Abstract
Zinc is an essential trace element and plays crucial roles in normal development, often as an integral structural component of transcription factors and enzymes. Recent evidence suggests that intracellular Zn2+ functions as a signaling molecule, mediating a variety of important physiological phenomena. However, the immediate effectors of intracellular Zn2+ signaling are not well known. We show here that intracellular Zn2+ potently and reversibly activates large-conductance voltage- and Ca2+-activated Slo1 K+ (BK) channels. The full effect of Zn2+ requires His365 in the RCK1 (regulator of conductance for K+) domain of the channel. Furthermore, mutation of two nearby acidic residues, Asp367 and Glu399, also reduced activation of the channel by Zn2+, suggesting a possible structural arrangement for Zn2+ binding by the aforementioned residues. Extracellular Zn2+ activated Slo1 BK channels when coexpressed with Zn2+-permeable TRPM7 (transient receptor potential melastatin 7) channels. The results thus demonstrate that Slo1 BK channels represent a positive and direct effector of Zn2+ signaling and may participate in sculpting cellular response to an increase in intracellular Zn2+ concentration.
Keywords: Calcium, Potassium Channels, Signal Transduction, TRP Channels, Zinc, BK Channel, Calcium-Activated Potassium Channels, Slo1
Introduction
Zinc is the second most abundant transition metal in the human body, playing a pivotal role in the normal development and growth. The utmost importance of zinc is evidenced by the diverse array of symptoms that could result from a chronic dietary deficiency of zinc (1). Biochemically, zinc serves as an essential structural and a catalytic component in many metalloproteins (2), in which the metal is typically coordinated by four or five ligands (3). Multiple zinc coordination geometries are known, but histidine and cysteine typically act as essential ligands (4).
In addition to its role as an integral structural and catalytic factor, Zn2+ is increasingly recognized as a potential intracellular signaling molecule, similar to Ca2+ (5, 6). Like intracellular Ca2+, intracellular Zn2+ is normally kept to a very low concentration, from pm to nm (5). Although measurements of free intracellular Zn2+ concentrations ([Zn2+]i) in living cells remain challenging, studies do suggest that [Zn2+]i may significantly increase under some conditions. For example, a robust release of Zn2+ from the endoplasmic reticulum, termed “zinc wave,” has been observed in response to extracellular stimuli, further suggesting that Zn2+ may act as an intracellular second messenger (7). In addition, local [Zn2+]i may be significantly higher near Zn2+-permeable channels (5, 8), analogous to the well known micro- and nano-domains of intracellular Ca2+ (9). Moreover, [Zn2+]i may increase concomitantly with [Ca2+]i under pathological conditions such as ischemia/hypoxia (5, 6, 10, 11), in which intracellular Ca2+ overload is suspected to contribute to cell death in these conditions (12). However, whether such increases in [Zn2+]i contribute to the deleterious effect or play a compensatory cell-protective effect is not clear (5, 11, 13–16).
Large-conductance voltage- and Ca2+-activated K+ (BKCa, Slo1 BK or KCa1.1) channels are distinguished by their allosteric activation by voltage and intracellular Ca2+ (17–19). Like other voltage-gated K+ channels, a BK channel complex includes four pore-forming α (Slo1) subunits, each of which contains a voltage sensor domain (S1–S4) and one-fourth of the ion conduction pore (S5–S6) (20). In addition, each Slo1 subunit possesses the transmembrane segment S0 (21) and a large cytoplasmic area harboring two homologous domains termed “regulators of conductance of potassium” (RCK1 and RCK2) essential for activation by Ca2+ for the channel (22, 23). Functionally, BK channels participate in many crucial physiological phenomena including vasoregulation, synaptic transmission, and hormone secretion mainly by affecting membrane excitability (17). In addition, as a feedback controller of intracellular Ca2+, BK channel activation has been demonstrated to have a potent cell protection effect by limiting the influx of Ca2+ during hypoxia/ischemia (24, 25).
The concomitant increases in [Zn2+]i and [Ca2+]i in ischemia/hypoxia and the cytoprotective role of the BK channel under the pathological conditions prompted us to examine whether Zn2+ is also a physiological activator of the channel. The Slo1 protein indeed contains multiple putative Zn2+-binding amino acid sequences such as HXXXH (X represents any amino acid) identified in other metal-binding proteins including S100 proteins, the largest subgroup of the EF-hand Ca2+-binding protein family (26–28). Our excised patch clamp measurements from heterologously expressed human Slo1 (hSlo1)3 BK channels revealed that intracellular Zn2+ robustly activates the channel and that mutation of one histidine residue in the RCK1 domain fully abolished the stimulatory effect of Zn2+. Our results therefore suggest that Slo1 coordinates Zn2+ using amino acid ligands in the RCK1 domain and that the Slo1 BK channel is a positive effector of intracellular Zn2+ signaling.
EXPERIMENTAL PROCEDURES
Channel Expression
Human Slo1 (KCNMA1; U11058) and its mutants in the expression vector pCI-neo (Promega), HA-tagged rat TRPM7 (XP_001056331) in pTracer-CMV vector (Invitrogen), and rat SK2 (KCNN2; U69882) in the expression vector pcDNA3 (Invitrogen) were transiently expressed in HEK tsA cells using FuGENE 6 (Roche) as described (29). In some experiments, hSlo1 and β1 (KCNMB1; U38907) in pEGFP-N1 (Clontech) were transfected together with a weight ratio of 1:1. The mutant channels were constructed using a PCR-based mutagenesis method (Agilent), and the sequences were verified (University of Pennsylvania DNA Sequencing Facility).
Electrophysiology and Data Analysis
Ionic currents were recorded using the cell-attached or excised inside-out configuration at room temperature. Patch electrodes (Warner) had a typical initial resistance of 1.5–2.0 megohms. The series resistance, up to 90% of the initial input resistance, was electronically compensated in the macroscopic current measurements. Macroscopic capacitive and leak currents were subtracted using a P/6 protocol. The current signal was filtered at 10 kHz through the built-in filter of the patch clamp amplifier (AxoPatch 200A; MDS Analytical Technologies) and digitized at 100 kHz using an ITC-16 AD/DA interface (HEKA). Conductance-voltage (G-V) curves were generated from tail currents and fitted with a Boltzmann equation as described (29). The resulting half-activation voltage (V0.5) was used to quantify the effect of Zn2+ on the channel. Both activation and deactivation time constants were obtained by fitting the currents with a single exponential excluding the initial 180 μs. The results were analyzed as described using IGOR Pro (WaveMetrics) (29). Statistical comparisons between two groups were performed using the unpaired or paired t test, as appropriate. Comparison of more than two groups was performed using analysis of variance followed by a Tukey HSD test as implemented in IGOR Pro. Statistical significance was assumed at p ≤ 0.05, and the data are presented as mean ± S.E. The number of samples in each group is shown in parentheses unless noted otherwise.
