auxotrophic for methionine (23, 24).
Glutaredoxins are small proteins that act as thiol oxidoreductases involved in the reduction of protein disulfides or glutathione-protein mixed disulfides (25). In S. cerevisiae, the dithiol glutaredoxins are encoded by GRX1 and GRX2 and contain CPYC at their active site (26). Cells lacking GRX1 are sensitive to superoxide generating agents and hydroperoxides, whereas cells lacking GRX2 are sensitive to hydroperoxides but not superoxides, indicating that the functions of GRX1 and GRX2 are only partially overlapping (26), and the two genes are differentially regulated (27).
S. cerevisiae contains five monothiol glutaredoxins. Grx3p, Grx4p, and Grx5p contain the CGFS motif in their active site. Grx3p and Grx4p regulate the nuclear localization of the Aft1p transcription factor involved in iron homeostasis (28, 29), whereas Grx5p participates at the late stages of the biosynthesis of Fe/S clusters in the mitochondrion (30, 31). GRX6 and GRX7 encode two monothiol glutaredoxins that contain active site sequences CSYS and CPYS, respectively. Both Grx6p and Grx7p are localized in the Golgi, but are not involved in oxidative protein folding (32). Cells lacking GRX6 and/or GRX7 were shown to be sensitive to oxidants hydrogen peroxide and diamide in one study (32), but showed no obvious phenotype, except for mild resistance to the glycosylation inhibitor tunicamycin in another (33). The biological role of Grx6p and Grx7p remains unknown.
Although the thioredoxins and glutaredoxins are similar in structure and have overlapping functions (22), they are regulated in a different manner. The oxidized disulfide form of thioredoxin is reduced directly by thioredoxin reductase using NADPH as the electron donor, whereas glutaredoxin is reduced by glutathione (GSH), and the oxidized glutathione (GSSG) is in turn reduced by glutathione reductase using electrons donated by NADPH (25). Glr1p is highly specific for the reduction of GSSG. Alternatively, while thioredoxins have been found to reduce a broad spectrum of substrates, GSSG was generally not considered to be a good substrate for thioredoxins (34, 35). However, cells deleted for TRR1 over-accumulate both GSH and GSSG (18). Furthermore, the trr1 glr1, and the trx1 trx2 glr1 mutants are inviable (18, 36), indicating a potential link between these two systems.
To further understand how cells maintain intracellular glutathione redox balance, we investigated the mechanisms of how cells respond to increased levels of GSSG in vivo. Surprisingly, we found that cells deleted for GLR1, encoding the only known glutathione reductase in S. cerevisiae, are able to convert GSSG to GSH. This led us to investigate possible alternative mechanisms that can reduce GSSG in cells.
EXPERIMENTAL PROCEDURES
Yeast Strains and Plasmids
The S. cerevisiae strains used (Table 1) were derived from BY4743 (Open Biosystems) or from W303 as described previously (14). Single gene deletion strains in the W303 background were generated using PCR to amplify the disrupted allele and flanking region (∼200–400 bp) from the strains available from the S. cerevisiae Genome Deletion Project (37) or using the appropriate plasmids from the PCR tool kit (38). The amplified DNA product was transformed into W303 with selection on YEPD medium containing 220 μg/ml geneticin (for KanMX selection, Invitrogen), 100 μg/ml nourseothricin (for natMT1 selection, Sigma) or 300 μg/ml hygromycin (for hphNT1 selection, Invitrogen). Transformation was performed using the lithium acetate-based method (39). Each deletant was confirmed by PCR analysis on genomic DNA isolated from the respective strains. Chromosomal HA-tagging of GSH1 was performed using the PCR-based targeted gene insertion method described (38). The resulting PCR fragment was transformed into W303 to generate strains containing an in-frame GSH1 allele with a C-terminal chromosomal HA tag. Multiple mutants were generated by mating and sporulation using standard yeast genetic methods.
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W303 | MATaura3–52 leu2–3,112 trp1–1 ade2–1 his3–11 | Chris M. Grant |
| JL3 | gsh1::LEU2 in W303 | Grant et al. (14) |
| CY7 | glr1::TRP1 in W303 | Grant et al. (17) |
| ST201 | gsh1::LEU2 glr1::TRP1 in W303 | This study |
| ST203 | glr1::TRP1 GSH1-HA::kanMX in W303 | This study |
| ST204 | grx6:: natMT1 grx7:: hphNT1 glr1:: TRP1 in W303 | This study |
| ST208 | grx1::LEU2 grx2::HIS3 glr1::TRP1 in W303 | This study |
| ST209 | grx1::LEU2 grx2::HIS3 grx6:: natMT1 grx7:: hphNT1 glr1:: TRP1 in W303 | This study |
| ST301 | trx1::hphNT1 glr1::TRP1 in W303 | This study |
| ST302 | trx2::hphNT1 glr1::TRP1 in W303 | This study |
| BY4743 | MATa/MATα his3Δ1/ his3Δ1 leu2Δ0/leu2Δ0 met15Δ0/MET15 LYS2/lysΔ0 ura3Δ0/ura3Δ0 | EUROSCARF |
| BY4743 gsh1) | gsh1::kanMX in BY4743 | EUROSCARF |
| BY4743 (glr1) | glr1::kanMX in BY4743 | EUROSCARF |
| BY4743 (hgt1) | hgt1::kanMX in BY4743 | EUROSCARF |
The N-terminally HA-tagged galactose-inducible TRR1, TRX1, and TRX2 constructs were generated using the Gateway system (Invitrogen) according to the manufacturer's instructions. Plasmids carrying the respective genes from the S. cerevisiae ORF collection (40) were shuffled into pDonr-221 using the BP reaction. The entry clones obtained were then used to introduce the respective genes into pAG416GAL1-ccdB (41) using the LR reaction. The resulting clones were sequenced for verification.