Chemicals and Solutions
All chemicals were from Sigma except for 2-aminoethyl methanethiosulfonate hydrobromide (MTSEA; Biotium). TPEN was dissolved in dimethyl sulfoxide and diluted with the internal recording solution to the final concentration of 10 μm. The final concentration of dimethyl sulfoxide (0.02%, v/v) did not affect Slo1 channel currents. For inside-out patch recording, the extracellular solution contained 140 mm KCl, 2 mm MgCl2, 10 mm HEPES, pH 7.2, with N-methyl-d-glucamine (NMDG). The intracellular solution contained 140 mm KF, 10 mm HEPES, pH 7.2 or 6.2, with NMDG and a different concentration of ZnCl2 or ZnSO4. The use of KF in the solution limited [Ca2+] < 20 nm (30). In the experiments with high concentrations of Ca2+, the intracellular solution did not contain any chelator and the pH was adjusted to 7.2 with NMDG. For the cell-attached patch experiment, the electrode solution contained 140 mm KCl, 2 mm MgCl2 or 2 mm ZnCl2, 10 mm HEPES, pH 7.2, with NMDG. The bath solution contained 130 mm NaCl, 4.0 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 10 mm HEPES, 15 mm glucose, pH 7.4, with NMDG.
RESULTS
Intracellular Zn2+ Activates hSlo1 Channels
To observe the effect of cytoplasmic Zn2+ on the Slo1 channel while maintaining a very low concentration of Ca2+, we used KF in the internal solution in which most of the contaminating Ca2+ precipitated due to the low solubility of CaF2. In such an internal solution, the free Ca2+ concentration has been estimated to be <20 nm (30). Consistently, we found that the activity of the hSlo1 channel remained unaltered when the inside-out patches were transferred from the KF internal solution (see “Experimental Procedures”) to the KCl internal solution with 11 mm EGTA in which [Ca2+] is calculated to be <10 nm (WEBMAXC STANDARD; data not shown). In addition, we found that up to 300 μm of Zn2+ in the KF solution failed to activate the small-conductance Ca2+-activated channel 2 (SK2), which has higher Ca2+ sensitivity than the Slo1 channel (9) (data not shown). These observations together affirmed that [Ca2+]i was appropriately buffered to a negligible level when Zn2+ was added into the KF internal solution.
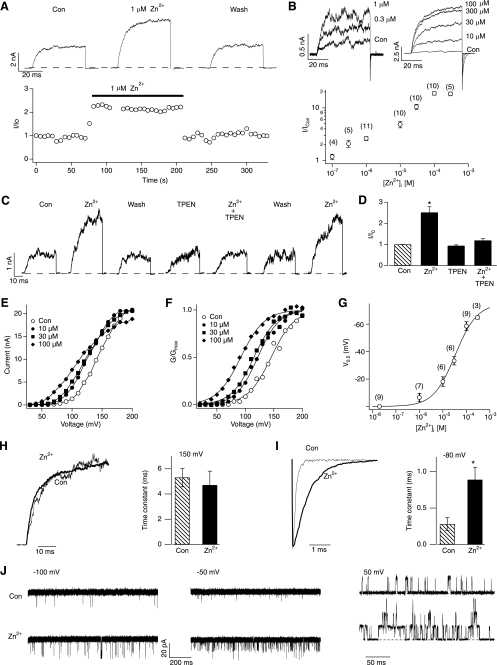
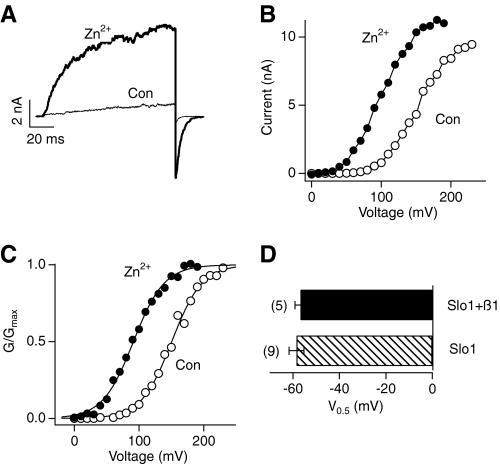
Addition of Zn2+ (0.3–300 μm) quickly and reversibly increased hSlo1 BK currents (Fig. 1, A and C) in a concentration-dependent manner (Fig. 1B). TPEN, a Zn2+ chelator with low affinity for Ca2+, fully antagonized the stimulatory effect of the Zn2+ addition to the intracellular solution (Fig. 1, C and D), further confirming that it was Zn2+ that increased the hSlo1 current. In contrast, extracellular Zn2+, up to 2 mm, was without any stimulatory effect (see Fig. 6B; see also Ref. 31).
FIGURE 1.
Application of Zn2+ to the cytoplasmic side activates hSlo1 channels. A, representative hSlo1 currents at 100 mV without and with 1 μm Zn2+ (top). The currents were elicited by pulses from 0 to 100 and then to 0 mV. The peak outward current size at 100 mV is plotted as a function time (bottom). B, representative hSlo1 currents (top) and values of normalized currents (I/ICon, bottom) at different concentrations of Zn2+ at 100 mV. The values of I/ICon were 1.17 ± 0.14, 2.09 ± 0.31, 2.62 ± 0.62, 4.88 ± 0.68, 10.50 ± 1.19, 18.90 ± 1.40, and 18.41 ± 1.52 at 0.1, 0.3, 1, 10, 30, 100, and 300 μm Zn2+, respectively. The currents were elicited by pulses from 0 to 100 and then to −80 mV. C, Zn2+ reversibly and repeatedly increased hSlo1 channel currents, but 10 μm TPEN abolished the effect of Zn2+. The currents were elicited as in A. D, fractional increase in the peak current size by Zn2+ (10 μm) in the absence and presence of TPEN (10 μm). The currents were elicited as in C. *, p < 0.01 compared with control group (n = 3 in each group). E, I-V curves of hSlo1 channels in the absence (open circles) and presence of 10 μm (filled circles), 30 μm (filled squares), and 100 μm (filled diamonds) Zn2+. F, G-V curves of hSlo1 channels with different concentrations of Zn2+: 0 μm (no Zn2+ added; open circles), 10 μm (filled circles), 30 μm (filled squares), and 100 μm (filled diamonds). The steepness of the G-V curves with Zn2+ was not different to that in the control condition (p > 0.69). G, V0.5 changes by different concentration of Zn2+. The concentration response was fitted by a Hill equation, ΔV0.5(x) = ΔV0.5(max)/[1 + (EC50/x)n] where n is the Hill coefficient, x is the Zn2+ concentration, and ΔV0.5(max) is the maximal shift in V0.5. H, representative normalized currents recorded at 150 mV before (thin trace) and after (thick trace) application of 100 μm Zn2+ (left). Time constants of activation before and after application of 100 μm Zn2+ (n = 7 in each group) (right). I, representative normalized currents recorded at −80 mV after 40-ms pulses of 150 mV in the absence (thin trace) and presence (thick trace) of 100 μm Zn2+ (left). Time constants of deactivation before and after application of 100 μm Zn2+ (n = 8 in each group) (right). *, p < 0.05 compared with control group. J, representative single-channel currents from different patches at −100, −50, and 50 mV before and after application of Zn2+. 10 μm and 100 μm Zn2+ were used at positive and negative voltages, respectively. Con, control.
FIGURE 6.
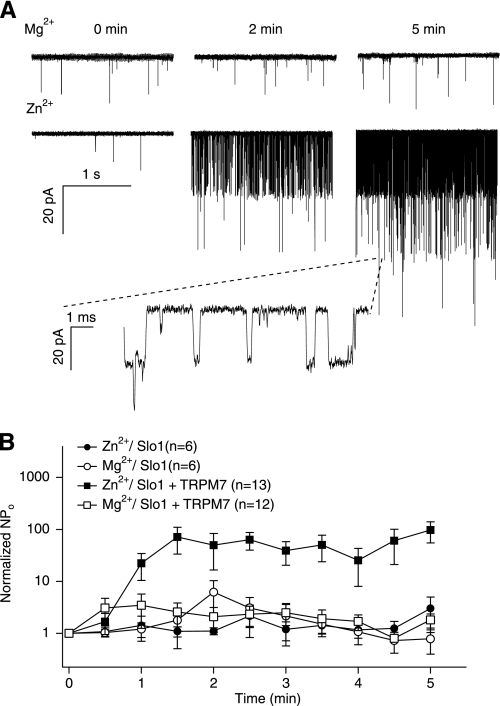
Coexpression with TRPM7 channels facilitates opening of hSlo1 channels. A, representative hSlo1 currents recorded at the time indicated in the presence of extracellular Mg2+ (top) or Zn2+ (bottom). Single-channel currents were recorded at −80 mV in the cell attached configuration from cells transfected with hSlo1 and TRPM7. The time 0 min indicates seal formation. Similar results were obtained in 8 of 13 cells. B, mean time courses of changes in channel open probability in the presence of extracellular Mg2+ (open symbols) or Zn2+ (filled symbols) with hSlo1 channels alone (circles) or with hSlo1 and TRPM7 channels together (squares). Single channel currents were recorded as in A. N, number of channels; Po, open probability.
The current-enhancing effect of Zn2+ was voltage-dependent (Fig. 1E) and accompanied by a shift in G-V to the hyperpolarized direction without any change in the steepness (Fig. 1F). Saturating concentrations of Zn2+ (≥100 μm) produced a shift in V0.5 of about −75 mV. The Zn2+-dependent shift in G-V V0.5 had an EC50 value of 33.6 ± 12.2 μm and a Hill coefficient of 0.93 ± 0.22 (Fig. 1G).
We noticed that high concentrations of Zn2+ slightly diminished the peak outward currents at extreme positive voltages (e.g. 200 mV in Fig. 1E) without decreasing the inward tail current size. This small inhibitory effect, most probably reflecting voltage-dependent block of the channel pore by Zn2+ (30), was not investigated any further. In addition to the shift of voltage dependence of activation to the hyperpolarized direction, Zn2+ slowed the deactivation kinetics without affecting the activation kinetics (Fig. 1, H and I).
The stimulatory effect of Zn2+ was also observed at the single-channel level. Zn2+ drastically increased single-channel open probability in a wide range of voltages, including a physiologically relevant negative voltage (−50 mV) and an extreme negative voltage where the primary voltage sensors of the channel are not activated (Fig. 1J). Zn2+ had no noticeable effect on the unitary current size (Fig. 1J).
Zn2+-dependent Activation of the hSlo1 BK Channel Did Not Require the Conserved Zinc-binding Motifs
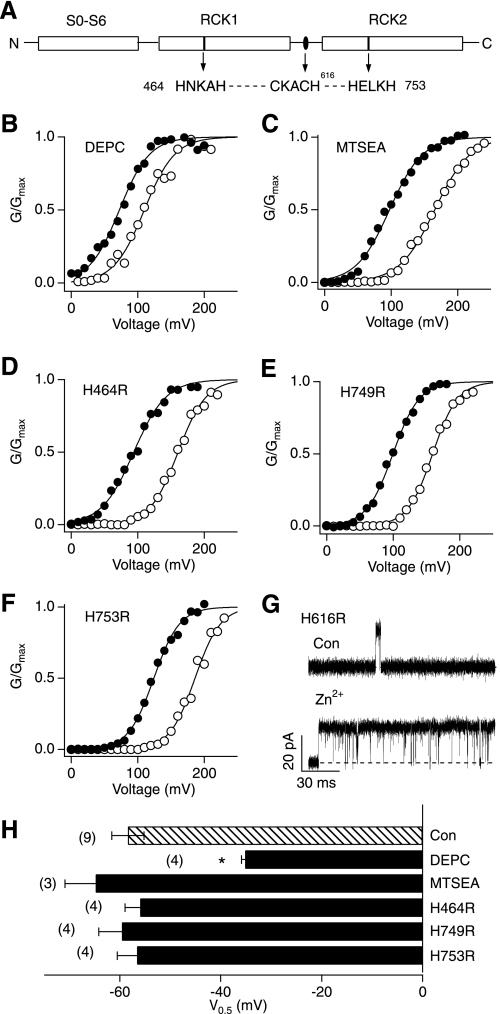
Structural studies suggest that histidine and cysteine are the two most frequently used zinc ligands in metalloproteins, in which zinc interacts with the imidazole nitrogen or thiol sulfur in the conserved zinc-binding motifs such as HXXXH, and CXXXH (X represents any amino acid) (2–4, 27, 28, 32). Mutation of either histidine residue in the conserved motif typically disrupts the Zn2+ coordination and reduces catalytic activity of metalloenzymes (26, 33). Inspection of the hSlo1 sequence shows that the cytoplasmic domain of the channel contains three putative zinc-binding motifs, 464HNKAH468, 749HELKH753, and 612CKACH616, localized in RCK1, RCK2, and the linker region between the two RCK domains, respectively (Fig. 2A). To assess the contributions of His and Cys to the Zn2+-induced Slo1 BK channel activation, we utilized diethyl pyrocarbonate, a histidine-modifying reagent (34), and a cysteine-modifying reagent, MTSEA (35). Our results showed that pretreatment of the channel with diethyl pyrocarbonate significantly attenuated the Zn2+-induced activation of the channel, decreasing the V0.5 shift to ∼50% of that observed in the control group (Fig. 2, B and H). In contrast, MTSEA failed to alter the Zn2+-induced channel activation (Fig. 2, C and H). We thus reasoned that the Slo1 protein interacts with Zn2+ using histidine residues, possibly in the aforementioned zinc motifs (Fig. 2A).
FIGURE 2.