Growth Conditions
Yeast strains were grown in YEPD medium containing 1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose, or synthetic defined medium (SD) containing 0.17% yeast nitrogen base without amino acids and ammonium sulfate, 0.5% ammonium sulfate, and supplemented with minimal auxotrophic requirements. For induction of genes under the control of the GAL1 promoter, cells were grown for 2 days in SD medium and re-inoculated to synthetic defined medium containing 2% (w/v) galactose (SD-GAL) instead of glucose. Auxotrophic supplements were added as follow: 76 mg/liter of l-tryptophan, l-isoleucine, and l-valine, 260 mg/liter l-leucine, 380 mg/liter l-histidine, 26 mg/liter uracil, and 18 mg/liter adenine. Appropriate supplements were omitted for the purpose of selecting plasmids.
Western Blot Analysis
Cells were grown as stated, and ∼40 ml of cells were harvested and lysed in lysis buffer (0.1 m Tris-HCl, pH 8, 20% (v/v) glycerol, 2 mm phenylmethylsulfonyl fluoride, 1% SDS). Protein content of cell lysate was determined and adjusted to 2 μg/μl in sample-loading buffer (5% (v/v) β-mercaptoethanol, 40% (v/v) glycerol, Tris-HCl, pH 6.8). Samples were boiled for 10 min, and 30 μg of each sample were loaded onto a 4–12% NuPAGE gel (Invitrogen). Anti-HA was diluted to 1:1000, and anti-Pgk1p was diluted to 1:5000.
β-Galactosidase Assay
Cells were grown to exponential phase (A600 ∼ 0.5), harvested, and analyzed for β-galactosidase activity as described (42). β-Galactosidase activity was expressed as units of ONPG (O-nitrophenyl-β-d-galactopyranoside) hydrolyzed (nmol) per min over total protein (mg), and the extent of induction was the ratio of the β-galactosidase activity of the treated sample to that of the untreated sample. For each strain, three independent transformants were assayed.
Determination of Intracellular Glutathione Levels
Reduced (GSH) and oxidized glutathione (GSSG) were estimated as described previously (14, 43). Cells were grown in SD medium (40 ml) to an A600 of 1 (∼2 × 107 cells/ml) and harvested by centrifugation (10,000 × g, 20 s), washed in 3 ml of ice-cold PBS buffer, resuspended in 350 μl of ice-cold 8 mm HCl containing 3% (w/v) 5-sulfosalicilic acid and disrupted using a mini-bead beater (1-min high speed, 4 °C). The lysate was clarified by centrifugation (5 min, 10,000 × g, 4 °C), and the supernatant used to determine total glutathione. GSSG was determined by reacting GSH in samples with 2-vinylpyridine prior to the assay. Levels of GSH and GSSG were calculated as described in Ref. 43.
Estimation of Intracellular Redox Environment
Intracellular values of the GSSG/2GSH half-cell redox potential (glutathione Eh′) were determined as an indicator of the cellular redox environment (1). These were calculated from intracellular concentrations of GSH and GSSG using the Nernst equation: Eh′ = E0 − 2.303(RT/nF)log10[(GSH)2/(GSSG)]. Glutathione Eh′ values are expressed in mV; E0 is the standard potential for reduced glutathione (−0.24 mV) at pH 7, R is the gas constant (8.31 J mol−1 K−1), T is the absolute temperature (303 K), n is the number of electrons transferred (2), and F is the Faraday constant (96,406 J V−1 mol−1). To estimate intracellular glutathione concentration, cell volumes were estimated from cell major (a) and minor (b) axes using the formula (44): V = πa2b/6. Cell dimensions were determined microscopically at ×100 magnification using a graticule calibrated against a slide micrometer grating. For each strain, at least 100 cells were measured and the average cell volume determined.
Analysis of Glutathione Reductase Activity in Cell Lysates
Cells were harvested according to the procedure used for Western blot analysis. Cell lysates were clarified by ultracentrifugation (100,000 × g, 4 °C, 20 min), and proteins present in each soluble fraction were concentrated using an Amicon Ultra centrifugal filters (3 kDa cutoff; Millipore) at 4 °C. Cell lysates were mixed with an equal volume of ice-cold phosphate-buffered saline and re-concentrated using the above columns. This process was repeated twice to reduce the levels of small molecular weight compounds including GSH and GSSG. Lysates prepared in this manner were assayed immediately. Glutathione reductase activity was determined by monitoring oxidation of NADPH (340 nm). Complete (total) reaction mixtures contained 20 μl of cell lysate in phosphate-buffered saline and NADPH (600 μm). Reactions were initiated by addition of 1 mm GSSG, and reduction of GSSG was estimated by monitoring GSSG-dependent NADPH oxidation, which was reflected by a decrease in absorbance at 340 nm. The background level of NADPH oxidation was also determined by monitoring NADPH oxidation by the corresponding cell lysate in the absence of added GSSG. GSSG-dependent oxidation of NADPH was calculated by subtracting the values obtained for GSSG-independent NADPH oxidation from the value obtained for the “complete” reaction mixture. Activity was normalized to the protein concentration present in each sample and expressed as nmol of NADPH oxidized per minute per mg of protein (nmol min−1 mg−1).
Reduction of GSSG by Purified Trx1p or Trx2p and Trr1p in Vitro
Plasmids expressing six-histidine residue-tagged versions of Trx1p (pBAD-YTRX1) and Trr1p (pBAD-YTRR1) were a kind gift from Barry Rosen (45). Trx2p was amplified by PCR and cloned into the pBAD expression vector (Invitrogen). Histidine-tagged proteins were purified by Ni2+-nitrilotriacetic acid chromatography and protein purity checked on SDS-PAGE gels. Glutathione reductase (Glr1p) derived from S. cerevisiae was purchased from Sigma. Reduction of GSSG by Glr1p or Trr1p was measured in vitro with purified proteins. Reaction mixes contained NADPH (600 μm) and either Glr1p (6.67 nm), or Trr1p (0.25 μm) and Trx1p (1.5 μm), or, Trr1p (0.25 μm) and Trx2p (1.5 μm). Reactions were started by addition of 1 mm GSSG and reduction of GSSG to GSH estimated by following the decrease in oxidation of NADPH (340 nm).
RESULTS
Treatment of Cells with Extracellular GSSG Leads to Accumulation of Intracellular GSH
To investigate if perturbation in cellular glutathione redox environment can lead to a change in cellular Eh′, intracellular GSSG concentration was increased by adding GSSG to the medium, and the changes in intracellular glutathione concentration and cellular Eh′ of the glutathione redox couple monitored over time.
In wild-type and glr1 cells, addition of 100 μm GSSG extracellularly led to an increase in intracellular glutathione concentration from 1 to 3 mm in 20 min and reaching a maximum of 6 mm after 2 h of treatment (Fig. 1A). Increased intracellular glutathione accumulation was dependent on the high affinity glutathione transporter Hgt1p/Opt1p (encoded by YJL212c), because cells lacking HGT1 exhibited a minimal increase in intracellular glutathione upon GSSG treatment (Fig. 1A). Upon GSSG treatment, an accumulation of total glutathione in wild-type and glr1 cells was therefore largely due to uptake of GSSG by Hgt1p. Previous studies found that Hgt1p is the sole high affinity GSH transporter in S. cerevisiae (46). Here it was demonstrated that Hgt1p could also transport GSSG into cells, because a minor increase in glutathione level was observed in the hgt1 mutant treated with GSSG. This indicates the presence of another less efficient route for GSSG uptake in S. cerevisiae.
FIGURE 1.
Effect of extracellular GSSG on intracellular glutathione redox homeostasis. The wild-type, hgt1 and glr1 cells were grown to exponential phase (A600, 0.5) in SD medium exposed to 100 μm GSSG, and intracellular glutathione was determined over the indicated time course. A, total intracellular glutathione; B, GSH; and C, GSSG of the indicated strains were determined. D, cellular glutathione Eh′ was determined over the time course. Error bars indicate the S.E. of four samples from two independent experiments.
Upon GSSG treatment, the glutathione accumulated by wild-type cells was predominantly in the reduced form GSH (Fig. 1B). This increase in GSH was likely to be due to the uptake of GSSG, followed by conversion of GSSG to GSH by GSSG reductase Glr1p, because glr1 cells accumulated predominantly GSSG upon GSSG treatment (Fig. 1C). Interestingly, the level of GSH in the glr1 mutant increased by up to 2-fold after exposure to GSSG for 1 h (Fig. 1B). Cells deleted for GLR1 have a more oxidizing intracellular environment as reflected from the Eh′ determination (Fig. 1D). Although exposure of wild-type and glr1 cells to GSSG led to a change in intracellular GSH and GSSG concentration (Fig. 1, B and C), cellular Eh′ remained relatively constant over the time of treatment. These data indicate that in the presence of excess GSSG, cells increase the level of GSH possibly to maintain cellular Eh′. Increased GSH accumulation in the glr1 cells treated with GSSG could have been due to elevated GSH synthesis and/or reduction of GSSG by a Glr1p-independent mechanism.
Treatment of Cells with Extracellular GSSG Leads to Accumulation of GSH in a Gsh1p- and Glr1p-independent Manner
The first committed step for de novo synthesis of GSH is catalyzed by γ-glutamylcysteine synthetase encoded by GSH1 (10). To determine whether elevated GSH accumulation in the glr1 cells treated with GSSG was associated with the expression of GSH1, GSH1 expression was monitored in the glr1 mutant over time using a GSH1::lacZ construct. The protein level of Gsh1p was also monitored using Western blot analysis of a glr1 strain harboring a chromosomally HA-tagged GSH1 under the control of its native promoter (Fig. 2, A and B).
FIGURE 2.