Zn2+ remains effective in mutant Slo1 channels with the conserved zinc-binding motifs disrupted. A, a schematic representation of the wild-type hSlo1 channel, showing three potential Zn2+-binding motifs in the RCK1 and RCK2 domains and the linker between of the two domains (top). B and C, representative G-V curves before (open circles) and after (filled circles) application of 100 μm Zn2+ following pretreatment with 2 mm diethyl pyrocarbonate for 5 min (B) and 1 mm MTSEA for 10 min (C). D–F, representative G-V curves of the His-to-Arg mutant channels indicated in the absence (open circle) and presence (filled circle) of 100 μm Zn2+. G, representative single-channel currents recorded from hSlo1 H616R in the absence (top) and presence (bottom) of 100 μm Zn2+. The single-channel openings were elicited by pulses to 100 mV from 0 mV. Similar results were observed in another three patches. H, changes in V0.5 by 100 μm Zn2+ in the wild-type channels with and without diethyl pyrocarbonate or MTSEA pretreatment and also in the His-to-Arg mutants. *, p < 0.001 compared with the no treatment wild-type group.
The potential involvement of the histidine residues in the zinc-binding motifs in the channel was further tested by mutation of His464, His616, His749, and His753. A robust stimulatory effect of Zn2+, indistinguishable from that in the wild-type channel, remained in these His-to-Arg mutants (Fig. 2, D–F). The mutant channel H616R (29) did not express well enough to record macroscopic currents; however, the mutant retained a Zn2+ sensitivity indistinguishable from that of the wild-type channel based on single-channel measurements (Fig. 2G). These results collectively indicated that the His residue(s) that coordinate Zn2+ are located elsewhere.
Zn2+ Is Less Effective at Low pH
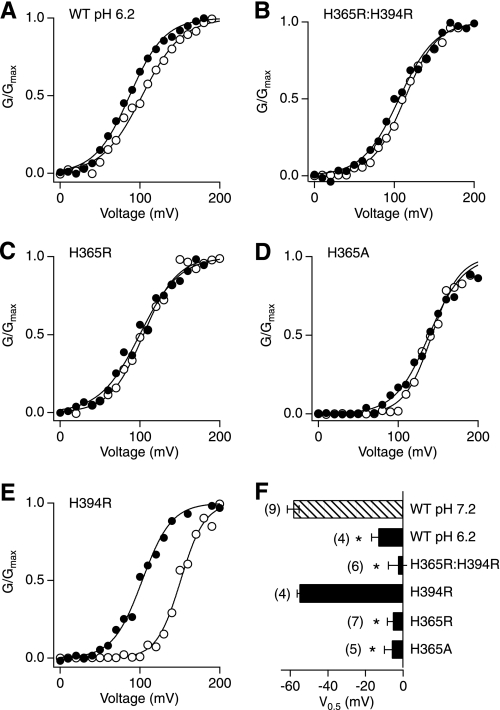
The bound Zn2+ can be removed from the metalloenzymes in low pH conditions, possibly owing to the protonation of imidazole nitrogens (36). We therefore examined whether intracellular H+ affected the action of Zn2+ on the Slo1 channel. The Zn2+-induced shift in V0.5 was indeed significantly reduced in the pH 6.2 internal solution to −25.1 ± 7.1 mV, less than a half of that at pH 7.2 (p < 0.01; Fig. 3, A and F).
FIGURE 3.
His365 in the RCK1 domain is required for Zn2+ action. A, G-V curves in the wild-type channel at pH 6.2 with (filled circles) and without (open circles) 100 μm Zn2+. B–E, typical G-V curves in the His mutants at pH 7.2 before (open circles) and after (filled circles) application of 100 μm Zn2+. F, changes in V0.5 in the wild-type channel at pH 7.2 and 6.2 and in the His mutants at pH 7.2. *, p < 0.001 compared with the wild-type (WT) channel at pH 7.2.
Mutation of His365 Abolishes the Zn2+ Effect
We previously demonstrated that two His residues, His365 and His394, in the RCK1 domain serve as the primary H+ sensors of the hSlo1 channel and mediate pH-dependent activation of the channel (37, 38). The antagonistic effect of low pH on the Zn2+-dependent activation suggests that the same His residues may be required for the Zn2+ action. Consistent with this possibility, the double mutation H365R/H394R completely abolished the effect of Zn2+ on V0.5; the ΔV0.5 value was −2.8 ± 5.1 mV (p < 0.001 compared with the wild-type channel; Fig. 3, B and F). Of the two His residues, His365 clearly plays the most important role, for the single mutation H365R alone eliminated the Zn2+ sensitivity (ΔV0.5 = −5.5 ± 2.9 mV; p < 0.0001 compared with the wild-type channel; Fig. 3, C and F). Mutation of His365 to neutral alanine (H365A) also completely disrupted the Zn2+ sensitivity of the channel (−6.0 ± 3.9 mV; p < 0.001 compared with the wild-type channel and p > 0.5 compared with H365R) (Fig. 3, D and F). In contrast, the mutant H394R remained fully Zn2+-sensitive (ΔV0.5 = −55.2 ± 1.2 mV; p > 0.5; Fig. 3, E and F). While both His365 and His394 in the RCK1 domain are important for pH-dependent activation of the hSlo1 channel (37, 38), only His365 is required for the Zn2+-dependent activation of the channel.
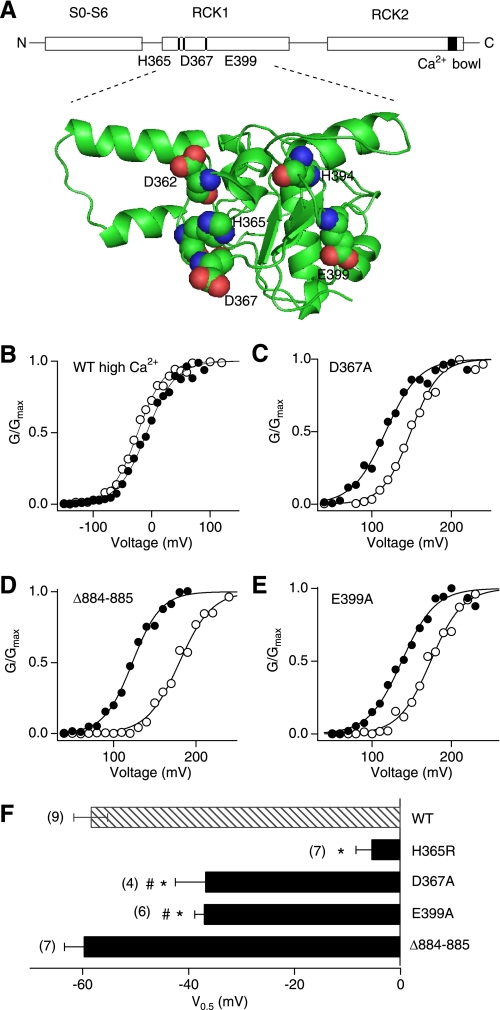
Select Acidic Residues in the RCK1 Domain Implicated in the Ca2+ Sensitivity Are also Important for the Zn2+ Action
His365, required for the Zn2+-dependent activation of the hSlo1 channel (see Fig. 3) also participates in both Ca2+- and H+-dependent activation of the Slo1 channel such that the stimulatory effect of H+ is diminished at higher concentrations of Ca2+ (37, 38). We hypothesized that Ca2+ may also interfere with the Zn2+-dependent activation of the channel. As predicted by this idea, we found that in the presence of 100 μm Ca2+, which is a saturating concentration for the high-affinity Ca2+ sensors of the Slo1 channel (39–42), Zn2+ failed to alter G-V (Fig. 4, B and F), indicative of a functional competition between Zn2+ and Ca2+.