Extracellular GSSG leads to an increase in intracellular GSH in a GSH1- and GLR1-independent manner. A, exponentially growing glr1 cells transformed with the GSH1::lacZ construct were treated with 100 μm GSSG and assayed for β-galactosidase activity. B, chromosomal copy of GSH1 in the glr1 mutant was tagged with HA. Exponentially growing cells were treated with 100 μm GSSG over the indicated time, and the cell lysate analyzed for Gsh1-HAp using anti-HA antibodies. Pgk1p was detected using anti-Pgk1p antibodies as a loading control. C, indicated mutants were grown to A600 0.5 in SD medium supplemented with 25 μm GSH. Cells were harvested and analyzed for intracellular GSH and GSSG levels. D, indicated strains were grown for 2 days and A600 adjusted to 1.0 before 5 μl of the diluted cultures were spotted onto SD plates containing no glutathione, 25 μm GSH, or 25 μm GSSG. E–G, cells of gsh1 glr1 were treated with 100 μm GSSG (diamond), 100 μm GSSG treated with 2-vinyl-pyridine (square), or untreated (triangle). E, intracellular total glutathione; F, GSH; and G, GSSG were determined at the indicated time. Error bars indicate the S.E. of four samples of two independent experiments. H, GSSG reductase activity in glr1 cell lysate. Wild-type and glr1 cells were grown to exponential phase, and the cell lysate of each strain was assayed for GSSG reductase activity.
The transcript level of GSH1 and the level of Gsh1-HAp did not increase in glr1 cells after exposure to GSSG for 60 min (Fig. 2, A and B), despite a significant increase in intracellular concentration of GSSG and GSH (Fig. 1). From these data, it is unlikely that transcriptional regulation of GSH1 is involved but it is not possible to exclude that elevated GSH accumulation in glr1 cells treated with GSSG may have been due to post-translational regulation of Gsh1p activity. To explore this issue and to determine whether increased GSH in glr1 cells treated with GSSG was dependent on GSH synthesis per se, GSH1 was deleted in the glr1 mutant.
The gsh1 glr1 mutant is auxotrophic for glutathione but was found to be capable of utilizing GSSG as a sole source of glutathione (Fig. 2D). In exponential phase, gsh1 glr1 cells grown in medium supplemented with 25 μm GSH accumulated intracellular GSSG to a similar level as that of glr1 cells (Fig. 2C). The gsh1 glr1 mutant treated with GSSG accumulated total glutathione (Fig. 2E), and GSSG increased by more than 4-fold relative to untreated cells at time 0 (Fig. 2G). Surprisingly, upon GSSG treatment the gsh1 glr1 mutant also displayed an accumulation of intracellular GSH (Fig. 2F). To exclude any possibility of GSH contamination being present in the GSSG stock solution used for this experiment, a separate GSSG stock solution was treated with 2-vinylpyridine to derivatize any GSH that may have been present at trace levels. Comparable data were obtained for GSSG pretreated with 2-vinylpyridine (Fig. 2, E and F). The lower level of GSH and total glutathione, but similar level of GSSG in the gsh1 glr1 mutant may indicate that cells degrade excess GSSG, and/or in the gsh1 glr1 mutant uptake of GSH cannot keep pace with oxidation of GSH to GSSG followed by degradation of GSSG. These data indicate that the increase in GSH level observed in the glr1 mutant treated with GSSG was due to the presence of a Glr1p-independent system capable of reducing GSSG to GSH.
Presence of Residual GSSG Reductase Activity in the glr1 Mutant
To further examine the Glr1p-independent GSSG reductase activity in vitro, whole cell lysates of the wild-type and glr1 strains were assayed for their ability to reduce GSSG using an NADPH oxidation assay.
The cell lysate derived from glr1 cells displayed some residual GSSG-dependent oxidation of NADPH (∼2% of wild-type cells; Fig. 2H), indicating the presence of residual GSSG reductase activity. This residual Glr1p-independent GSSG reductase activity was NADPH-dependent because a GSSG-dependent oxidation of NADH was not observed (data not shown). Cell lysates derived from glr1 cells that were subjected to heating (100 °C, 10 min) did not exhibit residual GSSG reduction activity (Fig. 2H), indicating that the residual Glr1p-independent GSSG reductase activity was heat labile and may be due to the presence of a protein in cells, other than Glr1p, with the capacity to reduce GSSG to GSH.
The in vivo data and the total cell lysate analysis (Fig. 2) indicate that S. cerevisiae has an alternative system to Glr1p that reduces GSSG in a NADPH-dependent manner. Although the residual GSSG reductase activity was relatively low, based on the in vitro assay, it still provided sufficient activity to reduce GSSG in vivo, as observed in the gsh1 glr1 cells (Fig. 2). We surmised that there are two Glr1p-independent systems that could possibly reduce GSSG to GSH in S. cerevisiae, both of which are dependent on NADPH. These are the glutaredoxin and the thioredoxin systems.
Grx1p, Grx2p, Grx6p, and Grx7p Do Not Contribute to Reduction of GSSG in Cells Deleted of GLR1
Recently, it was demonstrated that mammalian glutaredoxin 2 (Grx2) can use thioredoxin reductase to reduce several substrates, including GSSG. This indicates that apart from the conventional glutathione reductase pathway, the glutaredoxin-thioredoxin reductase pathway may be able to act as an alternate system to regenerate GSH from GSSG. Grx2 has a CSYC motif at its active site, and mutation of the second cysteine residue to serine (CSYC to CSYS) does not abolish Grx2 activity (47). In S. cerevisiae, GRX6 encodes a monothiol glutaredoxin containing the CSYS motif. Furthermore it was shown that glutaredoxins Grx1p, Grx2p, Grx6p, and Grx7p displayed activity toward the artificial disulfide substrate hydroxyethyl disulfide (HEDS) (26, 32), indicating that these proteins may be able to reduce GSSG. Therefore, to investigate the possible involvement of these glutaredoxins in reducing GSSG, their respective genes were deleted in the glr1 strain background. Monothiol glutaredoxins Grx3p, Grx4p, and Grx5p were not included in these analyses because they do not contain the CSYS motif and do not display activity toward HEDS (48, 49).
The grx6 grx7 glr1, grx1 grx2 glr1, and grx1 grx2 grx6 grx7 glr1 cells did not show any obvious growth defect in SD medium (supplemental Fig. S1), and cell lysates of these strains still retained GSSG reductase activity at a level comparable to that of the glr1 strain (supplemental Fig. S1) indicating that the Grx1p, Grx2p, Grx6p, and Grx7p do not contribute significantly to the residual reductase activity observed in glr1 cells.
The Thioredoxin-Thioredoxin Reductase System Can Reduce GSSG to GSH
Cells deleted for TRR1 or TRX1 and TRX2 overaccumulate oxidized as well as reduced glutathione (18, 36). Furthermore, the trr1 glr1 and the trx1 trx2 glr1 mutants are inviable (18, 50), indicating that there is a close relationship between the thioredoxin and the glutathione systems. It was recently demonstrated that Trx1p of S. cerevisiae is able to reduce GSSG in vitro (51), indicating a potential role of the thioredoxin in reducing GSSG to GSH in vivo. To investigate the possible role of the thioredoxin-thioredoxin reductases in GSSG reduction in vivo, the TRX1, TRX2, or TRR1 genes were separately placed under the control of the GAL1 promoter, overexpressed in the glr1 mutant, and cell lysates were analyzed for GSSG reductase activity, intracellular GSH/GSSG levels, and the cellular glutathione Eh′ were estimated.
Overexpression of TRR1, TRX1, and TRX2 was each verified using Western blot analysis (Fig. 3A). Overexpression of TRR1, TRX1, or TRX2 led to a significant (p value < 0.05) reduction in cellular GSSG level, and there was a significant increase (p value < 0.05) in GSH levels when TRX1 and TRX2 were overexpressed (Fig. 3B). The glr1 cells overexpressing TRX1 or TRX2 displayed a more reducing environment as indicated by the glutathione Eh′ (Fig. 3B, bottom panel).
FIGURE 3.
The thioredoxin-thioredoxin reductase system can reduce GSSG in vivo. A and B, overexpression of TRR1, TRX1, and TRX2 in glr1 cells reduces intracellular GSSG. The glr1 mutant was transformed with pGAL1-TRR1–3XHA, pGAL1-TRX1–3XHA, or pGAL1-TRX2–3XHA. The transformed cells were grown to stationary phase in SD minus uracil medium and re-inoculated into SD-GAL minus uracil medium, and the cultures were allowed to grow for 18 h to A600 0.5–1.0 before harvesting. A, Western blot analysis (top) of cell lysate using anti-HA antibody. B, intracellular concentration of GSSG, GSH, and glutathione Eh′ of the indicated strains. Error bars indicate S.E. from six replicates from two independent experiments. Asterisks (*) indicate significant differences (p value <0.05) compared with the glr1 mutant harboring the control vector.
To further examine the relative contribution of Trx1p and Trx2p in reduction of GSSG in vivo, the glr1, trx1 glr1, and trx2 glr1 mutants were treated with extracellular GSSG and examined for intracellular glutathione concentration. The trx1 glr1 and the trx2 glr1 mutant had a small but significantly higher level of intracellular GSSG relative to the glr1 mutant in untreated conditions (Time 0, Fig. 4A). Upon GSSG treatment, the trx1 glr1 and the trx2 glr1 mutant accumulated slightly more GSSG at 60 min (Fig. 4A) and displayed less GSH accumulation compared with the glr1 mutant at 20 and 60 min (Fig. 4B). Furthermore, glr1 cells deleted for TRX1 or TRX2 displayed a more oxidizing environment as determined using the glutathione Eh′ compared with the glr1 single mutant (Fig. 4C).
FIGURE 4.
The trx1 glr1 and trx2 glr1 strains have a decreased capacity to convert GSSG to GSH compared with the glr1 strain in vivo. The indicated strains were grown to exponential phase (A600, 0.5), in SD medium treated with 100 μm GSSG, and intracellular glutathione was determined over the indicated time. A, GSSG and B, GSH of the indicated strains were determined. C, cellular glutathione Eh′ was determined over the indicated time. Error bars indicate the S.E. of six biological replicates from two independent experiments. Asterisks (*) indicate significant differences (p value < 0.05) compared with the glr1 mutant at the indicated time.
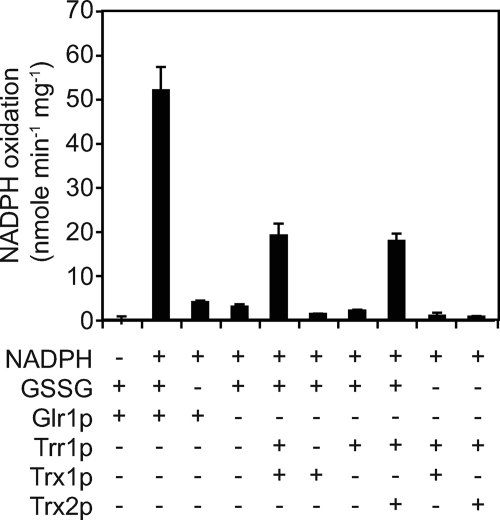
To investigate the relative efficacy of Trx1p and Trx2p in GSSG reduction, in vitro reduction of GSSG was examined in the presence of purified Trr1p, NADPH, Trx1p, or Trx2p. To compare the efficacy of GSSG reduction by the thioredoxin-thioredoxin reductase system with Glr1p, purified Glr1p was also examined.