FIGURE 4.
Negatively charged residues in the RCK1 domain contribute to the Zn2+-dependent activation of the Slo1 channel. A, a schematic representation of the potential Zn2+/Ca2+ sites in the wild-type hSlo1 channel (top) and a homology model of the mouse Slo1 (mSlo1) RCK1 domain (bottom) based on the structure of MthK channel (62). The residues required for the effects of Zn2+, Ca2+, and Mg2+ are highlighted. The mSlo1 sequence is identical to that of hSlo1 in the RCK1 domain. The images were prepared with MacPyMOL. B, a representative G-V curve in the wild-type channel in the presence of 200 μm Ca2+ alone (open circles) and of 200 μm Ca2+ and 100 μm Zn2+ together (filled circles). C–E, representative G-V curves in the Ca2+ sensor mutants in the absence (open circles) and presence (filled circles) of 100 μm Zn2+. F, changes in V0.5 caused by 100 μm Zn2+ in the wild-type (WT) and the mutant channels. The mutation Δ884–885 impairs the Ca2+ bowl function (39). *, p < 0.001 compared with the wild-type channel and #, p < 0.01 compared with the H365R channel.
Previous mutagenesis studies suggest the presence of at least three potential divalent cation sensors in each Slo1 subunit (18, 41–43) (Fig. 4A); a high-affinity sensor in the RCK1 domain, a high-affinity Ca2+ bowl sensor, and a low affinity sensor in the RCK1 domain that also mediates Mg2+-dependent activation of the channel (42). The charge-neutralization mutation D367A in the RCK domain is known to disrupt the high-affinity Ca2+-sensing by the RCK1 domain (41). We found that the mutation significantly decreased the shift in V0.5 by 100 μm Zn2+ by ∼35% to −36.9 ± 5.6 mV (p < 0.01 compared with the wild-type channel; Fig. 4, C and F). The function of the high-affinity Ca2+ bowl sensor in the RCK2 domain is disrupted by the deletion mutation Δ884–885 (39). This deletion mutation, however, failed to alter the stimulatory effect of Zn2+ on the channel (Fig. 4, D and F).
The low-affinity divalent cation sensitivity of the Slo1 channel is in part mediated by Glu399 in the RCK1 domain (43). The mutation E399A, which impairs the stimulatory action of mm levels of Mg2+ on the channel (42, 43), noticeably attenuated the Zn2+-dependent shift in V0.5 by ∼35% to −37.2 ± 1.8 mV (p < 0.01 compared with the wild-type channel; Fig. 4, E and F). The shifts in V0.5 by Zn2+ in the D367A and E399A mutants were statistically indistinguishable (Fig. 4F).
Other transition metals, such as Mn2+, also activate the Slo1 channel (30, 42). We found that the effect of Mn2+ was completely disrupted by the mutation E399A but not by the mutation H365A, which eliminates the Zn2+ sensitivity (supplemental Fig. S1).
Coexpression of β1 Subunit Does Not Alter the Effect of Zn2+
In addition to the four pore-forming Slo1 subunits, a native BK channel complex may also include auxiliary β subunits in a tissue-dependent manner (44). Heterologous coexpression of the auxiliary subunit β1, predominantly expressed in the cardiovascular system, dramatically increases the overall Ca2+ sensitivity and slows both the activation and deactivation kinetics of the channel complex (44). The underlying mechanism is postulated to involve an increase in the Ca2+ affinity of the high-affinity Ca2+ sensors in the RCK1 domain and the Ca2+ bowl in the RCK2 domain (45). Because the stimulatory effect of Zn2+ on the Slo1 channel was in part dependent on Asp367, an established component in the high-affinity RCK1 Ca2+ sensor (41), we examined whether coexpression of β1 enhanced the effectiveness of Zn2+. Functional coexpression of β1 was verified by the characteristically slower activation and deactivation kinetics. We found that Zn2+ remained effective in enhancing the Slo1 current. The shift in V0.5 (−56.8 ± 2.4 mV) was indistinguishable from that without coexpression of β1 (Fig. 5; p > 0.5).
FIGURE 5.
Coexpression of β1 does not alter the effectiveness of Zn2+. A, representative hSlo1+β1 currents at 100 mV before (thin trace) and after (thick trace) application of 100 μm Zn2+. The currents were elicited by pulses from 0 to 100 and then to −80 mV. B and C, typical I-V curves and G-V curves of hSlo1+β1 channels in the absence (open circles) and presence (filled circles) of 100 μm Zn2+. D, changes in V0.5 caused by 100 μm Zn2+ in Slo1 channel with or without the β1 subunit.
Extracellular Zn2+ Activates Slo1 BK Channel when Coexpressed with TRPM7
Many membrane transport proteins including ion channels mediate translocation of the extracellular Zn2+ into intracellular space. Extracellular Zn2+ did not affect the Slo1 channel activity; however, it robustly activated the channels when they were coexpressed with TRPM7, a nonselective cation channel permeable to Zn2+ (46, 47). In contrast, extracellular Mg2+ did not alter Slo1 channel open probability (Fig. 6).
DISCUSSION
Zn2+ is well known for its structural role in a large number of metalloproteins, including some voltage-gated K+ channels in which the metal ion mediates tetramerization of the channel proteins (48, 49). As an important intracellular messenger, Zn2+ also modulates multiple signaling pathways, but yet only a small number of its direct effectors have been clearly identified (5, 7). Among ion channels, recent studies show that the TRPA1 (transient receptor potential channel A1) (50, 51) and the ATP-sensitive K+ channel (KATP) (52) are activated by intracellular Zn2+ at nm and μm concentrations, respectively. Our study now adds the Slo1 channel as a new member of the Zn2+ signaling cascades. Heterologously expressed Slo1 BK channels are robustly activated by μm levels of intracellular Zn2+ in cell-free membrane patches, independently of the auxiliary subunit β1. Moreover, mutation of His365 in the RCK1 domain or nearby Asp367 or Glu399 involved in the Ca2+ sensing fully or partially abolished the channel activation by Zn2+.
Our finding that Zn2+ activates heterologously expressed Slo1 channels is in contrast with a previous report that Zn2+ had no effect on rat skeletal muscle BK channels incorporated in planar lipid bilayers (30). The reason for the apparent discrepancy is not clear. It may be noted that the authors also failed to observe any stimulatory effect of Mg2+, an established activator of Slo1 channels (43, 53) in the same study (30).