The data from Fig. 5 indicate that Trr1p cannot reduce GSSG without the presence of Trx1p or Trx2p. The Trx1p-Trr1p and Trx2p-Trr1p combinations reduced GSSG with similar efficacy (∼18–19 nmol/min/mg), although at a much lower rate compared with Glr1p (52 nmol/min/mg). The respective Vmax (μmol/min/mg) and Km (mm) values for GSSG reduction were as follows: Trx1p (Vmax = 101.4; Km 2.6), Trx2p (Vmax = 101; Km = 2.5), and Glr1p (Vmax = 118; Km 0.08). These data indicate that the thioredoxin-thioredoxin system is able to reduce GSSG, although Glr1p is much more efficient in doing so.
FIGURE 5.
Trx1p and Trx2p can facilitate reduction of GSSG to a similar level in vitro. Purified proteins were reconstituted with the indicated compounds, and the rate of NADPH oxidation to NADP+ was monitored at 340 nm. Error bars indicate the S.D. of three replicates.
The above data support the role of thioredoxin in reducing GSSG in vivo. A single thioredoxin is able to support the conversion of GSSG to GSH in vivo, because both the trx1 glr1 and the trx2 glr1 mutants were still able to accumulate GSH in the presence of excess GSSG, and the in vitro data indicate that Trx1p and Trx2p reduces GSSG at a similar efficacy.
DISCUSSION
Glutathione is the major low molecular weight redox buffer in most cells (1). Understanding the mechanism that controls the production of GSH and the recycling of GSSG in cells will provide further insight into how cellular redox homeostasis is maintained. Here, it was demonstrated that cells have robust mechanisms to maintain cellular glutathione redox homeostasis. Excess oxidized glutathione is readily converted to GSH, mainly through the activity of the glutathione reductase Glr1p. The in vivo data presented here also strongly indicate that excess GSSG leads to an increase in cellular GSH level, and this increase is independent of GSH1 and can occur in the absence of glutathione reductase encoded by GLR1. The presence of an alternative system to reduce GSSG in S. cerevisiae was demonstrated, and this system is likely to be the cytosolic thioredoxin-thioredoxin reductase system.
Several pieces of evidence support the role of GSSG reduction to GSH by the thioredoxin-thioredoxin reductase system. First, it was demonstrated that overexpression of either of the cytosolic thioredoxins Trx1p or Trx2p, or the thioredoxin reductase Trr1p led to a decrease in intracellular GSSG level in the glr1 mutant in vivo. Second, deletion of TRX1 or TRX2 in the glr1 mutant treated with GSSG reduced the accumulation of GSH compared with the glr1 mutant alone. Furthermore, it was previously shown that in S. cerevisiae, the trx1 trx2 double mutant and the trr1 mutant overaccumulate both oxidized and reduced glutathione. In addition, the trr1 glr1 double mutant and the trx1 trx2 glr1 triple mutants are inviable (18), indicating that the thioredoxin-thioredoxin reductase system overlaps with the glutathione reductase system. These data indicate that the cytosolic thioredoxin-thioredoxin reductase system can prevent the overaccumulation of GSSG in cells lacking the glutathione reductase Glr1p. The synthetic lethality associated with simultaneous disruption of the thioredoxin-thioredoxin reductase and glutathione reductase systems makes it difficult to determine the extent to which, or indeed whether or not, other systems contribute in reduction of GSSG in vivo. In addition the role that additional factors including GSH synthesis, GSH/GSSG degradation, transport of GSH/GSSG between cellular compartments, and/or formation/turnover of glutathione-protein mixed disulfides play in maintenance of overall cellular and organellar redox homeostasis requires further investigation.
Although thioredoxins have been shown to reduce a broad spectrum of substrates, GSSG is generally not considered to be a good substrate for thioredoxins (19, 35). It has been demonstrated that the thioredoxin-thioredoxin reductase system in the malaria parasite Plasmodium falciparum and fruit fly Drosophila melanogaster can convert GSSG to GSH in vitro (52, 53). Recently the second order rate constant of GSSG reduction by thioredoxin 1 and thioredoxin reductases in S. cerevisiae was determined by in vitro reconstitution of the purified proteins (51). It was shown that purified yeast thioredoxin 1 and thioredoxin reductase can reduce GSSG with a second order rate constant of 6 mm−1 min−1. This rate constant of the thioredoxin-thioredoxin reductase system in S. cerevisiae is similar to that established for the analogous system in Escherichia coli and comparable to that of D. melanogaster (10 mm−1 min−1) (51–53). These rate constants are at least ∼100–400 lower when comparing the reduction of GSSG to the reduction of insulin as a substrate by the thioredoxin-thioredoxin reductase system (19). However, given that GSSG may accumulate in the millimolar range, it was proposed that this rate is physiologically significant, and the reaction may occur in vivo (53). Additionally, the activity for GSSG reduction by the thioredoxins versus glutathione reductase measured in vitro using recombinant forms of the enzymes, or native forms isolated from cells may also differ to that of the native forms of the respective enzymes in intact cells. Differences in the availability of NADPH, GSSG, and/or other factors, and the presence of competing side reactions may also influence the relative contribution of the glutathione reductase and thioredoxin systems for reduction of GSSG in vivo. The contribution of the Trr1p/Trx1,2p system is likely to become more important when cells experience certain stress conditions (e.g. oxidative) where the burden on the glutathione redox system is known to increase and GSSG overaccumulation may ensue (7, 8, 14, 15). The observations that: 1) the gsh1 glr1 mutant can utilize GSSG as the sole source of glutathione; 2) the gsh1 glr1 strain accumulates GSH when challenged with exogenous GSSG and that overexpression of thioredoxins or thioredoxin reductase can reduce GSSG accumulation in glr1 cells; and 3) deletion of a single thioredoxin reduces the accumulation of GSH in the presence of excess GSSG all provide further support that the thioredoxin-thioredoxin reductase system can function to reduce GSSG to GSH in vivo. In a recent study of Arabidopsis, it was demonstrated that mutants lacking an isoform of glutathione reductase still exhibit dynamic reduction of GSSG in vivo under oxidative stress conditions (54). It was also demonstrated that the Arabidopsis thioredoxins were capable of reducing GSSG in vitro. These findings led to the proposal that the thioredoxin-thioredoxin reductase system may function as a backup for cytosolic glutathione reductase in Arabidopsis. Therefore, it is likely that the thioredoxins-thioredoxin reductase system and the glutathione reductase system function to reduce GSSG not only in plants and yeast, but also in a similar manner in other species.