The Mechanism of Channel Activation by Zn2+
The functional competition between Zn2+ and Ca2+ in activation of the Slo1 channel observed in this study is in line with the mutagenesis result that Asp367, essential for the normal high-affinity Ca2+ sensing of the channel (41), is also required for Zn2+ action. Accordingly, the mechanism of channel activation by Zn2+ may be similar to that by Ca2+. Although physical measurements of Ca2+ binding to the RCK1 sensor and the Ca2+ bowl sensor are preliminary (54–56), conformational changes in an isolated hSlo1 cytoplasmic domain induced by Ca2+ have been detected (54). Structural and functional studies of MthK and Slo1 suggest that Ca2+-dependent activation of the Slo1 channel may be accompanied by an expansion of the cytoplasmic domain termed a “gating ring” (22), the mechanical energy of which is further coupled to the channel pore (19, 57). Like Ca2+, Zn2+ may induce a similar expansion of the gating ring to promote activation of the channel. However, some differences between the effects of Ca2+ and Zn2+ exist. The maximal shift in V0.5 by Zn2+, about −75 mV, is clearly smaller than that by Ca2+, which can produce a shift of −200 mV at 300 μm (42). One readily discernible reason for the difference is that the Ca2+ action is supported by both the sensor in the RCK1 domain and the Ca2+ bowl sensor in the RCK2 domain (39, 41, 54, 58). Even in the absence of the Ca2+ bowl sensor, 300 μm Ca2+ can produce a −125 mV shift (41), still greater than that by Zn2+. The maximal V0.5 shift by Zn2+ is similar to that caused by H+, which also works via the RCK1 sensor and functionally competes with Ca2+ (37). The smaller shift by H+ is attributed to weaker allosteric coupling between the gate of the channel and the RCK1 sensor when H+ is bound as compared with that with Ca2+ bound (38). Thus, the coupling strength in the presence of Zn2+ may be similarly lower than that with Ca2+. Another difference between the effects of Ca2+ and Zn2+ relates to Glu399 in the RCK1 domain, a critical component in the low-affinity divalent ion sensing of the channel, and its neutralization impairs the channel activation by mm levels of Mg2+ (43). Whereas the stimulatory effect of μm levels of Ca2+ does not depend on Glu399, the effect of Zn2+ is diminished when Glu399 is neutralized (Fig. 4). The action of Zn2+ is thus influenced by the residues involved in both the high-affinity and low affinity divalent cation sensing mechanism (19). The biophysical mechanism of the channel activation by Zn2+ may be similar to that by Ca2+ because, unlike effect of Mg2+ (59), the Zn2+ action remains effective even at negative voltages where the voltage sensors of the channel are not activated. Finally, coexpression with β1 enhances the shift in V0.5 by Ca2+ but does not alter that by Zn2+ (Fig. 5). A similar β1-indepenent effect is observed with intracellular H+ (37, 38) further supporting the idea that Zn2+ and H+ may share a similar mechanism in Slo1 channel activation.
Zinc Coordination by Slo1
In many metalloproteins that contain zinc as a stable cofactor, the metal is coordinated by a water molecule and three to four ligands provided by the amino acid residues, typically the side chains of His, Glu, Asp, and Cys (4). Some proteins coordinate zinc using His, Asp, and Glu (4). In Slo1, at least His365, Asp367, and Glu399 contribute to the stimulatory effect of Zn2+ and His365 is required. The lack of a high-resolution atomic structure of the channel, however, precludes a detailed inference on the zinc coordination geometry. Furthermore, unlike most other zinc-containing proteins, binding of Zn2+ to the channel is rapid and readily reversible, and it is not clear how applicable the structural information obtained from the metalloproteins that contain zinc as a stable cofactor may be to the Slo1 protein. Many intracellular EF-hand Ca2+-binding proteins also reversibly bind to Zn2+ at concentrations similar to those used to activate Slo1 BK channels (28). Structural studies suggest that Zn2+ is often located in close proximity to Ca2+ sites and that the two ions reciprocally modulate binding of the other (60, 61), in agreement with our finding that Ca2+ and Zn2+ competitively activate Slo1 BK channel. The RCK1 domain, which contains the His residue essential for the Zn2+ action, was once postulated to contain an EF-hand-like domain (55). However, subsequent structural studies on the prokaryotic channel MthK, which shares a high level of sequence similarity in this area with the Slo1 BK channel, did not support this idea (22, 23). The homology model of Slo1 (Fig. 4A) (62) based on the MthK structure clearly shows that Asp367 and Glu399, are located in the vicinity of His365, forming a potential ligand binding pocket that accommodates a Ca2+, H+, or carbon monoxide (37, 63). The requirement for His and the contributions from Asp and Glu in Zn2+ activation of the Slo1 channel are in line with the zinc coordination schemes found in metalloproteins such as an Escherichia coli rhamnose isomerase (4, 64). We therefore suggest that His365, Asp367, and Glu399 in the RCK1 sensor coordinate Zn2+, and the conformational change of the sensor promotes opening of the gate. In TRPA1 channels, which are also activated by intracellular Zn2+, His and Cys residues located some distance away in the primary sequence appear to play a critical role in the Zn2+ sensitivity (51).
Physiological and Pathophysiological Implications
Our study demonstrated that human Slo1 BK channels were activated by high nm to μm of intracellular Zn2+. Similar concentrations were also used in Zn2+ modulation of other intracellular proteins such as mitochondrial enzymes (65–67) and ion channels (52). For instance, the EC50 for activation of recombinant KATP channels, sulfonylurea receptor (SUR)1/Kir6.2 and SUR2A/Kir6.2 are 1.8 and 60 μm, respectively (52). Such [Zn2+]i may not be observed physiologically in the bulk intracellular compartment. However, local [Zn2+]i may reach higher levels near intracellular Zn2+ stores or Zn2+ permeable channels and it plays important roles in normal neuronal transmission and immune response (5, 7, 68). Interestingly, some Zn2+-permeable ion channels may physically colocalize with Slo1 BK channels, potentially exposing the latter to a locally high level of Zn2+ (69). Although quantitative studies of such local Zn2+ domains are unavailable, functional analyses of local Ca2+ domains suggest that the [Ca2+]i near Slo1 BK channels can be a few orders of magnitude greater than the mean bulk concentration (9, 69). Thus it is plausible that the local [Zn2+]i increases transiently to a μm level to activate Slo1 BK channels. Our results (Fig. 6) show that such an increase in [Zn2+]i could occur through an influx of Zn2+ from the extracellular compartments mediated by Zn2+-permeable TRPM7 channels (46, 47, 70). The extracellular concentration of Zn2+ in confined compartments such as synaptic clefts may reach several hundred μm (5). Because both TRPM7 and Slo1 BK channels are widely expressed, TRPM7 channels could inject enough Zn2+ to activate Slo1 BK channels. Along with TRPA1 (50, 51) and KATP channels (52), Slo1 BK channels now represent a family of intracellular Zn2+-activated ion channels that could play physiological roles. Increases in [Zn2+]i may be even greater under some pathological conditions such as brain ischemia/reperfusion and epilepsy (5, 12). For example, in the experimental seizures induced by kainic acid, [Zn2+]i may increase to hundreds of nm and several μm in hippocampal and cortical neurons, respectively (71, 72). A recent study suggests that the actual increase in [Zn2+]i during brain ischemia and reperfusion may be significantly more than previously estimated because the divalent cation overload traditionally thought to be from Ca2+, is actually from Zn2+ (10). This interpretation and the observation that [Ca2+]i may reach 30 μm during ischemia (12) together indicate the actual [Zn2+]i may be in the μm range, sufficient to activate Slo1 BK channels, suggesting that Zn2+-dependent activation of Slo1 BK channels may play a role during cerebral ischemia. The finding that pharmacological activation of BK channels is cell protective during ischemic stroke (24, 25) indicates that the Zn2+-dependent activation of the channel probably represents a compensatory and adaptive response.