Supplementary Material
Acknowledgments
We thank Anita Ayer for providing the grx6 and grx7 mutants and Geoff Kornfeld for helpful suggestions.
This work was supported by grants from the Australian Research Council and the Cancer Institute New South Wales.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- GSH
- reduced glutathione
- GSSG
- oxidized glutathione
- HA
- hemagglutinin.
REFERENCES
- 1.Schafer F. Q., Buettner G. R. (2001) Free Radic Biol. Med. 30, 1191–1212 [DOI] [PubMed] [Google Scholar]
- 2.Perrone G. G., Tan S. X., Dawes I. W. (2008) Biochim. Biophys. Acta 1783, 1354–1368 [DOI] [PubMed] [Google Scholar]
- 3.Rietsch A., Beckwith J. (1998) Annu. Rev. Genet. 32, 163–184 [DOI] [PubMed] [Google Scholar]
- 4.Meister A., Anderson M. E. (1983) Annu. Rev. Biochem. 52, 711–760 [DOI] [PubMed] [Google Scholar]
- 5.Hwang C., Sinskey A. J., Lodish H. F. (1992) Science 257, 1496–1502 [DOI] [PubMed] [Google Scholar]
- 6.Drakulic T., Temple M. D., Guido R., Jarolim S., Breitenbach M., Attfield P. V., Dawes I. W. (2005) FEMS Yeast Res. 5, 1215–1228 [DOI] [PubMed] [Google Scholar]
- 7.Ng C. H., Tan S. X., Perrone G. G., Thorpe G. W., Higgins V. J., Dawes I. W. (2008) Free Radic Biol. Med. 44, 1131–1145 [DOI] [PubMed] [Google Scholar]
- 8.Tan S. X., Teo M., Lam Y. T., Dawes I. W., Perrone G. G. (2009) Mol. Biol. Cell 20, 1493–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perrone G. G., Grant C. M., Dawes I. W. (2005) Mol. Biol. Cell 16, 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohtake Y., Yabuuchi S. (1991) Yeast 7, 953–961 [DOI] [PubMed] [Google Scholar]
- 11.Inoue Y., Sugiyama K., Izawa S., Kimura A. (1998) Biochim. Biophys. Acta 1395, 315–320 [DOI] [PubMed] [Google Scholar]
- 12.Grant C. M., MacIver F. H., Dawes I. W. (1997) Mol. Biol. Cell 8, 1699–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izawa S., Inoue Y., Kimura A. (1995) FEBS Lett. 368, 73–76 [DOI] [PubMed] [Google Scholar]
- 14.Grant C. M., MacIver F. H., Dawes I. W. (1996) Curr. Genet. 29, 511–515 [DOI] [PubMed] [Google Scholar]
- 15.Grant C. M., Perrone G., Dawes I. W. (1998) Biochem. Biophys. Res. Commun. 253, 893–898 [DOI] [PubMed] [Google Scholar]
- 16.Stephen D. W., Jamieson D. J. (1996) FEMS Microbiol. Lett. 141, 207–212 [DOI] [PubMed] [Google Scholar]
- 17.Grant C. M., Collinson L. P., Roe J. H., Dawes I. W. (1996) Mol. Microbiol. 21, 171–179 [DOI] [PubMed] [Google Scholar]
- 18.Muller E. G. (1996) Mol. Biol. Cell 7, 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmgren A. (1985) Annu. Rev. Biochem. 54, 237–271 [DOI] [PubMed] [Google Scholar]
- 20.Muller E. G. (1991) J. Biol. Chem. 266, 9194–9202 [PubMed] [Google Scholar]
- 21.Kuge S., Jones N. (1994) EMBO J. 13, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draculic T., Dawes I. W., Grant C. M. (2000) Mol. Microbiol. 36, 1167–1174 [DOI] [PubMed] [Google Scholar]
- 23.Machado A. K., Morgan B. A., Merrill G. F. (1997) J. Biol. Chem. 272, 17045–17054 [DOI] [PubMed] [Google Scholar]
- 24.Pearson G. D., Merrill G. F. (1998) J. Biol. Chem. 273, 5431–5434 [DOI] [PubMed] [Google Scholar]
- 25.Holmgren A. (1989) J. Biol. Chem. 264, 13963–13966 [PubMed] [Google Scholar]
- 26.Luikenhuis S., Perrone G., Dawes I. W., Grant C. M. (1998) Mol. Biol. Cell 9, 1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant C. M., Luikenhuis S., Beckhouse A., Soderbergh M., Dawes I. W. (2000) Biochim. Biophys. Acta 1490, 33–42 [DOI] [PubMed] [Google Scholar]
- 28.Ojeda L., Keller G., Muhlenhoff U., Rutherford J. C., Lill R., Winge D. R. (2006) J. Biol. Chem. 281, 17661–17669 [DOI] [PubMed] [Google Scholar]