In summary, this study demonstrates that hSlo1 BK channels are intracellular Zn2+-activated channels and represent a new effector of intracellular Zn2+ signaling. The stimulatory effect of Zn2+ requires His, Asp and Glu in the RCK1 domain. As a member of the Zn2+-signaling cascade, Slo1 BK channels may participate in many phenomena mediated by intracellular Zn2+, particularly in some diseases associated with a significant increase in [Zn2+]i.
Supplementary Material
Acknowledgments
We thank Mark F. Reynolds (Saint Joseph's University, Philadelphia, PA) and Frank T. Horrigan (Baylor College of Medicine, Houston, TX) for discussion and Terry Dean and Yuan Wen for reading of the manuscript.
This work was supported in part by National Institutes of Health Grants GM057654 (to T. H.), MH084691 (to M. L.), and GM078579 (to M. L. and T. H.). This work was also supported by Deutsche Forschungsgemeinschaft (HE2993/8).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- hSlo1
- human Slo1
- G-V
- conductance-voltage
- I-V
- current-voltage
- TPEN
- N,N,N′N′-tetrakis(2-pyridylmethyl)ethylenediamine
- MTSEA
- 2-aminoethyl methanethiosulfonate hydrobromide
- NMDG
- N-methyl-d-glucamine.
REFERENCES
- 1.Sandstead H. H. (2000) J. Nutr. 130, 347S–349S [DOI] [PubMed] [Google Scholar]
- 2.Vallee B. L., Falchuk K. H. (1993) Physiol. Rev. 73, 79–118 [DOI] [PubMed] [Google Scholar]
- 3.Rulísek L., Vondrásek J. (1998) J. Inorg. Biochem. 71, 115–127 [DOI] [PubMed] [Google Scholar]
- 4.Auld D. S. (2001) Biometals 14, 271–313 [DOI] [PubMed] [Google Scholar]
- 5.Frederickson C. J., Koh J. Y., Bush A. I. (2005) Nat. Rev. Neurosci. 6, 449–462 [DOI] [PubMed] [Google Scholar]
- 6.Sensi S. L., Paoletti P., Bush A. I., Sekler I. (2009) Nat. Rev. Neurosci. 10, 780–791 [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki S., Sakata-Sogawa K., Hasegawa A., Suzuki T., Kabu K., Sato E., Kurosaki T., Yamashita S., Tokunaga M., Nishida K., Hirano T. (2007) J. Cell Biol. 177, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Hough C. J., Frederickson C. J., Sarvey J. M. (2001) J. Neurosci. 21, 8015–8025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakler B., Adelman J. P. (2008) Neuron 59, 873–881 [DOI] [PubMed] [Google Scholar]
- 10.Stork C. J., Li Y. V. (2006) J. Neurosci. 26, 10430–10437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi D. W., Koh J. Y. (1998) Annu. Rev. Neurosci. 21, 347–375 [DOI] [PubMed] [Google Scholar]
- 12.Lipton P. (1999) Physiol. Rev. 79, 1431–1568 [DOI] [PubMed] [Google Scholar]
- 13.Karagulova G., Yue Y., Moreyra A., Boutjdir M., Korichneva I. (2007) J. Pharmacol. Exp. Ther. 321, 517–525 [DOI] [PubMed] [Google Scholar]
- 14.Chanoit G., Lee S., Xi J., Zhu M., McIntosh R. A., Mueller R. A., Norfleet E. A., Xu Z. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H1227–H1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S., Chanoit G., McIntosh R. A., Zvara D. A., Xu Z. (2009) Am. J. Physiol. Heart Circ. Physiol. 297, H569–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell S. R., Hall D., Aiuto L., Wapnir R. A., Teichberg S., Tortolani A. J. (1994) Am. J. Physiol. 266, H2497–2507 [DOI] [PubMed] [Google Scholar]
- 17.Salkoff L., Butler A., Ferreira G., Santi C., Wei A. (2006) Nat. Rev. Neurosci. 7, 921–931 [DOI] [PubMed] [Google Scholar]
- 18.Magleby K. L. (2003) J. Gen. Physiol. 121, 81–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J., Yang H., Lee U. S. (2009) Cell Mol. Life Sci. 66, 852–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adelman J. P., Shen K. Z., Kavanaugh M. P., Warren R. A., Wu Y. N., Lagrutta A., Bond C. T., North R. A. (1992) Neuron 9, 209–216 [DOI] [PubMed] [Google Scholar]
- 21.Meera P., Wallner M., Song M., Toro L. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 14066–14071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y., Lee A., Chen J., Cadene M., Chait B. T., MacKinnon R. (2002) Nature 417, 515–522 [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y., Pico A., Cadene M., Chait B. T., MacKinnon R. (2001) Neuron 29, 593–601 [DOI] [PubMed] [Google Scholar]
- 24.Gribkoff V. K., Starrett J. E., Jr., Dworetzky S. I., Hewawasam P., Boissard C. G., Cook D. A., Frantz S. W., Heman K., Hibbard J. R., Huston K., Johnson G., Krishnan B. S., Kinney G. G., Lombardo L. A., Meanwell N. A., Molinoff P. B., Myers R. A., Moon S. L., Ortiz A., Pajor L., Pieschl R. L., Post-Munson D. J., Signor L. J., Srinivas N., Taber M. T., Thalody G., Trojnacki J. T., Wiener H., Yeleswaram K., Yeola S. W. (2001) Nat. Med. 7, 471–477 [DOI] [PubMed] [Google Scholar]
- 25.Xu W., Liu Y., Wang S., McDonald T., Van Eyk J. E., Sidor A., O'Rourke B. (2002) Science 298, 1029–1033 [DOI] [PubMed] [Google Scholar]
- 26.Rawlings N. D., Barrett A. J. (1995) Methods Enzymol. 248, 183–228 [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R., Dock-Bregeon A. C., Romby P., Caillet J., Springer M., Rees B., Ehresmann C., Ehresmann B., Moras D. (1999) Cell 97, 371–381 [DOI] [PubMed] [Google Scholar]
- 28.Korndörfer I. P., Brueckner F., Skerra A. (2007) J. Mol. Biol. 370, 887–898 [DOI] [PubMed] [Google Scholar]
- 29.Tang X. D., Xu R., Reynolds M. F., Garcia M. L., Heinemann S. H., Hoshi T. (2003) Nature 425, 531–535 [DOI] [PubMed] [Google Scholar]