- 29.Pujol-Carrion N., Belli G., Herrero E., Nogues A., de la Torre-Ruiz M. A. (2006) J. Cell Sci. 119, 4554–4564 [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Manzaneque M. T., Tamarit J., Bellí G., Ros J., Herrero E. (2002) Mol. Biol. Cell 13, 1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mühlenhoff U., Gerber J., Richhardt N., Lill R. (2003) EMBO J. 22, 4815–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesecke N., Spang A., Deponte M., Herrmann J. M. (2008) Mol. Biol. Cell 19, 2673–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izquierdo A., Casas C., Mühlenhoff U., Lillig C. H., Herrero E. (2008) Eukaryot. Cell 7, 1415–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmgren A., Björnstedt M. (1995) Methods Enzymol. 252, 199–208 [DOI] [PubMed] [Google Scholar]
- 35.Arnér E. S., Zhong L., Holmgren A. (1999) Methods Enzymol. 300, 226–239 [DOI] [PubMed] [Google Scholar]
- 36.Trotter E. W., Grant C. M. (2002) Mol. Microbiol. 46, 869–878 [DOI] [PubMed] [Google Scholar]
- 37.Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., Chu A. M., Connelly C., Davis K., Dietrich F., Dow S. W., El Bakkoury M., Foury F., Friend S. H., Gentalen E., Giaever G., Hegemann J. H., Jones T., Laub M., Liao H., Liebundguth N., Lockhart D. J., Lucau-Danila A., Lussier M., M'Rabet N., Menard P., Mittmann M., Pai C., Rebischung C., Revuelta J. L., Riles L., Roberts C. J., Ross-MacDonald P., Scherens B., Snyder M., Sookhai-Mahadeo S., Storms R. K., Véronneau S., Voet M., Volckaert G., Ward T. R., Wysocki R., Yen G. S., Yu K., Zimmermann K., Philippsen P., Johnston M., Davis R. W. (1999) Science 285, 901–906 [DOI] [PubMed] [Google Scholar]
- 38.Janke C., Magiera M. M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E., Knop M. (2004) Yeast 21, 947–962 [DOI] [PubMed] [Google Scholar]
- 39.Ito H., Fukuda Y., Murata K., Kimura A. (1983) J. Bacteriol. 153, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelperin D. M., White M. A., Wilkinson M. L., Kon Y., Kung L. A., Wise K. J., Lopez-Hoyo N., Jiang L., Piccirillo S., Yu H., Gerstein M., Dumont M. E., Phizicky E. M., Snyder M., Grayhack E. J. (2005) Genes Dev. 19, 2816–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberti S., Gitler A. D., Lindquist S. (2007) Yeast 24, 913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose M., Botstein D. (1983) Methods Enzymol. 101, 167–180 [DOI] [PubMed] [Google Scholar]
- 43.Vandeputte C., Guizon I., Genestie-Denis I., Vannier B., Lorenzon G. (1994) Cell Biol. Toxicol. 10, 415–421 [DOI] [PubMed] [Google Scholar]
- 44.Wheals A. E. (1982) Mol. Cell Biol. 2, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukhopadhyay R., Shi J., Rosen B. P. (2000) J. Biol. Chem. 275, 21149–21157 [DOI] [PubMed] [Google Scholar]
- 46.Bourbouloux A., Shahi P., Chakladar A., Delrot S., Bachhawat A. K. (2000) J. Biol. Chem. 275, 13259–13265 [DOI] [PubMed] [Google Scholar]
- 47.Johansson C., Lillig C. H., Holmgren A. (2004) J. Biol. Chem. 279, 7537–7543 [DOI] [PubMed] [Google Scholar]
- 48.Tamarit J., Belli G., Cabiscol E., Herrero E., Ros J. (2003) J. Biol. Chem. 278, 25745–25751 [DOI] [PubMed] [Google Scholar]
- 49.Herrero E., de la Torre-Ruiz M. A. (2007) Cell Mol. Life Sci. 64, 1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trotter E. W., Grant C. M. (2003) EMBO Rep. 4, 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bao R., Zhang Y., Lou X., Zhou C. Z., Chen Y. (2009) Biochim. Biophys. Acta 1794, 1218–1223 [DOI] [PubMed] [Google Scholar]
- 52.Kanzok S. M., Fechner A., Bauer H., Ulschmid J. K., Müller H. M., Botella-Munoz J., Schneuwly S., Schirmer R., Becker K. (2001) Science 291, 643–646 [DOI] [PubMed] [Google Scholar]
- 53.Kanzok S. M., Schirmer R. H., Turbachova I., Iozef R., Becker K. (2000) J. Biol. Chem. 275, 40180–40186 [DOI] [PubMed] [Google Scholar]
- 54.Marty L., Siala W., Schwarzländer M., Fricker M. D., Wirtz M., Sweetlove L. J., Meyer Y., Meyer A. J., Reichheld J. P., Hell R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9109–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.