- 30.Oberhauser A., Alvarez O., Latorre R. (1988) J. Gen. Physiol. 92, 67–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Z., Wong K. Y., Horrigan F. T. (2008) J. Gen. Physiol. 131, 483–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volbeda A., Fontecilla-Camps J. C., Frey M. (1996) Curr. Opin. Struct. Biol. 6, 804–812 [DOI] [PubMed] [Google Scholar]
- 33.McGwire B. S., Chang K. P. (1996) J. Biol. Chem. 271, 7903–7909 [DOI] [PubMed] [Google Scholar]
- 34.Miles E. W. (1977) Methods Enzymol. 47, 431–442 [DOI] [PubMed] [Google Scholar]
- 35.Karlin A., Akabas M. H. (1998) Methods Enzymol. 293, 123–145 [DOI] [PubMed] [Google Scholar]
- 36.Pantoliano M. W., Valentine J. S., Burger A. R., Lippard S. J. (1982) J. Inorg. Biochem. 17, 325–341 [DOI] [PubMed] [Google Scholar]
- 37.Hou S., Xu R., Heinemann S. H., Hoshi T. (2008) Nat. Struct. Mol. Biol. 15, 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou S., Horrigan F. T., Xu R., Heinemann S. H., Hoshi T. (2009) Channels 3, 249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber M., Salkoff L. (1997) Biophys. J. 73, 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao L., Rapin A. M., Holmstrand E. C., Cox D. H. (2002) J. Gen. Physiol. 120, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia X. M., Zeng X., Lingle C. J. (2002) Nature 418, 880–884 [DOI] [PubMed] [Google Scholar]
- 42.Zeng X. H., Xia X. M., Lingle C. J. (2005) J. Gen. Physiol. 125, 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J., Krishnamoorthy G., Yang Y., Hu L., Chaturvedi N., Harilal D., Qin J., Cui J. (2002) Nature 418, 876–880 [DOI] [PubMed] [Google Scholar]
- 44.Torres Y. P., Morera F. J., Carvacho I., Latorre R. (2007) J. Biol. Chem. 282, 24485–24489 [DOI] [PubMed] [Google Scholar]
- 45.Sweet T. B., Cox D. H. (2009) J. Gen. Physiol. 133, 139–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Runnels L. W., Yue L., Clapham D. E. (2001) Science 291, 1043–1047 [DOI] [PubMed] [Google Scholar]
- 47.Monteilh-Zoller M. K., Hermosura M. C., Nadler M. J., Scharenberg A. M., Penner R., Fleig A. (2003) J. Gen. Physiol. 121, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bixby K. A., Nanao M. H., Shen N. V., Kreusch A., Bellamy H., Pfaffinger P. J., Choe S. (1999) Nat. Struct. Biol. 6, 38–43 [DOI] [PubMed] [Google Scholar]
- 49.Strang C., Kunjilwar K., DeRubeis D., Peterson D., Pfaffinger P. J. (2003) J. Biol. Chem. 278, 31361–31371 [DOI] [PubMed] [Google Scholar]
- 50.Andersson D. A., Gentry C., Moss S., Bevan S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8374–8379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu H., Bandell M., Petrus M. J., Zhu M. X., Patapoutian A. (2009) Nat. Chem. Biol. 5, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prost A. L., Bloc A., Hussy N., Derand R., Vivaudou M. (2004) J. Physiol. 559, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi J., Cui J. (2001) J. Gen. Physiol. 118, 589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yusifov T., Savalli N., Gandhi C. S., Ottolia M., Olcese R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braun A. P., Sy L. (2001) J. Physiol. 533, 681–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bao L., Kaldany C., Holmstrand E. C., Cox D. H. (2004) J. Gen. Physiol. 123, 475–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu X., Qian X., Magleby K. L. (2004) Neuron 42, 745–756 [DOI] [PubMed] [Google Scholar]
- 58.Qian X., Niu X., Magleby K. L. (2006) J. Gen. Physiol. 128, 389–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horrigan F. T., Cui J., Aldrich R. W. (1999) J. Gen. Physiol. 114, 277–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostendorp T., Heizmann C. W., Kroneck P. M., Fritz G. (2005) Acta Crystallogr. Sect. F. Struct. Biol. Cryst. Commun. 61, 673–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch M., Bhattacharya S., Kehl T., Gimona M., Vasák M., Chazin W., Heizmann C. W., Kroneck P. M., Fritz G. (2007) Biochim. Biophys. Acta 1773, 457–470 [DOI] [PubMed] [Google Scholar]
- 62.Latorre R., Brauchi S. (2006) Biol. Res. 39, 385–401 [DOI] [PubMed] [Google Scholar]
- 63.Hou S., Xu R., Heinemann S. H., Hoshi T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 4039–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korndörfer I. P., Fessner W. D., Matthews B. W. (2000) J. Mol. Biol. 300, 917–933 [DOI] [PubMed] [Google Scholar]
- 65.Mills D. A., Schmidt B., Hiser C., Westley E., Ferguson-Miller S. (2002) J. Biol. Chem. 277, 14894–14901 [DOI] [PubMed] [Google Scholar]
- 66.Link T. A., von Jagow G. (1995) J. Biol. Chem. 270, 25001–25006 [DOI] [PubMed] [Google Scholar]
- 67.Sharpley M. S., Hirst J. (2006) J. Biol. Chem. 281, 34803–34809 [DOI] [PubMed] [Google Scholar]
- 68.Permyakov E. (2009) Metalloproteomics, 1st Ed., pp. 283–338, John Wiley & Sons, Inc., Hoboken, NJ [Google Scholar]
- 69.Berkefeld H., Sailer C. A., Bildl W., Rohde V., Thumfart J. O., Eble S., Klugbauer N., Reisinger E., Bischofberger J., Oliver D., Knaus H. G., Schulte U., Fakler B. (2006) Science 314, 615–620 [DOI] [PubMed] [Google Scholar]
- 70.Fonfria E., Murdock P. R., Cusdin F. S., Benham C. D., Kelsell R. E., McNulty S. (2006) J. Recept. Signal Transduct. Res. 26, 159–178 [DOI] [PubMed] [Google Scholar]
- 71.Côté A., Chiasson M., Peralta M. R., 3rd, Lafortune K., Pellegrini L., Tóth K. (2005) J. Physiol. 566, 821–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sensi S. L., Yin H. Z., Carriedo S. G., Rao S. S., Weiss J. H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2414–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.